Extraction of Total Anthocyanins from Sicana odorifera Black Peel Fruits Growing in Paraguay for Food Applications
Abstract
:Featured Application
Abstract
1. Introduction
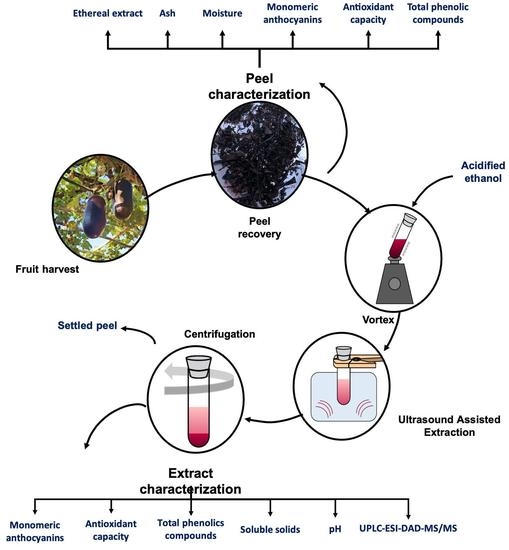
2. Materials and Methods
2.1. Collection and Samples Preparation
2.2. Morphological and Physicochemical Characteristics of Pulp
2.3. Composition Analysis on Kurugua’s Pulp, Peel, and Extracts
2.4. Antioxidant Activity
2.5. Efficiency of Anthocyanins Extraction from Peels
2.5.1. Effect of the Ultrasound-Assisted Extraction Process Variables
2.5.2. Characterization of the Anthocyanin Extract
2.6. UPLC-ESI-MS/MS Profiling of the Peel Fruits Extract from S. odorifera Naudim Vell. “kurugua”: UPLC-ESI-DAD-MS/MS Peel Extract Analyses
2.7. Statistical Analysis
3. Results
3.1. Morphological and Physicochemical Characters of Ripe S. odorifera Peel
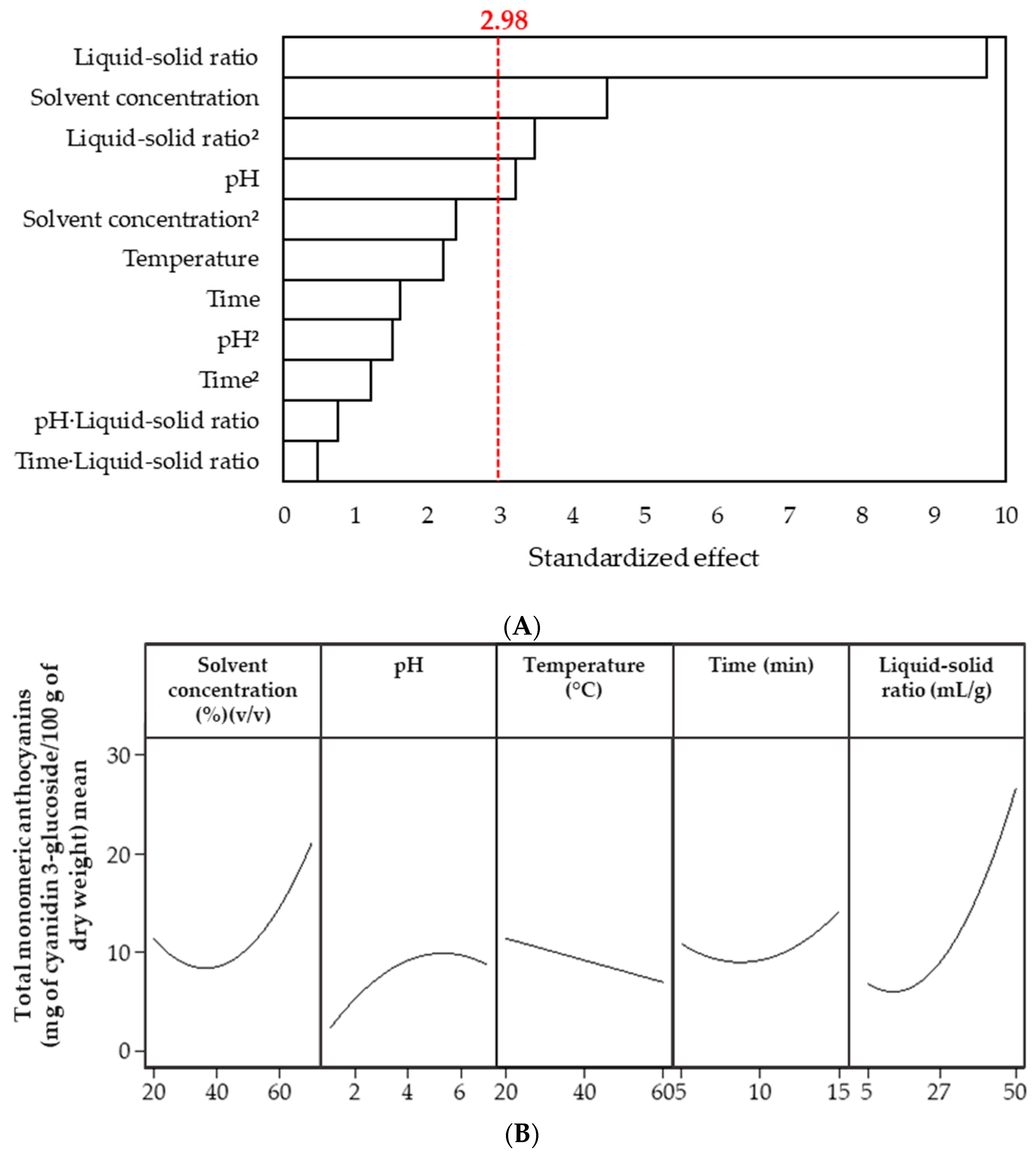
3.2. Efficiency of Anthocyanins Extraction from Peels
3.2.1. Effect of the Ultrasound-Assisted Extraction Process Variables
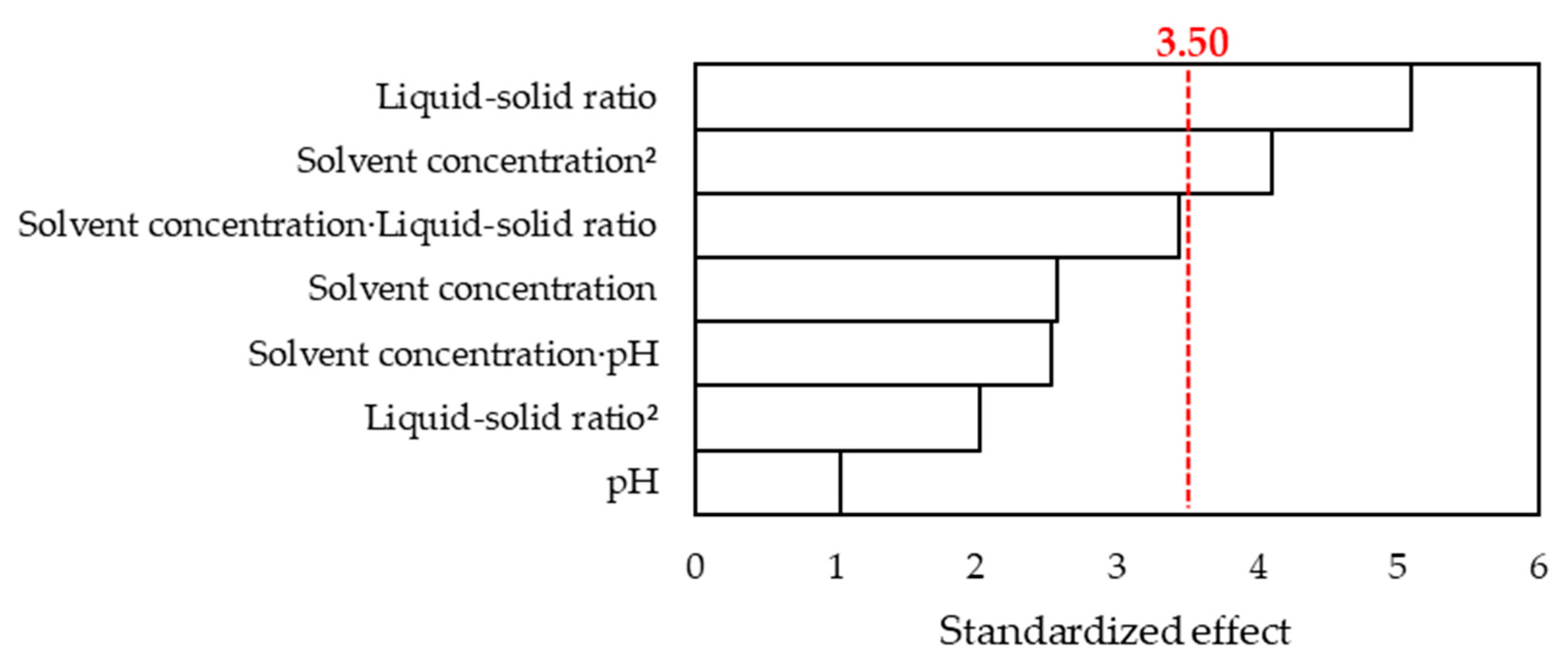
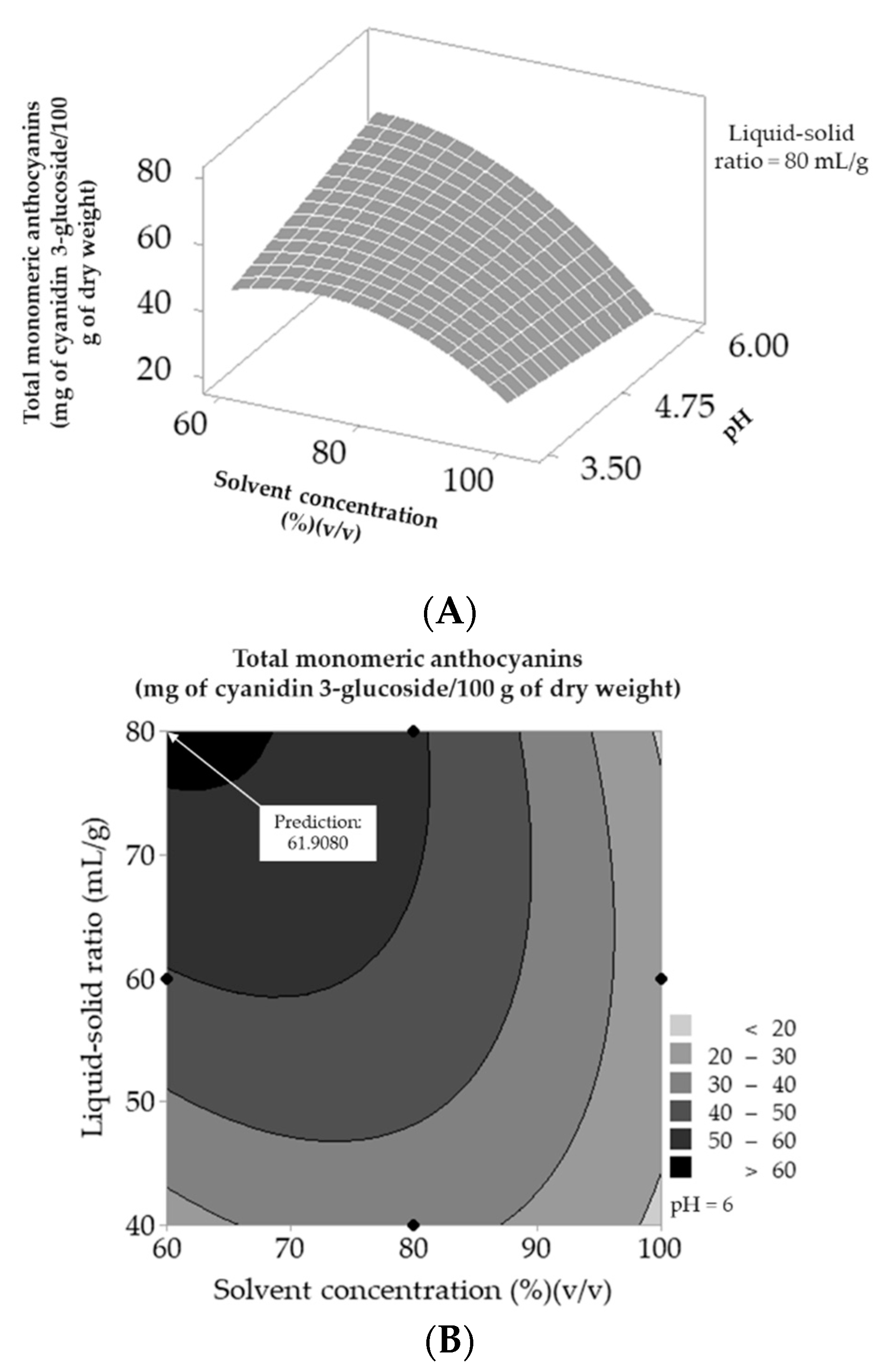
3.2.2. Development of the Response Surface Methodology
3.2.3. Characterization of the Anthocyanin Extract
3.3. UPLC-ESI-DAD-MS/MS Peel Extract Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Paula Filho, G.X.; Barreira, T.F.; Pinheiro, S.S.; de Morais Cardoso, L.; Duarte Martino, H.S.; Pinheiro-Sant’Ana, H.M. ‘Melão croá’ (Sicana sphaerica Vell.) and ‘maracujina’ (Sicana odorifera Naud.): Chemical composition, carotenoids, vitamins and minerals in native fruits from the Brazilian Atlantic forest. Fruits 2015, 70, 341–349. [Google Scholar] [CrossRef]
- Jaramillo, K.; Dawid, C.; Hofmann, T.; Fujimoto, Y.; Osorio, C. Identification of Antioxidative Flavonols and Anthocyanins inSicana odoriferaFruit Peel. J. Agric. Food Chem. 2011, 59, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Secretaría Técnica de Planificación. República del Paraguay. Quieren Revalorizar Identidad Histórica a Través del Kurugua. Available online: https://www.stp.gov.py/v1/quieren-revalorizar-identidad-historica-a-traves-del-kurugua/ (accessed on 25 April 2021).
- Kienteka, S.S.; Corrêa-Ferreira, M.L.; Petkowicz, C. Characterization of cell wall polysaccharides from Sicana odorifera fruit and structural analysis of a galactan-rich fraction pectins as side chains. Carbohydr. Polym. 2018, 197, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, B.R.; Dias, M.I.; Pereira, C.; Petrović, J.; Soković, M.; Calhelha, R.C.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R.; Barros, L. Valorization of Sicanaodorifera (Vell.) Naudin Epicarp as a Source of Bioactive Compounds: Chemical Characterization and Evaluation of Its Bioactive Properties. Foods 2021, 10, 700. [Google Scholar] [CrossRef]
- Parada, F.; Duque, C.; Fujimoto, Y. Free and bound volatile composition and characterization of some glucoconjugates as aroma precursors in melón de olor fruit pulp (Sicana odorifera). J. Agric. Food Chem. 2000, 48, 6200–6204. [Google Scholar] [CrossRef]
- Nakano, S.; Fujimoto, Y.; Takaishi, Y. Cucurbita-5, 23-diene-3β, 25-diol from Sicana odorifera. Fitoterapia 2004, 75, 609–611. [Google Scholar] [CrossRef]
- Contreras-Calderón, J.; Calderón-Jaimes, L.; Guerra-Hernández, E.; García-Villanova, B. Antioxidant capacity, phenolic content and vitamin C in pulp, peel and seed from 24 exotic fruits from Colombia. Food Res. Int. 2011, 44, 2047–2053. [Google Scholar] [CrossRef]
- Albuquerque, B.R.; Pinela, J.; Barros, L.; Oliveira, M.B.P.; Ferreira, I.C. Anthocyanin-rich extract of jabuticaba epicarp as a natural colorant: Optimization of heat- and ultrasound-assisted extractions and application in a bakery product. Food Chem. 2020, 316, 126364. [Google Scholar] [CrossRef]
- Domínguez, R.; Zhang, L.; Rocchetti, G.; Lucini, L.; Pateiro, M.; Munekata, P.E.S.; Lorenzo, J.M. Elderberry (Sambucus nigra L.) as potential source of antioxidants. Characterization, optimization of extraction parameters and bioactive properties. Food Chem. 2020, 330, 127266. [Google Scholar] [CrossRef]
- Ghada, B.; Pereira, E.; Pinela, J.; Prieto, M.A.; Pereira, C.; Calhelha, R.C.; Stojković, D.; Sokóvić, M.; Zaghdoudi, K.; Barros, L.; et al. Recovery of Anthocyanins from Passion Fruit Epicarp for Food Colorants: Extraction Process Optimization and Evaluation of Bioactive Properties. Molecules 2020, 25, 3203. [Google Scholar] [CrossRef]
- Ochoa, S.; Durango-Zuleta, M.M.; Osorio-Tobón, J.F. Techno-economic evaluation of the extraction of anthocyanins from purple yam (Dioscorea alata) using ultrasound-assisted extraction and conventional extraction processes. Food Bioprod. Process. 2020, 122, 111–123. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef] [PubMed]
- Farooq, S.; Shah, M.A.; Siddiqui, M.W.; Dar, B.N.; Mir, S.A.; Ali, A. Recent trends in extraction techniques of anthocyanins from plant materials. J. Food Meas. Charact. 2020, 14, 3508–3519. [Google Scholar] [CrossRef]
- Yao, Y.; Pan, Y.; Liu, S. Power ultrasound and its applications: A state-of-the-art review. Ultrason. Sonochem. 2020, 62, 104722. [Google Scholar] [CrossRef] [PubMed]
- Saratale, G.D.; Saratale, R.G.; Varjani, S.; Cho, S.-K.; Ghodake, G.S.; Kadam, A.; Mulla, S.I.; Bharagava, R.N.; Kim, D.-S.; Shin, H.S. Development of ultrasound aided chemical pretreatment methods to enrich saccharification of wheat waste biomass for polyhydroxybutyrate production and its characterization. Ind. Crop. Prod. 2020, 150, 112425. [Google Scholar] [CrossRef]
- Mongomery, D.C. Montgomery: Design and Analysis of Experiments; John Willy & Sons: Hoboken, NJ, USA, 2017; p. 78. [Google Scholar]
- Lee, J.; Rennaker, C.; Wrolstad, R.E. Correlation of two anthocyanin quantification methods: HPLC and spectrophotometric methods. Food Chem. 2008, 110, 782–786. [Google Scholar] [CrossRef]
- Jiang, H.-L.; Yang, J.-L.; Shi, Y.-P. Optimization of ultrasonic cell grinder extraction of anthocyanins from blueberry using response surface methodology. Ultrason. Sonochem. 2017, 34, 325–331. [Google Scholar] [CrossRef]
- Yin, Y.; Jia, J.; Wang, T.; Wang, C. Optimization of natural anthocyanin efficient extracting from purple sweet potato for silk fabric dyeing. J. Clean. Prod. 2017, 149, 673–679. [Google Scholar] [CrossRef]
- Mereles, L.; Ferro, E. Physical characteristics, composition and minerals content in Macadamia integrifolia Maiden & Betche nuts, harvested in Cordillera Department, Paraguay. Rojasiana 2015, 14, 55–68. [Google Scholar]
- Horwitz, W. (Ed.) Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- IICA. Protocolos Estandarizados para la Valoración de Frutos Nativos Del Procisur Frente a la Creciente Demanda por Ingredientes y Aditivos Especializados (Carotenoides, Antocianinas y Polifenoles); Procisur: Montevideo, Uruguay, 2018. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Lao, F.; Giusti, M.M. Extraction of purple corn (Zea mays L.) cob pigments and phenolic compounds using food-friendly solvents. J. Cereal Sci. 2018, 80, 87–93. [Google Scholar] [CrossRef]
- Zapata, L.M. Obtención de extracto de antocianinas a partir de arándanos para ser utilizado como antioxidante y colorante en la industria alimentaria [Unpublished doctoral thesis]. Universitat Politècnica de València. 2014. Available online: https://doi.org/10.4995/Thesis/10251/39105 (accessed on 25 April 2021).
- Assous, M.; Abdel-Hady, M.; Medany, G.M. Evaluation of red pigment extracted from purple carrots and its utilization as antioxidant and natural food colorants. Ann. Agric. Sci. 2014, 59, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Arrazola, G.; Herazo, I.; Alvis, A. Microencapsulación de antocianinas de berenjena (Solanum melongena L.) mediante Secado por aspersión y evaluación de la estabilidad de su color y capacidad antioxidante. Inf. Tecnol. 2014, 25, 31–42. [Google Scholar] [CrossRef] [Green Version]
- Andersen, Ø.M.; Jordheim, M. Anthocyanins. In Encyclopedia of Life Sciences [Internet]; John Wiley & Sons, Ltd.: Chichester, UK, 2010. [Google Scholar] [CrossRef]
- Martínez Zambrano, J.J.; Rojas Sarmiento, H.A.; Borda Guerra, G.d.L.; Hastamorir Caro, A.N.; Medina Riaño, F.M. Revista Facultad Nacional de Agronomía-Medellín. Rev. Fac. Nac. Agron. 2011, 64, 6015–6022. Available online: http://www.redalyc.org/articulo.oa?id=179922364024 (accessed on 25 June 2021).
- Ngamwonglumlert, L.; Devahastin, S.; Chiewchan, N. Natural colorants: Pigment stability and extraction yield enhancement via utilization of appropriate pretreatment and extraction methods. Crit. Rev. Food Sci. Nutr. 2017, 57, 3243–3259. [Google Scholar] [CrossRef]
- Belwal, T.; Huang, H.; Li, L.; Duan, Z.; Zhang, X.; Aalim, H.; Luo, Z. Optimization model for ultrasonic-assisted and scale-up extraction of anthocyanins from Pyrus communis ‘Starkrimson’ fruit peel. Food Chem. 2019, 297, 124993. [Google Scholar] [CrossRef]
- Kumar, V.; Pathak, P.; Bhardwaj, N.K. Facile chemo-refining approach for production of micro-nanofibrillated cellulose from bleached mixed hardwood pulp to improve paper quality. Carbohydr. Polym. 2020, 238, 116186. [Google Scholar] [CrossRef]
- Fan, G.; Han, Y.; Gu, Z.; Chen, D. Optimizing conditions for anthocyanins extraction from purple sweet potato using response surface methodology (RSM). LWT 2008, 41, 155–160. [Google Scholar] [CrossRef]
- Paez-Cartaya, I.; Rodríguez-Sánchez, J.L.; Cruz-Viera, L. Optimización de la extracción de antocianinas de Hibiscus Sabdariffa L. y su caracterización cromática. Cienc. Tecnol. Aliment. 2018, 28, 17–21. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. In Methods in Enzymology; Academic Press: London, UK, 1998; Volume 299, pp. 152–178. [Google Scholar]
- Giusti, M.M.; Wrolstad, R.E. Characterization and measurement of anthocyanins by UV-visible spectroscopy. In Current Protocols in Food Analytical Chemistry; Wrolstad, R.E., Acree, T.E., An, H., Decker, E.A., Penner, M.H., Reid, D.S., Schwartz, S.J., Shoemaker, C.F., Sporns, P., Eds.; John Wiley & Sons: New York, NY, USA, 2001; pp. F1.2.1–F1.2.13. [Google Scholar] [CrossRef]
- Aspee, F.J.; Theoduloz, C.; Vieira, M.N.; Rodríguez-Werner, M.A.; Schmalfuss, E.; Winterhalter, P.; Schmeda-Hirschmann, G. Phenolics from the Patagonian currants Ribes spp.: Isolation, characterization and cytoprotective effect in human AGS cells. J. Funct. Foods 2016, 26, 11–26. [Google Scholar] [CrossRef]
- Chen, G.; Li, X.; Saleri, F.; Guo, M. Analysis of Flavonoids in Rhamnus davurica and Its Antiproliferative Activities. Molecules 2016, 21, 1275. [Google Scholar] [CrossRef] [PubMed]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [Green Version]
- Cacace, J.; Mazza, G. Mass transfer process during extraction of phenolic compounds from milled berries. J. Food Eng. 2003, 59, 379–389. [Google Scholar] [CrossRef]
- Lao, F.; Giusti, M.M. Quantification of Purple Corn (Zea mays L.) Anthocyanins Using Spectrophotometric and HPLC Approaches: Method Comparison and Correlation. Food Anal. Methods 2016, 9, 1367–1380. [Google Scholar] [CrossRef]
- Maran, J.P.; Manikandan, S.; Nivetha, C.V.; Dinesh, R. Ultrasound assisted extraction of bioactive compounds from Nephelium lappaceum L. fruit peel using central composite face centered response surface design. Arab. J. Chem. 2017, 10, S1145–S1157. [Google Scholar] [CrossRef] [Green Version]
- Hiranrangsee, L.; Kumaree, K.K.; Sadiq, M.B.; Anal, A.K. Extraction of anthocyanins from pericarp and lipids from seeds of mangosteen (Garcinia mangostana L.) by Ultrasound-assisted extraction (UAE) and evaluation of pericarp extract enriched functional ice-cream. J. Food Sci. Technol. 2016, 53, 3806–3813. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, F.A.N.; Fonteles, T.V.; Rodrigues, S.; de Brito, E.S.; Tiwari, B.K. Ultrasound-assisted extraction of anthocyanins and phenolics from jabuticaba (Myrciaria cauliflora) peel: Kinetics and mathematical modeling. J. Food Sci. Technol. 2020, 57, 2321–2328. [Google Scholar] [CrossRef] [PubMed]
- Dranca, F.; Oroian, M. Optimization of ultrasound-assisted extraction of total monomeric anthocyanin (TMA) and total phenolic content (TPC) from eggplant (Solanum melongena L.) peel. Ultrason. Sonochem. 2016, 31, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Meregalli, M.M.; Puton, B.M.S.; Camera, F.D.; Amaral, A.U.; Zeni, J.; Cansian, R.L.; Mignoni, M.L.; Backes, G.T. Conventional and ultrasound-assisted methods for extraction of bioactive compounds from red araçá peel (Psidium cattleianum Sabine). Arab. J. Chem. 2020, 13, 5800–5809. [Google Scholar] [CrossRef]
- Burgos-Edwards, A.; Jiménez-Aspee, F.; Thomas-Valdés, S.; Schmeda-Hirschmann, G.; Theoduloz, C. Qualitative and quantitative changes in polyphenol composition and bioactivity of Ribes magellanicum and R. punctatum after in vitro gastrointestinal digestion. Food Chem. 2017, 237, 1073–1082. [Google Scholar] [CrossRef]
- Karimi, A.; Krähmer, A.; Herwig, N.; Schulz, H.; Hadian, J.; Meiners, T. Variation of Secondary Metabolite Profile of Zataria multiflora Boiss. Populations Linked to Geographic, Climatic, and Edaphic Factors. Front. Plant Sci. 2020, 11, 969. [Google Scholar] [CrossRef] [PubMed]


| Independent Variable | Level | References | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Solvent concentration (%) (v/v) | 20 | 45 | 70 | [10,27,30] |
| pH | 1 | 4 | 7 | [31] |
| Temperature (°C) | 20 | 40 | 60 | [32,33] |
| Time (min) | 5 | 10 | 15 | [34,35] |
| Liquid–solid ratio (mL/g) | 5 | 27.5 | 50 | [10,36,37] |
| External Characteristics of S. odorifera Ripe Fruits. | ||
|---|---|---|
| Weight (g) | 1970 ± 51 | |
| Longitudinal diameter (cm) | 26.9 ± 1.4 | |
| Transverse diameter (cm) | 10.4 ± 0.7 | |
| Mesocarp + exocarp (cm) | 0.31± 0.02 | |
| Physicochemical Characters | Pulp | Peel |
| Moisture (g/100 g) | 88.0 ± 0.1 | 8.84 ± 0.15 |
| Ash (g/100 g) | 0.15 ± 0.00 | 3.95 ± 0.44 |
| Total protein (g/100 g) | 1.07 ± 0.08 | - |
| Total carbohydrate (g/100 g) | 5.55 ± 0.31 | - |
| Total Lipids (g/100 g) | Nd | 10.58 ± 1.28 |
| Dietary fiber (g/100 g) | 2.92 ± 0.00 | - |
| pH | 6.69 ± 0.04 | 6.19 ± 0.01 |
| Soluble solids (°Brix) * | 8.2 ± 0.2 | 18.4 ± 0.00 |
| Phenolics Compounds, Anthocyanins, and Total Antioxidant Capacity | Pulp | Peel |
| Total phenols compounds (mg GAE/100 g) | 37.2 ± 4.84 a | 100 ± 3.35 b |
| Monomeric anthocyanins (mg/g of cyanidin 3-glucoside) | 2.64 ± 0.10 a | 19.7 ± 2.69 b |
| Total antioxidant capacity ABTS (μM TEAC/g) | 4.39 ± 0.55 a | 0.201 ± 0.03 b |
| Solvent Concentration (%) (v/v) | pH | Temperature (°C) | Time (min) | Liquid–Solid Ratio (mL/g) | Total Monomeric Anthocyanins (mg C3G/100 g DW) |
|---|---|---|---|---|---|
| 20 | 1 | 40 | 5 | 50 | 21.0 |
| 20 | 1 | 40 | 5 | 50 | 20.0 |
| 20 | 1 | 60 | 15 | 5 | 6.3 |
| 20 | 1 | 60 | 15 | 5 | 7.2 |
| 20 | 4 | 20 | 15 | 50 | 41.6 |
| 20 | 4 | 20 | 15 | 50 | 33.3 |
| 20 | 7 | 20 | 10 | 5 | 9.1 |
| 20 | 7 | 20 | 10 | 5 | 5.5 |
| 20 | 7 | 60 | 5 | 27.5 | 13.6 |
| 20 | 7 | 60 | 5 | 27.5 | 11.9 |
| 45 | 1 | 20 | 5 | 5 | 5.8 |
| 45 | 1 | 20 | 5 | 5 | 7.6 |
| 45 | 4 | 40 | 10 | 27.5 | 8.4 |
| 45 | 4 | 40 | 10 | 27.5 | 10.0 |
| 45 | 7 | 60 | 15 | 50 | 27.6 |
| 45 | 7 | 60 | 15 | 50 | 26.1 |
| 70 | 1 | 20 | 15 | 27.5 | 19.6 |
| 70 | 1 | 20 | 15 | 27.5 | 18.0 |
| 70 | 1 | 60 | 10 | 50 | 37.4 |
| 70 | 1 | 60 | 10 | 50 | 23.1 |
| 70 | 4 | 60 | 5 | 5 | 16.7 |
| 70 | 4 | 60 | 5 | 5 | 13.2 |
| 70 | 7 | 40 | 15 | 5 | 23.7 |
| 70 | 7 | 40 | 15 | 5 | 26.2 |
| 70 | 7 | 20 | 5 | 50 | 45.8 |
| 70 | 7 | 20 | 5 | 50 | 41.2 |
| Independent Variable | Level | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| Solvent concentration (%) (v/v) | 60 | 80 | 100 |
| pH | 3.50 | 4.75 | 6.00 |
| Liquid–solid ratio (mL/g) | 40 | 60 | 80 |
| Solvent Concentration (%) (v/v) | pH | Liquid–Solid Ratio (mL/g) | TMA (mg C3G/100 g DW) |
|---|---|---|---|
| 60 | 3.5 | 60 | 33.8 |
| 60 | 4.75 | 40 | 19.2 |
| 60 | 4.75 | 80 | 56.0 |
| 60 | 6 | 60 | 43.1 |
| 80 | 3.5 | 40 | 25.3 |
| 80 | 3.5 | 80 | 44.5 |
| 80 | 4.75 | 60 | 45.2 |
| 80 | 4.75 | 60 | 43.5 |
| 80 | 4.75 | 60 | 45.8 |
| 80 | 6 | 40 | 37.7 |
| 80 | 6 | 80 | 54.8 |
| 100 | 3.5 | 60 | 40.1 |
| 100 | 4.75 | 40 | 18.9 |
| 100 | 4.75 | 80 | 20.3 |
| 100 | 6 | 60 | 23.2 |
| Characterization | Value |
|---|---|
| Total monomeric anthocyanins (mg C3G/100 g DW) | 60.3 ± 0.3 |
| Antioxidant capacity (mmol TEs/g) | 0.246 ± 0.00 |
| Total phenols (mg GAEs/100 g) | 9558 ± 522 |
| pH | 6.19 ± 0.01 |
| Total soluble solids (°Brix) | 18.4 ± 0.00 |
| Peak | Rt (min) | UVmax | [M−H]−/[M+H]+ | Polarity | MS/MS Fragments | Tentative Identification |
|---|---|---|---|---|---|---|
| 1 | 1.91 | 517, 275 | 449.44 | positive | 287.42 (100) | Cyanidin hexoside |
| 2 | 1.91 | 517, 276 | 595.54 | positive | 287.29 (100) | Cyanidin rutinoside |
| 3 | 3.33 | 515, 275 | 595.58 | positive | 449.12 (10), 287.46 (100) | Cyanidin hexoside rhamnoside |
| 4 | 3.34 | 579.52 | positive | 433.82 (10), 271.33 (100) | Pelargonidin hexoside rhamnoside | |
| 5 | 5.15 | 633.48 | positive | 633.48 (100), 487.02 (10), 331.39 (15) | Malvidin rhamnoside shikimate | |
| 6 | 5.21 | 349, 263 | 609.55 | negative | 609.59 (100), 300.87 (35) | Quercetin rutinoside |
| 7 | 5.21 | 349, 262 | 463.52 | negative | 300.69 (100) | Quercetin hexoside |
| 8 | 5.76 | 347, 264 | 593.58 | negative | 593.33 (100), 285.53 (50) | Kaempferol rutinoside |
| 9 | 5.76 | 347, 265 | 563.55 | negative | 463.20 (70), 301.71 (100) | Quercetin hexoside succinate |
| 10 | 5.79 | 347, 265 | 447.55 | negative | 284.72 (100), 255.41 (40) | Kaempferol hexoside |
| 11 | 6.37 | 369, 255 | 301.34 | negative | 255.18 (10), 177.74 (50), 151.39 (85), 107.27 (100) | Quercetin |
| 12 | 6.89 | 453.81 | positive | 210.48 (100) | Unknown | |
| 13 | 6.95–7.03 | 365, 265 | 285.29 | negative | 285.10 (70), 256.17 (30), 239.55 (100), 229.53 (7 0) | Kaempferol |
| 14 | 7.35 | 567.06 | positive | 453.76 (55), 381.64 (20), 210.77 (100) | Unknown |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mereles, L.; Caballero, S.; Burgos-Edwards, A.; Benítez, M.; Ferreira, D.; Coronel, E.; Ferreiro, O. Extraction of Total Anthocyanins from Sicana odorifera Black Peel Fruits Growing in Paraguay for Food Applications. Appl. Sci. 2021, 11, 6026. https://doi.org/10.3390/app11136026
Mereles L, Caballero S, Burgos-Edwards A, Benítez M, Ferreira D, Coronel E, Ferreiro O. Extraction of Total Anthocyanins from Sicana odorifera Black Peel Fruits Growing in Paraguay for Food Applications. Applied Sciences. 2021; 11(13):6026. https://doi.org/10.3390/app11136026
Chicago/Turabian StyleMereles, Laura, Silvia Caballero, Alberto Burgos-Edwards, Macarena Benítez, Danya Ferreira, Eva Coronel, and Omayra Ferreiro. 2021. "Extraction of Total Anthocyanins from Sicana odorifera Black Peel Fruits Growing in Paraguay for Food Applications" Applied Sciences 11, no. 13: 6026. https://doi.org/10.3390/app11136026
APA StyleMereles, L., Caballero, S., Burgos-Edwards, A., Benítez, M., Ferreira, D., Coronel, E., & Ferreiro, O. (2021). Extraction of Total Anthocyanins from Sicana odorifera Black Peel Fruits Growing in Paraguay for Food Applications. Applied Sciences, 11(13), 6026. https://doi.org/10.3390/app11136026