Abstract
Cement-based materials decay with exposure to aggressive agents, a development that raises infrastructure operation and maintenance costs substantially. This paper analyses the inclusion of ultrafine construction and demolition (UC&DW) and biomass-fuelled power plant (BA) waste as pozzolanic additions to cement in pursuit of more sustainable and eco-respectful binders and assesses the durability of the end materials when exposed to seawater, chlorides (0.5 M NaCl) or sulphates (0.3 M Na2SO4). The effect of adding silica fume (SF) at a replacement ratio of 5% was also analysed. Durability was determined using the methodology proposed by Koch and Steinegger, whilst microstructural changes were monitored with mercury intrusion porosimetry (MIP), X-ray diffraction (XRD) and scanning electron microscopy (SEM) for a fuller understanding of decay processes. According to the findings, the new blended cements containing 20%UC&DW + 10%BA or 20%UC&DW + 20%BA + 5%SF resist the attack by the aggressive media studied, with a 56-d corrosion index of over 0.7. The composition of the reaction products generated with the attack is essentially the same in OPC and the SCM-bearing materials. The results show that the optimal replacement ratio for SCM is 30%.
1. Introduction
The physical, chemical, mechanical and biological actions affecting concrete structures throughout their service life compromises their durability and compliance with the ultimate limit state and service standards to which they were designed. The result is substantial yearly repair and maintenance costs, which in 2016 came to ~$123 × 109 worldwide according to ASCE 2017 data on infrastructures [1]. Islam et al. [2], in turn, reported that maintenance costs amount to 14% to 20% of design costs in the UK, Hong Kong and Australia.
The presence of physical-chemical pathological processes in such structures is associated with parameters (choice of materials, type of environmental exposure, w/c ratio in concrete, structural shapes, etc.) defined in project stages ranging from design and construction to operation [3]. When choosing materials, the type of binder (cement), as the concrete component that interacts most readily with aggressive media (sulphates, chlorides, seawater, etc.) in the environment is of particular relevance to durability. Such interaction directly and indirectly induces the formation of expansive products that prompt cracking and/or surface spalling to the detriment of the bearing section of the concrete. Given that cement susceptibility to such action depends on the chemical composition, crystalline or gelatinous nature and porosity of cement hydration products, those factors play a decisive role in the reactions with aggressive agents. The presence of calcium silicate or aluminate hydrates and especially portlandite is therefore a determinant in cement durability [4].
Along those lines, blending supplementary cementitious materials (SCMs) into cement or concrete is known to improve concrete structure durability [5]. Pozzolanic SCMs in particular favour durability due to their effect on pore system refinement and reduction of pore solution alkalinity and portlandite content in cement matrices [4,6]. In addition to those technical advantages, the use of SCMs carries social and environmental benefits associated with lower natural resource consumption and the valorisation of industrial by-products, as well as a significant decline in CO2 emissions. CEM II/A-LL or L manufacture generates 16.2% and CEM III/B manufacture 73.0% less of that greenhouse gas than CEM I [7].
Against that backdrop, the scientific community has shown a growing interest in the last 10 years in identifying SCMs that may serve as alternatives to the silica fume, fly ash, blast furnace slag, natural or calcined pozzolans and limestone envisaged in European standard EN 197-1 [8]. One of the most prominent lines of research underway involves the assessment of the mechanical viability of new SCMs sourced from construction and demolition (C&DW) and agroforestry waste in the formulation of more sustainable binary eco-cements.
In 2016, the European Union alone generated 923.9 Mt of C&DW, accounting for around 35% of all the solid waste produced by its 28 members [9]. Those by-products can be separated, crushed and sieved to yield recycled aggregate (RA), in turn classified under three categories depending on concrete (Rc) and masonry (Rb) content [10]: concrete RA (Rc ≥ 90%; Rb ≤ 10%), mixed RA (Rc < 90%; Rb ≤ 30%) and mixed masonry RA (Rc < 70%; Rb > 30%). Whilst the coarse (>4 mm), and especially the concrete, fraction have been extensively researched [11,12] for use as the granular skeleton in recycled concrete, little scientific–technical knowledge has been collected on the fine (<4 mm) and ultrafine (<0.125 µm) fractions. The valorisation of those materials, comprising 30% to 40% of all C&DW [13], is a challenge to be confronted if circular economy principles are to be instituted in the construction industry.
The use of C&DW ultrafines as SCMs in new cements is an innovative line of work undertaken in recent years by a number of authors, whose interest has focused primarily on the mechanical performance of pastes and/or mortars bearing those materials as partial (3% to 30%) cement replacements. The consensus conclusion reached [14,15,16,17,18] is that GRC contains SiO2, Al2O3 and Fe2O3 (44% ≤ SiO2 + Al2O3 + Fe2O3 ≤ 59%), along with CaO (42% to 16%) and exhibits low pozzolanicity and hydraulicity. Its filler effect likewise-identified would favour hydration, providing nucleation sites that would densify the cement matrix microstructure. Masonry and mixed C&DW ultrafines [19,20,21,22] comprise essentially SiO2, Al2O3 and Fe2O3 (SiO2 + Al2O3 + Fe2O3 ≥ 84%), with pozzolanicity at least as high as fly ash and similar to that of other masonry materials, such as masonry [4,23] and fired clay sanitary ware [24] rubble. Further to research on mechanical behaviour, GRC has no adverse effect on new eco-cement performance at low replacement ratios (≤7%), whereas masonry ultrafines induce no significant decline in compressive or flexural strength at percentages of up to 25%.
Another line of research that has evoked considerable interest, as noted earlier, is the valorisation of agroforestry waste as an SCM. Two types of such waste are distinguished, depending on the origin [25]: laboratory-calcined (500 °C ≤ T ≤ 700 °C) biomass agroforestry waste (primarily bagasse, rice husk and to a lesser extent bamboo ash) [26,27,28]; and biomass or bottom ash, BA, from power and/or heat plants [29,30,31,32], which generate around 10 Mt of BA yearly [33]. SiO2, Al2O3 and Fe2O3 (SiO2 + Al2O3 + Fe2O3 ≥ 70%) are the majority compounds in such waste, which exhibits high early and late age lime fixation capacity (=pozzolanicity). No common pattern has been identified in the effect of including such SCMs on end product mechanical performance, however, which has on the contrary been observed to depend on the origin and nature of the waste and the replacement ratio [30,34,35,36].
In that regard, no prior study has been published in the international literature assessing the durability of ternary or quaternary eco-cements, simultaneously bearing mixed C&DW ultrafines (UC&DW) and BA or both with silica fume (UC&DW + BA + SF).
The research described hereunder used the Koch and Steinegger method [37] to analyse the seawater-, chloride- and sulphate-resistance of cement pastes bearing 30% or 45% SCMs (UC&DW + BA or UC&DW + BA + SF). Secondarily, the processes governing cement decay were determined on the grounds of XRD-identified mineralogical variations and the morphological changes observed in the exposed materials with scanning electron microscopy. The variations in pore size distribution detected with mercury porosimetry were also explored.
2. Materials and Methods
For ready and more convenient reference, the materials and methods deployed in this study are described schematically below.
2.1. Materials
Waste material: biomass ultrafines (BA)
Supplier: Spanish power plant
Origin: herbaceous + woody (eucalyptus, fruit, pine tree, etc.)
Chemical composition: (Table 1) CaO + MgO + SiO2 ~70 wt%; alkali content ~12 wt%; Al2O3 + Fe2O3 content ~7 wt%

Table 1.
SCM chemical composition.
XRD-determined mineralogical composition: amorphous hump at 2θ = 20°–35° indicative of amorphous silica; diffraction lines characteristic of cristobalite and quartz; other crystalline phases: sylvite (KCl), calcite (CaCO3), mullite (Al6Si2O13), hematite (Fe2O3), and alkaline orthoclase-like alkaline feldspars (KAlSiO3); all reported earlier as components in similar biomass waste [32].
Specific surface: 2800 cm2/g; density: 2.75 g/cm3.
Waste material: mixed C&DW ultrafines (UC&DW)
Origin: C&DW management plant in the Spanish province of Cáceres
Chemical composition: (Table 1) SiO2 + Al2O3 + Fe2O3 ~66 wt%; CaO ~13 wt%; alkalis ~5 wt%; trace elements
Mineralogical composition (Figure 1): majority phases (as in other mixed C&DW recycled aggregates [38]) = quartz, feldspars (albite and orthoclase), phyllosilicates (chamosite and biotite) and hematite; traces of calcite.
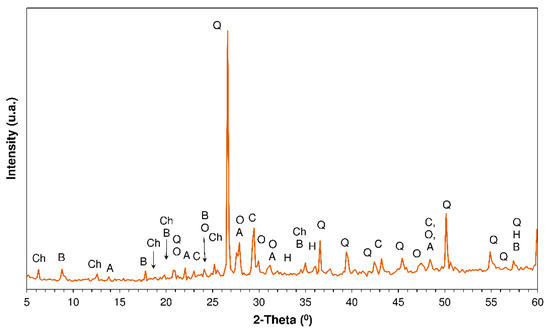
Figure 1.
XRD pattern of the mixed construction and demolition waste ultrafine (Note—Chamosite; B: biotite; A: Albite; O: orthoclase; H: hematite; Q: quartz; and C: calcite).
Specific surface: 5700 cm2/g; density: 2.88 g/cm3.
Standardized supplementary cementitious materials: silica fume
Origin: commercial (Elkem Microwhite®)
Characteristics: white; SiO2 content ~98%, specific surface area ≥15 m2/g
Material: cement (OPC)
Class/strength: EN 197-1 [8]-compliant CEM I 42.5 R
Manufacturer: Lafarge plant, Villaluenga de la Sagra, Spanish province of Toledo
Specific surface: 3560 cm2/g; density: 3.1 g/cm3.
2.2. Blended Cements
M1
Components: 70%OPC + 20%UC&DW + 10%BA, yielding a type II/B blended portland cement (21 % to 35 % total additions) or a type IV/A pozzolanic cement (11 % to 35 % total additions) in different proportions in keeping with European standard EBA, at replacement ratios of 10 % to 20 % according to the classification set out in standard EN 197-1 [8]
Physical, mechanical characteristics: Table 2

Table 2.
New cement physical and mechanical properties.
EN 197-1-compliant: yes, for 28 d 42.5 MPa and 32.5 MPa ordinary cements
M2
Components: 55%OPC + 20%UC&DW + 20%BA + 5%SF, yielding a type IV/B pozzolanic cement (36% to 55% total additions) in different proportions in keeping with European standards, at replacement ratios of 10% to 20% according to the classification set out in standard EN 197-1 [8]
Physical, mechanical characteristics: Table 2
EN 197-1-compliant: yes, for 28 d 32.5 MPa ordinary cements
2.3. Method
Paste preparation (OPC, M1, M2)
Water: deionised, w/c ratio = 0.5
Specimen number, shape, dimensions; 12 per blend, medium (seawater; chloride, sulphate solutions) and exposure time; prismatic; 1 × 1 × 6 cm
Moulding, curing: removal from moulds 24 h after casting, cured for 21 d at 100% RH and T = 20 ± 1 °C
Treatment
Soaking media: seawater (ASTM-D1141 [39]); 0.5 M sodium chloride, 0.3 M sodium sulphate; reference: deionised water (Koch–Steinegger method, [37,40]), deemed optimal for assessing blended cement resistance to such agents as it simultaneously accommodates the pozzolanic reaction and the assessment of its most prominent advantages [41]
Soaking time; T: 21 d, 56 d or 90 d; 20 °C
Procedure: specimens triple-washed in deionized water to remove any excess salt and dried in a laboratory kiln at 40 °C to a constant weight prior to characterisation at each exposure time
Mechanical and microstructural analysis
Parameters measured: flexural strength, chemical resistance and XRD-identification of new compounds at each age
Determination of chemical resistance to aggressive media: Koch–Steineger corrosion index, calculated as (Equation (1)):
where CI = corrosion index; FSX = flexural strength at exposure time ‘i’ to medium ‘X’; (sw: seawater; ch: 0.3 M NaCl; s: 0.5 M Na2SO4); FSW = flexural strength in water-soaked specimens, same exposure times
Pore size distribution, SEM/EDX: in 56-d specimens
2.4. Instrumental Techniques
XRD (mineralogy)
Instrument: Bruker AXS D8 X-ray powder diffractometer
Specifications: 3-kW (Cu Ka1.2) copper anode; wolfram cathode X-ray generator
Scanning characteristics: 2θ angles of 5° to 60°; scanning rate, 2°/min
Voltage generator tube settings: 40 kV, 30 mA
SEM (microstructural analysis)
Instrument: Hitachi S4800 electron microscope
Specifications: coupled to a Bruker Nano XFlash 5030 silicon drift detector for EDX determination of chemical composition
MIP (porosity)
Instrument: Micromeritics Autopore IV 9500 mercury porosimeter
Specifications: measuring range, 0.006 μm to 175 μm; operating pressure: ≤33 000 psi (227.5 MPa) [42]
Mean pore size calculation (Equation (2)):
where V = median pore diameter (volume); A = median pore diameter (area)
Test frame (mechanical strength)
Instrument: IBERTEST AUTOTEST 200/10-SW test frame fitted with an adapter for 1 × 1 × 6 cm specimens.
3. Results
3.1. Mechanical Properties
The decline in flexural strength values of the water-soaked cement and the materials exposed to seawater, sodium chloride (0.5 M NaCl) or sodium sulphate (0.3 M Na2SO4), was an indication that the effect of the dilution resulting from the lower amount of clinker present in new blends M1 and M2 was greater than the effect of the pozzolanicity of this type of SCMs (UC&DW and BA). That finding was consistent with results reported by other authors who recorded declines in strength associated with smaller proportions of C-S-H gel and greater porosity in pastes prepared with SCMs processed from industrial by-products [36,43,44].
On the whole, strength rose with exposure time in the pastes exposed to seawater and 0.3 M Na2SO4. That pattern was associated with the formation of salts (see point 3.4) that would initially fill pores to generate a more compact matrix. Subsequent salt expansion, however, would raise inner stress and induce microcracking [41,45].
The rise in strength in the pastes exposed to 0.5 M NaCl may be attributed to: (i) the formation of more hydration product [46], favoured by the enhancement of Ca(OH)2 solubility in NaCl solutions; (ii) greater C-S-H gel stability [47] with the uptake of some of the Na+ into the C-S-H interlayer spaces [48]; or (iii) Friedel’s salt precipitation in the pores [49].
A comparison of the behaviour of the blends exposed to the three types of aggressive media is provided in Table 3. OPC and M1 were observed to follow a similar pattern except when soaked in seawater, where the simultaneous inclusion of the new SCMs (BA and UC&DW) lowered strength by ~5 % relative to the water-soaked 90 d pastes. Of the pastes exposed to sodium chloride, performance declined the least in 90 d M1, to ~1 % below its water-soaked counterpart, compared to the ~9% decline observed for OPC. Strength rose in the same materials in the 90-d attack by Na2SO4, by 72% in OPC and 13% in M1.

Table 3.
Flexural strength (MPa) of pastes soaked in water or aggressive media.
The M2 blends (OPC + UC&DW + BA + SF) exhibited lower performance than the other two pastes irrespective of the exposure medium. In the samples exposed to seawater, the 90 d values for M2 were ~38% lower than for OPC ~8% lower where the medium was 0.5 M NaCl and ~2% lower when attacked by 0.3 M Na2SO4. Those findings infer that including SF with the Ba + UC&DW combination had no beneficial effect, possibly because the amount of portlandite present in the binder/SCM system was insufficient to support the pozzolanic reaction, which would be expected to generate a more compact and stronger matrix [50].
3.2. Porosity
Macro- and mesopore size distribution in the 56 d OPC, M1 and M2 pastes is graphed in Figure 2. The refinement in water-soaked paste M1 and M2 pore systems relative to OPC was attributable to the pozzolanic reaction between the SCMs and the portlandite generated during cement hydration. C-S-H gel, the predominant reaction product, would fill the pores, reducing the volume of macro- and raising the volume of mesopores [51].
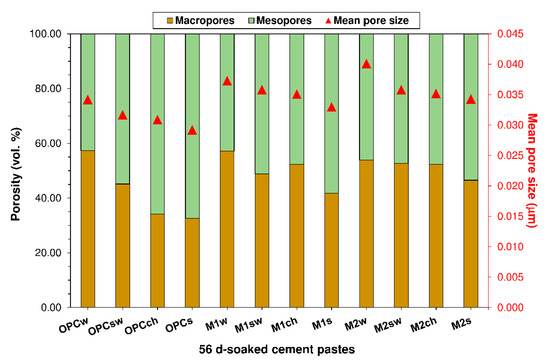
Figure 2.
Pore systems in 56-d pastes exposed to distilled water (w), seawater (sw), 0.5 M NaCl (ch) or 0.3 M Na2SO4 (s).
The pore system in the OPC exposed to aggressive media was refined more intensely than the systems in pastes M1 or M2, enhancing its durability and inducing higher Kock & Steinegger indices (see Section 3.3). That finding is consistent with prior accelerated testing for durability in cement-based materials bearing fly ash and either silica/alumina nanoparticles [52] or masonry industry sludge [4].
The total porosity (Pt) values for the 56-d water- or aggressive medium-soaked pastes listed in Table 4 show that porosity was higher in SCM-bearing pastes M1 and M2 than in OPC due to the intrinsic effect of the blend of SCMs on that parameter. The decline in total porosity observed in the pastes exposed to the aggressive media relative to the water-soaked specimens was attributed to the precipitation of new products such as Friedel’s salt, ettringite, gypsum and brucite resulting from the chemical attack on the pore system. Their presence induced a rise in mesopore volume [53,54,55] (Figure 2), lowering permeability and retarding decay [56]. The common denominator in all the pastes analysed was that the decline in Pt was steepest in 0.3 M Na2SO4 (followed by seawater and 0.5 M NaCl pastes in that order) and steeper than in the distilled water-soaked samples. That finding was closely associated with the nature of the products of the chemical attack [6].

Table 4.
Total porosity (vol.%) in 56-d pastes.
Those patterns were consistent with prior reports of the behaviour of cement pastes bearing pozzolans such as blast furnace slag, fly ash, silica fume, C&DW or biomass waste after exposure to aggressive agents [20,25,56,57].
3.3. Corrosion Index
The OPC, M1 and M2 corrosion index/exposure time (texp) curves in Figure 3 show that in all the pastes, with the exception of seawater-soaked M2, an initial rise in the index (texp ≤ 56 d) was followed by a decline after 90 d of exposure. Further to Koch–Steinegger criteria, pastes OPC, M1 and M2 could be deemed sulphate-resistant, for their 56-d corrosion index was greater than or equal to 0.70.
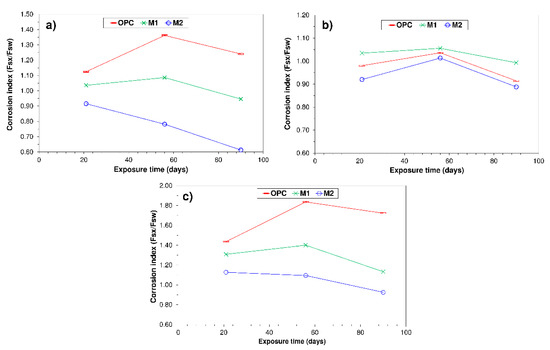
Figure 3.
Corrosion indices over time in pastes OPC, M1 and M2 exposed to aggressive media: (a) seawater; (b) 0.5 M NaCl; (c) 0.3 M Na2SO4.
In this respect also, the inclusion of SF to the BA + UC&DW blend was observed to have no beneficial effect on paste durability where the M1 corrosion index was consistently lower than the OPC value, irrespective of test age and aggressive medium.
3.4. X-ray Diffration Findings
The XRD patterns for pastes OPC, M1 (70%OPC + 20%UC&DW + 10%BA) and M2 (55%OPC + 20%UC&DW + 20%BA + 5%SF) soaked in water or exposed to the aggressive media analysed are reproduced in Figure 4, Figure 5, Figure 6 and Figure 7. The crystalline compounds identified in the water-soaked OPC specimens (Figure 3) included primarily calcium monocarboaluminate (CH22Al2Ca4O20), ettringite (CaO)6(Al2O3)(SO3)3·32H2O), portlandite (Ca(OH)2), calcite (CaCO3) and anhydrous dicalcium silicate (C2S) in the form of larnite, a mineral present in portland cement detected by XRD after soaking in water because it hydrates more slowly than other mineralogical phases in the cement [58].
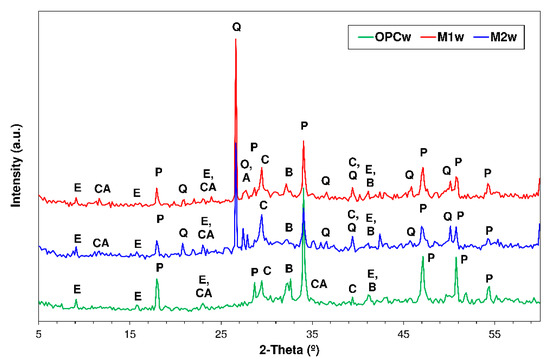
Figure 4.
XRD patterns for 56-d-soaked pastes: OPC, M1 and M2 (Note—E: ettringite; CA: calcium monocarboaluminato; P: portlandite; Q: quartz; O: orthoclase; A: Albite; C: calcite; and B: belite).
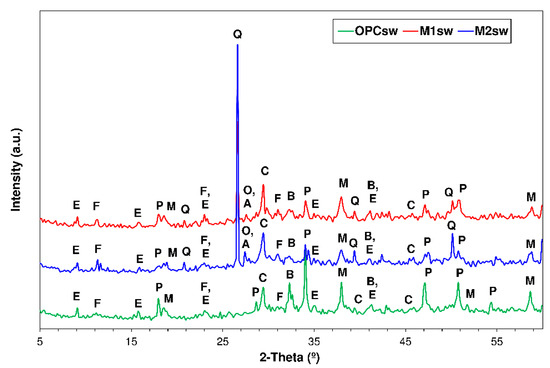
Figure 5.
XRD patterns for 56 d OPC, M1 and M2 exposed to seawater (Note—E: ettringite; M: brucite; P: portlandite; Q: quartz; O: orthoclase; A: Albite; C: calcite; and B: belite).
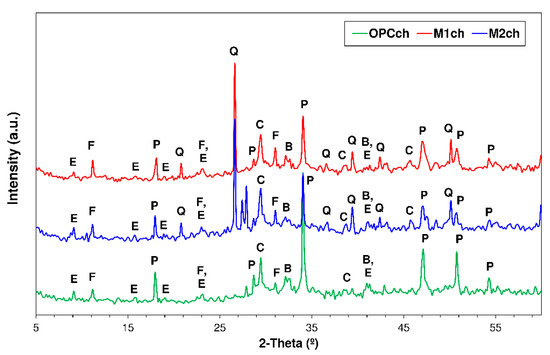
Figure 6.
XRD patterns for 56-d OPC, M1 and M2 exposed to 0.5 M NaCl (Note—E: ettringite; F: Friedel’s salt; P: portlandite; Q: quartz; O: orthoclase; A: Albite; C: calcite; and B: belite).
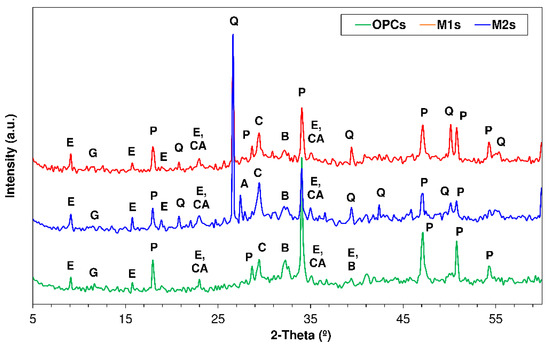
Figure 7.
XRD patterns for 56 d OPC, M1 and M2 exposed to 0.3 M Na2SO4 (Note—E: ettringite; G: gypsum; CA: calcium monocarboaluminato; P: portlandite; Q: quartz; O: orthoclase; A: Albite; C: calcite; and B: belite).
The same phases as observed in OPC were identified in phases M1 and M2, along with quartz and the feldspars albite and orthoclase present in the SCMs used, as discussed in Section 2.1. The intensity of the portlandite reflections declined as a result not only of lower cement content, but also of the pozzolanic reaction induced by the UC&DW, BA and SF added.
The calcium monocarboaluminate observed in the water-soaked specimens was absent in the pastes exposed to seawater (Figure 5). The diffractograms for the latter also contained lines for other crystalline compounds resulting from the reaction between hydration products and the sulphate and chloride ions in seawater. The Cl− ions diffusing through paste pores were partially retained by the phases bearing Al2O3, giving rise to calcium monochloraluminate hydrate or Friedel’s salt (3CaO⋅AI2O3⋅CaCl2⋅AI2O3⋅10H2O) [59,60], from which ettringite may have formed via reaction with SO42− ions [4]. Brucite (Mg(OH)2) also formed as the product of the reaction between the Mg2+ in the seawater and the OH- ions present in portlandite [61]. The brucite diffraction lines were more intense in the OPC than in M1 or M2 (see Section 3.4). The lines for portlandite were significantly less intense inn the seawater- than in the water-soaked specimens, from which seawater may be inferred to have attacked that hydration product directly. In contrast, the ettringite and calcite lines were more intense due to the interaction between the phases in the paste and the sulphates and carbonates present in seawater, resulting in the formation of secondary ettringite [62] and calcite [61].
The pastes exposed to 0.5 M NaCl (Figure 6) were observed to form Friedel’s salt as a product of the reaction between phases C3A and C4AF, thereby limiting the secondary formation of ettringite, an expansive mineral with a consequently adverse effect on cement-based materials. The intensity of the respective diffraction lines was greater in pastes M1 and M2 than in OPC. The intensity of the lines for ettringite remained unchanged relative to the water-soaked pastes, a finding consistent with Ekolu et al. [63] reports to the effect that ettringite remains stable at NaCl concentrations of 0.5 M or lower.
Specimen exposure to the action of Na2SO4 (Figure 7) translated primarily into a rise in the intensity of the diffraction line for ettringite. The gypsum (Ca2SO4⋅2H2O) formation identified with SEM/EDX (see Section 3.4), could not be corroborated via XRD because the respective diffraction line overlapped with the reflections for other phases present in the pastes, such as the monocarboaluminates (2θ = 11.7°) and quartz (2θ = 20.8°) [64].
3.5. SEM/EDX Microstructural Analysis
Micrographs of the C-S-H gels in water-soaked pastes are reproduced in Figure 8a–c. The EDX analysis (ten analyses/gel) showed that the Ca/Si ratio in the OPC gel was 2.0, compared to 1.7 in M1 and 1.9 in M2. The findings also showed that adding SCMs induced aluminium uptake in the gel, as reported earlier by other authors who used pozzolanic additions to partially replace clinker [22,24,36,57]. Inasmuch as the aluminium may have replaced Si in the gel structure, those findings would explain the lower Ca/Si ratio in the pastes bearing SCMs. Compact portlandite plates were observed in all the pastes analysed, although less frequently and in smaller mean sizes in paste M1 and M2 than in OPC, due to the pozzolanicity of the former. Those findings were consistent with the XRD data.
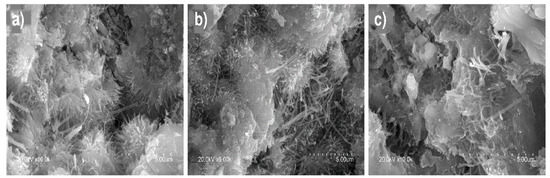
Figure 8.
Calcium silicate hydrates (C-S-H gels) in 56-d water-soaked pastes: (a) OPC (×6000); (b) M1 (×6000); (c) M2 (×10,000).
Magnesium in the form of brucite predominated in the surface layer observed to form in all the 56-d pastes exposed to seawater (Figure 9a–c), corroborating the XRD findings. Brucite, the result of the reaction between portlandite and the MgCl2 in seawater, was present on specimen surfaces only. The layer was thicker in the OPC (e = 107.1 μm) than in blend M1 (e = 82.4 μm) or M2 (e = 83.5 μm). Friedel’s salt formation was also observed in seawater-soaked pastes OPC, M1 and M2, corroborating the XRD findings (Section 3.3).
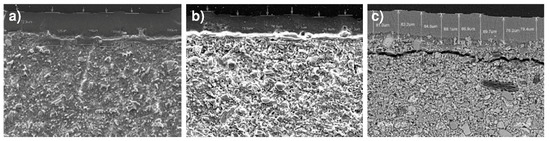
Figure 9.
Brucite formation in 56-d seawater-soaked pastes: (a) OPC (×200); (b) M1 (×200); (c) M2 (×250).
Plates identified as Friedel’s salt on the grounds of composition (Al, Cl and Ca) and morphology [60,65] were observed in the 56-d pastes exposed to 0.5 M NaCl (Figure 10).
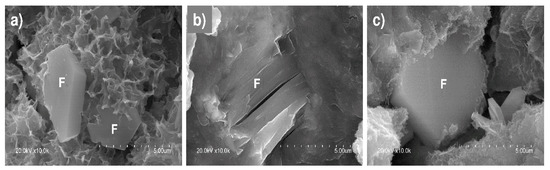
Figure 10.
Friedel’s salt in 56-d 0.5 M NaCl-soaked pastes (×10,000): (a) OPC; (b) M1; (c) M2.
The pastes exposed to the 0.3 M Na2SO4 solution were observed to form gypsum deposits clustering primarily in pores (Figure 11a), where the conditions for nucleation were most favourable [66,67]. Typically elongated needle-like ettringite (Figure 11b) was also identified [68,69], primarily inside pores [70], favoured by pressure conditions and the presence of the necessary ions [71,72]. For those very reasons, pores, together with cracks, are the sites where ettringite normally crystallises.
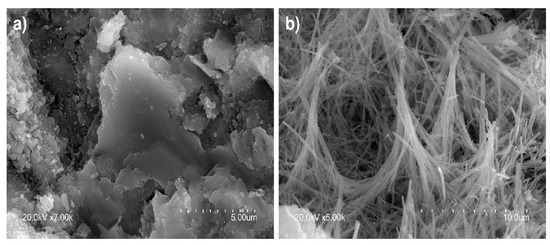
Figure 11.
(a) Gyspum deposits (×7000) and (b) needle-like ettringite (×5000).
4. Conclusions
The following conclusions can be drawn from this study.
- Pastes bearing 30% (20% UC&DW + 10%BA) SCMs exhibit 10% lower flexural strength than OPC and those with 45% (20%UC&DW + 20%BA + 5%SF) supplementary cementitious materials 11% lower strength than the 56-d water-soaked pastes.
- As the pozzolanic reaction between SCMs and portlandite does not suffice to offset the dilution induced by a lower proportion of cement, the new pastes exhibit a larger mean pore size and greater porosity than OPC.
- Including UC&DW + BA or UC&DW + BA + SF yields cements able to resist the chemical attacks analysed, with a 56-d corrosion index >0.7 in all cases.
- Resistance to aggressive media attack is not enhanced by raising the percentage of SCM added, for the cements bearing the binary blend UC&DW + BA proved to be more resistant than those to which silica fume was added.
- Paste M1 bearing 30% SCMs had a higher 56-d corrosion index, at 1.06, for the 0.5 M NaCl solution than both OPC (CI = 1.04) and M2 (CI = 1.02).
- OPC paste resists seawater and 0.3 M Na2SO4 better than M1, which in turn had a higher corrosion index than M2 irrespective of the medium.
The addition of UC&DW + BA or UC&DW + BA + SF prompts no significant change in cement paste morphology or in the compounds generated during the chemical attack.
Author Contributions
Conceptualisation, I.F.S.d.B., M.I.S.d.R., G.M., S.B., and C.M.; methodology, I.F.S.d.B., and G.M.; formal analysis, I.F.S.d.B., and G.M.; investigation, S.B., I.F.S.d.B., and G.M.; writing—original draft preparation, I.F.S.d.B., and G.M.; writing—review and editing, I.F.S.d.B., G.M., M.I.S.d.R., and C.M.; supervision, I.F.S.d.B., M.I.S.d.R. and C.M.; project administration, I.F.S.d.B. and G.M.; funding acquisition, M.I.S.d.R., and C.M. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Spanish Ministry for Science and Innovation under project PID2019-107238RB-C21/AEI/10.13039/501100011033. It benefited as well from funding furnished by the Consejería de Economía, Ciencia y Agenda Digital from Junta de Extremadura under grant GR 18122 awarded to the MATERIA research group.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Acknowledgments
The cement supplied by the Lafarge Group plant Villaluenga de la Sagra, Toledo, Spain, is gratefully acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Guo, H.; Dong, Y.; Gu, X. Durability assessment of reinforced concrete structures considering global warming: A performance-based engineering and experimental approach. Constr. Build. Mater. 2020, 233, 117251. [Google Scholar] [CrossRef]
- Islam, R.; Nazifa, T.H.; Mohammed, S.F.; Zishan, M.A.; Yusof, Z.M.; Mong, S.G. Impacts of design deficiencies on maintenance cost of high-rise residential buildings and mitigation measures. J. Build. Eng. 2021, 39, 10221. [Google Scholar]
- Yang, K.-H.; Lim, H.S.; Kwon, S.J.; Kim, J.H. Repair cost estimation techniques for reinforced concrete structures located at the seashore: Considering various probabilistic service life functions and actual mix proportions. Constr. Build. Mater. 2020, 256, 119469. [Google Scholar] [CrossRef]
- Sánchez de Rojas, M.I.; Frías, M.; Sabador, E.; Asensio, E.; Rivera, J.; Medina, C. Durability and chromatic behavior in cement pastes containing ceramic industry milling and glazing by-products. J. Am. Ceram. Soc. 2019, 102, 1971–1981. [Google Scholar] [CrossRef]
- Claisse, P.A. Cements and cement replacement materials. In Civil Engineering Materials; Butterworth-Heinemann: Oxford, UK, 2016; pp. 163–176. [Google Scholar]
- Kumar Metha, P.; Monteiro, P.J.M. Concrete: Microstructure, Properties and Materials, 3rd ed.; McGraw-Hill: New York, NY, USA, 2006; p. 659. [Google Scholar]
- UK Quality Ash Association. Fact Sheet 18—Embodied CO2e of UK cement, additions and cementitious materials. Available online: https://cement.mineralproducts.org/documents/Factsheet_18.pdf (accessed on 15 June 2021).
- European Committee for Standardization. EN 197-1. Cement. Composition, Specifications and Conformity Criteria for Common Cements; European Committee for Standardization: Geneva, Switzerland, 2011. [Google Scholar]
- Villoria-Sáez, P.; Porras-Amores, C.; del Río Merino, M. Estimation of construction and demolition waste. In Advances in Construction and Demolition Waste Recycling; Woodhead Publishing: Sawston, UK, 2020; pp. 13–30. [Google Scholar]
- Velardo, P.; Sáez del Bosque, I.F.; Matías, A.; Sánchez de Rojas, M.I.; Medina, C. Properties of concretes bearing mixed recycled aggregate with polymer-modified surfaces. J. Build. Eng. 2021, 38, 102211. [Google Scholar] [CrossRef]
- Gonzalez-Fonteboa, B.; Seara-Paz, S.; de Brito, J.; Gonzalez-Taboada, I.; Martinez-Abella, F.; Vasco-Silva, R. Recycled concrete with coarse recycled aggregate. An overview and analysis. Mater. Constr. 2018, 68, e151. [Google Scholar] [CrossRef] [Green Version]
- Plaza, P.; Sáez del Bosque, I.F.; Frías, M.; Sánchez de Rojas, M.I.; Medina, C. Use of recycled coarse and fine aggregates in structural eco-concretes. Physical and mechanical properties and CO2 emissions. Constr. Build. Mater. 2021, 285, 122926. [Google Scholar] [CrossRef]
- Moreno-Juez, J.; Vegas, I.J.; Gebremariam, A.T.; García-Cortés, V.; di Maio, F. Treatment of end-of-life concrete in an innovative heating-air classification system for circular cement-based products. J. Clean Prod. 2020, 263, 121515. [Google Scholar] [CrossRef]
- Letelier, V.; Tarela, E.; Muñoz, P.; Moriconi, G. Combined effects of recycled hydrated cement and recycled aggregates on the mechanical properties of concrete. Constr. Build. Mater. 2017, 132, 365–375. [Google Scholar] [CrossRef]
- Xiao, J.; Xiao, Y.; Liu, Y.; Ding, T. Carbon emission analyses of concretes made with recycled materials considering CO2 uptake through carbonation absorption. Struct. Concr. 2021, 22, E58–E73. [Google Scholar] [CrossRef]
- Sun, C.; Chen, L.; Xiao, J.; Singh, A.; Zeng, J. Compound utilization of construction and industrial waste as cementitious recycled powder in mortar. Resour. Conserv. Recycl. 2021, 170, 105561. [Google Scholar] [CrossRef]
- Cantero, B.; Bravo, M.; de Brito, J.; Sáez del Bosque, I.F.; Medina, C. Assessment of the Permeability to Aggressive Agents of Concrete with Recycled Cement and Mixed Recycled Aggregate. Appl. Sci. 2021, 11, 3856. [Google Scholar] [CrossRef]
- Caneda-Martínez, L.; Monasterio, M.; Moreno-Juez, J.; Martínez-Ramírez, S.; García, R.; Frías, M. Behaviour and Properties of Eco-Cement Pastes Elaborated with Recycled Concrete Powder from Construction and Demolition Wastes. Materials 2021, 14, 1299. [Google Scholar] [CrossRef] [PubMed]
- Asensio, E.; Medina, C.; Frías, M.; Sánchez de Rojas, M.I. Use of clay-based construction and demolition waste as additions in the design of new low and very low heat of hydration cements. Mater. Struct. 2018, 51, 101. [Google Scholar] [CrossRef]
- Asensio, E.; Medina, C.; Frías, M.; Sánchez de Rojas, M.I. Clay-based construction and demolition waste as a pozzolanic addition in blended cements. Effect on sulfate resistance. Constr. Build. Mater. 2016, 127, 950–958. [Google Scholar] [CrossRef]
- Asensio, E.; Medina, C.; Frías, M.; Sánchez de Rojas, M.I. Characterization of Ceramic-Based Construction and Demolition Waste: Use as Pozzolan in Cements. J. Am. Ceram. Soc. 2016, 99, 4121–4127. [Google Scholar] [CrossRef]
- Asensio, E.; Medina, C.; Frías, M.; Sánchez de Rojas, M.I. Fired clay-based construction and demolition waste as pozzolanic addition in cements. Design of new eco-efficient cements. J. Clean Prod. 2020, 265, 121610. [Google Scholar] [CrossRef]
- Sánchez de Rojas, M.I.; Marin, F.; Rivera, J.; Frias, M. Morphology and properties in blended cements with ceramic wastes as a pozzolanic material. J. Am. Ceram. Soc. 2006, 89, 3701–3705. [Google Scholar] [CrossRef]
- Medina, C.; Saez del Bosque, I.F.; Asensio, E.; Frias, M.; Sanchez de Rojas, M.I. Mineralogy and Microstructure of Hydrated Phases During the Pozzolanic Reaction in the Sanitary Ware Waste/Ca(OH)2 System. J. Am. Ceram. Soc. 2016, 99, 340–348. [Google Scholar] [CrossRef]
- Medina, J.M.; Sánchez de Rojas, M.I.; Sáez del Bosque, I.F.; Frías, M.; Medina, C. Sulfate Resistance in Cements Bearing Bottom Ash from Biomass-Fired Electric Power Plants. Appl. Sci. 2020, 10, 8982. [Google Scholar] [CrossRef]
- Medina, C.; Sáez del Bosque, I.F.; Frías, M.; Sánchez de Rojas, M.I. Design and characterisation of ternary cements containing rice husk ash and fly ash. Constr. Build. Mater. 2018, 187, 65–76. [Google Scholar] [CrossRef]
- Frías, M.; Savastano, H.; Villar, E.; Sánchez de Rojas, M.I.; Santos, S. Characterization and properties of blended cement matrices containing activated bamboo leaf wastes. Cem. Concr. Compos. 2012, 34, 1019–1023. [Google Scholar] [CrossRef]
- Gonzalez-Kunz, R.N.; Pineda, P.; Bras, A.; Morillas, L. SPlant biomass ashes in cement-based building materials. Feasibility as eco-efficient structural mortars and grouts. Sust. Cities Soc. 2017, 31, 151–172. [Google Scholar] [CrossRef]
- Rajamma, R.; Senff, L.; Ribeiro, M.J.; Labrincha, J.A.; Ball, R.J.; Allen, G.C.; Ferreira, V.M. Biomass fly ash effect on fresh and hardened state properties of cement based materials. Compos. Pt. B Eng. 2015, 77, 1–9. [Google Scholar] [CrossRef]
- Ohenoja, K.; Wigren, V.; Osterbacka, J.; Illikainen, M. Mechanically Treated Fly Ash from Fluidized Bed Combustion of Peat, Wood, and Wastes in Concrete. Waste Biomass Valorization 2020, 11, 3071–3079. [Google Scholar] [CrossRef] [Green Version]
- Sáez del Bosque, I.F.; Sánchez de Rojas, M.I.; Asensio, E.; Frías, M.; Medina, C. Industrial waste from biomass-fired electric power plants as alternative pozzolanic materials. In Waste and By-Products in Cement-Based Materials; Woodhead Publishing: Sawston, UK, 2021; pp. 243–282. [Google Scholar]
- Medina, J.M.; Sáez del Bosque, I.F.; Frías, M.; Sánchez de Rojas, M.I.; Medina, C. Characterisation and valorisation of biomass waste as a possible addition in eco-cement design. Mater. Struct. 2017, 50, 207. [Google Scholar] [CrossRef]
- Carevic, I.; Baricevic, A.; Stirmer, N.; Bajto, J.S. Correlation between physical and chemical properties of wood biomass ash and cement composites performances. Constr. Build. Mater. 2020, 256, 119450. [Google Scholar] [CrossRef]
- Medina, J.M.; Sáez del Bosque, I.F.; Frías, M.; Sánchez de Rojas, M.I.; Medina, C. Design and properties of eco-friendly binary mortars containing ash from biomass-fuelled power plants. Cem. Concr. Compos. 2019, 104, 103372. [Google Scholar] [CrossRef]
- Agrela, F.; Cabrera, M.; Morales, M.M.; Zamorano, M.; Alshaaer, M. Biomass fly ash and biomass bottom ash. In New Trends in Eco-Efficient and Recycled Concrete; Woodhead Publishing: Sawston, UK, 2019; pp. 23–58. [Google Scholar]
- Sáez del Bosque, I.F.; Medina, J.M.; Frías, M.; Sánchez de Rojas, M.I.; Medina, C. Use of biomass-fired power plant bottom ash as an addition in new blended cements: Effect on the structure of the C-S-H gel formed during hydration. Constr. Build. Mater. 2019, 228, 117081. [Google Scholar] [CrossRef]
- Koch, A.; Steinegger, U. A rapid test for cements for their behaviour under sulphate attack. Zem-Kalk-Gips 1960, 7, 317–324. [Google Scholar]
- Cantero, B.; Sáez del Bosque, I.F.; Matías, A.; Sánchez de Rojas, M.I.; Medina, C. Effect of Recycled Aggregate on Performance of Granular Skeleton. ACI Mater. J. 2020, 117, 113–124. [Google Scholar] [CrossRef]
- American Society for Testing and Material. ASTM D1141. Standard Practice for the Preparation of Substitute Ocean Water; ASTM: West Conshohocken, PA, USA, 2013. [Google Scholar]
- Medina, G.; Sáez del Bosque, I.F.; Frias, M.; Sánchez de Rojas, M.I.; Medina, C. Sulfate Resistance in Cements Bearing Ornamental Granite Industry Sludge. Materials 2020, 13, 4081. [Google Scholar] [CrossRef]
- Irassar, E.F. Sulfate resistance of blended cement: Prediction and relation with flexural strength. Cem. Concr. Res. 1990, 20, 209–218. [Google Scholar] [CrossRef]
- American Society for Testing and Material. D 4404-84: Test Method for Determination of Pore Volume and Pore Volume Distribution of Soil and Rock by Mercury Intrusion Porosimetry; ASTM: West Conshohocken, PA, USA, 2004. [Google Scholar]
- Medina, G.; Sáez del Bosque, I.F.; Frías, M.; Sánchez de Rojas, M.I.; Medina, C. Effect of Granite Waste on Binary Cement Hydration and Paste Performance: Statistical Analysis. ACI Mater. J. 2019, 116, 63–72. [Google Scholar] [CrossRef]
- Medina, G.; Sáez del Bosque, I.F.; Frías, M.; Sánchez de Rojas, M.I.; Medina, C. Mineralogical study of granite waste in a pozzolan/Ca(OH)2 system: Influence of the activation process. Appl. Clay Sci. 2017, 135, 362–371. [Google Scholar] [CrossRef]
- Scherer, G.W. Stress from crystallization of salt. Cem. Concr. Res. 2004, 34, 1613–1624. [Google Scholar] [CrossRef]
- Li, G.; Zhang, A.; Song, Z.; Liu, S.; Zhang, J. Ground granulated blast furnace slag effect on the durability of ternary cementitious system exposed to combined attack of chloride and sulfate. Constr. Build. Mater. 2018, 158, 640–648. [Google Scholar] [CrossRef]
- Li, S.; Roy, D.M. Investigation of relations between porosity, pore structure, and C1—Diffusion of fly ash and blended cement pastes. Cem. Concr. Res. 1986, 16, 749–759. [Google Scholar] [CrossRef]
- Florea, M.V.A.; Brouwers, H.J.H. Modelling of chloride binding related to hydration products in slag-blended cements. Constr. Build. Mater. 2014, 64, 421–430. [Google Scholar] [CrossRef] [Green Version]
- Goni, S.; Frias, M.; Vigil de la Villa, R.; Garcia, R. Sodium chloride effect on durability of ternary blended cement. Microstructural characterization and strength. Compos. Pt. B Eng. 2013, 54, 163–168. [Google Scholar] [CrossRef]
- Villar-Cociña, E.; Morales, E.V.; Santos, S.F.; Savastano, H.; Frías, M. Pozzolanic behavior of bamboo leaf ash: Characterization and determination of the kinetic parameters. Cem. Concr. Compos. 2011, 33, 68–73. [Google Scholar] [CrossRef]
- McCarthy, M.J.; Dyer, T.D. Pozzolanas and Pozzolanic Materials. In Lea’s Chemistry of Cement and Concrete, 5th ed.; Butterworth-Heinemann: Oxfrod, UK, 2019; pp. 363–467. [Google Scholar]
- Bassuoni, M.T.; Rahman, M.M. Response of concrete to accelerated physical salt attack exposure. Cem. Concr. Res. 2016, 79, 395–408. [Google Scholar] [CrossRef]
- Frias, M.; Goni, S.; Garcia, R.; Vigil, R. Seawater effect on durability of ternary cements. Synergy of chloride and sulphate ions. Compos. Pt. B Eng. 2013, 46, 173–178. [Google Scholar] [CrossRef]
- Ikumi, T.; Cavalaro, S.H.P.; Segura, I. The role of porosity in external sulphate attack. Cem. Concr. Compos. 2019, 97, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Ikumi, T.; Segura, I. Numerical assessment of external sulfate attack in concrete structures. A review. Cem. Concr. Res. 2019, 121, 91–105. [Google Scholar] [CrossRef]
- Liu, K.; Sun, D.; Wang, A.; Zhang, G.; Tang, J. Long-Term Performance of Blended Cement Paste Containing Fly Ash against Sodium Sulfate Attack. J. Mater. Civ. Eng. 2018, 30, 04018309. [Google Scholar] [CrossRef]
- Taylor, H.F.W. Cement Chemistry, 2nd ed.; Thomas Telford Publishing: London, UK, 1997. [Google Scholar]
- Richardson, I.G. The nature of the hydration products in hardened cement pastes. Cem. Concr. Compos. 2000, 22, 97–113. [Google Scholar] [CrossRef]
- Saez del Bosque, I.F.; van den Heede, P.; de Belie, N.; Sanchez de Rojas, M.I.; Medina, C. Freeze-thaw resistance of concrete containing mixed aggregate and construction and demolition waste-additioned cement in water and de-icing salts. Constr. Build. Mater. 2020, 259, 119772. [Google Scholar] [CrossRef]
- Friedel, P.M. Sur un chloro-aluminate de calcium hydraté se maclant par compression. Bull. Soc. Franç. Minéral. 1987, 19, 122–136. [Google Scholar]
- De Weerdt, K.; Lothenbach, B.; Geiker, M.R. Comparing chloride ingress from seawater and NaCl solution in Portland cement mortar. Cem. Concr. Res. 2019, 115, 80–89. [Google Scholar] [CrossRef]
- Geng, J.; Easterbrook, D.; Li, L.; Mo, L. The stability of bound chlorides in cement paste with sulfate attack. Cem. Concr. Res. 2015, 68, 211–222. [Google Scholar] [CrossRef]
- Ekolu, S.O.; Thomas, M.D.A.; Hooton, R.D. Pessimum effect of externally applied chlorides on expansion due to delayed ettringite formation: Proposed mechanism. Cem. Concr. Res. 2006, 36, 688–696. [Google Scholar] [CrossRef]
- Caneda-Martínez, L.; Kunther, W.; Medina, C.; Sánchez de Rojas, M.I.; Frías, M. Exploring sulphate resistance of coal mining waste blended cements through experiments and thermodynamic modelling. Cem. Concr. Compos. 2021, 121, 104086. [Google Scholar] [CrossRef]
- Birnin-Yauri, U.A.; Glasser, F.P. Friedel’s salt, Ca2Al(OH)6(Cl,OH)·2H2O: Its solid solutions and their role in chloride binding. Cem. Concr. Res. 1998, 28, 1713–1723. [Google Scholar] [CrossRef]
- Santhanam, M.; Cohen, M.D.; Olek, J. Mechanism of sulfate attack: A fresh look: Part 2. Proposed mechanisms. Cem. Concr. Res. 2003, 33, 341–346. [Google Scholar] [CrossRef]
- Xiong, C.; Jiang, L.; Xu, Y.; Song, Z.; Chu, H.; Guo, Q. Influences of exposure condition and sulfate salt type on deterioration of paste with and without fly ash. Constr. Build. Mater. 2016, 113, 951–963. [Google Scholar] [CrossRef]
- Komatsu, R.; Mizukoshi, N.; Makida, K.; Tsukamoto, K. In-situ observation of ettringite crystals. J. Cryst. Growth 2009, 311, 1005–1008. [Google Scholar] [CrossRef]
- Hewlett, P.C. Lea’s Chemistry of Cement and Concrete, 4th ed.; Butterworth-Heinemann: Oxfrod, UK, 1998; p. 1053. [Google Scholar]
- Neville, A.M. Properties of Concrete, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 2008; p. 844. [Google Scholar]
- Liu, Z.; Deng, D.; de Schutter, G. Does concrete suffer sulfate salt weathering? Constr. Build. Mater. 2014, 66, 692–701. [Google Scholar] [CrossRef]
- Taylor, H.F.W.; Famy, C.; Scrivener, K.L. Delayed ettringite formation. Cem. Concr. Res. 2001, 31, 683–693. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).