Fabrication of Conductive and Gas-Sensing Microstructures Using Focused Deposition of Copper Nanoparticles Synthesized by Spark Discharge
Abstract
1. Introduction
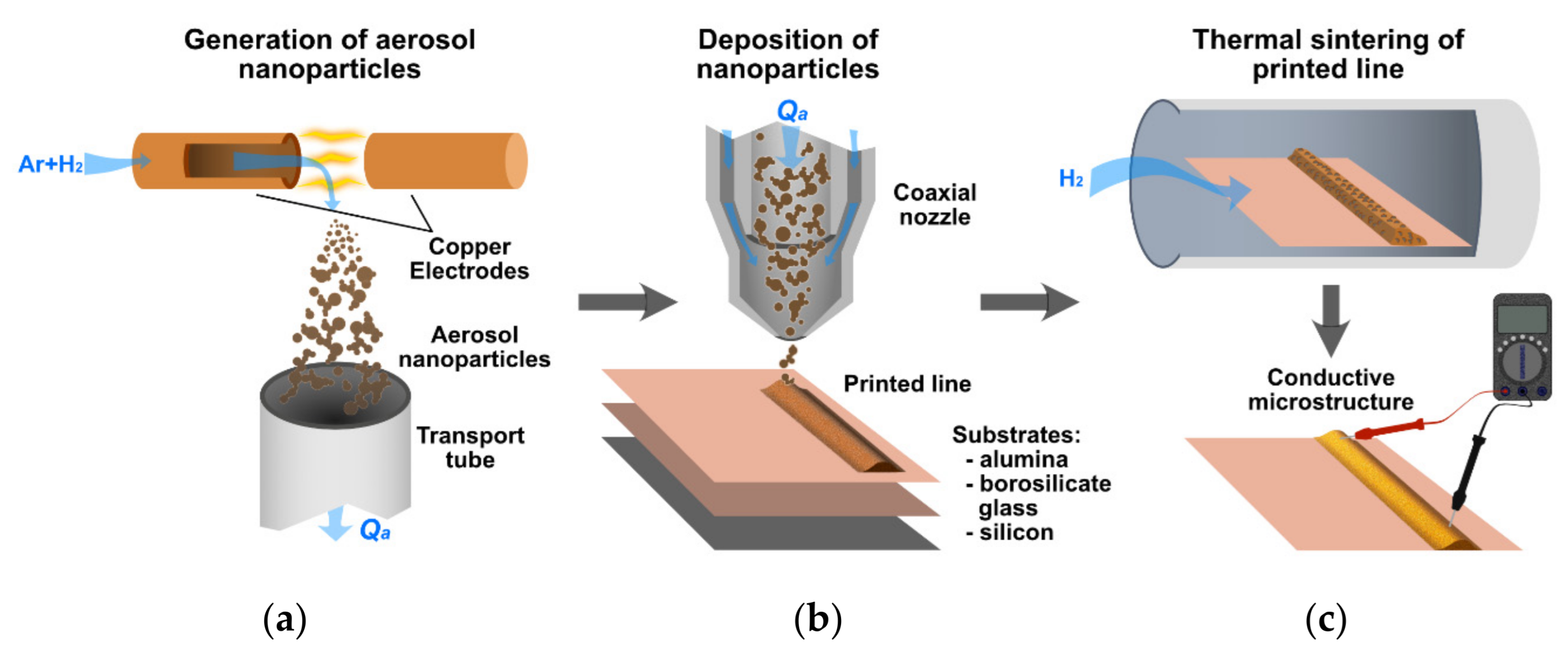
2. Materials and Methods
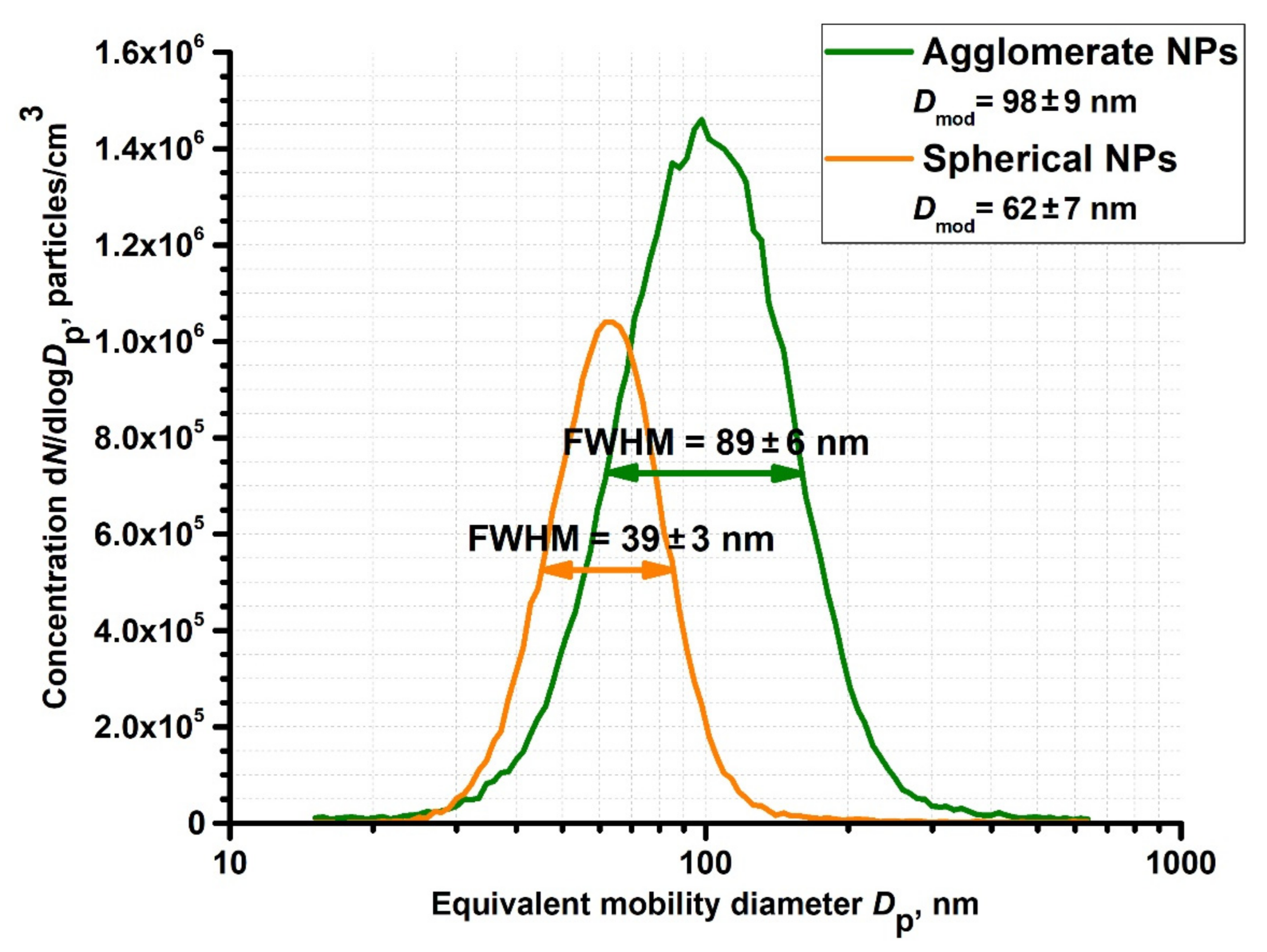
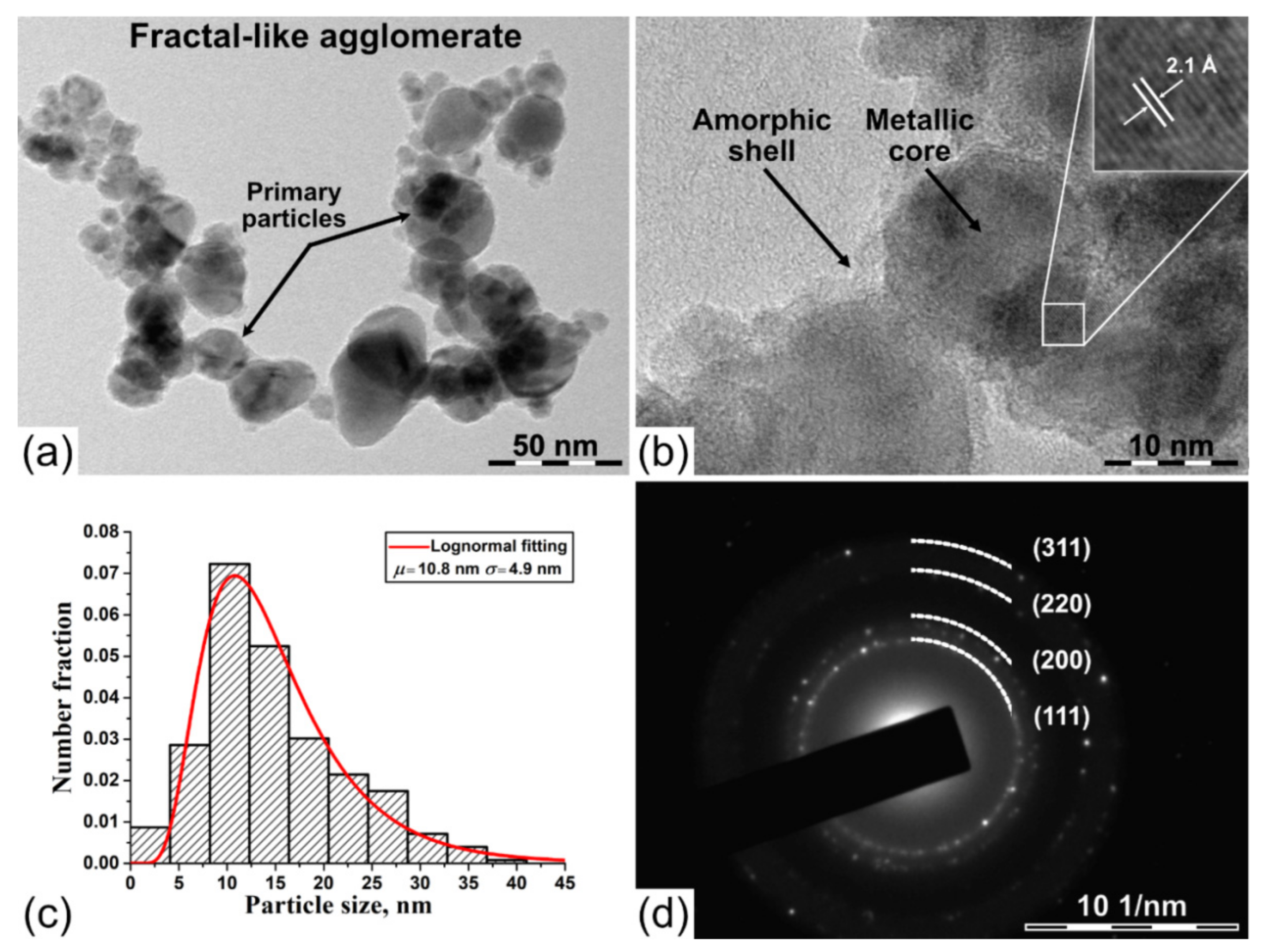
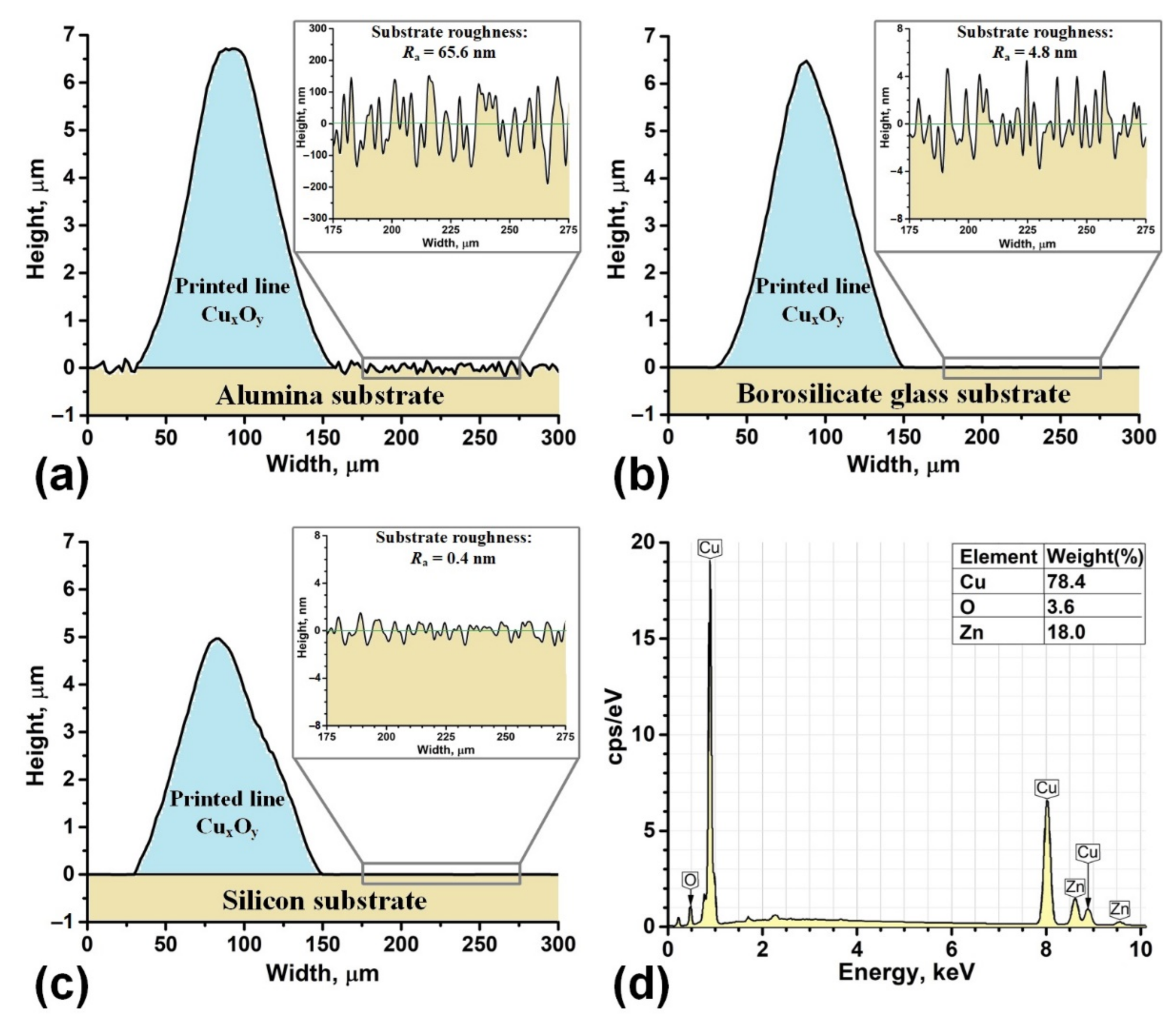
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Kassem, O.; Saadaoui, M.; Rieu, M.; Viricelle, J.-P. A Novel Approach to a Fully Inkjet Printed SnO2-Based Gas Sensor on a Flexible Foil. J. Mater. Chem. C 2019, 7, 12343–12353. [Google Scholar] [CrossRef]
- Singh, R.; Singh, E.; Nalwa, H.S. Inkjet Printed Nanomaterial Based Flexible Radio Frequency Identification (RFID) Tag Sensors for the Internet of Nano Things. RSC Adv. 2017, 7, 48597–48630. [Google Scholar] [CrossRef]
- Han, G.D.; Bae, K.; Kang, E.H.; Choi, H.J.; Shim, J.H. Inkjet Printing for Manufacturing Solid Oxide Fuel Cells. ACS Energy Lett. 2020, 5, 1586–1592. [Google Scholar] [CrossRef]
- Zhu, H.; Shin, E.-S.; Liu, A.; Ji, D.; Xu, Y.; Noh, Y.-Y. Printable Semiconductors for Backplane TFTs of Flexible OLED Displays. Adv. Funct. Mater. 2020, 30, 1904588. [Google Scholar] [CrossRef]
- Wu, C.-J.; Sheng, Y.-J.; Tsao, H.-K. Copper Conductive Lines on Flexible Substrates Fabricated at Room Temperature. J. Mater. Chem. C 2016, 4, 3274–3280. [Google Scholar] [CrossRef]
- Arsenov, P.V.; Efimov, A.A.; Ivanov, V.V. Optimizing Aerosol Jet Printing Process of Platinum Ink for High-Resolution Conductive Microstructures on Ceramic and Polymer Substrates. Polymers 2021, 13, 918. [Google Scholar] [CrossRef]
- Nayak, L.; Mohanty, S.; Nayak, S.K.; Ramadoss, A. A Review on Inkjet Printing of Nanoparticle Inks for Flexible Electronics. J. Mater. Chem. C 2019, 7, 8771–8795. [Google Scholar] [CrossRef]
- Wilkinson, N.J.; Smith, M.A.A.; Kay, R.W.; Harris, R.A. A Review of Aerosol Jet Printing—a Non-Traditional Hybrid Process for Micro-Manufacturing. Int. J. Adv. Manuf. Technol. 2019. [Google Scholar] [CrossRef]
- Suikkola, J.; Björninen, T.; Mosallaei, M.; Kankkunen, T.; Iso-Ketola, P.; Ukkonen, L.; Vanhala, J.; Mäntysalo, M. Screen-Printing Fabrication and Characterization of Stretchable Electronics. Sci. Rep. 2016, 6, 25784. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, F.S.; Simonenko, N.P.; Trouillet, V.; Volkov, I.A.; Plugin, I.A.; Rupasov, D.P.; Mokrushin, A.S.; Nagornov, I.A.; Simonenko, T.L.; Vlasov, I.S.; et al. Microplotter-Printed On-Chip Combinatorial Library of Ink-Derived Multiple Metal Oxides as an “Electronic Olfaction” Unit. ACS Appl. Mater. Interfaces 2020, 12, 56135–56150. [Google Scholar] [CrossRef]
- Gao, D.; Zhou, J. Designs and Applications of Electrohydrodynamic 3D Printing. Int. J. Bioprinting 2019, 5. [Google Scholar] [CrossRef] [PubMed]
- Wegner, K.; Vinati, S.; Piseri, P.; Antonini, A.; Zelioli, A.; Barborini, E.; Ducati, C.; Milani, P. High-Rate Production of Functional Nanostructured Films and Devices by Coupling Flame Spray Pyrolysis with Supersonic Expansion. Nanotechnology 2012, 23, 185603. [Google Scholar] [CrossRef]
- Kim, H.; Kim, J.; Yang, H.; Suh, J.; Kim, T.; Han, B.; Kim, S.; Kim, D.S.; Pikhitsa, P.V.; Choi, M. Parallel Patterning of Nanoparticles via Electrodynamic Focusing of Charged Aerosols. Nat. Nanotechnol. 2006, 1, 117–121. [Google Scholar] [CrossRef]
- Lee, G.-Y.; Park, J.-I.; Kim, C.-S.; Yoon, H.-S.; Yang, J.; Ahn, S.-H. Aerodynamically Focused Nanoparticle (AFN) Printing: Novel Direct Printing Technique of Solvent-Free and Inorganic Nanoparticles. ACS Appl. Mater. Interfaces 2014, 6, 16466–16471. [Google Scholar] [CrossRef]
- Efimov, A.A.; Potapov, G.N.; Nisan, A.V.; Ivanov, V.V. Controlled Focusing of Silver Nanoparticles Beam to Form the Microstructures on Substrates. Results Phys. 2017, 7, 440–443. [Google Scholar] [CrossRef]
- Aghajani, S.; Accardo, A.; Tichem, M. Aerosol Direct Writing and Thermal Tuning of Copper Nanoparticle Patterns as Surface-Enhanced Raman Scattering Sensors. ACS Appl. Nano Mater. 2020, 3, 5665–5675. [Google Scholar] [CrossRef]
- Khabarov, K.; Kornyushin, D.; Masnaviev, B.; Tuzhilin, D.; Saprykin, D.; Efimov, A.; Ivanov, V. The Influence of Laser Sintering Modes on the Conductivity and Microstructure of Silver Nanoparticle Arrays Formed by Dry Aerosol Printing. Appl. Sci. 2020, 10, 246. [Google Scholar] [CrossRef]
- Meuller, B.O.; Messing, M.E.; Engberg, D.L.J.; Jansson, A.M.; Johansson, L.I.M.; Norlén, S.M.; Tureson, N.; Deppert, K. Review of Spark Discharge Generators for Production of Nanoparticle Aerosols. Aerosol Sci. Technol. 2012, 46, 1256–1270. [Google Scholar] [CrossRef]
- Teo, B.H.; Khanna, A.; Shanmugam, V.; Aguilar, M.L.O.; Delos Santos, M.E.; Chua, D.J.W.; Chang, W.-C.; Mueller, T. Development of Nanoparticle Copper Screen Printing Pastes for Silicon Heterojunction Solar Cells. Sol. Energy 2019, 189, 179–185. [Google Scholar] [CrossRef]
- Norita, S.; Kumaki, D.; Kobayashi, Y.; Sato, T.; Fukuda, K.; Tokito, S. Inkjet-Printed Copper Electrodes Using Photonic Sintering and Their Application to Organic Thin-Film Transistors. Org. Electron. 2015, 25, 131–134. [Google Scholar] [CrossRef]
- Li, W.; Zhang, H.; Gao, Y.; Jiu, J.; Li, C.-F.; Chen, C.; Hu, D.; Goya, Y.; Wang, Y.; Koga, H.; et al. Highly Reliable and Highly Conductive Submicron Cu Particle Patterns Fabricated by Low Temperature Heat-Welding and Subsequent Flash Light Sinter-Reinforcement. J. Mater. Chem. C 2017, 5, 1155–1164. [Google Scholar] [CrossRef]
- Rizwan, M.; Kutty, A.A.; Kgwadi, M.; Drysdale, T.D.; Sydänheimo, L.; Ukkonen, L.; Virkki, J. Possibilities of Fabricating Copper-Based RFID Tags With Photonic-Sintered Inkjet Printing and Thermal Transfer Printing. IEEE Antennas Wirel. Propag. Lett. 2017, 16, 1828–1831. [Google Scholar] [CrossRef]
- Li, W.; Sun, Q.; Li, L.; Jiu, J.; Liu, X.-Y.; Kanehara, M.; Minari, T.; Suganuma, K. The Rise of Conductive Copper Inks: Challenges and Perspectives. Appl. Mater. Today 2020, 18, 100451. [Google Scholar] [CrossRef]
- Yablokov, M.; Vasiliev, A.; Varfolomeev, A.; Zavyalov, S. Room Temperature Gas Multisensor System Based on a Novel Polymer Nanocomposite Material. Procedia Eng. 2014, 87, 152–155. [Google Scholar] [CrossRef][Green Version]
- Mylnikov, D.; Efimov, A.; Ivanov, V. Measuring and Optimization of Energy Transfer to the Interelectrode Gaps during the Synthesis of Nanoparticles in a Spark Discharge. Aerosol Sci. Technol. 2019, 1393–1403. [Google Scholar] [CrossRef]
- Khabarov, K.; Urazov, M.; Lizunova, A.; Kameneva, E.; Efimov, A.; Ivanov, V. Influence of Ag Electrodes Asymmetry Arrangement on Their Erosion Wear and Nanoparticle Synthesis in Spark Discharge. Appl. Sci. 2021, 11, 4147. [Google Scholar] [CrossRef]
- Binder, S.; Glatthaar, M.; Rädlein, E. Analytical Investigation of Aerosol Jet Printing. Aerosol Sci. Technol. 2014, 48, 924–929. [Google Scholar] [CrossRef]
- Secor, E.B. Principles of Aerosol Jet Printing. Flex. Print. Electron. 2018, 3, 035002. [Google Scholar] [CrossRef]
- On the Approval of Sanitary Rules and Norms SanPiN 1.2.3685-21 “Hygienic Norms and Requirements for Ensuring Safety and (Or) Harmlessness for Human Environmental Factors”—Docs.Cntd.Ru. Available online: https://docs.cntd.ru/document/573500115 (accessed on 10 June 2021).
- Ivanov, V.V.; Efimov, A.A.; Myl’nikov, D.A.; Lizunova, A.A. Synthesis of Nanoparticles in a Pulsed-Periodic Gas Discharge and Their Potential Applications. Russ. J. Phys. Chem. A 2018, 92, 607–612. [Google Scholar] [CrossRef]
- Goudeli, E. Chapter 4—Formation and growth of fractal-like agglomerates and aggregates in the gas phase. In Frontiers of Nanoscience; Computational Modelling of Nanomaterials; Grammatikopoulos, P., Ed.; Elsevier: London, UK, 2020; Volume 17, pp. 41–60. [Google Scholar]
- Lizunova, A.A.; Efimov, A.A.; Arsenov, P.V.; Ivanov, V.V. Influence of the Sintering Temperature on Morphology and Particle Size of Silver Synthesized by Spark Discharge. IOP Conf. Ser. Mater. Sci. Eng. 2018, 307, 012081. [Google Scholar] [CrossRef]
- Fu, Q.; Stein, M.; Li, W.; Zheng, J.; Kruis, F.E. Conductive Films Prepared from Inks Based on Copper Nanoparticles Synthesized by Transferred Arc Discharge. Nanotechnology 2019, 31, 025302. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Lee, G.; Rhee, C.; Lee, M. Expeditious Low-Temperature Sintering of Copper Nanoparticles with Thin Defective Carbon Shells. Nanoscale 2015, 7, 6627–6635. [Google Scholar] [CrossRef] [PubMed]
- Beedasy, V.; Smith, P.J. Printed Electronics as Prepared by Inkjet Printing. Materials 2020, 13, 704. [Google Scholar] [CrossRef] [PubMed]
- Efimov, A.A.; Arsenov, P.V.; Borisov, V.I.; Buchnev, A.I.; Lizunova, A.A.; Kornyushin, D.V.; Tikhonov, S.S.; Musaev, A.G.; Urazov, M.N.; Shcherbakov, M.I.; et al. Synthesis of Nanoparticles by Spark Discharge as a Facile and Versatile Technique of Preparing Highly Conductive Pt Nano-Ink for Printed Electronics. Nanomaterials 2021, 11, 234. [Google Scholar] [CrossRef]
- Rydosz, A. The Use of Copper Oxide Thin Films in Gas-Sensing Applications. Coatings 2018, 8, 425. [Google Scholar] [CrossRef]
- Kim, I.; Kim, J. The Effect of Reduction Atmospheres on the Sintering Behaviors of Inkjet-Printed Cu Interconnectors. J. Appl. Phys. 2010, 108, 102807. [Google Scholar] [CrossRef]
- Magdassi, S.; Grouchko, M.; Kamyshny, A. Copper Nanoparticles for Printed Electronics: Routes Towards Achieving Oxidation Stability. Materials 2010, 3, 4626–4638. [Google Scholar] [CrossRef]
- Reisman, A.; Berkenblit, M.; Ghez, R.; Chan, S.A. The Equilibrium Constant for the Reaction of ZnO + H2 and the Chemical Vapor Transport of Zno via the Zn + H2O Reaction. J. Electron. Mater. 1972, 1, 395–419. [Google Scholar] [CrossRef]
- Qi, J.; Hu, X. The Loss of ZnO as the Support for Metal Catalysts by H2 Reduction. Phys. Chem. Chem. Phys. 2020, 22, 3953–3958. [Google Scholar] [CrossRef]
- German, R.M. Chapter Thirteen—Nanoscale Sintering. In Sintering: From Empirical Observations to Scientific Principles; German, R.M., Ed.; Butterworth-Heinemann: Boston, MA, USA, 2014; pp. 413–432. ISBN 978-0-12-401682-8. [Google Scholar]
- Kornyushin, D.V.; Efimov, A.A.; Khabarov, K.M.; Ivanov, V.V. The Influence of the Morphology of Oxidized Copper Nanoparticles on the Electrical Properties of Microstructures Obtained by Dry Aerosol Printing. J. Phys. Conf. Ser. 2020, 1695, 012032. [Google Scholar] [CrossRef]
- Ragulya, A.V. Consolidation of Ceramic Nanopowders. Adv. Appl. Ceram. 2008, 107, 118–134. [Google Scholar] [CrossRef]
- Ferkel, H.; Hellmig, R.J. Effect of Nanopowder Deagglomeration on the Densities of Nanocrystalline Ceramic Green Bodies and Their Sintering Behaviour. Nanostruct. Mater. 1999, 11, 617–622. [Google Scholar] [CrossRef]
- Giuntini, D.; Bordia, R.K.; Olevsky, E.A. Feasibility of in Situ De-Agglomeration during Powder Consolidation. J. Am. Ceram. Soc. 2019, 102, 628–643. [Google Scholar] [CrossRef]
- Steinhauer, S. Gas Sensors Based on Copper Oxide Nanomaterials: A Review. Chemosensors 2021, 9, 51. [Google Scholar] [CrossRef]
- Lin, T.; Lv, X.; Hu, Z.; Xu, A.; Feng, C. Semiconductor Metal Oxides as Chemoresistive Sensors for Detecting Volatile Organic Compounds. Sensors 2019, 19, 233. [Google Scholar] [CrossRef] [PubMed]
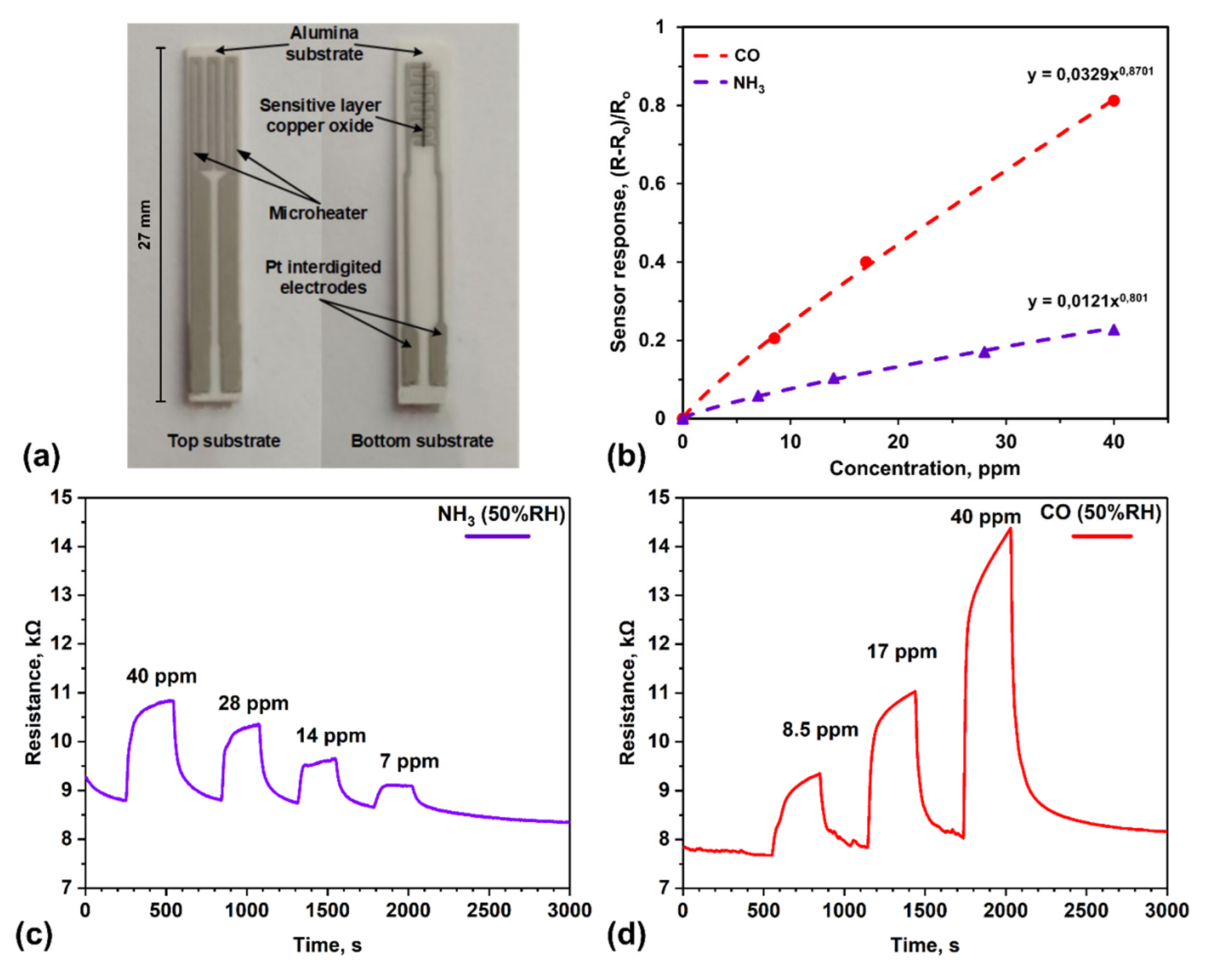
| Working Temperature, °C | Target Gas | Concentration, ppm | Relative Humidity, % |
|---|---|---|---|
| 270 | CO | 8.5, 17 and 40 | 50 |
| NH3 | 7, 14, 28 and 40 |
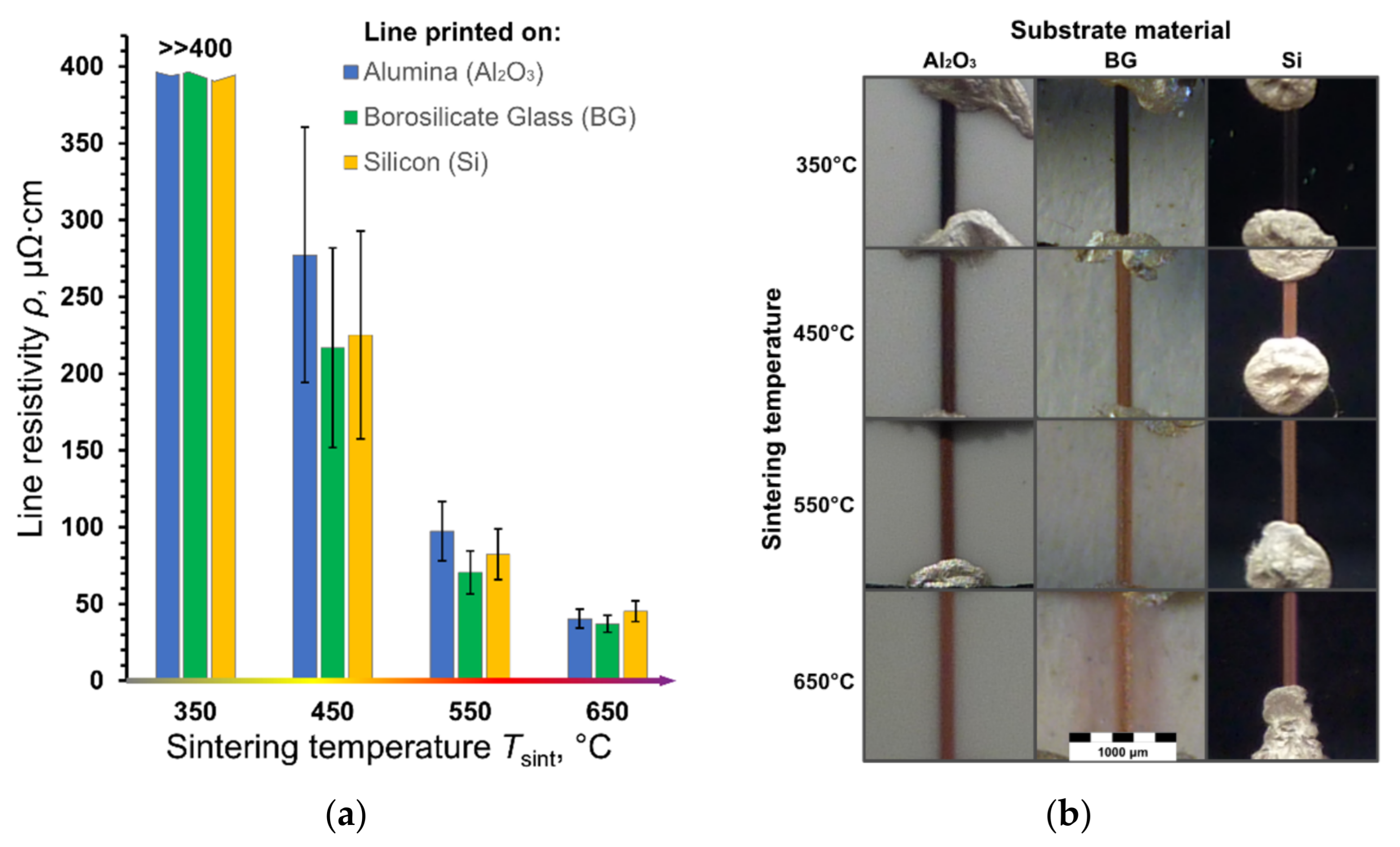
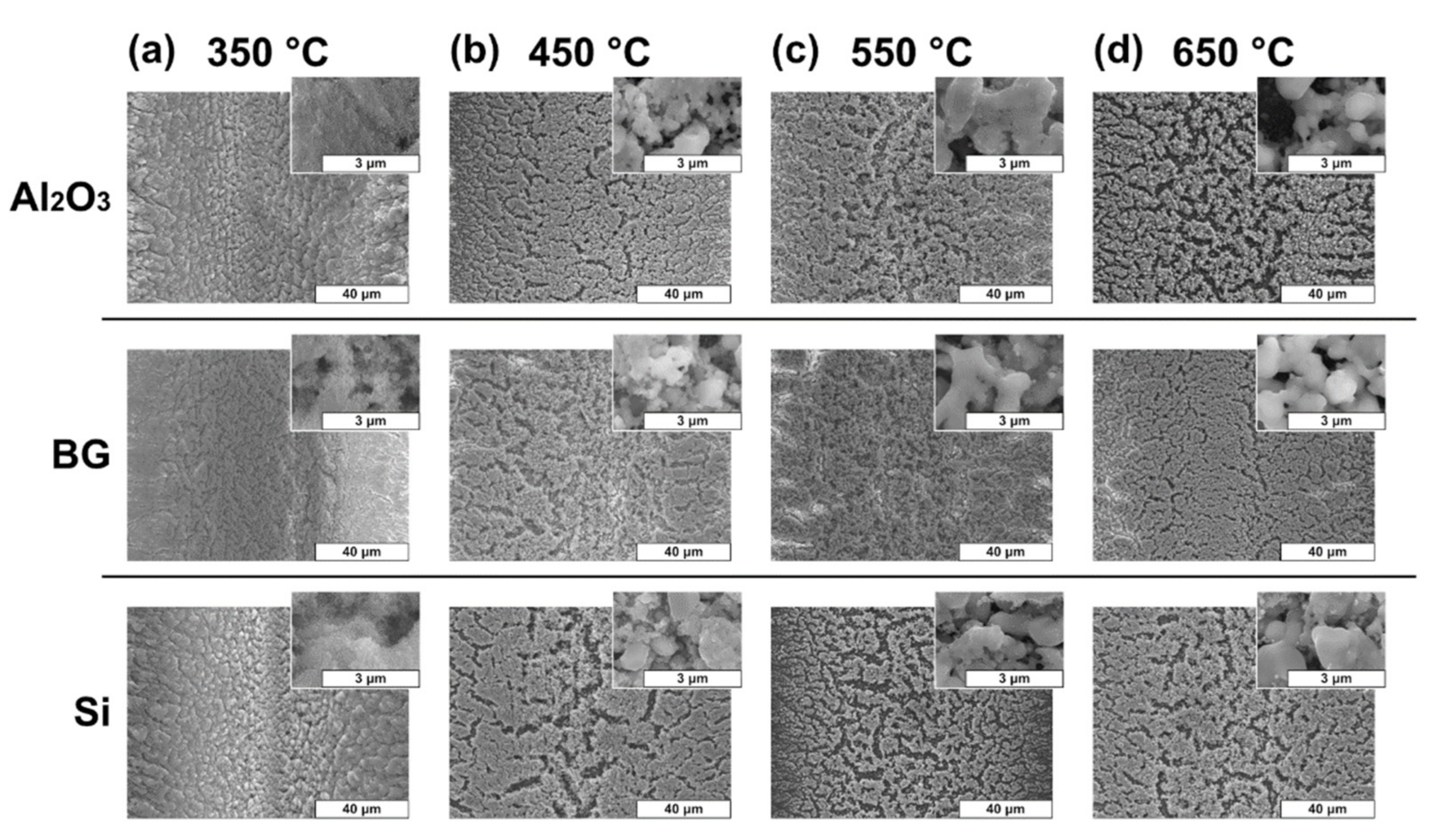
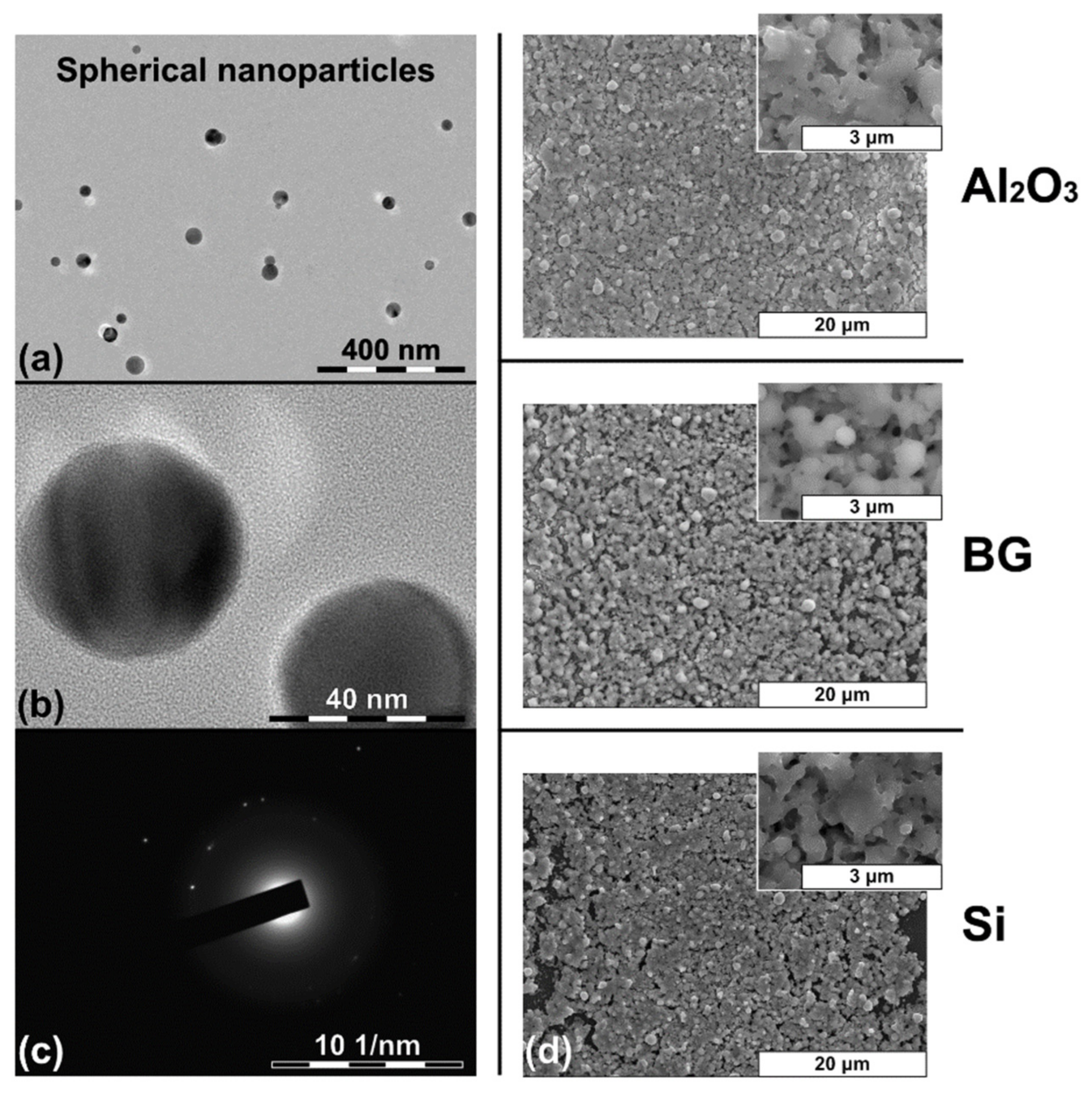
| Sintering Temperature Tsint, °C | Alumina (Al2O3) | Borosilicate Glass (BG) | Silicon (Si) |
|---|---|---|---|
| Shrinkage S, % | |||
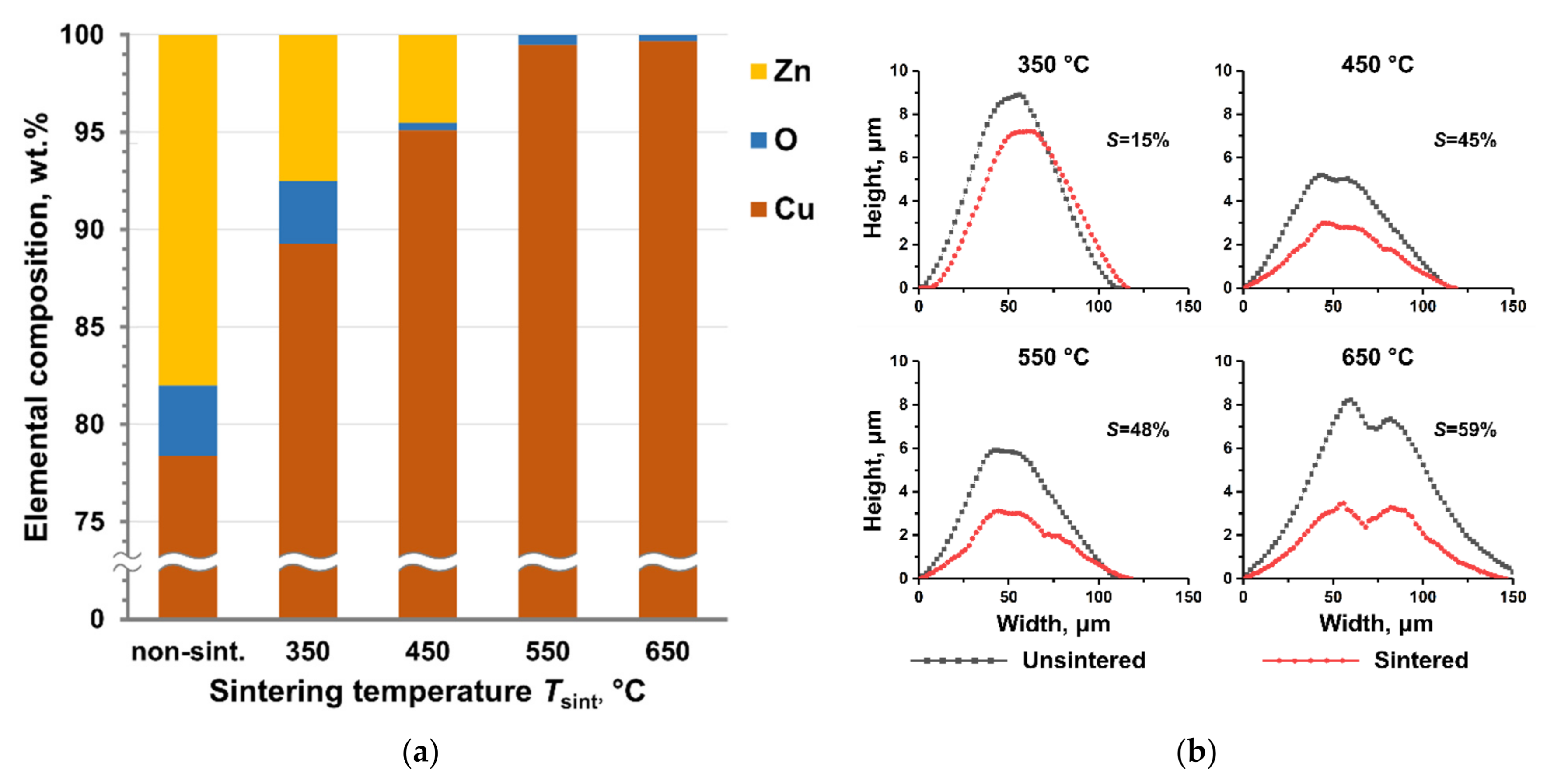
| 350 | 15 ± 7 | 12 ± 7 | 14 ± 7 |
| 450 | 45 ± 8 | 59 ± 8 | 54 ± 6 |
| 550 | 48 ± 5 | 65 ± 7 | 61 ± 7 |
| 650 | 59 ± 6 | 69 ± 9 | 63 ± 8 |
| Alumina (Al2O3) | Borosilicate Glass (BG) | Silicon (Si) | |
|---|---|---|---|
| Electrical resistivity ρ, µΩ·cm | 5.1 ± 0.6 | 5.3 ± 0.7 | 5.1 ± 0.8 |
| Shrinkage S, % | 44 ± 10 | 38 ± 9 | 32 ± 8 |
| Elemental composition of sintered lines, wt.% | Cu—98.6 Ag—1.0 O—0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Efimov, A.A.; Kornyushin, D.V.; Buchnev, A.I.; Kameneva, E.I.; Lizunova, A.A.; Arsenov, P.V.; Varfolomeev, A.E.; Pavzderin, N.B.; Nikonov, A.V.; Ivanov, V.V. Fabrication of Conductive and Gas-Sensing Microstructures Using Focused Deposition of Copper Nanoparticles Synthesized by Spark Discharge. Appl. Sci. 2021, 11, 5791. https://doi.org/10.3390/app11135791
Efimov AA, Kornyushin DV, Buchnev AI, Kameneva EI, Lizunova AA, Arsenov PV, Varfolomeev AE, Pavzderin NB, Nikonov AV, Ivanov VV. Fabrication of Conductive and Gas-Sensing Microstructures Using Focused Deposition of Copper Nanoparticles Synthesized by Spark Discharge. Applied Sciences. 2021; 11(13):5791. https://doi.org/10.3390/app11135791
Chicago/Turabian StyleEfimov, Alexey A., Denis V. Kornyushin, Arseny I. Buchnev, Ekaterina I. Kameneva, Anna A. Lizunova, Pavel V. Arsenov, Andrey E. Varfolomeev, Nikita B. Pavzderin, Alexey V. Nikonov, and Victor V. Ivanov. 2021. "Fabrication of Conductive and Gas-Sensing Microstructures Using Focused Deposition of Copper Nanoparticles Synthesized by Spark Discharge" Applied Sciences 11, no. 13: 5791. https://doi.org/10.3390/app11135791
APA StyleEfimov, A. A., Kornyushin, D. V., Buchnev, A. I., Kameneva, E. I., Lizunova, A. A., Arsenov, P. V., Varfolomeev, A. E., Pavzderin, N. B., Nikonov, A. V., & Ivanov, V. V. (2021). Fabrication of Conductive and Gas-Sensing Microstructures Using Focused Deposition of Copper Nanoparticles Synthesized by Spark Discharge. Applied Sciences, 11(13), 5791. https://doi.org/10.3390/app11135791






