A Review on the Advancements in the Field of Metal Complexes with Schiff Bases as Antiproliferative Agents
Abstract
:1. Introduction
2. Antiproliferative Activity of Schiff Bases Complexed with Transition Metals
3. Antiproliferative Activity of Schiff Bases Complexed with Platinum Group Metals (PGM)
4. Antiproliferative Activity of Schiff Base Complexed with Lanthanides
5. Summary
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Schiff, H. Mittheilungen aus dem universitätslaboratorium in Pisa: Eine neue reihe organischer basen. Justus Liebigs Ann. Chem. 1864, 131, 118–119. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, P.; Dey, S.K.; Ara, M.H.; Karim, K.; Islam, A.B.M. A review on synthesis and versatile applications of some selected Schiff bases with their transition metal complexes. Egypt. J. Chem. 2020, 63, 5–6. [Google Scholar] [CrossRef]
- Hameed, A.; al-Rashida, M.; Uroos, M.; Ali, S.A.; Khan, K.M. Schiff bases in medicinal chemistry: A patent review (2010–2015). Expert Opin. Ther. Pat. 2017, 27, 63–79. [Google Scholar] [CrossRef]
- Chimento, A.; Saturnino, C.; Iacopetta, D.; Mazzotta, R.; Caruso, A.; Plutino, M.R.; Mariconda, A.; Ramunno, A.; Sinicropi, M.S.; Pezzi, V.; et al. Inhibition of human topoisomerase I and II and anti-proliferative effects on MCF-7 cells by new titanocene complexes. Bioorg. Med. Chem. 2015, 23, 7302–7312. [Google Scholar] [CrossRef]
- Sirignano, E.; Saturnino, C.; Botta, A.; Sinicropi, M.S.; Caruso, A.; Pisano, A.; Lappano, R.; Maggiolini, M.; Longo, P. Synthesis, characterization and cytotoxic activity on breast cancer cells of new half-titanocene derivatives. Bioorg. Med. Chem. Lett. 2013, 23, 3458–3462. [Google Scholar] [CrossRef]
- Ceramella, J.; Mariconda, A.; Iacopetta, D.; Saturnino, C.; Barbarossa, A.; Caruso, A.; Rosano, C.; Sinicropi, M.S.; Longo, P. From coins to cancer therapy: Gold, silver and copper complexes targeting human topoisomerases. Bioorg Med. Chem. Lett. 2020, 30, 126905. [Google Scholar] [CrossRef] [PubMed]
- Iacopetta, D.; Rosano, C.; Sirignano, M.; Mariconda, A.; Ceramella, J.; Ponassi, M.; Saturnino, C.; Sinicropi, M.S.; Longo, P. Is the way to fight cancer paved with gold? metal-based carbene complexes with multiple and fascinating biological features. Pharmaceuticals 2020, 13, 91. [Google Scholar] [CrossRef] [PubMed]
- Catalano, A.; Iacopetta, D.; Sinicropi, M.S.; Franchini, C. Diarylureas as antitumor agents. Appl. Sci. 2021, 11, 374. [Google Scholar] [CrossRef]
- Catalano, A.; Iacopetta, D.; Pellegrino, M.; Aquaro, S.; Franchini, C.; Sinicropi, M.S. Diarylureas: Repositioning from antitumor to antimicrobials or multi-target agents against new pandemics. Antibiotics 2021, 10, 92. [Google Scholar] [CrossRef] [PubMed]
- Kajal, A.; Bala, S.; Kamboj, S.; Sharma, N.; Saini, V. Schiff bases: A versatile pharmacophore. J. Catal. 2013. [Google Scholar] [CrossRef] [Green Version]
- Iacopetta, D.; Ceramella, J.; Catalano, A.; Saturnino, C.; Bonomo, M.G.; Franchini, C.; Sinicropi, M.S. Schiff bases: Interesting scaffolds with promising antitumoral properties. Appl. Sci. 2021, 11, 1877. [Google Scholar] [CrossRef]
- Kaya, S.; Erkan, S.; Karakaş, D. Computational investigation of molecular structures, spectroscopic properties and antitumor-antibacterial activities of some Schiff bases. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 244, 118829. [Google Scholar] [CrossRef]
- Kordestani, N.; Rudbari, H.A.; Bruno, G.; Rosario, S.; Braun, J.D.; Herbert, D.E.; Blacque, O.; Correia, I.; Zaman, M.A.; Bindu, M.M.; et al. Solid-state to solution helicity inversion of pseudotetrahedral chiral copper (II) complexes with 2, 4-dihalo-salicylaldiminate ligands. Dalton Transact. 2020, 49, 8247–8264. [Google Scholar]
- Miroslaw, B. Homo-and Hetero-Oligonuclear Complexes of Platinum Group Metals (PGM) Coordinated by Imine Schiff Base Ligands. Int. J. Mol. Sci. 2020, 21, 3493. [Google Scholar] [CrossRef]
- Pilichos, E.; Font-Bardia, M.; Escuer, A.; Mayans, J. Structural and magnetic studies of mononuclear lanthanide complexes derived from N-rich chiral Schiff bases. Dalton Transact. 2021, 50, 1746–1753. [Google Scholar] [CrossRef]
- Paswan, S.; Jaiswal, N.; Modanawal, V.K.; Patel, M.K.; Singh, R.K.P. An experimental and theoretical investigation of lanthanide complexes [Ln = Nd, Yb, Eu, Dy and tb] with 4-((2-hydroxy-naphthalen-1-yl) methylene amino) benzenesulfonamide ligand. Inorg. Chim. Acta 2020, 513, 119955. [Google Scholar] [CrossRef]
- Tsantis, S.T.; Tzimopoulos, D.I.; Holynska, M.; Perlepes, S.P. Oligonuclear Actinoid Complexes with Schiff Bases as Ligands—Older Achievements and Recent Progress. Int. J. Mol. Sci. 2020, 21, 555. [Google Scholar] [CrossRef] [Green Version]
- Omar, Z.T.; Jadhav, S.; Mohsin, M.; Faizaa, A.S.; Rai, M. Complexation study of synthesized pharmacological organic ligands with samarium. Rus. J. Inorg. Chem. 2020, 65, 2046–2052. [Google Scholar] [CrossRef]
- Rodríguez, M.R.; Balsa, L.M.; Piro, O.E.; Etcheverría, G.A.; García-Tojal, J.; Pis-Diez, R.; León, I.E.; Parajón-Costa, B.P.; González-Baró, A.C. Synthesis, crystal structure, spectroscopic characterization, DFT calculations and cytotoxicity assays of a new Cu(II) complex with an acylhydrazone ligand derived from thiophene. Inorganics 2021, 9, 9. [Google Scholar] [CrossRef]
- Sun, Y.; Lu, Y.; Bian, M.; Yang, Z.; Ma, X.; Liu, W. Pt (II) and Au (III) complexes containing Schiff-base ligands: A promising source for antitumor treatment. Eur. J. Med. Chem. 2021, 211, 113098. [Google Scholar] [CrossRef] [PubMed]
- Horozić, E.; Suljagić, J.; Suljkanovic, M. Synthesis, characterization, antioxidant and antimicrobial activity of Copper (II) complex with Schiff base derived from 2,2-dihydroxyindane-1, 3-dione and Tryptophan. Am. J. Org. Chem. 2019, 9, 9–13. [Google Scholar]
- Ejidike, I. Cu(II) Complexes of 4-[(1E)-N-{2-[(Z)-Benzylidene-amino]ethyl}ethanimidoyl]benzene-1,3-diol Schiff base: Synthesis, spectroscopic, in-vitro antioxidant, antifungal and antibacterial studies. Molecules 2018, 23, 1581. [Google Scholar] [CrossRef] [Green Version]
- Eltayeb, N.E.; Lasri, J.; Soliman, S.M.; Mavromatis, C.; Hajjar, D.; Elsilk, S.E.; Babgi, B.A.; Hussien, M.A. Crystal structure, DFT, antimicrobial, anticancer and molecular docking of (4E)-4-((aryl)methyleneamino)-1,2-dihydro-2,3-dimethyl-1-phenylpyrazol-5-one. J. Mol. Struct. 2020, 1213, 128185. [Google Scholar] [CrossRef]
- Al Zoubi, W. Biological activities of Schiff bases and their complexes: A review of recent works. Int. J. Org. Chem. 2013, 3, 73. [Google Scholar] [CrossRef] [Green Version]
- de Fátima, Â.; de P. Pereira, C.; Olímpio, C.R.S.D.G.; de Freitas Oliveira, B.G.; Franco, L.L.; da Silva, P.H.C. Schiff bases and their metal complexes as urease inhibitors—A brief review. J. Adv. Res. 2018, 13, 113–126. [Google Scholar]
- Shah, S.S.; Shah, D.; Khan, I.; Ahmad, S.; Ali, U.; ur Rahman, A. Synthesis and antioxidant activities of schiff bases and their complexes: An updated review. Biointerf. Res. Appl. Chem. 2020, 10, 6936–6963. [Google Scholar]
- Ibrahim, M.M.; Fathy, A.M.; Al-Harbi, S.A.; Sallam, S.A.; Al-Juaid, S.; Ramadan, A.E.M.M. Palladium(II) based imines; synthesis, characterization, X-ray structural analysis; DFT and catalytic hydrogenation study. J. Organometall. Chem. 2021, 939, 121764. [Google Scholar] [CrossRef]
- Chen, S.L.; Liu, X.Y.; Li, S.C.; Wu, C.; Li, Z.Y.; Li, T.T. Synthesis and crystal structure of a new Zn (II) complex with anti-leukemia activity. Inorg. Nano-Met. Chem. 2021, 51, 224–229. [Google Scholar] [CrossRef]
- Nurmamat, M.; Yan, H.; Wang, R.; Zhao, H.; Li, Y.; Wang, X.; Nurmaimaiti, K.; Kurmanjiang, T.; Luo, D.; Baodi, J.; et al. Novel copper(II) complex with a 4-acylpyrazolone derivative and coligand induce apoptosis in liver cancer cells. ACS Med. Chem. Lett. 2021, 12, 467–476. [Google Scholar] [CrossRef]
- Yadamani, S.; Neamati, A.; Homayouni-Tabrizi, M.; Beyramabadi, S.A.; Yadamani, S.; Gharib, A.; Morsali, A.; Khashi, M. Treatment of the breast cancer by using low frequency electromagnetic fields and Mn(II) complex of a Schiff base derived from the pyridoxal. Breast 2018, 41, 107–112. [Google Scholar] [CrossRef]
- Chen, S.; Liu, X.; Ge, X.; Wang, Q.; Xie, Y.; Hao, Y.; Zhang, Y.; Zhang, L.; Shang, W.; Liu, Z. Lysosome-targeted iridium(III) compounds with pyridine-triphenylamine Schiff base ligands: Syntheses, antitumor applications and mechanisms. Inorg. Chem. Front. 2020, 7, 91–100. [Google Scholar] [CrossRef]
- Wang, N.; Zeng, Q.; Zhang, R.; Xing, D.; Zhang, T. Eradication of solid tumors by chemodynamic theranostics with H2O2-catalyzed hydroxyl radical burst. Theranostics 2021, 11, 2334. [Google Scholar] [CrossRef] [PubMed]
- Oiye, É.N.; Ribeiro, M.F.M.; Katayama, J.M.T.; Tadini, M.C.; Balbino, M.A.; Eleotério, I.C.; Magalhães, J.; Castro, A.S.; Silva, R.S.M.; da Cruz Júnior, J.W.; et al. Electrochemical Sensors Containing Schiff Bases and their Transition Metal Complexes to Detect Analytes of Forensic, Pharmaceutical and Environmental Interest. A Review. Crit. Rev. Anal. Chem. 2019, 49, 488–509. [Google Scholar] [CrossRef]
- Wang, Z.G.; Ding, X.J.; Huang, Y.Y.; Yan, X.J.; Ding, B.; Li, Q.Z.; Xie, C.Z.; Xu, J.Y. The development of coumarin Schiff base system applied as highly selective fluorescent/colorimetric probes for Cu2+ and tumor biomarker glutathione detection. Dyes Pigments 2020, 175, 108156. [Google Scholar] [CrossRef]
- Song, X.-Q.; Wang, Z.-G.; Wang, Y.; Huang, Y.-Y.; Sun, Y.-X.; Ouyang, Y.; Xie, C.-Z.; Xu, J.-Y. Syntheses, characterization, DNA/HSA binding ability and antitumor activities of a family of isostructural binuclear lanthanide complexes containing hydrazine Schiff base. J. Biomol. Struct. Dyn. 2020, 38, 733–743. [Google Scholar] [CrossRef]
- Kaczmarek, M.T.; Zabiszak, M.; Nowak, M.; Jastrzab, R. Lanthanides: Schiff base complexes, applications in cancer diagnosis, therapy, and antibacterial activity. Coord. Chem. Rev. 2018, 370, 42–54. [Google Scholar] [CrossRef]
- Malik, M.A.; Dar, O.A.; Gull, P.; Wani, M.Y.; Hashmi, A.A. Heterocyclic Schiff base transition metal complexes in antimicrobial and anticancer chemotherapy. MedChemComm 2018, 9, 409–436. [Google Scholar] [CrossRef] [PubMed]
- Tadele, K.T.; Tsega, T.W. Schiff bases and their metal complexes as potential anticancer candidates: A review of recent works. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem. Anti-Cancer Agents) 2019, 19, 1786–1795. [Google Scholar] [CrossRef] [PubMed]
- Sakthivel, A.; Jeyasubramanian, K.; Thangagiri, B.; Raja, J.D. Recent advances in Schiff base metal complexes derived from 4-amoniantipyrine derivatives and their potential applications. J. Mol. Struct. 2020, 1222, 128885. [Google Scholar] [CrossRef]
- Matela, G. Schiff bases and complexes: A review on anti-cancer activity. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem. Anti-Cancer Agents) 2020, 20, 1908–1917. [Google Scholar] [CrossRef]
- Chaudhary, N.K.; Guragain, B.; Chaudhary, S.K.; Mishra, P. Schiff base metal complex as a potential therapeutic drug in medical science: A critical review. BIBECHANA 2021, 18, 214–230. [Google Scholar] [CrossRef]
- Islam, R.; Uddin, E.; Bitu, N.A.; Asraf, A.; Hossen, F.; Haque, M.; Mannan, A. Recent Advances in Biological and Catalytic Activities of Schiff base containing Acetylacetone and their Metal Complexes-A Short Overview. Asian J. Res. Chem. 2020, 13, 395–406. [Google Scholar] [CrossRef]
- Emam, S.; El Sayed, I.; Ayad, M.; Hathout, H. Synthesis, characterization and anticancer activity of new Schiff bases bearing neocryptolepine. J. Mol. Struct. 2017, 1146, 600–619. [Google Scholar] [CrossRef]
- Fetoh, A.; Asla, K.A.; El-Sherif, A.A.; El-Didamony, H.; El-Reash, G.M.A. Synthesis, structural characterization, thermogravimetric, molecular modelling and biological studies of Co(II) and Ni(II) Schiff bases complexes. J. Mol. Struct. 2019, 1178, 524–537. [Google Scholar] [CrossRef]
- Shi, S.; Yu, S.; Quan, L.; Mansoor, M.; Chen, Z.; Hu, H.; Liu, D.; Liang, Y.; Liang, F. Synthesis and antitumor activities of transition metal complexes of a bis-Schiff base of 2-hydroxy-1-naphthalenecarboxaldehyde. J. Inorg. Biochem. 2020, 210, 111173. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.M.; Heakal, B.H.; Younis, A.; Abdelmoaz, M.A.; Abdrabou, M.M. Conventional and Microwave-Assisted Synthesis, Antimicrobial and Antitumor Studies of Tridentate Schiff Base Derived from O-vanillin and Phenyl Urea and its Complexes. Adv. J. Chem. Sect. A 2020, 3, 621–638. [Google Scholar]
- Al-Serwi, R.H.; Othman, G.; Attia, M.A.; Enan, E.T.; El-Sherbiny, M.; Mahmoud, S.; Elsherbiny, N. Enhancement of cisplatin cytotoxicity by Cu(II)–Mn(II) schiff base tetradentate complex in human oral squamous cell carcinoma. Molecules 2020, 25, 4688. [Google Scholar] [CrossRef]
- Alyar, S.; Özmen, Ü.Ö.; Adem, Ş.; Alyar, H.; Bilen, E.; Kaya, K. Synthesis, spectroscopic characterizations, carbonic anhydrase II inhibitory activity, anticancer activity and docking studies of new Schiff bases of sulfa drugs. J. Mol. Sci. 2021, 1223, 128911. [Google Scholar] [CrossRef]
- Ismail, B.A.; Nassar, D.A.; Abd El–Wahab, Z.H.; Ali, O.A. Synthesis, characterization, thermal, DFT computational studies and anticancer activity of furfural-type Schiff base complexes. J. Mol. Struct. 2021, 1227, 129393. [Google Scholar] [CrossRef]
- Wongsuwan, S.; Chatwichien, J.; Pinchaipat, B.; Kumphune, S.; Harding, D.J.; Harding, P.; Boonmak, J.; Youngme, S.; Chotima, R. Synthesis, characterization and anticancer activity of Fe (II) and Fe(III) complexes containing N-(8-quinolyl) salicylaldimine Schiff base ligands. JBIC J. Biol. Inorg. Chem. 2021, 1–13. [Google Scholar]
- Naureen, B.; Miana, G.A.; Shahid, K.; Asghar, M.; Tanveer, S.; Sarwar, A. Iron(III) and zinc(II) monodentate Schiff base metal complexes: Synthesis, characterisation and biological activities. J. Mol. Struct. 2021, 1231, 129946. [Google Scholar] [CrossRef]
- Alkış, M.E.; Keleştemür, Ü.; Alan, Y.; Turan, N.; Buldurun, K. Cobalt and ruthenium complexes with pyrimidine based Schiff base: Synthesis, characterization, anticancer activities and electrochemotherapy efficiency. J. Mol. Struct. 2021, 1226, 129402. [Google Scholar] [CrossRef]
- Chen, S.Y.; Jiang, X.H.; Liu, R.X.; Huang, Y.; Shen, W.Y.; Jiang, Y.H.; Huamg, K.B.; Liu, Y.C. New cytotoxic zinc(II) and copper(II) complexes of Schiff base ligands derived from homopiperonylamine and halogenated salicylaldehyde. Inorg. Chim. Acta 2021, 516, 120171. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; El-Metwaly, N.M.; Alzahrani, S.O.; Bawazeer, A.M.; Shaaban, S.; Adam, M.S.S. Targeting ctDNA binding and elaborated in-vitro assessments concerning novel Schiff base complexes: Synthesis, characterization, DFT and detailed in-silico confirmation. J. Mol. Liq. 2021, 322, 114977. [Google Scholar] [CrossRef]
- Liao, W.H.; Song, X.Q.; Kong, Y.J.; Bao, R.D.; Li, F.F.; Zhou, J.; Zhao, Q.H.; Xu, J.Y.; Xie, N.; Xie, M.J. A novel Schiff base cobalt(III) complex induces a synergistic effect on cervical cancer cells by arresting early apoptosis stage. BioMetals 2021, 34, 277–289. [Google Scholar] [CrossRef]
- Mbugua, S.N.; Sibuyi, N.R.; Njenga, L.W.; Odhiambo, R.A.; Wandiga, S.O.; Meyer, M.; Lancelette, R.A.; Onani, M.O. New palladium(II) and platinum(II) complexes based on pyrrole Schiff bases: Synthesis, characterization, X-ray structure, and anticancer activity. ACS Omega 2020, 5, 14942–14954. [Google Scholar] [CrossRef]
- Özdemir, Ö.; Gürkan, P.; Demir, Y.D.Ş.; Ark, M. Novel palladium (II) complexes of N-(5-nitro-salicylidene)-Schiff bases: Synthesis, spectroscopic characterization and cytotoxicity investigation. J. Mol. Struct. 2020, 1207, 127852. [Google Scholar] [CrossRef]
- Zhang, Z.; Du, G.; Gong, G.; Sheng, Y.; Lu, X.; Cai, W.; Wang, F.; Zhao, G. A novel ferrocene-palladium metal complex: Synthesis, single crystal structure, in vitro cytotoxicity study and molecular docking. J. Mol. Struct. 2021, 1232, 130021. [Google Scholar] [CrossRef]
- Acharya, S.; Maji, M.; Chakraborty, M.P.; Bhattacharya, I.; Das, R.; Gupta, A.; Mukherjee, A. Disruption of the microtubule network and inhibition of VEGFR2 phosphorylation by cytotoxic N, O-coordinated Pt(II) and Ru(II) complexes of trimethoxy aniline-based Schiff bases. Inorg. Chem. 2021, 60, 3418–3430. [Google Scholar] [CrossRef]
- Moubeen, S.; El-Shahat, M.; Aziz, A.; Attia, A. Synthesis, characterization and biological evaluation of novel octahedral Ru (III) complexes containing pentadentate Schiff base ligands. Curr. Chem. Lett. 2021, 10, 17–32. [Google Scholar] [CrossRef]
- Andiappan, K.; Sanmugam, A.; Deivanayagam, E.; Karuppasamy, K.; Kim, H.-S.; Vikraman, D. In vitro cytotoxicity activity of novel Schiff base ligand–lanthanide complexes. Sci. Rep. 2018, 8, 3054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sathiyanarayanan, V.; Prasath, P.V.; Sekhar, P.C.; Ravichandran, K.; Easwaramoorthy, D.; Mohammad, F.; Al-Lohedan, H.A.; Oh, W.C.; Sagadevan, S. Docking and in vitro molecular biology studies of p-anisidine-appended 1-hydroxy-2-acetonapthanone Schiff base lanthanum(III) complexes. RSC Adv. 2020, 10, 16457–16472. [Google Scholar] [CrossRef]
- Hua, L.; Li, W.; Chen, Y.; Liang, K.; Cai, H.; Wang, J.; Wang, S.; Yin, T.; Liang, L. A hexanuclear Nd (III) complex derived from a Schiff base with significant antitumor and antifungal activity. Appl. Organometall. Chem. 2021, 35, e6081. [Google Scholar] [CrossRef]
| Structure | Compd, Activity | Ref |
|---|---|---|
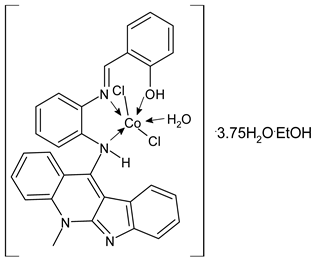 | [Co2(HL2)Cl2(H2O)].3.75H2O.EtOH (1) percentage inhibition = 83.22% (HT29) | Emam et al. (2017) [43] |
 | [Ni2(L1)Cl2.6H2O].2H2O (2) | Fetoh et al. (2019) [44] |
 | [Co2(H2L2)Cl4.2H2O].H2O (3) | Fetoh et al. (2019) [44] |
 | Cu3L2(OAc)2 (4) IC50 = 0.83 μM (MGC80-3) IC50 = 0.59 μM (HeLa) IC50 = 0.47 μM (T-24) IC50 = 0.81 μM (HepG2) IC50 = 0.98 μM (MDA) IC50 = 5.69 μM (A549) IC50 = 0.99 μM (Bel-7402) IC50 = 1.41 μM (Wi-38) | Shi et al. (2020) [45] |
 | Ni3L2(OAc)2 (5) IC50 = 5.11 μM (MGC80-3) IC50 = 5.04 μM (HeLa) IC50 = 3.81 μM (T-24) IC50 = 7.94 μM (HepG2) IC50 = 12.41 μM (MDA) IC50 = 9.59 μM (A549) IC50 = 17.00 μM (Bel-7402) IC50 = 12.52 μM (Wi-38) | Shi et al. (2020) [45] |
 | Fe3L2(OAc)2 (6) IC50 = 3.75 μM (MGC80-3) IC50 = 3.67 μM (HeLa) IC50 = 2.99 μM (T-24) IC50 = 4.90 μM (HepG2) IC50 = 1.87 μM (MDA) IC50 = 3.30 μM (A549) IC50 = 5.69 μM (Bel-7402) IC50 = 2.71 μM (Wi-38) | Shi et al. (2020) [45] |
 | Mn-L (7) IC50 = 70.3 µg/mL (MCF-7) IC50 = 49.9 µg/mL (HCT-116) | Hassan et al. (2020) [46] |
 | Cu-L (8) IC50 = 61.1 µg/mL (MCF-7) IC50 = 52.7 µg/mL (HCT-116) | Hassan et al. (2020) [46] |
 | Zn-L (9) IC50 = 61.3 µg/mL (MCF-7) IC50 = 51.9 µg/mL (HCT-116) | Hassan et al. (2020) [46] |
 | [Cu(1-(biphenyl)-2-hydroxyimino-2-(4-chloroanilino)-1-ethanone)(H2O)Mn(phen)2](ClO4)2 (10) IC50 = 250 µM (SCC) | Al-Serwi et al. (2020) [47] |
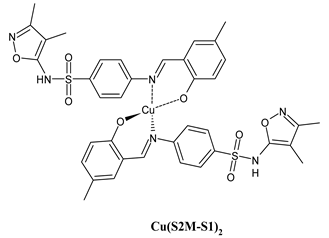 | Cu(S2M-S1)2 (11) IC50 = 40 µM (MCF-7) | Alyar et al. (2021) [48] |
 | Cu(S1M-S1)2 (12) IC50 = 40 µM (MCF-7) | Alyar et al. (2021) [48] |
 | [AgL]NO3 (13) IC50 = 12.9 μg/mL | Ismail et al. (2021) [49] |
 | [CrLNO3(H2O)]2NO3.2H2O (14) IC50 = 14.8 μg/mL, | Ismail et al. (2021) [49] |
 | [FeLCl2]Cl (15) IC50 = 7.31 μg/mL | Ismail et al. (2021) [49] |
 | [CoLCl2] (16) IC50 = 8.53 μg/mL, | Ismail et al. (2021) [49] |
 | [CuLCl2] (17) IC50 = 17.1 μg/mL | Ismail et al. (2021) [49] |
 | [CdL(NO3)]NO3 (18) IC50 = 1.95 μg/mL | Ismail et al. (2021) [49] |
 | [Fe(qsal-Cl2)2]Cl (19) IC50 = 10 µM (A549) | Wongsuwan et al. (2021) [50] |
 | (L1)2Zn(Ac)2 (20) IC50 = 6.81 µg/mL | Naureen et al. (2021) [51] |
 | (L2)2Zn(Ac)2 (21) IC50 = 7.58 µg/mL | Naureen et al. (2021) [51] |
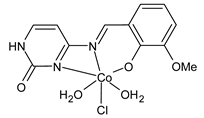 | [CoCl·L(H2O)2]·2H2O (22) IC50 = 610 µM (Caco-2) | Alkiş et al. (2021) [52] |
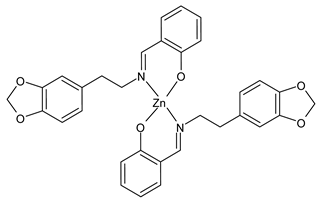 | [Zn(La)2] (23) IC50 = 40.31 µM (T-24) IC50 = 14.68 µM (HepG2) IC50 = >50 µM (SK-OV-3) | Chen et al. (2021) [53] |
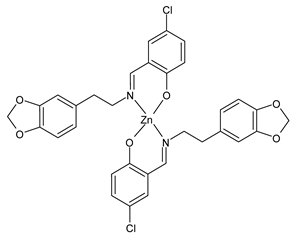 | [Zn(Lb)2] (24) IC50 = 13.77 µM (T-24) IC50 = 9.00 µM (HepG2) IC50 = 36.64 µM (SK-OV-3) | Chen et al. (2021) [53] |
 | [Cu(Lc)2] (25) IC50 = 22.63 µM (T-24) IC50 = 46.72 µM (HepG2) IC50 = 24.72 µM (SK-OV-3) | Chen et al. (2021) [53] |
 | DSHNZn (26) IC50 = 30.20 (HCT-116) IC50 = 13.90 (MCF-7) IC50 = 21.10 µM (HepG2) | Abu-Dief et al. (2021) [54] |
 | DSHNVO (27) IC50 = 31.90 µM (HCT-116) IC50 = 15.30 µM (MCF-7) IC50 = 22.80 µM (HepG2) | Abu-Dief et al. (2021) [54] |
 | 28 IC50 = 12.40 µM (HeLa) IC50 = 18.96 µM (LoVo) IC50 = 22.68 µM (A549) IC50 = 18.03 µM (A549/cis) | Liao et al. (2021) [55] |
| Structure | Compd, Activity | Ref |
|---|---|---|
 | 29 IC50 = 0.3 µg/mL (HepG2) IC50 = 16.63 µg/mL (CaCo-2) | Mbugua et al. (2017) [56] |
 | 30 IC50 = 13 µg/mL (HepG2) IC50 = 15.81 µg/mL (CaCo-2) | Mbugua et al. (2017) [56] |
 | 31 At 1 µM kills 10% of HeLa cells | Özdemir et al. (2020) [57] |
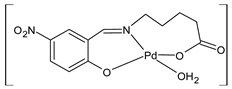 | 32 At 1 µM kills 20% of HeLa cells | Özdemir et al. (2020) [57] |
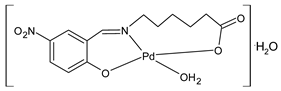 | 33 At 1 µM kills 90% of HeLa cells | Özdemir et al. (2020) [57] |
 | 34 IC50 = 0.88 μM (Sk-Hep-1) IC50 = 8.28 μM (MHCC97-L) IC50 = 8.35 μM (NIH3T3) IC50 = 22.9 μM (MDA-MB-231) IC50 = 22.4 μM (A549) IC50 = 15.8 μM (A549/Taxol) | Zhang et al. (2021) [58] |
 | [PtII(L1)(DMSO)Cl] (35) IC50 = 2.6 µM (MIAPaCa-2) IC50 = 2.5 µM (HepG2) IC50 = 2.2 µM (MDA-MB-231) IC50 = 3.7 µM (HEK-293) IC50 = 3.8 µM (HFF-1) | Acharya et al. (2021) [59] |
 | [RuII(L2)(p-cymene)Cl] (36) IC50 = 6.1 µM (MIAPaCa-2) IC50 = 7.5 µM (HepG2) IC50 = 5.5 µM (MDA-MB-231) IC50 = 8.7 µM (HEK-293) IC50 = 7.7 µM (HFF-1) | Acharya et al. (2021) [59] |
 | [PtII(L2)(DMSO)Cl] (37) IC50 = 1.2 µM (MIAPaCa-2) IC50 = 1.4 µM (HepG2) IC50 = 1.1 µM (MDA-MB-231) IC50 = 2.0 µM (HEK-293) IC50 = 1.8 µM (HFF-1) | Acharya et al. (2021) [59] |
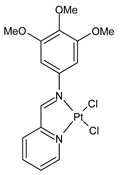 | [PtII(L3)Cl2] (38) IC50 = 20.2 µM (MIAPaCa-2) IC50 = 21.4 µM (HepG2) IC50 = 19.4 µM (MDA-MB-231). | Acharya et al. (2021) [59] |
 | [Ru(L1)Cl] (39) 8.5 µM < IC50 < 9.0 µM (HeLa) IC50 ~ 9.0 µM (MCF-7) | Moubeen et al. (2021) [60] |
 | [Ru(L2)Cl] (40) IC50 ~ 9.5 µM (HeLa) IC50 ~ 11 µM (MCF-7) | Moubeen et al. (2021) [60] |
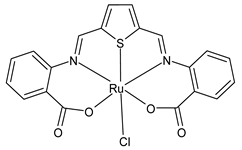 | [Ru(L3)Cl] (41) IC50 ~ 6.5 µM (HeLa) IC50 ~ 10 µM (MCF-7) | Moubeen et al. (2021) [60] |
 | [RuCl(p-cymene)L] (42) IC50 = 510 µM (Caco-2) | Alkiş et al. (2021) [52] |
| Structure | Compd, Activity | Ref |
|---|---|---|
 | SBLEr (43) 50% viability reduction at 25 µg/mL | Andiappan et al. (2018) [61] |
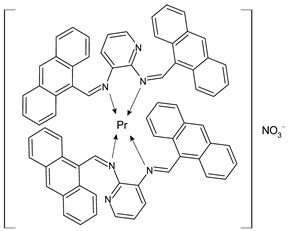 | SBLPr (44) 50% viability reduction at 25 µg/ml | Andiappan et al. (2018) [61] |
 | [PrL(NO3)2(H2O)]NO3 (45) Cell viability: 49.26% (HeLa) | Sathiyanarayanan et al. (2020) [62] |
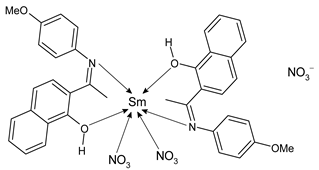 | [SmL(NO3)2]NO3 (46) Cell viability: 20.15% (HeLa) IC50 = 34 µg/mL (HeLa) | Sathiyanarayanan et al. (2020) [62] |
 | [YbL(NO3)2(H2O)]NO3 (47) Cell viability: 55.16% (HeLa) | Sathiyanarayanan et al. (2020) [62] |
 | {[Nd6(HL)2L2X2(NO3)8(EtOH)6](EtOH)(H2O)3} (48) IC50 = 4.42 µM (SMMC-7721) | Hua et al. (2021) [63] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catalano, A.; Sinicropi, M.S.; Iacopetta, D.; Ceramella, J.; Mariconda, A.; Rosano, C.; Scali, E.; Saturnino, C.; Longo, P. A Review on the Advancements in the Field of Metal Complexes with Schiff Bases as Antiproliferative Agents. Appl. Sci. 2021, 11, 6027. https://doi.org/10.3390/app11136027
Catalano A, Sinicropi MS, Iacopetta D, Ceramella J, Mariconda A, Rosano C, Scali E, Saturnino C, Longo P. A Review on the Advancements in the Field of Metal Complexes with Schiff Bases as Antiproliferative Agents. Applied Sciences. 2021; 11(13):6027. https://doi.org/10.3390/app11136027
Chicago/Turabian StyleCatalano, Alessia, Maria Stefania Sinicropi, Domenico Iacopetta, Jessica Ceramella, Annaluisa Mariconda, Camillo Rosano, Elisabetta Scali, Carmela Saturnino, and Pasquale Longo. 2021. "A Review on the Advancements in the Field of Metal Complexes with Schiff Bases as Antiproliferative Agents" Applied Sciences 11, no. 13: 6027. https://doi.org/10.3390/app11136027
APA StyleCatalano, A., Sinicropi, M. S., Iacopetta, D., Ceramella, J., Mariconda, A., Rosano, C., Scali, E., Saturnino, C., & Longo, P. (2021). A Review on the Advancements in the Field of Metal Complexes with Schiff Bases as Antiproliferative Agents. Applied Sciences, 11(13), 6027. https://doi.org/10.3390/app11136027












