Processed Scutellaria baicalensis Georgi Extract Alleviates LPS-Induced Inflammatory and Oxidative Stress through a Crosstalk between NF-κB and KEAP1/NRF2 Signaling in Macrophage Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of SGE and PSGE
2.2. Liquid Chromatography–Mass Spectrometry (LC–MS) Analysis
2.3. Materials and Chemicals
2.4. Cell Culture
2.5. Cell Viability Assay
2.6. Nitric Oxide (NO) Assay
2.7. IL-6, TNF-α and PGE2 Assay
2.8. Western Blotting
2.9. Cellular Reactive Oxygen Species (ROS) Assay
2.10. Real-Time qPCR (RT-qPCR) Analysis
2.11. Statistical Analysis
3. Results
3.1. LC-MS Analysis
3.2. PSGE Modulated the Release of NO and PGE2
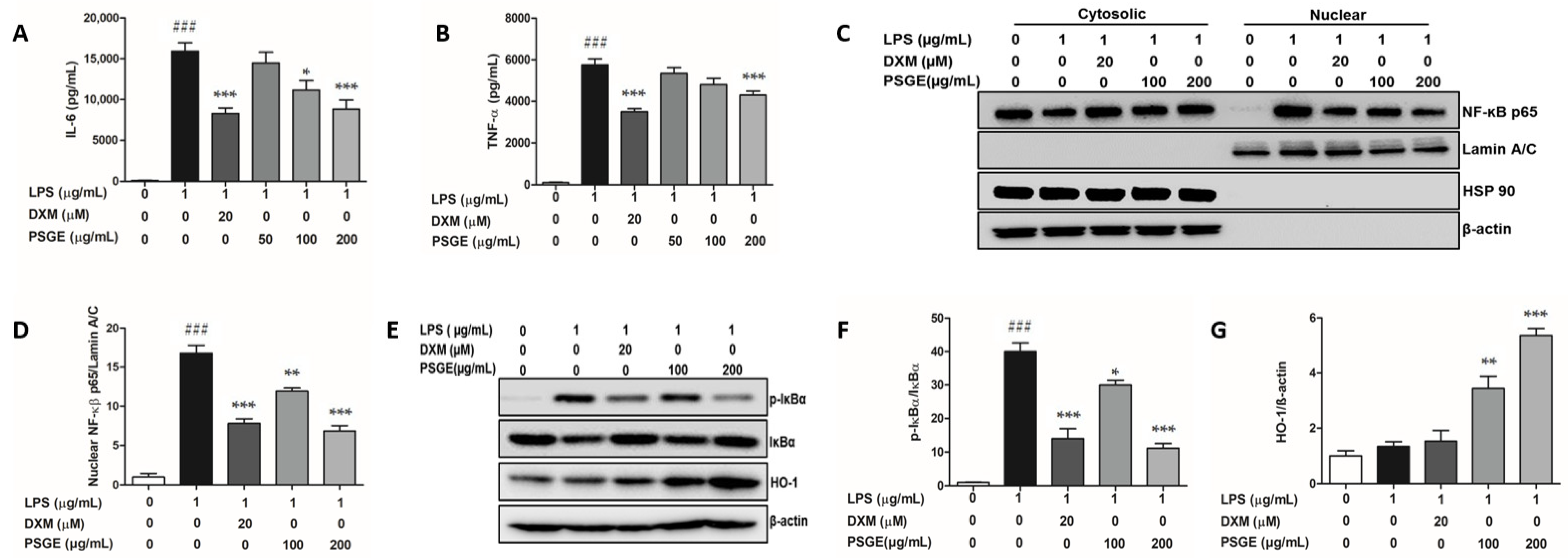
3.3. PSGE Regulated the NF-κB-Mediated Inflammatory Responses
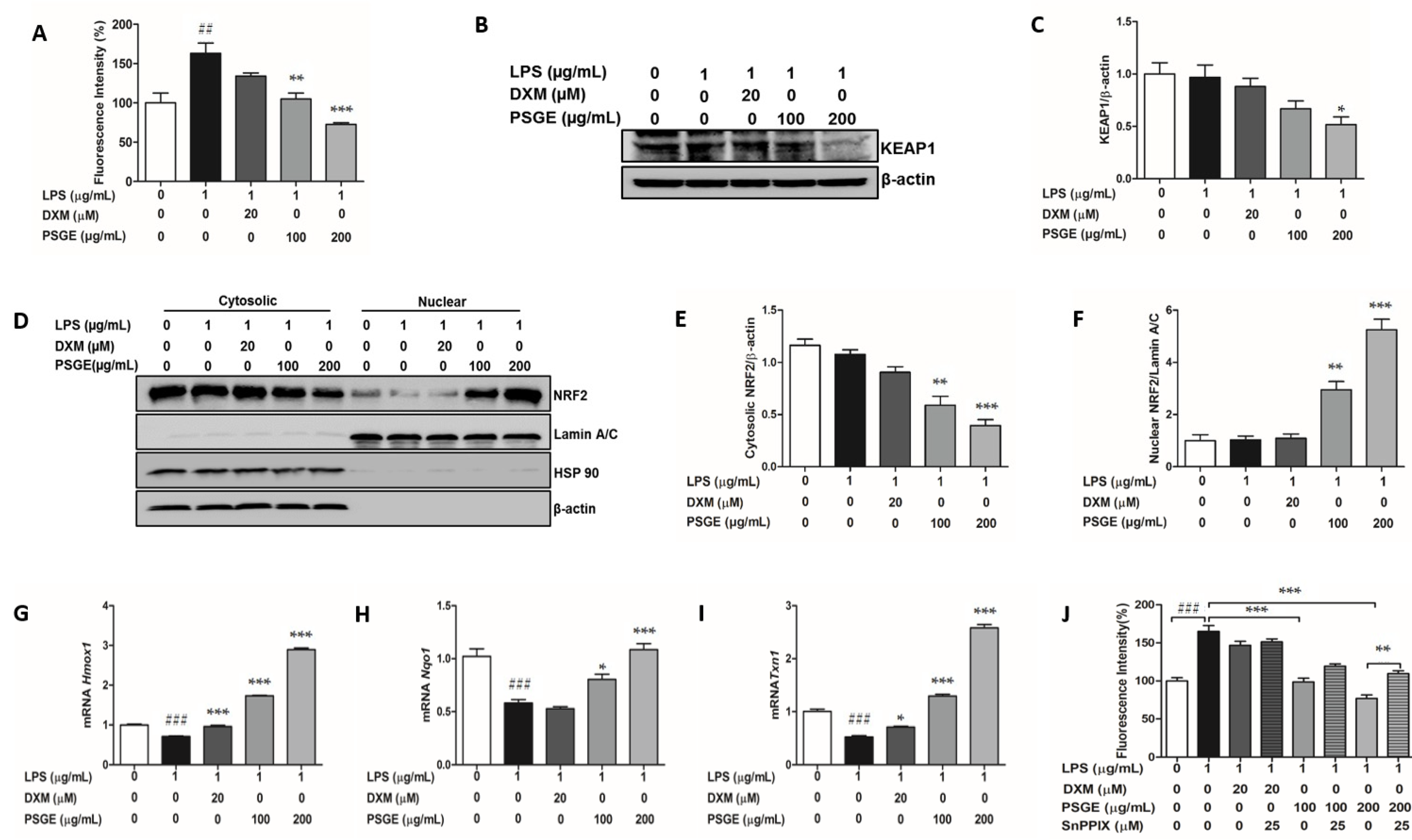
3.4. PSGE Activated the NRF2-Mediated Antioxidant Enzymes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jabbour, H.N.; Sales, K.J.; Catalano, R.D.; Norman, J.E. Inflammatory pathways in female reproductive health and disease. Reproduction 2009, 138, 903–919. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, A.U. An overview of inflammation: Mechanism and consequences. Front. Biol. 2011, 6, 274. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free. Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [Green Version]
- Lima, A.; Alvim, H. Review on non-steroid antiinflammatory: Acetylsalicylic acid. Rev. Inic. Ciente. Ext. 2018, 1, 169–174. [Google Scholar]
- Meek, I.L.; Van de Laar, M.A.; E Vonkeman, H. Non-steroidal anti-inflammatory drugs: An overview of cardiovascular risks. Pharmaceuticals 2010, 3, 2146–2162. [Google Scholar] [CrossRef] [Green Version]
- Moghadam-Kia, S.; Werth, V.P. Prevention and treatment of systemic glucocorticoid side effects. Int. J. Dermatol. 2010, 49, 239–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wongrakpanich, S.; Wongrakpanich, A.; Melhado, K.; Rangaswami, J. A comprehensive review of non-steroidal anti-inflammatory drug use in the elderly. Aging Dis. 2018, 9, 143–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, H. Medicinal plants in light of history: Recognized therapeutic modality. J. Evid. Based Complement. Altern. Med. 2014, 19, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Koparde, A.; Doijad, R.; Magdum, C. Natural products in drug discovery. In Pharmacognosy-Medicinal Plants; IntechOpen: London, UK, 2019. [Google Scholar]
- Zhao, T.; Tang, H.; Xie, L.; Zheng, Y.; Ma, Z.; Sun, Q.; Li, X. Scutellaria baicalensis georgi. (lamiaceae): A review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. J. Pharm. Pharmacol. 2019, 71, 1353–1369. [Google Scholar] [CrossRef] [Green Version]
- Shao, Z.H.; Li, C.Q.; Vanden Hoek, T.L.; Becker, L.B.; Schumacker, P.T.; Wu, J.A.; Attele, A.S.; Yuan, C.S. Extract from scutellaria baicalensis georgi attenuates oxidant stress in cardiomyocytes. J. Mol. Cell. Cardiol. 1999, 31, 1885–1895. [Google Scholar] [CrossRef] [PubMed]
- Ikemoto, S.; Sugimura, K.; Yoshida, N.; Yasumoto, R.; Wada, S.; Yamamoto, K.; Kishimoto, T. Antitumor effects of scutellariae radix and its components baicalein, baicalin, and wogonin on bladder cancer cell lines. Urology 2000, 55, 951–955. [Google Scholar] [CrossRef]
- Wang, H.H.; Liao, J.F.; Chen, C.F. Anticonvulsant effect of water extract of scutellariae radix in mice. J. Ethnopharmacol. 2000, 73, 185–190. [Google Scholar] [CrossRef]
- Chu, M.; Chu, Z.Y.; Wang, D.D. The extract of compound radix scutellariae on mrna replication and ifn expression of influenza virus in mice. Zhong Yao Cai = Zhongyaocai = J. Chin. Med. Mater. 2007, 30, 63–65. [Google Scholar]
- Yoon, S.B.; Lee, Y.J.; Park, S.K.; Kim, H.C.; Bae, H.; Kim, H.M.; Ko, S.G.; Choi, H.Y.; Oh, M.S.; Park, W. Anti-inflammatory effects of scutellaria baicalensis water extract on lps-activated raw 264.7 macrophages. J. Ethnopharmacol. 2009, 125, 286–290. [Google Scholar] [CrossRef]
- Li, C.; Lin, G.; Zuo, Z. Pharmacological effects and pharmacokinetics properties of radix scutellariae and its bioactive flavones. Biopharm. Drug Dispos. 2011, 32, 427–445. [Google Scholar] [CrossRef]
- Commission, C.P. Chinese Pharmacopoeia; China Medical Science Press: Beijing, China, 2015; Volume 1, pp. 191–193. [Google Scholar]
- Sheridan, H.; Kopp, B.; Krenn, L.; Guo, D.; Sendker, J. Traditional chinese herbal medicine preparation: Invoking the butterfly effect. Science 2015, 350, S64–S66. [Google Scholar]
- Choi, Y.A.; Kang, O.H.; Park, H.J.; Tae, J.; Kim, D.K.; Kang, C.S.; Choi, S.C.; Yun, K.J.; Choi, S.J.; Nah, Y.H.; et al. Effect of processed scutellaria baicalensis on dextran sulfate sodium-induced colitis in mice. Int. J. Mol. Med. 2005, 16, 667–672. [Google Scholar]
- Shin, Y.O.; Park, C.H.; Lee, G.H.; Yokozawa, T.; Roh, S.S.; Rhee, M.H. Heat-processed scutellariae radix enhances anti-inflammatory effect against lipopolysaccharide-induced acute lung injury in mice via nf- kappa b signaling. Evid. Based Complement. Altern. Med. eCAM 2015, 2015, 456846. [Google Scholar] [CrossRef] [Green Version]
- Shin, S.; Shin, Y.; Lee, J.; Lee, A.; Kim, M.; Park, C.; Seo, B.; Roh, S. Ethanol-heated processed scutellariae radix improve inflammatory response through an inhibitory effect against oxidative stress in mice with the lipopolysaccharide-induced intestine injury of mice. Korea J. Herbol. 2015, 30, 81–88. [Google Scholar] [CrossRef]
- Yamamoto, M.; Kensler, T.W.; Motohashi, H. The keap1-nrf2 system: A thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol. Rev. 2018, 98, 1169–1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative stress and inflammation: What polyphenols can do for us? Oxidative Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phan, M.A.T.; Paterson, J.; Bucknall, M.; Arcot, J. Interactions between phytochemicals from fruits and vegetables: Effects on bioactivities and bioavailability. Crit. Rev. Food Sci. Nutr. 2018, 58, 1310–1329. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.L.; Wang, S.; Kuang, Y.; Hu, Z.M.; Qiao, X.; Ye, M. A comprehensive review on phytochemistry, pharmacology, and flavonoid biosynthesis of scutellaria baicalensis. Pharm. Biol. 2018, 56, 465–484. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Wang, S.; Lu, J.; Jing, Y.; Li, M.; Cao, J.; Bian, B.; Hu, C. Seeing the unseen of chinese herbal medicine processing (paozhi): Advances in new perspectives. Chin. Med. 2018, 13, 4. [Google Scholar] [CrossRef]
- Cai, B.; Qin, K.; Wu, H.; Cai, H.; Lu, T.; Zhang, X. Chemical mechanism during chinese medicine processing. Prog. Chem. 2012, 24, 637. [Google Scholar] [CrossRef]
- Ham, I.H.; Maeng, W.M.; Yang, G.S.; Kim, D.H.; Kim, D.H.; Cho, J.H.; Choi, H.Y. Study on the variation of components from scutellariae radix by processing and storage condition. Korea J. Herbol. 2007, 22, 189–199. [Google Scholar]
- Levvy, G.A. Baicalinase, a plant beta-glucuronidase. Biochem. J. 1954, 58, 462–469. [Google Scholar] [CrossRef]
- Ku, S.; Zheng, H.; Park, M.S.; Ji, G.E. Optimization of β-glucuronidase activity from lactobacillus delbrueckii rh2 and and its use for biotransformation of baicalin and wogonoside. J. Korean Soc. Appl. Biol. Chem. 2011, 54, 275–280. [Google Scholar] [CrossRef]
- Park, C.H.; Shin, M.-R.; An, B.K.; Joh, H.W.; Lee, J.C.; Roh, S.-S.; Yokozawa, T. Heat-processed scutellariae radix protects hepatic inflammation through the amelioration of oxidative stress in lipopolysaccharide-induced mice. Am. J. Chin. Med. 2017, 45, 1233–1252. [Google Scholar] [CrossRef]
- Korhonen, R.; Lahti, A.; Kankaanranta, H.; Moilanen, E. Nitric oxide production and signaling in inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 471–479. [Google Scholar] [CrossRef]
- Finetti, F.; Travelli, C.; Ercoli, J.; Colombo, G.; Buoso, E.; Trabalzini, L. Prostaglandin e2 and cancer: Insight into tumor progression and immunity. Biology 2020, 9, 424. [Google Scholar] [CrossRef]
- Kim, E.H.; Shim, B.; Kang, S.; Jeong, G.; Lee, J.S.; Yu, Y.B.; Chun, M. Anti-inflammatory effects of scutellaria baicalensis extract via suppression of immune modulators and map kinase signaling molecules. J. Ethnopharmacol. 2009, 126, 320–331. [Google Scholar] [CrossRef]
- Jeong, K.; Shin, Y.C.; Park, S.; Park, J.S.; Kim, N.; Um, J.Y.; Go, H.; Sun, S.; Lee, S.; Park, W.; et al. Ethanol extract of scutellaria baicalensis georgi prevents oxidative damage and neuroinflammation and memorial impairments in artificial senescense mice. J. Biomed. Sci. 2011, 18, 14. [Google Scholar] [CrossRef] [Green Version]
- Hazra, S.; Batra, R.K.; Tai, H.H.; Sharma, S.; Cui, X.; Dubinett, S.M. Pioglitazone and rosiglitazone decrease prostaglandin e2 in non-small-cell lung cancer cells by up-regulating 15-hydroxyprostaglandin dehydrogenase. Mol. Pharmacol. 2007, 71, 1715–1720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oeckinghaus, A.; Ghosh, S. The nf-kappab family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef] [PubMed]
- Juvekar, A.; Manna, S.; Ramaswami, S.; Chang, T.P.; Vu, H.Y.; Ghosh, C.C.; Celiker, M.Y.; Vancurova, I. Bortezomib induces nuclear translocation of ikappabalpha resulting in gene-specific suppression of nf-kappab--dependent transcription and induction of apoptosis in ctcl. Mol. Cancer Res. MCR 2011, 9, 183–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, G.H.; Qu, J.; Shen, X. Nf-kappab/p65 antagonizes nrf2-are pathway by depriving cbp from nrf2 and facilitating recruitment of hdac3 to mafk. Biochim. Biophys. Acta 2008, 1783, 713–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.S.; Kim, K.S.; Ko, W.; Li, B.; Keo, S.; Jeong, G.S.; Oh, H.; Kim, Y.C. The neoflavonoid latifolin isolated from meoh extract of dalbergia odorifera attenuates inflammatory responses by inhibiting nf-kappab activation via nrf2-mediated heme oxygenase-1 expression. Phytother. Res. PTR 2014, 28, 1216–1223. [Google Scholar] [CrossRef]
- Jiang, L.; Jiang, Q.; Yang, S.; Huang, S.; Han, X.; Duan, J.; Pan, S.; Zhao, M.; Guo, S. Gyy4137 attenuates lps-induced acute lung injury via heme oxygenase-1 modulation. Pulm. Pharmacol. Ther. 2019, 54, 77–86. [Google Scholar] [CrossRef]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An overview of nrf2 signaling pathway and its role in inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef] [PubMed]
- Campbell, N.K.; Fitzgerald, H.K.; Dunne, A. Regulation of inflammation by the antioxidant haem oxygenase 1. Nat. Rev. Immunol. 2021, 21, 411–425. [Google Scholar]
- Lee, Y.M.; Cheng, P.Y.; Chim, L.S.; Kung, C.W.; Ka, S.M.; Chung, M.T.; Sheu, J.R. Baicalein, an active component of scutellaria baicalensis georgi, improves cardiac contractile function in endotoxaemic rats via induction of heme oxygenase-1 and suppression of inflammatory responses. J. Ethnopharmacol. 2011, 135, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.S.; Kim, O.S.; Kim, B.Y.; Jeong, S.J. Apigetrin from scutellaria baicalensis georgi inhibits neuroinflammation in bv-2 microglia and exerts neuroprotective effect in ht22 hippocampal cells. J. Med. Food 2016, 19, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Hu, L.; Li, W. Baicalin ameliorates lipopolysaccharide-induced acute lung injury in mice by suppressing oxidative stress and inflammation via the activation of the nrf2-mediated ho-1 signaling pathway. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2019, 392, 1421–1433. [Google Scholar] [CrossRef]


| Parameters | Conditions | |||
|---|---|---|---|---|
| Analytical column | TSK-gell ODS-80Ts (4.6 mm × 150 mm) | |||
| Column temperature | 35 ℃ | |||
| Injection volume | 10 µL | |||
| UV Wavelength | 277 nm | |||
| Mobile phase | Final time (min) | Water in 0.1% formic acid | Acetonitrile | |
| 0.0 | 80 | 20 | ||
| 30.0 | 20 | 80 | ||
| 30.1 | 0 | 100 | ||
| 40.0 | 0 | 100 | ||
| 40.1 | 80 | 20 | ||
| Flow rate | 1.0 mL/min | |||
| Voltages | Capillary | 3.00 kV | ||
| Cone | 40 V | |||
| Extractor | 2 V | |||
| RF Lens | 0.2 V | |||
| Source Temperature | 120 ℃ | |||
| Desolvation Temperature | 400 ℃ | |||
| Gas flow | Desolvation | 600 L/h | ||
| Cone | 30 L/h | |||
| Compounds | Linear Range (µg/mL) | Regression Equation | R2 | LOD (µg/mL) | LOQ (µg/mL) |
|---|---|---|---|---|---|
| baicalin | 62.5–1000 | y = 428.15x + 4238.5 | 1 | 13.09 | 39.67 |
| baicalein | 12.5–200 | y = 529.06x − 8488.3 | 0.9883 | 47.94 | 145.27 |
| wogonin | 6.25–100 | y = 983.39x − 328.62 | 0.9999 | 1.66 | 5.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, A.; Kim, E.H.; Lee, J.-H.; Leem, K.-H.; Seong, S.; Kim, W. Processed Scutellaria baicalensis Georgi Extract Alleviates LPS-Induced Inflammatory and Oxidative Stress through a Crosstalk between NF-κB and KEAP1/NRF2 Signaling in Macrophage Cells. Appl. Sci. 2021, 11, 6055. https://doi.org/10.3390/app11136055
Ali A, Kim EH, Lee J-H, Leem K-H, Seong S, Kim W. Processed Scutellaria baicalensis Georgi Extract Alleviates LPS-Induced Inflammatory and Oxidative Stress through a Crosstalk between NF-κB and KEAP1/NRF2 Signaling in Macrophage Cells. Applied Sciences. 2021; 11(13):6055. https://doi.org/10.3390/app11136055
Chicago/Turabian StyleAli, Akhtar, En Hyung Kim, Jong-Hyun Lee, Kang-Hyun Leem, Shin Seong, and Wonnam Kim. 2021. "Processed Scutellaria baicalensis Georgi Extract Alleviates LPS-Induced Inflammatory and Oxidative Stress through a Crosstalk between NF-κB and KEAP1/NRF2 Signaling in Macrophage Cells" Applied Sciences 11, no. 13: 6055. https://doi.org/10.3390/app11136055
APA StyleAli, A., Kim, E. H., Lee, J.-H., Leem, K.-H., Seong, S., & Kim, W. (2021). Processed Scutellaria baicalensis Georgi Extract Alleviates LPS-Induced Inflammatory and Oxidative Stress through a Crosstalk between NF-κB and KEAP1/NRF2 Signaling in Macrophage Cells. Applied Sciences, 11(13), 6055. https://doi.org/10.3390/app11136055





