Abstract
Amino sugars are key microbial biomarkers for determining the contribution of microbial residues in soil organic matter (SOM). However, it remains largely unclear as to what extent inorganic nitrogen (N) fertilization can lead to the significant degradation of SOM in alkaline agricultural soils. A six-year field experiment was conducted from 2013 to 2018 to evaluate the effects of chronic N enrichment on microbial residues, amino sugars, and soil biochemical properties under four nitrogen (urea, 46% N) fertilization scenarios: 0 (no-N, control), 75 (low-N), 225 (medium-N), and 375 (high-N) kg N ha−1. The results showed that chronic N enrichment stimulated microbial residues and amino sugar accumulation over time. The medium-N treatment increased the concentration of muramic acid (15.77%), glucosamine (13.55%), galactosamine (18.84%), bacterial residues (16.88%), fungal residues (11.31%), and total microbial residues (12.57%) compared to the control in 2018; however, these concentrations were comparable to the high-N treatment concentrations. The ratio of glucosamine to galactosamine and of glucosamine to muramic acid decreased over time due to a larger increase in bacterial residues as compared to fungal residues. Microbial biomass, soil organic carbon, and aboveground plant biomass positively correlated with microbial residues and amino sugar components. Chronic N enrichment improved the soil biochemical properties and aboveground plant biomass, which stimulated microbial residues and amino sugar accumulation over time.
1. Introduction
Amino sugars (AS) are important constituents of microbial cell walls in soil, and their accretion influences the contribution of microbial residues to soil organic matter (SOM) stability [1]. More than 90% of AS are found in dead cells [2], with the characteristic function of quantifying microbial residues, which is not a surrogate for animate microbial activity and biomass [3]. Thus, the amount of AS in the soil correlates with the amount of microbial residues accumulated in the SOM. Soil microbes use amino sugars as an alternative nitrogen (N) source in the absence of N due to their nutrient-dependent bioavailability. In addition, AS serves as a stable nitrogen pool in soils with high N content or as a labile nitrogen pool in N-limited soils [4], and they contribute significantly to bioavailable organic nitrogen in the soil [5]. Amino sugars account for about 5.0% to 12.0% of soil organic nitrogen [6,7], and 6.2% to 8.3% of soil organic carbon (SOC) [8] in the surface layer of most soils. In general, twenty-six (26) AS have been discovered in microorganisms and their synthesized products, of which only four are abundant in soils, namely D-glucosamine (GluN), D-muramic acid (MurN), D-galactosamine (GalN), and D-mannosamine (ManN) [9]. On average, GluN accounts for about 60% of the total AS in soils, followed by GalN (30%), MurN acid (7%), and ManN (4%) [10]. Muramic acid and glucosamine are primarily derived from bacteria and fungi, respectively, and their accumulation correlates with metabolic activities and microbial growth [10,11]. Galactosamine is derived from bacteria [12], but other authors assume that it is derived from fungi [13]. Ratios of AS can be used to quantify the accumulation of bacterial and fungal residues in soils [2]. The ratio of glucosamine to galactosamine is used to determine the contribution of fungi to SOM stability, while the ratio of glucosamine to muramic acid is used to determine the contribution of bacteria to AS accumulation [3,14].
Inorganic N enrichment influences the retention of microbial residues and regulates key processes in SOC turnover [15]. Microbial residues are the main carbon resource for persistent soil carbon pool, accounting for about 50% of SOC [16]. The balance between the synthesis and decomposition of microbial metabolites is directly related to the accumulation of microbial residues in the soil [10]. The amount of microbial residue C in SOC is used to quantify the contribution of microbe-derived carbon to SOC [16]. Microbial residues serve as an important source of stable carbon; thus, its contribution to SOC pool variations will influence predictions of global change on SOC pools. Therefore, studying the accumulation of microbial residues in soils is important in understanding the influence of microbes on SOC stabilization and the global C cycle [17]. Moreover, N enrichment leads to a shift from fungi to bacteria-dominated microbial communities [18]; this affects the ratio of fungal to bacterial residues, which in turn affects AS accumulation in soils. Nitrogen enrichment has also been reported to reduce microbial residues and amino sugar accumulation in N-limited acidic soils as a result of soil acidification or N saturation [19,20]. Soils with low pH (acidic soils) suppress microbial activities and growth [21,22,23], which negatively affects microbial residues and amino sugar accumulation. Soil pH is an important factor regulating the degradation of microbial cell walls, with a low pH favoring the degradation of chintin, while a high pH stimulates the degradation of peptidoglycan [5]. The response of microbial residues and amino sugar accumulation to inorganic N enrichment in acidic soils has been extensively studied [24,25]. Nevertheless, the effects of chronic N enrichment on microbial residues and amino sugar found in alkaline soils are poorly understood. We hypothesized that chronic N enrichment would promote microbial growth and activity as well as improve the biochemical properties of the alkaline soil, which in turn would stimulate microbial residues and AS accumulation over time. Adequate N enrichment enhances the ability of soil microbes to sequester N or C into their residues, which consequently increases net microbial AS accumulation over time [26]. Hence, it is important to investigate how different chronic N enrichment rates affect carbon and nitrogen sequestration in agricultural alkaline soils in order to understand the contribution of microbial residues and amino sugars to SOM stability. The study was therefore conducted to determine the effects of chronic N enrichment on microbial residues, amino sugar concentrations, and biochemical properties of an alkaline loess soil.
2. Materials and Methods
2.1. Site and Treatment Description
The study was conducted from May 2013 to October 2018 at the Xiangquan experimental site in Dingxi, Gansu Province, China. The site is located at latitude 35°27′ N and longitude 104°30′ E. It has a mean annual precipitation of 400 mm, a mean temperature of 6.9 °C, and a frost-free period of 140 days. The soil biochemical properties at the site are presented in Table 1. In this study, four nitrogen (urea, 46% N) application rates were investigated, namely: no-N application (0 kg N ha−1, control), low-N application (75 kg N ha−1), medium-N application (225 kg N ha−1), and high-N application (375 kg N ha−1).

Table 1.
Soil properties before the experiment in 2013.
2.2. Soil Sampling and Analysis
Post-harvest soil samples were collected from five sampling points at a depth of 0–20 cm in each plot and then bulked to form a composite sample. Stones, animal and plant debris, and visually detectable fauna were removed, and each sample was divided into two parts. One part was kept in the refrigerator (4 °C) for microbial biomass and available nitrogen (nitrate and ammonium) analyses, while the other part was air-dried for 14 days and ground through 1 mm and 0.15 mm meshes before soil chemical analysis.
Soil pH (1:2.5 soil:water suspension) was determined using a portable pH meter (Shanghai Precision and Scientific Instrument Company Limited, China). Total nitrogen content was measured using a modified Kjeldahl Method, as described by Bremner [27]. Soil organic carbon content was determined using the potassium dichromate digestion method [28]. Ammonium (NH4+-N) and nitrate (NO3−-N) concentrations in the soil were extracted with 2M KCl at a ratio of 1:10. The concentrations of NH4+-N and NO3−-N in the filtrate were measured using the Continuous-Flow Analysis—AA3 analyzer, Seal, Germany. Microbial biomass carbon (MBC) and microbial biomass nitrogen (MBN) were determined using the chloroform fumigation extraction method [29], with slight modifications. Briefly, 10 g (oven-dry basis) of fresh soil was weighed in duplicates; one part was fumigated with ethanol-free chloroform (CHCI3), while the other part was not fumigated. A total of 40 mL of 0.5 mol L−1 K2SO4 was added to the fumigated and non-fumigated soil samples, and then shaken at 300 r min−1 for 60 min. The solutions were then filtered and the carbon and nitrogen content in the filtrates were determined using a TOC analyzer (German Jena, Multi N/C 3000). The MBC and MBN concentrations were calculated using the following formulas:
where kEC = 0.45 [30] and kEN = 0.54 [31].
Microbial biomass C = Fumigated C content - Non fumigated C content/kEC
Microbial biomass N = Fumigated N content - Non fumigated N content/kEN
The carbon and nitrogen contents in the non-fumigated samples were used to calculate dissolved organic carbon (DOC) and dissolved organic nitrogen (DON), respectively, as described by Boyer and Groffman [32].
2.3. Microbial Residues and Amino Sugars Analysis
The concentration of amino sugars (GluN, GalN, and MurN) in the soil was determined according to the method described by Zhang and Amelung [33]. Briefly, soil samples were hydrolyzed at 105 °C with 6 M HCl for 8 h. The solutions were filtered and centrifuged after adjusting their pH to a level between 6.6 and 6.8. The supernatant solutions were then freeze-dried, and methanol was used to wash out the AS from the residues. The recovered AS were converted to aldononitrile derivatives and the excess derivatization reagents were removed by a two-step reaction. In the first step, a derivatization reagent containing 40 mg mL−1 4-(dimethylamino) pyridine in pyridine methanol (4:1 v/v) and 32 mg mL−1 hydroxylamine hydrochloride was added to the dry sample in the airlock, while in the second step, acetic anhydride was added. Dichloromethane was used to extract the derivatives, and 1M HCl and distilled water were used to remove excess anhydride. The final AS derivatives were then dissolved in 300 µL ethyl acetate-hexane (v:v = 1:1) for analysis. Internal standard (myo-inositol) was added before hydrolysis and the derivatives of AS were determined using an Agilent 6890A GC (Agilent Tech. Co., Wilmington, DE, USA) equipped with a flame ionization detector and an HP-5 (25 m × 0.32 mm × 0.25 μm) fused silica column. Fungal residues in SOC were calculated as the difference between total GluN and bacterial GluN [13,34], while the bacterial residues in SOC were determined by multiplying the MurN content by 45 [34]. The total microbial residues in SOC were calculated as the sum of bacterial and fungal residues.
2.4. Aboveground Plant Biomass Determination
Aboveground plant biomass was determined after potato plants were harvested in the 2018 season. In each experimental unit, six plants were cut from the soil surface, excluding the border plants. The shoots were then oven-dried at a constant temperature of 80 °C until a constant weight was reached and recorded as aboveground biomass (g m−2).
2.5. Data Analysis
A one-way analysis of variance was conducted using SPSS software version 21 for Windows (IBM Corp., Chicago, IL, USA). Treatment means were compared by the least significant difference test at a 0.05 probability level. Regression analysis was conducted using SPSS to determine the functional relationships among N rate, aboveground plant biomass, SOC, and total N. The correlation matrix analysis was performed using SPSS to determine the relationship between microbial amino sugar components and soil biochemical properties. Principal component analysis (PCA) was conducted using Canoco 5 (Microcomputer Power, Ithaca, NY, USA) to explore the effects of nitrogen enrichment on microbial residues, amino sugars, and soil biochemical properties. Figures and tables were drawn using GraphPad Prism 6 software (GraphPad Software, Inc., San Diego, CA, USA) and Microsoft Word version 365 (Microsoft Corporation, Redmond, Washington, USA), respectively.
3. Results
3.1. Effects of Chronic Nitrogen Enrichment on Amino Sugar and Microbial Residue Components from 2014 to 2018
Chronic N enrichment significantly affected the concentration of amino sugar components in the soil (Figure 1). Glucosamine content followed a linear trend from 2014 to 2018 and significantly varied by N enrichment (Figure 1a). The GluN content ranged from 148.73 to 18.90 mg kg−1 and increased with increasing N rate; however, the GluN content under the high-N and medium-N treatments was at par throughout the study period. The medium-N treatment stimulated GluN content by 8.33%, 11.18%, and 13.55% compared to the control in 2016, 2017, and 2018, respectively. Galactosamine content was significantly affected by N enrichment from 2016 and 2018 (Figure 1b). The GalN content ranged from 67.44 to 90.30 mg kg−1 and increased with an increase in N rate. The high-N and the medium-N treatments had statistically similar GalN content throughout the experimental period. Conversely, the GalN content was 13.13%, 18.68%, and 18.84% higher under the medium-N treatment than the control in 2016, 2017, and 2018, respectively. Moreover, N enrichment significantly influenced muramic acid content from 2016 to 2018 (Figure 1c). The MurN content ranged from 7.37 to 9.84 mg kg−1, and increased with increasing N rate; however, the high-N and medium-N treatments had statistically similar MurN content from 2014 to 2018. Compared to the control, the medium-N treatment increased MurN content by 8.17% in 2016, 14.11% in 2017, and 15.77% in 2018. Similarly, total amino sugar content followed a linear trend and varied significantly by N enrichment from 2016 to 2018 (Figure 1d). The total AS content increased with increasing N rate, and it ranged from 223.54 to 281.04 mg kg−1. There was no significant difference between the high-N and medium-N treatments in terms of total AS content. The total AS content in the soil was higher under the medium-N treatment than the control by 9.82%, 13.71%, and 15.31% in 2016, 2017, and 2018, respectively. The ratio of glucosamine to galactosamine (GluN/GalN) varied significantly by the nitrogen enrichment only in 2017 and 2018, and it decreased over time (Table 2). The GluN/GalN ratio ranged from 1.99 to 2.25 and decreased with increasing N application rate. Compared to the control, the high-N treatment decreased the GluN/GalN ratio by 8.29% and 7.41% in 2017 and 2018, respectively. Similar to the GluN/GalN ratio, the ratio of glucosamine to muramic acid (GluN/MurN) was significantly affected by the nitrogen enrichment only in 2017 and 2018, and it decreased over time (Table 2). The Glu/MurN ratio ranged from 18.38 to 20.79 and decreased with increasing N application rate. The GluN/MurN ratio was 5.64% and 6.18% higher under the control than under the high-N treatment in 2017 and 2018, respectively.
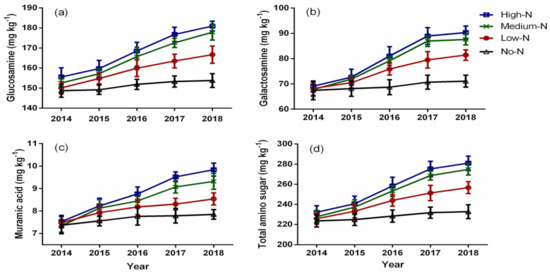
Figure 1.
Effects of chronic nitrogen enrichment on glucosamine (a), galactosamine (b), muramic acid (c), and total amino sugar (d). Bars represent ± standard error of means with four replications.

Table 2.
Effects of nitrogen enrichment on the glucosamine and galactosamine ratio (GluN/GalN), the glucosamine and muramic acid ratio (GluN/MurN), and soil organic carbon and pH content from 2014 to 2018.
Nitrogen enrichment significantly affected the bacterial residues in SOC from 2015 to 2018 (Figure 2a). The bacterial residues in SOC ranged from 3.78 to 4.83 mg kg−1, and it increased with increasing N rate; however, the high-N and medium-N treatments had statistically similar bacterial residues in SOC throughout the study period. Compared to the control, the medium-N treatment increased bacterial residues in SOC by 4.73% in 2015, 11.49% in 2016, 16.02% in 2017, and 16.88% in 2018. The fungal residues in SOC were also significantly varied by the N enrichment from 2015 to 2018 (Figure 2b). Fungal residues in SOC ranged from 14.15 to 16.40 mg kg−1 and it increased with an increase in N rate; but the fungal residues recorded by the high-N and medium-N treatments were statistically similar throughout the study period. The medium-N treatment stimulated fungal residues in SOC by 3.01%, 6.16%, 10.75%, and 11.31% in 2015, 2016, 2017, and 2018, respectively, compared to the control. Moreover, N enrichment significantly affected total microbial residues in SOC from 2015 to 2018 (Figure 2c). The total microbial residues in SOC ranged from 17.93 to 21.23 mg kg−1 and it increased with increasing N rate. The high-N and medium-N rates had statistically similar total microbial residues throughout the experiment; however, the medium-N treatment significantly stimulated total microbial residues in SOC by 3.38%, 7.35%, 11.94%, and 12.57% compared to the control in 2015, 2016, 2017, and 2018, respectively. The ratio of fungal residues to bacterial residues in SOC significantly varied by nitrogen enrichment from 2016 to 2018 and decreased with an increase in N rate (Figure 2d). The ratio of fungal residues to bacterial residues ranged from 3.40 to 3.74 and decreased over time. The ratio decreased by 6.25% in 2016, 6.28% in 2017, and 6.85% in 2018 under the high-N treatment compared to the control.
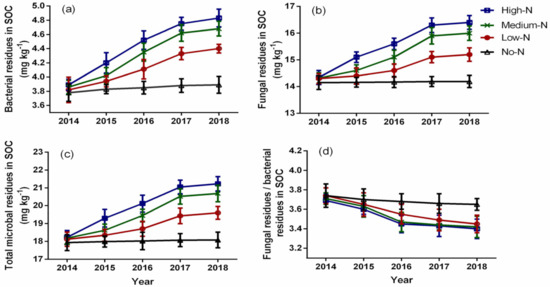
Figure 2.
Effects of nitrogen enrichment on bacterial residues in SOC (a), fungal residues in SOC (b), total microbial residues in SOC (c), and ratio of fungal residues to bacterial residues in SOC (d). Bars represent ± standard error of means with four replications.
3.2. Effects of Chronic Nitrogen Enrichment on Microbial Biomass, and Dissolved Organic Carbon and Nitrogen Concentrations in the Soil from 2014 to 2018
Nitrogen enrichment significantly affected microbial biomass (MBN and MBC) from 2015 to 2018 (Figure 3a,b). The MBN concentration in the soil ranged from 5.66 to 13.1 mg kg−1, while the MBC concentration ranged from 79.36 to 117.11 mg kg−1. Microbial biomass followed a linear trend and increased with increasing N rate; however, the high-N and medium-N treatments had statistically similar microbial biomass throughout the study period. Compared to the control, the medium-N treatment stimulated MBN by 28.63%, 38.17%, 45.98%, and 47.86% in 2015, 2016, 2017, and 2018, respectively. Similarly, the MBC content increased under the medium-N treatment by 7.59% in 2015, 15.30% in 2016, 20.56% in 2017, and 23.58% in 2018 compared to the control. Similar to microbial biomass, the ratio of MBC to SOC varied significantly by N enrichment from 2014 to 2018 (Figure 3c). The MBC/SOC ratio ranged from 9.18 to 12.19 and ranked as: high-N > medium-N > low-N > no-N, throughout the experiment. The effect of N enrichment on the MBC/MBN ratio was also significant from 2014 to 2018 and decreased over time (Figure 3d). The ratio of MBC to MBN ranged from 8.59 to 14.47, and it decreased with increasing N rate and followed this trend: no-N > low-N > medium-N > high-N, respectively, throughout the study. Dissolved organic carbon (DOC) and dissolved organic nitrogen (DON) were significantly stimulated by N enrichment and increased from 2015 to 2018 (Figure 4a,b). The DOC and DON contents followed a linear trend and ranged from 179.3 to 266.0 mg kg−1 and 129.5 to 223.8 mg kg−1, respectively. Similar to MBC and MBN, DOC and DON increased with an increasing N application rate. The DOC and DON contents were ranked as: high-N > medium-N > low-N > no-N, throughout the study period. Similar to the MBC/SOC ratio, the ratio of dissolved organic carbon to soil organic carbon (DOC/SOC) varied significantly from 2016 to 2018 by nitrogen enrichment (Figure 4c). All treatments increased the DOC/SOC ratio over time; however, it increased with an increase in N rate. The DOC/SOC ratio ranged from 20.78 to 27.68. The high-N and medium-N treatments had statistically similar ratio throughout the experimental period; however, the DOC/SOC ratio was stimulated by 6.60% in 2016, 9.12% in 2017, and 11.07% in 2018 under the medium-N treatment compared to the control. The ratio of dissolved organic carbon to dissolved organic nitrogen (DOC/DON) was significantly affected by nitrogen enrichment and decreased with increasing N rate (Figure 4d). The DOC/DON ratio ranged from 1.09 to 1.45 and decreased over time. The DOC/DON ratio was ranked as: no-N > low-N > medium-N > high-N, respectively.
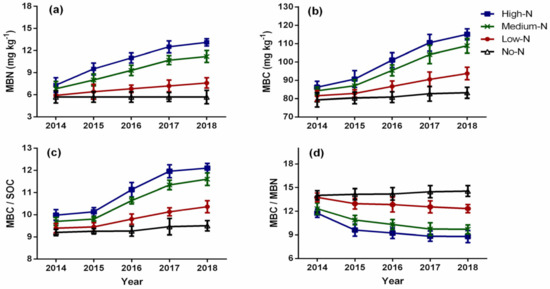
Figure 3.
Effects of nitrogen enrichment on microbial biomass nitrogen (a), microbial biomass carbon (b), microbial biomass carbon and soil organic carbon ratio (c), microbial biomass carbon and microbial biomass nitrogen ratio (d). MBN = microbial biomass nitrogen; MBC = microbial biomass carbon; SOC = soil organic carbon. Bars represent ± standard error of means, with four replications.
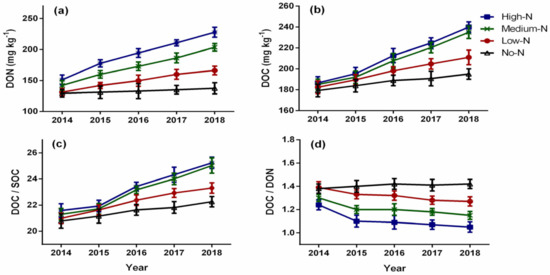
Figure 4.
Effects of nitrogen enrichment on dissolved organic nitrogen (a), dissolved organic carbon (b), dissolved organic carbon and soil organic carbon ratio (c), dissolved organic nitrogen and dissolved organic nitrogen ratio (d). DON = dissolved organic nitrogen; DOC = dissolved organic carbon; SOC = soil organic carbon. Bars represent ± standard error of means, with four replications.
Soil organic carbon was significantly affected by nitrogen enrichment from 2016 to 2018 (Table 2). The SOC content ranged from 8.63 to 9.51 g kg−1 and it increased with increasing N application rate; however, the low-N, high-N, and medium-N treatments had statistically similar SOC throughout the study period. Compared to the control, the medium-N treatment significantly increased the SOC content by 6.71% in 2018. Soil pH was also significantly varied by N enrichment from 2016 to 2018, and it decreased over time (Table 2). Soil pH ranged from 8.01 to 8.31 and it decreased with an increase in the N application rate. Compared to the control, soil pH significantly decreased by 1.71% and 2.32% under the medium-N and high-N treatments, respectively, in 2018. Moreover, total soil nitrogen significantly varied by N enrichment from 2016 to 2018 (Table 3). The total N content in the soil ranged from 0.95 to 1.28 g kg−1. All the treatments increased total N content over time, but it increased significantly with an increase in N application rate. However, the medium-N and the high-N treatments had statistically similar total N content throughout the experiment. The medium-N treatment significantly increased total N content by 11.11%, 14.04%, and 17.89% in the 2016, 2017, and 2018 seasons, respectively, compared to the control. Soil available nitrogen (NH4+-N and NO3−-N) concentration was significantly increased by N enrichment from 2014 to 2018 (Table 3). The NH4+-N concentration ranged from 0.76 to 1.25 mg kg−1, whereas NO3−-N concentration ranged from 4.68 to 19.18 mg kg−1. The concentrations of NH4+-N and NO3−-N in the soil were ranked as high-N > medium-N > low-N > no-N throughout the experiment. In 2018, the high-N treatment increased NH4+-N and NO3−-N concentration by 38.40% and 75.50%, respectively, compared to the control.

Table 3.
Effects of inorganic nitrogen enrichment on total nitrogen, ammonium nitrogen (NH4+-N), and nitrate nitrogen (NO3−-N) from 2014 to 2018.
The functional relationships between the N rate and aboveground plant biomass (APB), SOC and APB, and total N and APB are showed in Figure 5. There was a significant relationship between the N rate and APB (R2 = 0.99). Similarly, APB significantly related to SOC (R2 = 0.97) and total N (R2 = 0.96).
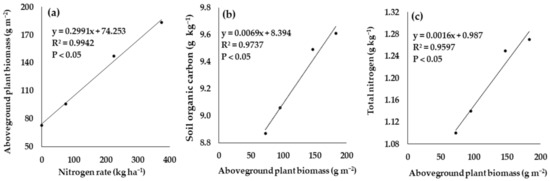
Figure 5.
Functional relationship between aboveground plant biomass and nitrogen rate (a); aboveground plant biomass and soil organic carbon (b); aboveground plant biomass and total nitrogen (c).
3.3. Relationships between Microbial Residues, Amino Sugars, and the Biochemical Properties of the Soil
The correlation matrix between microbial necromass and soil biochemical properties is shown in Table 4. Soil pH showed a significant and negative correlation with GluN (r = −0.967), MurN (r = −0.996), total AS (r = −0.963), bacterial residues (r = 0.996), and total microbial residues (r = −0.974); however, it correlated only slightly with galactosamine (r = −0.937) and fungal residues (r = −0.939). Microbial biomass (MBC and MBN) correlated significantly and positively with GalN, GluN, MurN, total AS, bacterial residues, fungal residues, and total microbial residues; except for fungal residues, with which it correlated only slightly (r = 0.936). Aboveground plant biomass also showed a significant and positive relationship with GluN (r = 0.956), MurN (r = 0.989), bacterial residues (r = 0.989), and total microbial residues (r = 0.964), but only slightly correlated with GalN (r = 0.913), total AS (r = 0.948), and fungal residues (r = 0.964). Moreover, DOC significantly and positively correlated with GalN (r = 0.953), GluN (r = 0.980), MurN (r = 0.998), total AS (r = 0.976), bacterial residues (r = 0.994), fungal residues (r = 0.954), and total microbial residues (r = 0.985). Similar to DOC, SOC showed a significant and positive relationship with GalN (r = 0.933), GluN (r = 0.983), MurN (r = 0.988), total AS (r = 0.971), bacterial residues (r = 0.989), fungal residues (r = 0.937), and total microbial residues (0.985). The principal component analysis (PCA) was conducted to explore the effects of nitrogen enrichment on microbial residues, amino sugars, and soil biochemical properties. Principal component 1 (PC1) explained 96.8%, while principal component 2 (PC2) explained 2.6% of the total variance (Figure 6). Microbial residues, amino sugars, and soil biochemical properties showed significant separations among the nitrogen treatments. The high-N treatment had the highest score, followed by the medium-N, low-N, and no-N treatments, respectively, along PC1; however, the low-N and medium-N treatments had higher scores than the high-N and no-N treatments along PC2. Three of the nitrogen treatments (high-N, low-N, and no-N) were far away from the origin, suggesting that nitrogen enrichment strongly influences microbial residues, amino sugars, and the biochemical properties of the soil.

Table 4.
Pearson’s correlation analysis between microbial amino sugar components and soil biochemical properties in 2018.
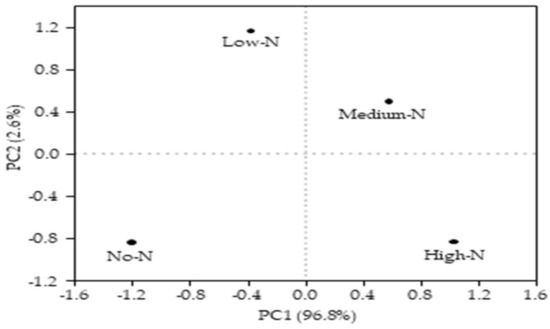
Figure 6.
Principal components analysis (PCA) based on microbial residues, amino sugars, and soil biochemical properties after 6 years of nitrogen enrichment (No-N, low-N, medium-N, and high-N).
4. Discussion
4.1. Effects of Chronic Nitrogen Enrichment on Microbial Residues and Amino Sugar Accumulation
Amino sugars are usually derived from the turnover of microbial residues in soils, as plants cannot produce a large amount of AS [34,35]. Previous studies reported that amino sugars decreased significantly with N enrichment due to calcium or magnesium deficiency resulting from soil acidification, N saturation, or altered carbon availability [19,20]. Thus, N enrichment decreases microbial AS accumulation in acidic soils. Griepentrog et al. [24] and Zhang et al. [25] reported that nitrogen enrichment had an insignificant effect on AS concentrations in soil. However, the results of the present study showed that chronic N enrichment increased microbial residues and amino sugar concentrations in the soil (Figure 1 and Figure 2), which is consistent with previous studies [17,26,36]. The discrepancy between the previous studies [24,25] and our study may be attributed to differences in soil pH. The pH of the soil used in the present study is above 8, while that of the previous studies was less than 5. Zhang et al. [25] documented that N enrichment lowered the pH of the acidic soil, which hindered microbial activities and production. Soils with low pH suppress the growth and activity of microbes, especially bacteria [22,37], thereby reducing amino sugar accumulation in N-fertilized soils [25,38]. In the present study, N enrichment reduced soil pH close to the neutral range (Table 2), which might have facilitated microbial growth and activity; consequently, this stimulated microbial residues and amino sugar accumulation. Soils with a neutral pH or a near-neutral range promote microbial growth and activity [22,23,39]. In addition, N enrichment stimulated microbial residues and AS probably due to its high microbial biomass, dissolved organic C, and SOC concentrations in the soil. Microbial residues and AS showed a positive correlation with microbial biomass, dissolved organic carbon, and SOC (Table 4). The accumulation of microbial residues and AS was higher under the high-N and medium-N treatments, where the N requirement was sufficient for microbial growth. The lower microbial residues and AS accumulation in the low-N and the control treatments probably occurred due to low N availability (Table 3), thereby reducing microbial growth and their residual metabolic production [39]. Ding et al. [26] found that adequate N enrichment facilitated soil microbes to sequester N or C into their residues, and consequently increased their net microbial AS accumulation over time. Moreover, aboveground plant biomass, which was returned to the soil annually after harvest, increased with an increasing N rate (Figure 5a); this in turn might have caused an increase in the microbial residues and AS accumulation under the high and medium N rates. The aboveground plant biomass also showed a positive and significant correlation with microbial AS components (Table 4). This finding concurs with Arnebrant et al. [40] and Corre et al. [41].
The concentration of the individual amino sugars (GluN, GalN, and MurN) increased with time and paralleled to the gradual accumulation of total N and SOC, which concurs with Pronk et al. [42]. The concentrations of the individual amino sugars were ranked as GluN > GalN > MurN and a similar trend was reported by previous studies [2,24,25,26]. The ratios of GluN to MurN and GluN to GalN decreased with time, since the increase in GalN and MurN over time was greater than the increase in GluN due to bacteria dominance in the alkaline soil. In a high pH environment, bacteria dominate over fungi because bacteria are preferred at high pH levels [23,39,43]. This could explain why the ratio of fungal to bacterial residues decreased over time (Figure 2d). In general, bacteria require more nutrients than fungi [44], and therefore dominate in soils where N is abundant. This could also explain why the medium-N and the high-N treatments had the lowest ratio of GluN to MurN and GluN to GalN. The lower ratios of GluN to MurN and GluN to GalN indicate that bacteria contribute significantly to soil organic matter stability and amino sugar accumulation in the alkaline soil. Our findings are in agreement with Zhang et al. [45], who postulated that nitrogen enrichment lowers the ratio of fungi to bacteria due to different reactions between bacterial biomass and fungal biomass.
4.2. Effects of Chronic Nitrogen Enrichment on Soil Biochemical Properties
Nitrogen enrichment improved the soil biochemical properties, which is in line with previous studies [46,47]. Soil microbial biomass (MBC and MBN) increased with an increasing N rate, which concurs with Raiesi [48]. The high-N and the medium-N treatments had the highest microbial biomass, probably due to their high available N concentration (Table 3), which enhanced the degradation of SOM, thereby stimulating microbial biomass concentration in the soil. However, our result contradicts previous studies that reported that N enrichment reduces microbial biomass in soils [45,49,50,51,52]. The discrepancy between our study and previous studies may be attributed to variations in soil pH [53], experimental duration and biome type [49], and nutrient availability [54]. The previous studies were conducted in forest and grassland ecosystems, while the present study was conducted in a cropland ecosystem; nitrogen enrichment decreases microbial biomass in forest and grassland ecosystems, while it increases microbial biomass in cropland and desert ecosystems [49]. Cropland and desert ecosystems are generally limited by nitrogen; therefore, nitrogen enrichment increases soil N availability and promotes microbial growth [49]. Soil DOC plays a key role in the formation of SOM and the movement of nutrients within ecosystems [55]. Nitrogen enrichment decreases soil DOC concentration due to the chemical stabilization of organic carbon [56] and the stimulation of the microbial mineralization rate, which increases DOC consumption [57]. Results of the current study, however, revealed that nitrogen enrichment stimulated soil DOC concentration, which is consistent with Shi et al. [58] and Wei et al. [52]; this could be attributed to the nutrient status of the soil [59,60]. Rosa et al. [60] pointed out that soil DOC significantly correlated with SOC and total N. Soil DOC increased with an increasing N rate (Figure 4b), probably due to aboveground plant biomass, which was returned to the soil annually. Increasing N rate significantly increased aboveground plant biomass (Figure 5a), which in turn stimulated DOC concentration in the soil. This finding corresponds with previous studies that reported that soil DOC increases as N rate increase due to litter production [61,62]. Nitrogen enrichment increased soil DON, which agrees with Currie et al. [63]. The soil DON content increased with an increasing N rate, probably due to the effect of nitrogen enrichment on soil pH. Felip and Rekasi [59] documented that soil pH negatively correlated with soil DON concentration. The MBC/MBN and DOC/DON ratios decreased with an increasing N rate (Figure 3d and Figure 4d), since the increase in DON and MBN along the N gradient was higher than that of DOC and MBC contents, respectively. The nitrate concentration in the soil was higher than that of ammonium throughout the experimental period, as nitrate is the main form of available N in soils [46,64]. High-N treatment had the highest nitrate (NO3−-N) concentration, partly due to its high rate exceeding the N requirement of the plants, which is consistent with previous studies [46,65,66]. However, the linear accumulation of residual soil NO3−-N content by the high-N treatment could pollute underground water, and thereby affect human health [66,67,68].
Nitrogen enrichment decreased soil pH over time and could be attributed to the process of nitrification, which resulted in the production and release of hydrogen ions into the soil solution [53]. Lu et al. [69] pointed out that N enrichment increases the leaching of basic cations such as Ca2+ and Mg2+ in the charge balance of the soil solution, thereby decreasing soil pH. An increase in N rate decreased soil pH, which agrees with previous studies [47,49,52,66,70]. The slight decrease in soil pH in the control occurred probably as a result of the depletion of basic cations by plants and the decomposition of organic matter [70]. The soil organic carbon content increased over time, including in the control, and this may be partly due to carbon sequestered from plant biomass returned to the soil, which concurs with previous studies [71,72,73]. The SOC content increased with an increasing N rate, which can partly be attributed to aboveground plant biomass. The increase in N rate increased aboveground plant biomass, which was returned to the soil after harvest (Figure 5b) and therefore might have supplied additional carbon to the soil after decomposition [74,75]. Li et al. [74] found a positive and significant correlation between SOC and aboveground plant biomass. Moreover, Fontaine et al. [76] observed that high N enrichment stores more carbon in the soil due to the prime effect. Our results are in agreement with previous studies where increasing N enrichment significantly increased SOC [37,70,74]. Total N content in the soil increased with an increasing N rate and could probably be attributed to N accumulation from the chronic N enrichment, as crop N depletion was less than N inputs over time. This finding is consistent with Sainju et al. [66], Aula et al. [70], and Anning et al. [77], who reported that total soil N content increased with increasing N fertilization.
5. Conclusions
The results of the study support our hypothesis that chronic nitrogen enrichment could stimulate microbial residue and amino sugar accumulation in alkaline soil. Microbial residue and amino sugar accumulation increased with an increasing N rate; however, the high-N and medium-N treatments had statistically similar accumulation. Total amino sugar was significantly higher under high-N and medium-N treatments, where N requirement for microbial growth was sufficient. The ratios of glucosamine to muramic acid and glucosamine to galactosamine decreased over time as a result of the more substantial increase in bacterial residues than that in fungal residues. Thus, bacteria contribute significantly to soil organic matter stability, microbial necromass, and AS accumulation in alkaline soil. Nitrogen enrichment improved the soil biochemical properties by increasing soil organic carbon, total nitrogen, available nitrogen, microbial biomass, and dissolved organic carbon concentrations, while lowering soil pH. Microbial residues and AS components showed a positive and significant correlation with microbial biomass, dissolved organic carbon, soil organic carbon, and aboveground plant biomass. However, soil pH showed a negative and significant correlation with glucosamine, muramic acid, total amino sugar, bacterial residues, and total microbial residues in soil organic carbon. Chronic nitrogen enrichment improved the soil biochemical properties and aboveground plant biomass, which stimulated microbial residue and amino sugar accumulation over time. Soil enzymes and microbial turnover have been reported to influence microbial residues and AS accumulation; however, these parameters were not measured in the present study. Therefore, we recommend future studies to focus on how chronic N enrichment affects soil enzyme activity and microbial turnover, and their contribution to microbial residues and AS accumulation in alkaline agricultural soils.
Author Contributions
Conceptualization, H.Q. and Q.S.; methodology, H.Q. and D.K.A.; software, D.K.A.; validation, H.Q., D.K.A., and Q.S.; formal analysis, D.K.A. and P.G.; investigation, D.K.A., Z.L., and D.D.; resources, H.Q.; data curation, D.K.A., D.D., and Z.L.; writing—original draft preparation, D.K.A.; writing—review and editing, H.Q. and D.K.A.; visualization, P.G. and C.Z.; supervision, H.Q. and C.Z.; project administration, Q.S. and H.Q.; funding acquisition, H.Q. and Q.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Key Basic Research Program of China, grant number No.2015CB150501.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We are grateful to Zhongjun Jia, Xingchen Dong, and Yongling Ji for their support during the manuscript preparation.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Roberts, P.; Bol, R.; Jones, D.L. Free amino sugar reactions in soil in relation to soil carbon and nitrogen cycling. Soil Biol. Biochem. 2007, 39, 3081–3092. [Google Scholar] [CrossRef]
- Van Groenigen, K.-J.; Bloem, J.; Bååth, E.; Boeckx, P.; Rousk, J.; Bode, S.; Forristal, D.; Jones, M.B. Abundance, production and stabilization of microbial biomass under conventional and reduced tillage. Soil Biol. Biochem. 2010, 42, 48–55. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, X.; Rubert, K.F.; Balser, T.C. Effect of plant materials on microbial transformation of amino sugars in three soil microcosms. Biol. Fertil. Soils 2007, 43, 631–639. [Google Scholar] [CrossRef]
- Li, L.; Wilson, C.B.; He, H.; Zhang, X.; Zhou, F.; Schaeffer, S.M. Physical, biochemical, and microbial controls on amino sugar accumulation in soils under long-term cover cropping and no-tillage farming. Soil Biol. Biochem. 2019, 135, 369–378. [Google Scholar] [CrossRef]
- Hu, Y.; Zheng, Q.; Zhang, S.; Noll, L.; Wanek, W. Significant release and microbial utilization of amino sugars and D-amino acid enantiomers from microbial cell wall decomposition in soils. Soil Biol. Biochem. 2018, 123, 115–125. [Google Scholar] [CrossRef]
- Amelung, W.; Lobe, I.; Du Preez, C.C. Fate of microbial residues in sandy soils of the South African Highveld as influenced by prolonged arable cropping. Eur. J. Soil Sci. 2002, 53, 29–35. [Google Scholar] [CrossRef]
- Appuhn, A.; Scheller, E.; Joergensen, R.G. Relationships between microbial indices in roots and silt loam soils forming a gradient in soil organic matter. Soil Biol. Biochem. 2006, 38, 2557–2564. [Google Scholar] [CrossRef]
- Ni, X.; Liao, S.; Tan, S.; Wang, D.; Peng, Y.; Yue, K.; Wu, F.; Yang, Y. A quantitative assessment of amino sugars in soil profiles. Soil Biol. Biochem. 2020, 143, 107762. [Google Scholar] [CrossRef]
- Amelung, W.; Brodowski, S.; Sandhage-Hofmann, A.; Bol, R. Combining biomarker with stable isotope analyses for assessing the transformation and turnover of soil organic matter. Adv. Agron. 2008, 100, 155–250. [Google Scholar]
- Joergensen, R.G. Amino sugars as specific indices for fungal and bacterial residues in soil. Biol. Fertil. Soils 2018, 54, 559–568. [Google Scholar] [CrossRef]
- Glaser, B.; Turrión, M.A.-B.; Alef, K. Amino sugars and muramic acid—biomarkers for soil microbial community structure analysis. Soil Biol. Biochem. 2004, 36, 399–407. [Google Scholar] [CrossRef]
- Amelung, W.; Miltner, A.; Zhang, X.; Zech, W. Fate of microbial residues during litter decomposition as affected by minerals. Soil Sci. 2001, 166, 598–606. [Google Scholar] [CrossRef]
- Engelking, B.; Flessa, H.; Joergensen, R.G. Shifts in amino sugar and ergosterol contents after addition of sucrose and cellulose to soil. Soil Biol. Biochem. 2007, 39, 2111–2118. [Google Scholar] [CrossRef]
- Lauer, F.; Kösters, R.; Du Preez, C.C.; Amelung, W. Microbial residues as indicators of soil restoration in South African secondary pastures. Soil Biol. Biochem. 2011, 43, 787–794. [Google Scholar] [CrossRef]
- Zak, D.R.; Pregitzer, K.S.; Kubiske, M.E.; Burton, A.J. Forest productivity under elevated CO2 and O3: Positive feedbacks to soil N cycling sustain decade-long net primary productivity enhancement by CO2. Ecol. Lett. 2011, 14, 1220–1226. [Google Scholar] [CrossRef][Green Version]
- Liang, C.; Amelung, W.; Lehmann, J.; Kästner, M. Quantitative assessment of microbial necromass contribution to soil organic matter. Glob. Chang. Biol. 2019, 25, 3578–3590. [Google Scholar] [CrossRef]
- Liang, C.; Gutknecht, J.; Balser, T. Microbial lipid and amino sugar responses to long-term simulated global environmental changes in a California annual grassland. Front. Microbiol. 2015, 6, 385. [Google Scholar] [CrossRef]
- Strickland, M.S.; Rousk, J. Considering fungal: Bacterial dominance in soils–methods, controls, and ecosystem implications. Soil Biol. Biochem. 2010, 42, 1385–1395. [Google Scholar] [CrossRef]
- Treseder, K.K. Nitrogen additions and microbial biomass: A meta-analysis of ecosystem studies. Ecol. Lett. 2008, 11, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Demoling, F.; Nilsson, L.O.; Bååth, E. Bacterial and fungal response to nitrogen fertilization in three coniferous forest soils. Soil Biol. Biochem. 2008, 40, 370–379. [Google Scholar] [CrossRef]
- Wang, A.S.; Angle, J.S.; Chaney, R.L.; Delorme, T.A.; McIntosh, M. Changes in soil biological activities under reduced soil pH during Thlaspi caerulescens phytoextraction. Soil Biol. Biochem. 2006, 38, 1451–1461. [Google Scholar] [CrossRef]
- Maier, R.M.; Pepper, I.L. Earth environments. In Environmental Microbiology; Elsevier: Amsterdam, The Netherlands, 2009; pp. 57–82. [Google Scholar]
- Neina, D. The role of soil pH in plant nutrition and soil remediation. Appl. Environ. Soil Sci. 2019, 2019, 5794869. [Google Scholar] [CrossRef]
- Griepentrog, M.; Bodé, S.; Boeckx, P.; Hagedorn, F.; Heim, A.; Schmidt, M.W. Nitrogen deposition promotes the production of new fungal residues but retards the decomposition of old residues in forest soil fractions. Appl. Environ. Soil Sci. 2014, 20, 327–340. [Google Scholar] [CrossRef]
- Zhang, W.; Cui, Y.; Lu, X.; Bai, E.; He, H.; Xie, H.; Liang, C.; Zhang, X. High nitrogen deposition decreases the contribution of fungal residues to soil carbon pools in a tropical forest ecosystem. Soil Biol. Biochem. 2016, 97, 211–214. [Google Scholar] [CrossRef]
- Ding, X.; Zhang, X.; He, H.; Xie, H. Dynamics of soil amino sugar pools during decomposition processes of corn residues as affected by inorganic N addition. J. Soils Sediments 2010, 10, 758–766. [Google Scholar] [CrossRef]
- Bremner, J.M. Nitrogen-total. In Methods of Soil Analysis: Part 3 Chemical Methods; Soil Science Society of America: Madison, WI, USA, 1996; Volume 5, pp. 1085–1121. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Wu, J.; Joergensen, R.; Pommerening, B.; Chaussod, R.; Brookes, P. Measurement of soil microbial biomass C by fumigation-extraction-an automated procedure. Soil Biol. Biochem. 1990, 22, 1167–1169. [Google Scholar] [CrossRef]
- Brookes, P.; Landman, A.; Pruden, G.; Jenkinson, D. Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Boyer, J.; Groffman, P. Bioavailability of water extractable organic carbon fractions in forest and agricultural soil profiles. Soil Biol. Biochem. 1996, 28, 783–790. [Google Scholar] [CrossRef]
- Zhang, X.; Amelung, W. Gas chromatographic determination of muramic acid, glucosamine, mannosamine, and galactosamine in soils. Soil Biol. Biochem. 1996, 28, 1201–1206. [Google Scholar] [CrossRef]
- Appuhn, A.; Joergensen, R.G. Microbial colonisation of roots as a function of plant species. Soil Biol. Biochem. 2006, 38, 1040–1051. [Google Scholar] [CrossRef]
- Dai, X.; Ping, C.-L.; Hines, M.E.; Zhang, X.; Zech, W. Amino sugars in arctic soils. Commun. Soil Sci. Plant Anal. 2002, 33, 789–805. [Google Scholar] [CrossRef]
- Chen, J.; Ji, C.; Fang, J.; He, H.; Zhu, B. Dynamics of microbial residues control the responses of mineral-associated soil organic carbon to N addition in two temperate forests. Sci. Total Environ. 2020, 748, 141318. [Google Scholar] [CrossRef]
- Kätterer, T.; Bolinder, M.; Berglund, K.; Kirchmann, H. Strategies for carbon sequestration in agricultural soils in northern Europe. Acta Agric. Scand. Sect. A Anim. Sci. 2012, 62, 181–198. [Google Scholar] [CrossRef]
- Zhang, N.; Wan, S.; Li, L.; Bi, J.; Zhao, M.; Ma, K. Impacts of urea N addition on soil microbial community in a semi-arid temperate steppe in northern China. Plant Soil 2008, 311, 19–28. [Google Scholar] [CrossRef]
- Rousk, J.; Brookes, P.C.; Bååth, E. Contrasting soil pH effects on fungal and bacterial growth suggest functional redundancy in carbon mineralization. Appl. Environ. Microbiol. 2009, 75, 1589–1596. [Google Scholar] [CrossRef] [PubMed]
- Arnebrant, K.; Bååth, E.; Söderström, B.; Nohrstedt, H.Ö. Soil microbial activity in eleven Swedish coniferous forests in relation to site fertility and nitrogen fertilization. Scand. J. Forest Res. 1996, 11, 1–6. [Google Scholar] [CrossRef]
- Corre, M.D.; Beese, F.O.; Brumme, R. Soil nitrogen cycle in high nitrogen deposition forest: Changes under nitrogen saturation and liming. Ecol. Appl. 2003, 13, 287–298. [Google Scholar] [CrossRef]
- Pronk, G.J.; Heister, K.; Kögel-Knabner, I. Is turnover and development of organic matter controlled by mineral composition? Soil Biol. Biochem. 2013, 67, 235–244. [Google Scholar] [CrossRef]
- Bååth, E.; Anderson, T.-H. Comparison of soil fungal/bacterial ratios in a pH gradient using physiological and PLFA-based techniques. Soil Biol. Biochem. 2003, 35, 955–963. [Google Scholar] [CrossRef]
- Güsewell, S.; Gessner, M.O. N: P ratios influence litter decomposition and colonization by fungi and bacteria in microcosms. Funct. Ecol. 2009, 23, 211–219. [Google Scholar] [CrossRef]
- Zhang, T.A.; Chen, H.Y.; Ruan, H. Global negative effects of nitrogen deposition on soil microbes. ISME J. 2018, 12, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Qiu, S.; Cao, C.; Zheng, C.; Zhou, W.; He, P. Responses of soil properties, microbial community and crop yields to various rates of nitrogen fertilization in a wheat–maize cropping system in north-central China. Agric. Ecosyst. Environ. 2014, 194, 29–37. [Google Scholar] [CrossRef]
- Yuan, X.; Niu, D.; Weber-Grullon, L.; Fu, H. Nitrogen deposition enhances plant-microbe interactions in a semiarid grassland: The role of soil physicochemical properties. Geoderma 2020, 373, 114446. [Google Scholar] [CrossRef]
- Raiesi, F. Soil properties and N application effects on microbial activities in two winter wheat cropping systems. Biol. Fertil. Soils 2004, 40, 88–92. [Google Scholar] [CrossRef]
- Jia, X.; Zhong, Y.; Liu, J.; Zhu, G.; Shangguan, Z.; Yan, W. Effects of nitrogen enrichment on soil microbial characteristics: From biomass to enzyme activities. Geoderma 2020, 366, 114256. [Google Scholar] [CrossRef]
- Ma, S.; Chen, G.; Du, E.; Tian, D.; Xing, A.; Shen, H.; Ji, C.; Zheng, C.; Zhu, J.; Zhu, J. Effects of nitrogen addition on microbial residues and their contribution to soil organic carbon in China’s forests from tropical to boreal zone. Environ. Pollut. 2021, 268, 115941. [Google Scholar] [CrossRef]
- Wang, C.; Lu, X.; Mori, T.; Mao, Q.; Zhou, K.; Zhou, G.; Nie, Y.; Mo, J. Responses of soil microbial community to continuous experimental nitrogen additions for 13 years in a nitrogen-rich tropical forest. Soil Biol. Biochem. 2018, 121, 103–112. [Google Scholar] [CrossRef]
- Du, W.; Li, Y.; He, P.; Zhang, J.; Jing, H.; Nie, C.; Liu, Y. Nitrogen Addition Decreases Soil Respiration without Changing the Temperature Sensitivity in a Semiarid Grassland. J. Res. Ecol. 2020, 11, 129–139. [Google Scholar]
- Geisseler, D.; Scow, K.M. Long-term effects of mineral fertilizers on soil microorganisms—A review. Soil Biol. Biochem. 2014, 75, 54–63. [Google Scholar] [CrossRef]
- Li, F.; Liu, M.; Li, Z.; Jiang, C.; Han, F.; Che, Y. Changes in soil microbial biomass and functional diversity with a nitrogen gradient in soil columns. Appl. Soil Ecol. 2013, 64, 1–6. [Google Scholar] [CrossRef]
- Neff, J.C.; Asner, G.P. Dissolved organic carbon in terrestrial ecosystems: Synthesis and a model. Ecosystems 2001, 4, 29–48. [Google Scholar] [CrossRef]
- Michel, K.; Matzner, E. Nitrogen content of forest floor Oa layers affects carbon pathways and nitrogen mineralization. Soil Biol. Biochem. 2002, 34, 1807–1813. [Google Scholar] [CrossRef]
- Gundersen, P.; Emmett, B.A.; Kjønaas, O.J.; Koopmans, C.J.; Tietema, A. Impact of nitrogen deposition on nitrogen cycling in forests: A synthesis of NITREX data. For. Ecol. Manag. 1998, 101, 37–55. [Google Scholar] [CrossRef]
- Shi, L.; Dech, J.P.; Yao, H.; Zhao, P.; Shu, Y.; Zhou, M. The effects of nitrogen addition on dissolved carbon in boreal forest soils of northeastern China. Sci. Rep. 2019, 9, 8274. [Google Scholar] [CrossRef]
- Filep, T.; Rékási, M. Factors controlling dissolved organic carbon (DOC), dissolved organic nitrogen (DON) and DOC/DON ratio in arable soils based on a dataset from Hungary. Geoderma 2011, 162, 312–318. [Google Scholar] [CrossRef]
- Rosa, E.; Debska, B. Seasonal changes in the content of dissolved organic matter in arable soils. J. Soils Sediments 2018, 18, 2703–2714. [Google Scholar] [CrossRef]
- Wang, X.-G.; Li, C.-S.; Luo, Y.; Hua, K.-K.; Zhou, M.-H. The impact of nitrogen amendment and crop growth on dissolved organic carbon in soil solution. J. Mount. Sci. 2016, 13, 95–103. [Google Scholar] [CrossRef]
- Wang, D.; Yi, W.; Zhou, Y.; He, S.; Tang, L.; Yin, X.; Zhao, P.; Long, G. Intercropping and N application enhance soil dissolved organic carbon concentration with complicated chemical composition. Soil Tillage Res. 2021, 210, 104979. [Google Scholar] [CrossRef]
- Currie, W.S.; Aber, J.D.; McDowell, W.H.; Boone, R.D.; Magill, A.H. Vertical transport of dissolved organic C and N under long-term N amendments in pine and hardwood forests. Biogeochemistry 1996, 35, 471–505. [Google Scholar] [CrossRef]
- Wu, T.-Y.; Ma, B.; Liang, B. Quantification of seasonal soil nitrogen mineralization for corn production in eastern Canada. Nutr. Cycl. Agroecosyst. 2008, 81, 279–290. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Y.; Zhang, J.; Liu, W.; Dang, Z.; Cao, W.; Qiang, Q. Effects of mulch, N fertilizer, and plant density on wheat yield, wheat nitrogen uptake, and residual soil nitrate in a dryland area of China. Nutr. Cycl. Agroecosyst. 2009, 85, 109–121. [Google Scholar] [CrossRef]
- Sainju, U.M.; Ghimire, R.; Pradhan, G.P. Nitrogen fertilization I: Impact on crop, soil, and environment. In Nitrogen Fixation; IntechOpen: London, UK, 2019. [Google Scholar]
- Mahvi, A.; Nouri, J.; Babaei, A.; Nabizadeh, R. Agricultural activities impact on groundwater nitrate pollution. Int. J. Environ. Sci. Technol. 2005, 2, 41–47. [Google Scholar] [CrossRef]
- Jamaludin, N.; Sham, S.M.; Ismail, S.N.S. Health risk assessment of nitrate exposure in well water of residents in intensive agriculture area. Am. J. Appl. Sci. 2013, 10, 442. [Google Scholar] [CrossRef]
- Lu, X.; Vitousek, P.M.; Mao, Q.; Gilliam, F.S.; Luo, Y.; Zhou, G.; Zou, X.; Bai, E.; Scanlon, T.M.; Hou, E. Plant acclimation to long-term high nitrogen deposition in an N-rich tropical forest. Proc. Natl. Acad. Sci. USA 2018, 115, 5187–5192. [Google Scholar] [CrossRef] [PubMed]
- Aula, L.; Macnack, N.; Omara, P.; Mullock, J.; Raun, W. Effect of fertilizer nitrogen (N) on soil organic carbon, total N, and soil pH in long-term continuous winter wheat (Triticum aestivum L.). Commun. Soil Sci. Plant Anal. 2016, 47, 863–874. [Google Scholar] [CrossRef]
- Dolan, M.; Clapp, C.; Allmaras, R.; Baker, J.; Molina, J. Soil organic carbon and nitrogen in a Minnesota soil as related to tillage, residue and nitrogen management. Soil Tillage Res. 2006, 89, 221–231. [Google Scholar] [CrossRef]
- Shen, M.-X.; Yang, L.-Z.; Yao, Y.-M.; Wu, D.-D.; Wang, J.; Guo, R.; Yin, S. Long-term effects of fertilizer managements on crop yields and organic carbon storage of a typical rice–wheat agroecosystem of China. Biol. Fertil. Soils 2007, 44, 187–200. [Google Scholar] [CrossRef]
- Hu, C.; Li, S.-L.; Qiao, Y.; Liu, D.-H.; Chen, Y.-F. Effects of 30 years repeated fertilizer applications on soil properties, microbes and crop yields in rice-wheat cropping systems. Exp. Agric. 2015, 51, 355. [Google Scholar] [CrossRef]
- Li, J.; Jian, S.; Lane, C.S.; Lu, Y.; He, X.; Wang, G.; Mayes, M.A.; Dzantor, K.E.; Hui, D. Effects of nitrogen fertilization and bioenergy crop type on topsoil organic carbon and total Nitrogen contents in middle Tennessee USA. PLoS ONE 2020, 15, e0230688. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.E.; Follett, R.F.; Pruessner, E.G.; Varvel, G.E.; Vogel, K.P.; Mitchell, R.B. N fertilizer and harvest impacts on bioenergy crop contributions to SOC. Glob. Chang. Biol. Bioenergy 2016, 8, 1201–1211. [Google Scholar] [CrossRef]
- Fontaine, S.; Hénault, C.; Aamor, A.; Bdioui, N.; Bloor, J.; Maire, V.; Mary, B.; Revaillot, S.; Maron, P.-A. Fungi mediate long term sequestration of carbon and nitrogen in soil through their priming effect. Soil Biol. Biochem. 2011, 43, 86–96. [Google Scholar] [CrossRef]
- Anning, D.K.; Qiu, H.; Zhang, C.; Ghanney, P.; Zhang, Y.; Guo, Y. Maize Straw Return and Nitrogen Rate Effects on Potato (Solanum tuberosum L.) Performance and Soil Physicochemical Characteristics in Northwest China. Sustainability 2021, 13, 5508. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).