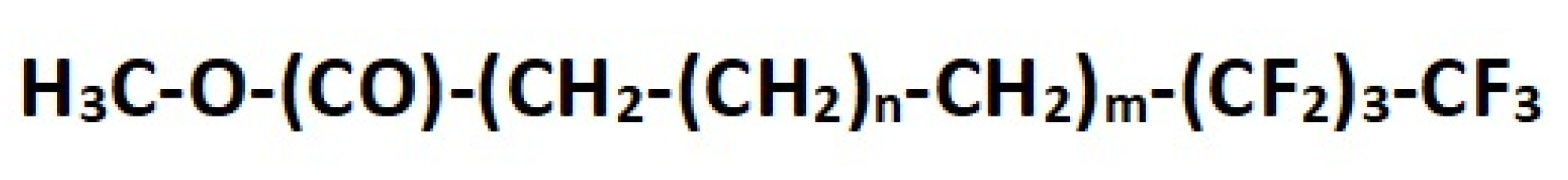1. Introduction
The protection of ancient stone monuments and objects is one of the topics of the recent research in the field of conservation of cultural heritage [
1]. Stone materials are affected by degradation processes such as black crusts, efflorescents, decoesions, etc., caused by several chemical, physical, and biological factors. Among others, water is one of the main causes of degradation, playing an important role in many decay mechanisms [
2]. It acts in a direct way through a solubilization action and in an indirect way through freeze/thaw cycles, pollutant transport, and soluble salt hydration. In addition, water may promote the growth of biological organisms which may cause material degradation.
It is well known that hydrophobic treatment is one of the most important interventions usually carried out in the conservation of stone artefacts and monuments, preserving them against the water [
3,
4]. The choice of hydrophobic products should be aimed at preventing liquid water penetration, to allow vapour leakage, delaying degradation processes, and to avoid chromatic alterations, leaving open at the same time the stone porosity for water vapour flow [
5,
6]. Nowadays, the use of hydrophobic polymers is generally accepted as being suitable due to their protective action, even if the direction of the research is also aimed at the development of innovative and inorganic materials [
7]. The evaluation of protection actions is based on the regulations by the European Committee for Standardization (CEN TC 346) [
8,
9].
Calcarenites are used in the most important testimony of ancient and modern monuments of Sicilian history. The different lithotypes, possessing different porosity and mineralogical features [
10], are present both in the east (Noto “white type” and Comiso) and in the west (Palermo, Mazara, Agrigento, Cusa).
Indeed, the performance of a polymeric protective can be different for different lithotypes, as demonstrated in our previous papers [
11,
12]. In most cases, the choice of the best protective is made based on a compromise between several factors, such as hydrophobicity and aesthetic appearance [
13].
The reason for the different behaviour of different protectives concerning one specific lithotype could be seen in the different microscopic interactions that occur, which could also be connected with the macroscopic behaviour [
14].
The interactions between the two materials (stone and polymer) can be responsible for the variations in the structural organization of the polymers when they are in contact with the stone surface, and these can depend not only on chemical factors but also on physical factors related to the porosity and dimensional distribution of pores [
15].
For this reason, the goal of this work is to verify the connections between the macroscale properties and the microscale properties of protected stone material. The work is carried out on Cusa’s stone, a calcarenite coming from the ancient and renowned quarries of Cusa near the acropolis of Selinunte (TP), used for the construction of most of the temples in the archaeological park of Selinunte, the largest in Europe [
16], in particular, Temple G, which is one of the most impressive temples of Magna Grecia.
In our previous paper, the effectiveness of the protective treatment of three formulations (the polysiloxane Wacker 290 and two fluorinated elastomers, Fluoline HY and Fluo AQ of CTS s.r.l.) was investigated using water absorption by capillarity, water vapour permeability and colorimetric tests [
12]. The obtained results show that all three formulations conferred protective properties, but Fluo AQ is the protective agent giving the best performance. The Fluo AQ guarantees a permeability similar to that of natural stone and drastically reduces the water absorption, thus giving it high water repellence. Conversely, the Fluoline HY significantly reduces the water vapour permeability of the stone and shows little water repellence.
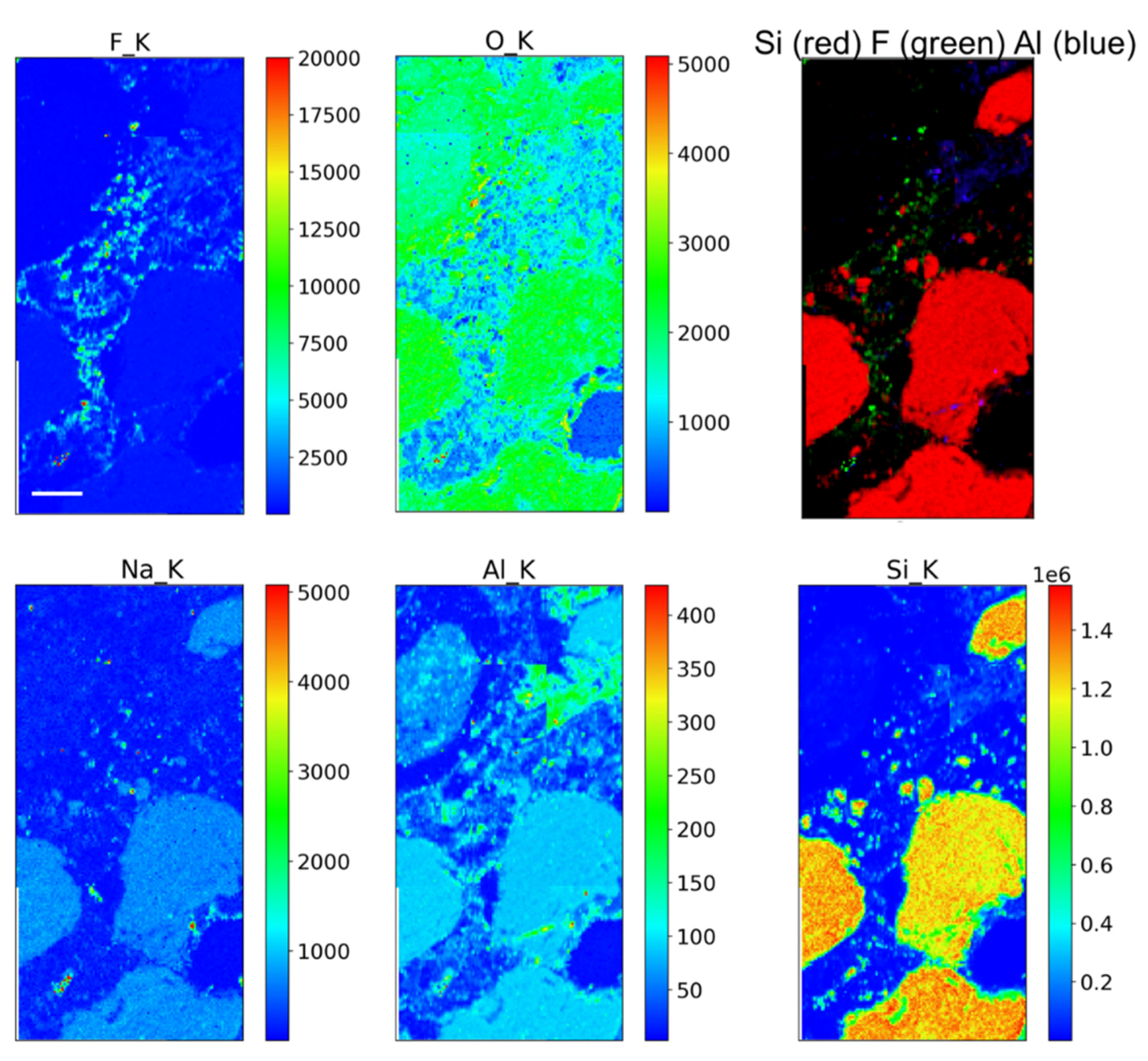
Here, the distribution of the polymer in the pore network was investigated by scanning electron microscopy and X-ray microscopy. Scanning electron microscopy studies provide relevant clues about the location of the products inside the porous matrix of stones [
17,
18]. However, in order to conduct a more in-depth investigation and obtain information concerning, for example, the adhesion of products to stone grains, the use of advanced techniques like X-ray microscopy allows us to improve the amount and quality of the acquired information in terms of the dimensional scale of investigation and identifiable chemical elements [
19,
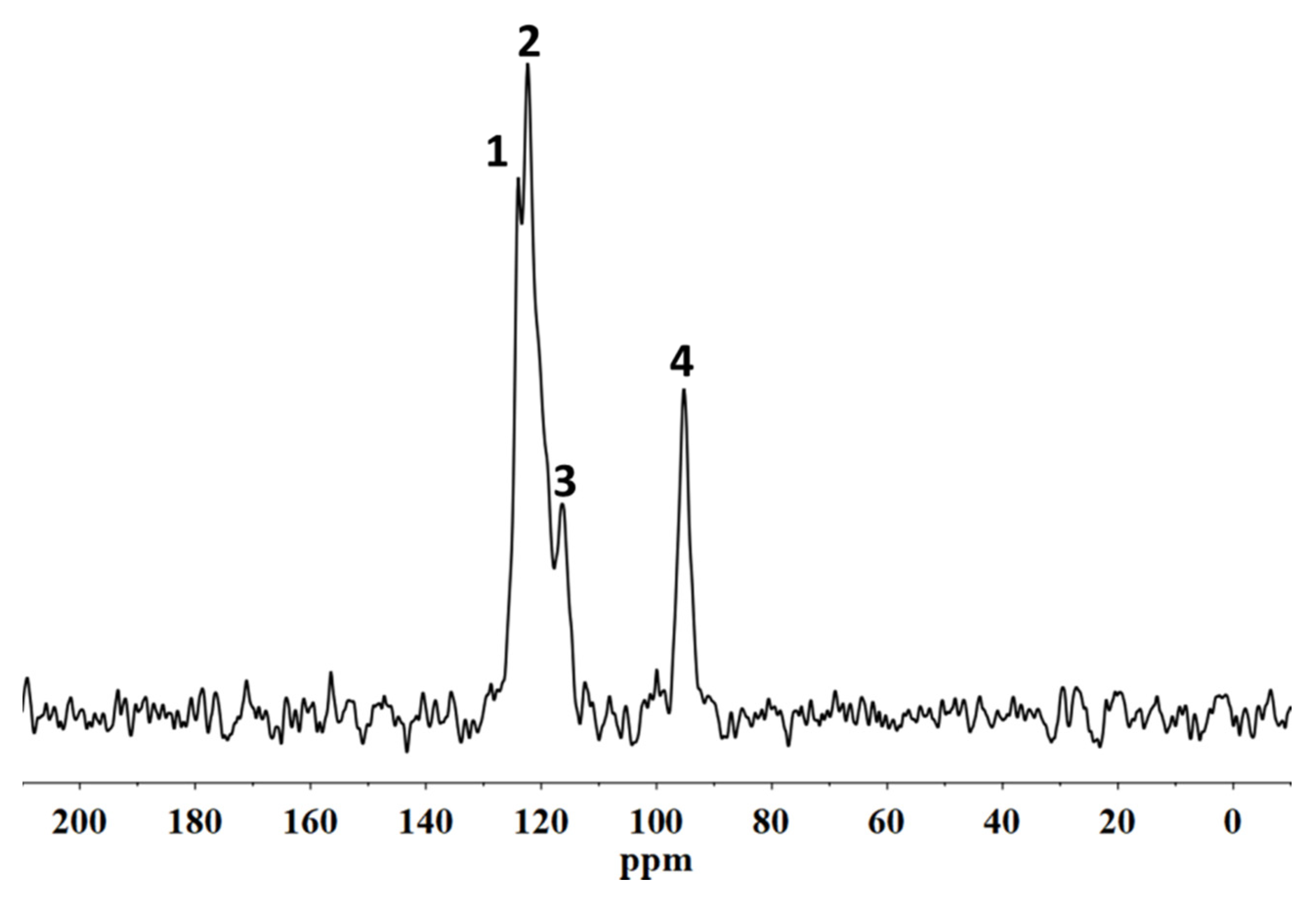
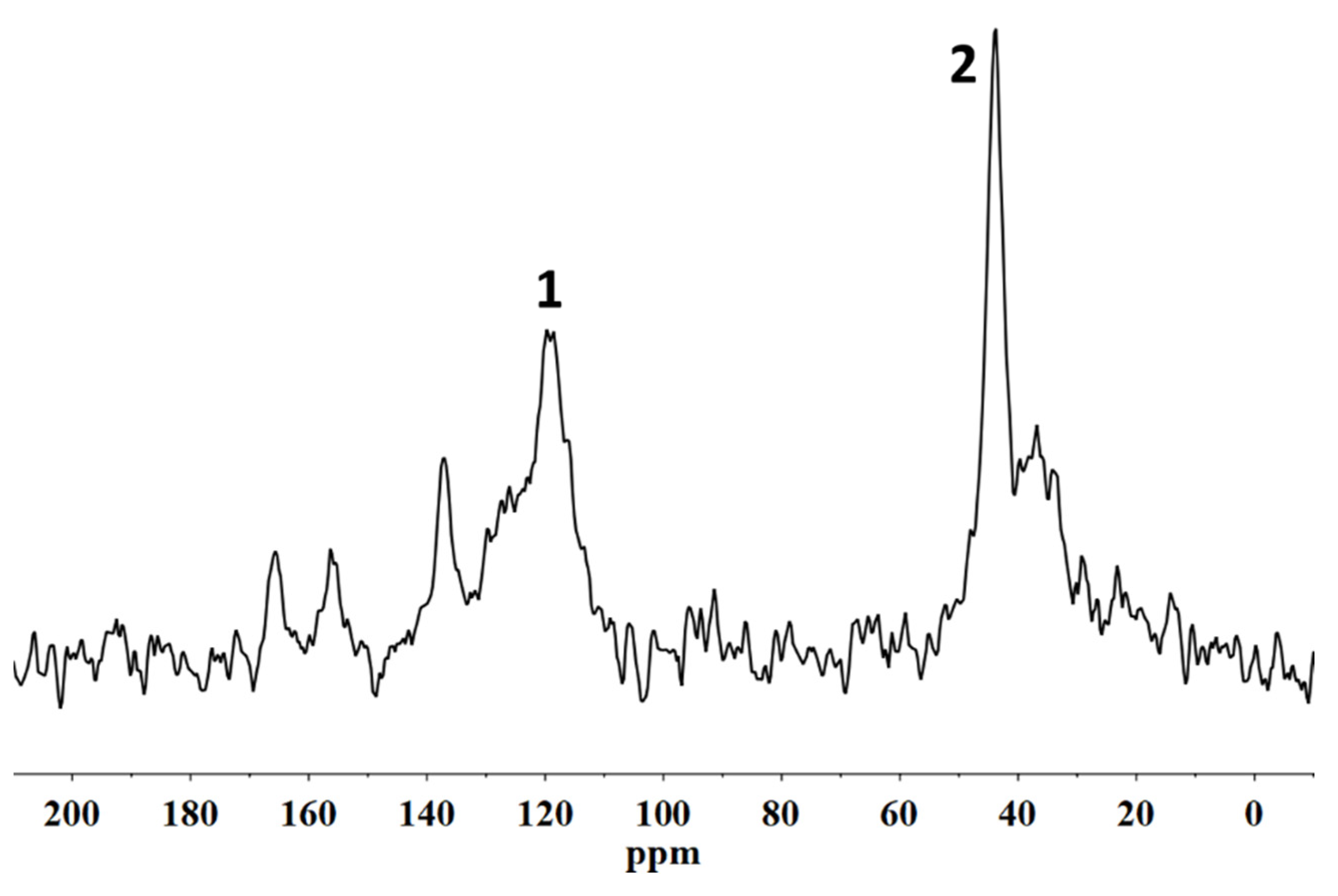
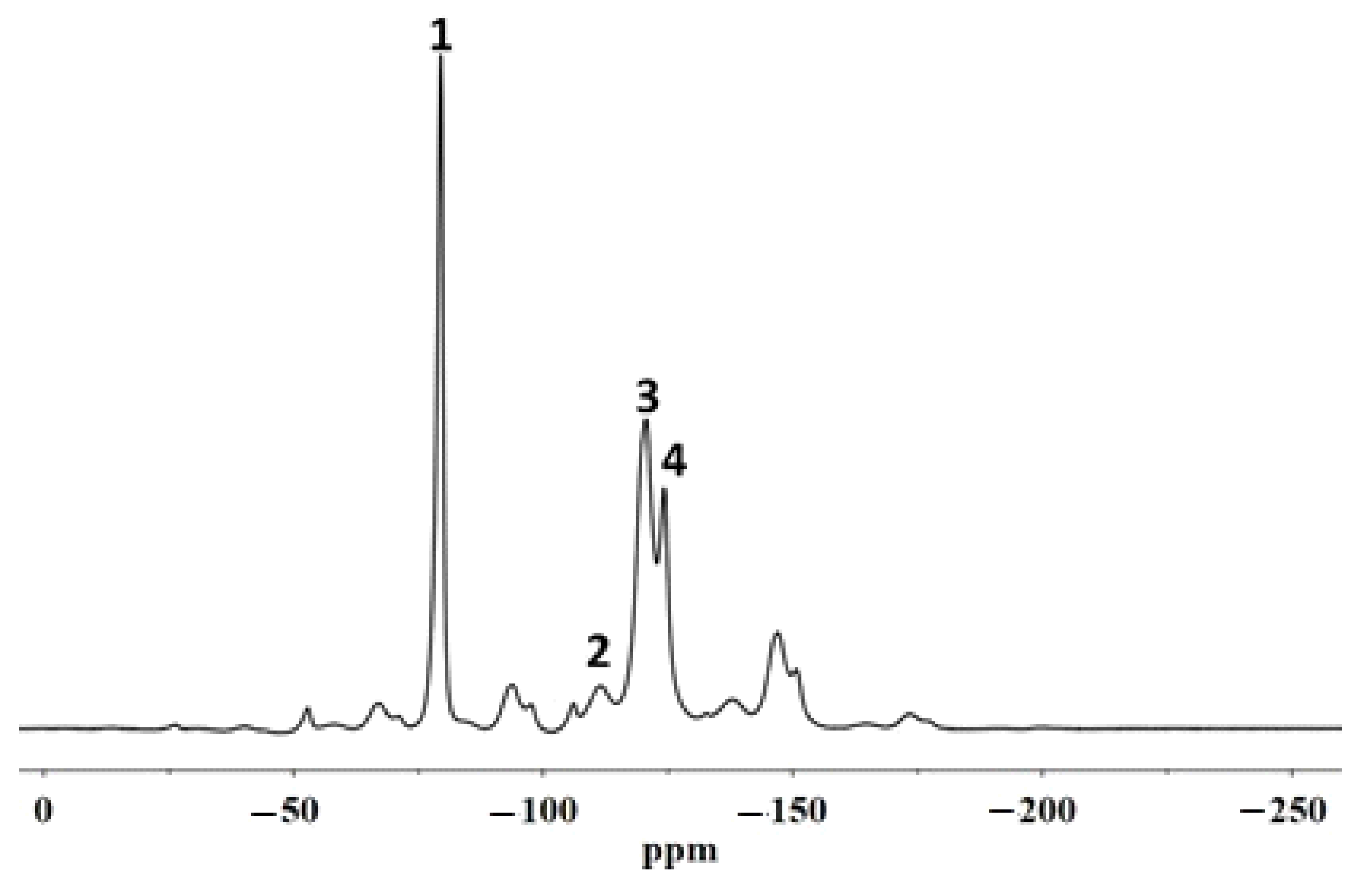
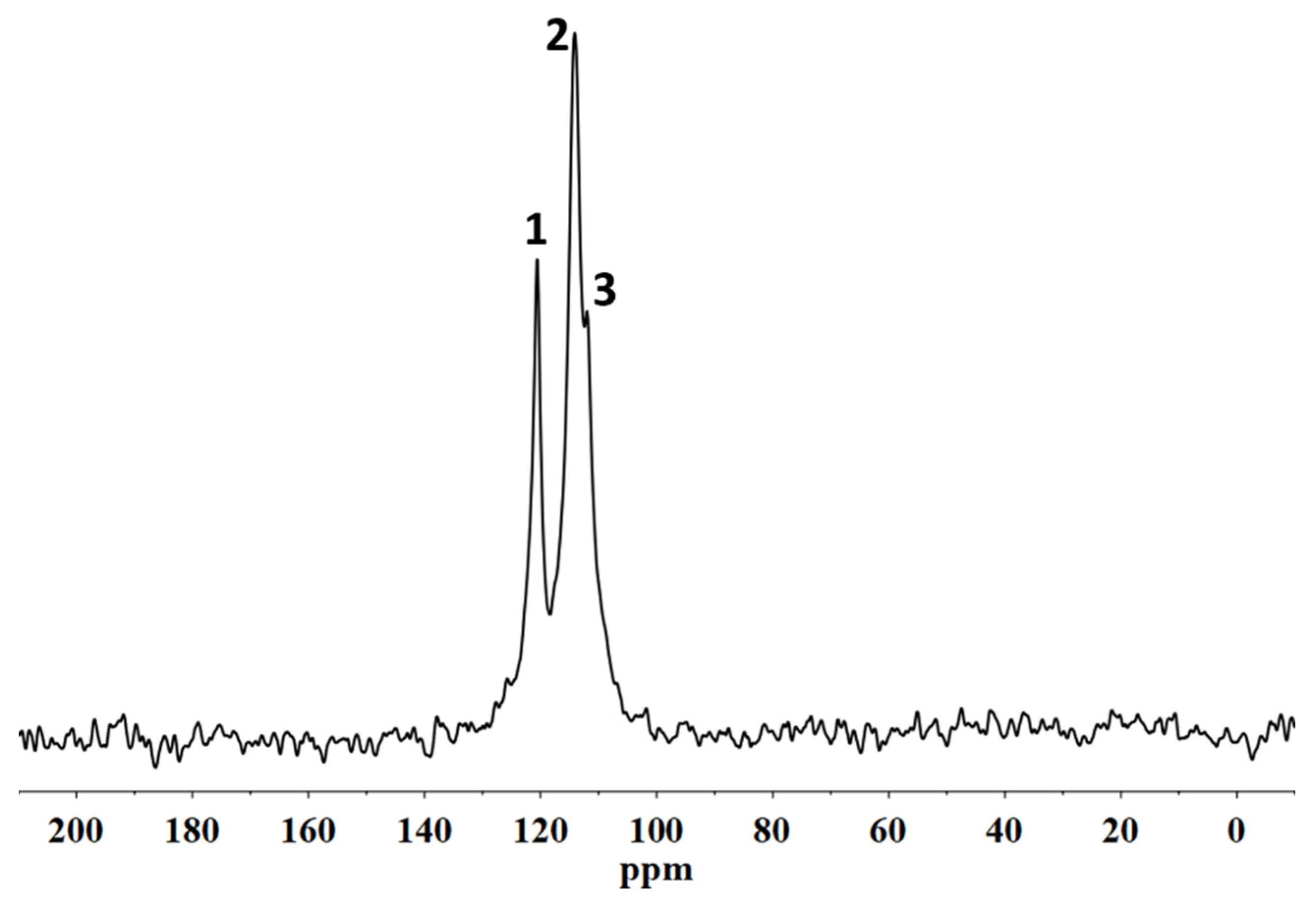
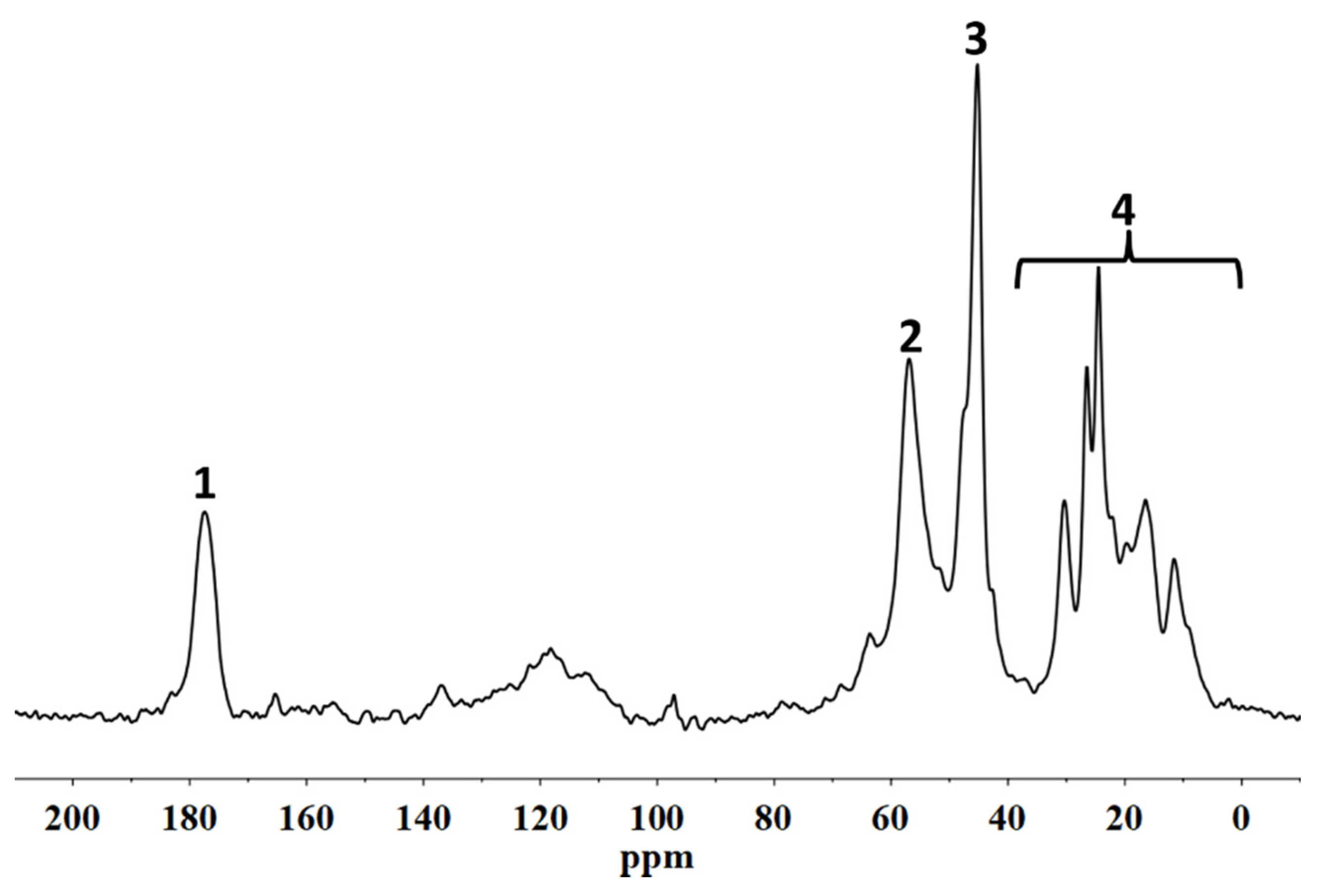
20]. The characterization of the fluorinated polymeric materials and their structural organization following the interactions with the stone substrate was performed using solid state NMR. In particular,
19F MAS NMR spectroscopies allowed us to analyse the molecular interactions involving the polymer directly on the coated stone surface. In addition, the spin–lattice relaxation times, T
1, and the spin–spin relaxation times, T
2, were evaluated in order to provide information on the variations of the mobility of both the backbone chains of the polymers and the side chains, respectively. In fact, the variation of the polymer chain mobility can act as evidence of their different structural organization when they are applied on the porous system [
21], suggesting the formation or not of a homogeneous surface film that justifies the water repellence or an accumulation of the material inside the pores that it could cause a significant reduction in the water vapour permeability of the stone.
2. Materials and Methods
2.1. Polymer Formulations
The two protective polymer formulations were provided by CTS s.r.l. The Fluoline HY® is a commercial protective agent based on a fluorinated elastomer consisting of vinylidene fluoride and hexafluoropropene copolymers at 3 wt.% in a 1:1 mixture (w/w) of acetone/butyl acetate. The Fluo AQ is a protective product under testing based on a fluorinated elastomer dispersed in water, with a concentration of the active content of about 5 wt.%.
2.2. Distribution of Polymers Inside the Porous Matrix of Stone
In order to investigate the distribution of the polymer inside the stone matrix by means of scanning electron microscopy and soft X-ray synchrotron microscopy, the specimens of the stone treated with fluorinated polymers were cut to obtain a 1 mm thick cross section cut perpendicularly to the treated surfaces, spanning to a depth of 5 mm, showing both external and internal parts of the stone. The dimensions were properly tailored to enable performing the correct placement in the sample stage of the equipment.
2.3. Scanning Electron Microscopy (SEM)
The cross sections were observed in scanning electron microscopy by using a Phenom Pro X, Phenom-World (Eindhoven, The Netherlands) with an optical magnification range of 20–135×, an electron magnification range of 80–130,000×, maximal digital zoom of 12×, acceleration voltages of 15 kV, a backscattered electron detector (BSD) and energy dispersive X-ray spectrometer (EDS) detectors, with a nominal resolution of 10 nm or less. The microscope was equipped with a temperature-controlled (25 °C) sample holder. The samples were positioned on an aluminium stub using adhesive carbon tape.
2.4. Soft X-ray Synchrotron Microscopy (Elettra)
The TwinMic microscope [
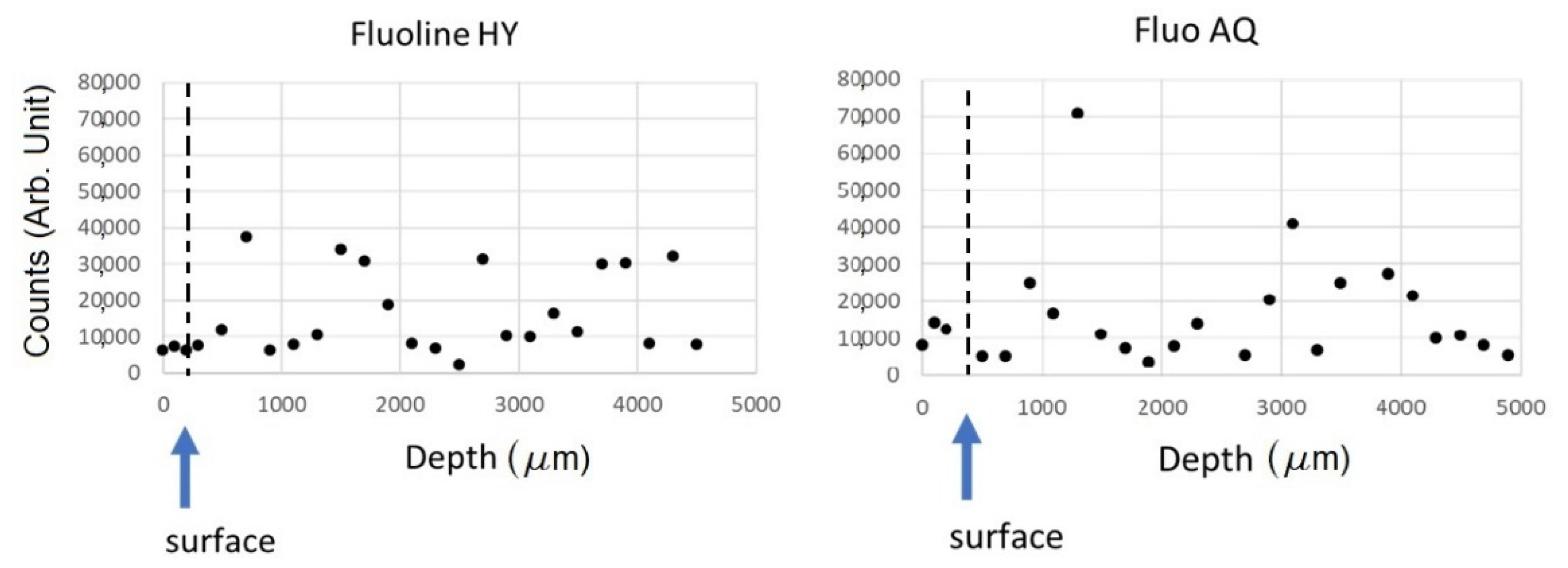
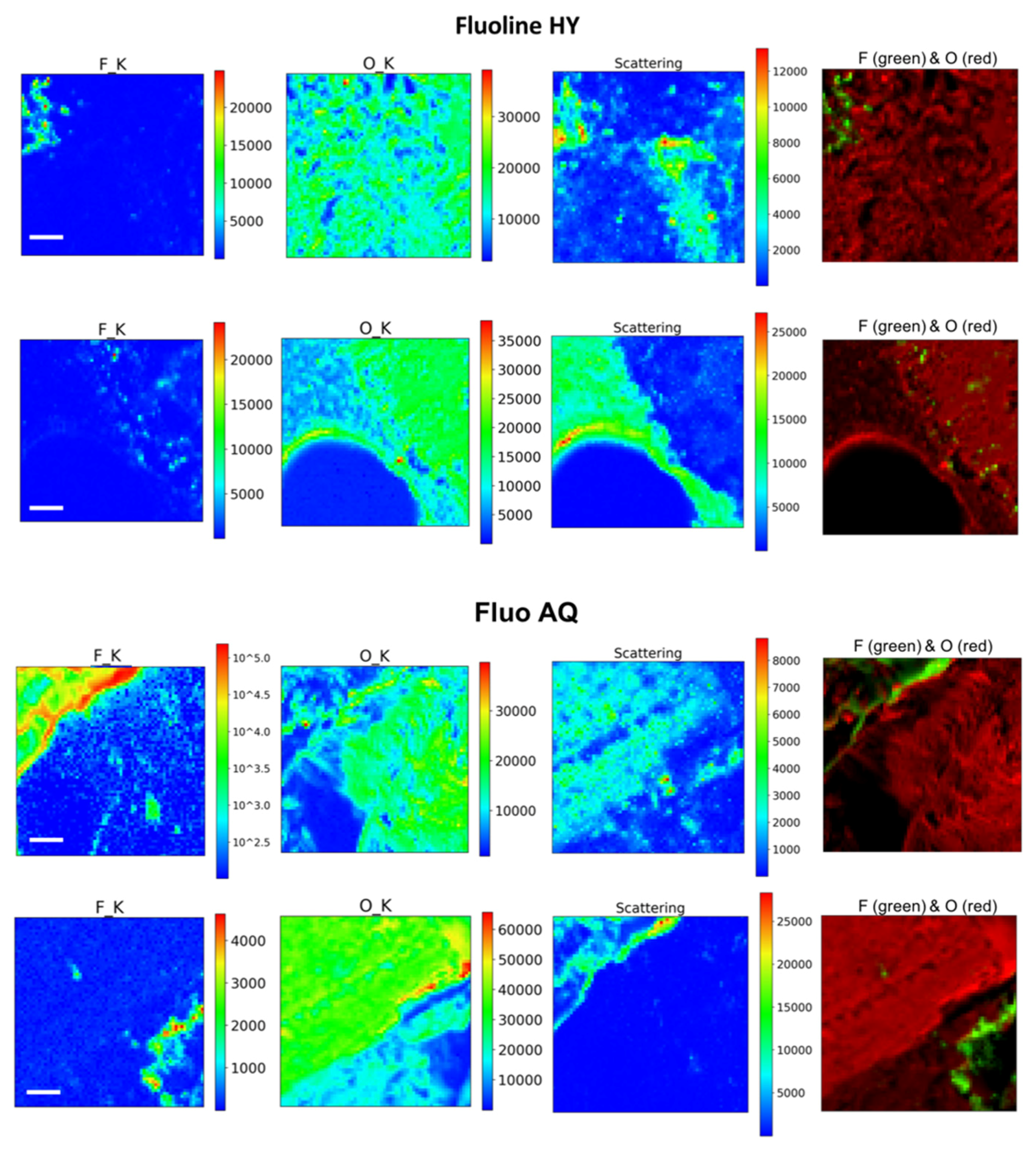
22] was set up in reflection mode by using the method proposed by Raneri et al. [
23]. The microscope operated in scanning mode. A zone plate with diffractive optics of 600 microns in diameter and a 50 nm outermost zone focused the incoming monochromatized X-ray photons on the sample plane, while the sample was raster scanned across the microprobe. Perpendicular profiles of the stone section were recorded at 1 keV energy X-ray source by collecting the X-ray fluorescence signal, where the highest sensitivity to fluorine was obtained. The X-ray fluorescence (XRF) spectra were acquired for areas at different depths from the surface. On one of the samples, XRF maps were acquired also at 2 keV on 18 adjacent zones, 80 × 80 µm
2 each, and then were post-processed by merging and stitching all of them into a single image, covering a total area of 240 × 480 µm
2. XRF maps were acquired in several zones, at different depth from the surface, at 1 and 2 keV with a spot size of 1.2 or 2 microns in diameter, respectively, and 4 s acquisition time per pixel. All XRF spectra were analysed by PyMCA software [
24], determining the areas of the elements’ peaks after background subtraction.
2.5. Characterization of Polymer Formulations
The two polymer formulations were characterized in the form of polymeric films in order to simulate the coating on the surface of the substrate stone. The polymeric films were obtained by pouring 30 g of each formulation into a capsule of a 10 cm diameter Petri dish and evaporating the solvent under a ventilated hood at room temperature.
2.6. Study of Interactions
The interactions between the stone substrate and each protective agent were investigated using two samples obtained by cutting the surface layer of two specimens treated with Fluoline HY and Fluo AQ, respectively, using a Dremel mini drill.
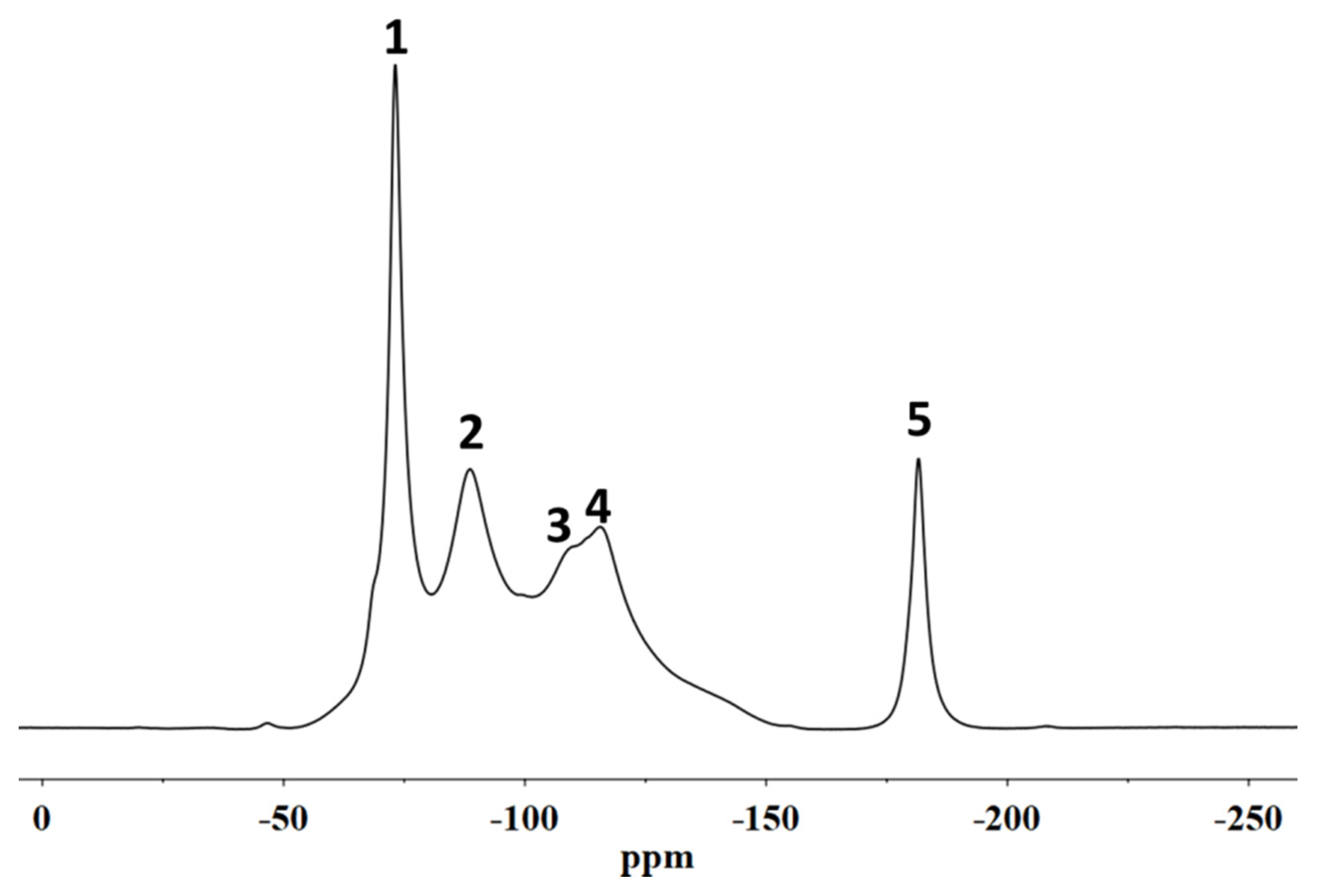
2.7. Solid-State NMR Spectroscopy
All spectra were acquired by using a Bruker Avance II 400 (9.4T) spectrometer operating at 400.15, 100.61 and 376.50 MHz for the 1H, 13C and 19F nuclides, respectively. Fifty milligrams of each sample were packed in 4 mm diameter zirconia rotors for the 13C and 29Si nuclide experiments and 2.5 mm for the 19F experiments. All rotors were sealed with KEL-F caps. 19F MAS NMR spectra were acquired at a rotation frequency of the magic angle of 10 kHz, a 90° pulse duration of 4 μs, 128 scans and a delay time between one scan and the next of 3 s. The external reference standard used was polytetrafluoroethylene (−123 ppm). 13C {19F} CP-MAS NMR spectra were acquired at a rotation frequency of the magic angle of 10 kHz, a pulse duration on 19F nuclei of 2.5 μs, contact time of 1 ms, a delay time of 3 s, with 7000 scans. The Hartmann–Hahn condition was optimized on a Teflon sample. 13C {1H} CP-MAS NMR spectra were acquired at a magic angle rotation frequency of 10 kHz, a pulse duration on 1H of 2.5 μs, contact time of 1.5 ms, a delay time of 3 s, with 5000 scans. The Hartman–Hahn condition was optimized on an adamantane sample which was also used as a chemical shift reference (29.5 and 38.6 ppm).
The spin–lattice relaxation times, T1F, were calculated by inversion recovery pulse sequence (180-τ-90), using a delay time in a range from 0 to 10 s; The spin–spin relaxation times, T2F, were calculated by pulse sequence Carr–Purcell–Meiboom–Gill (CPMG) (90-τ-180), using a delay time ranging from 0.1 to 10 ms.
4. Conclusions
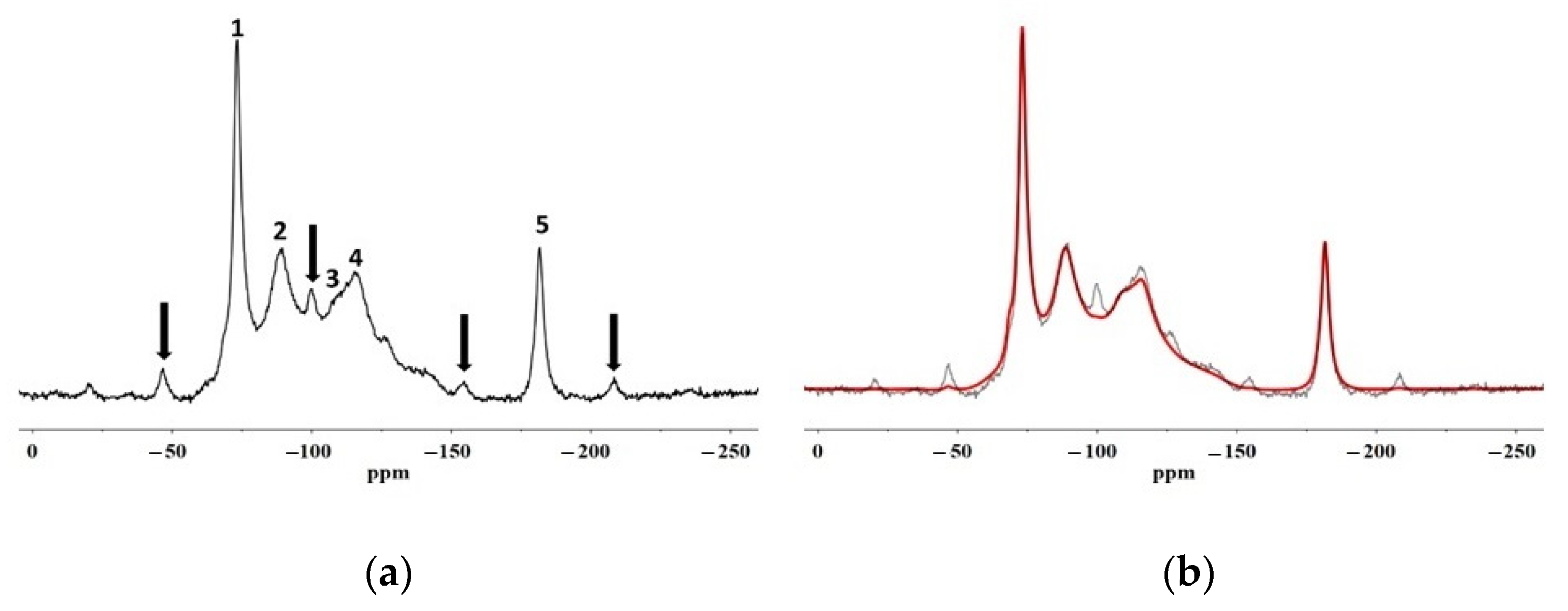
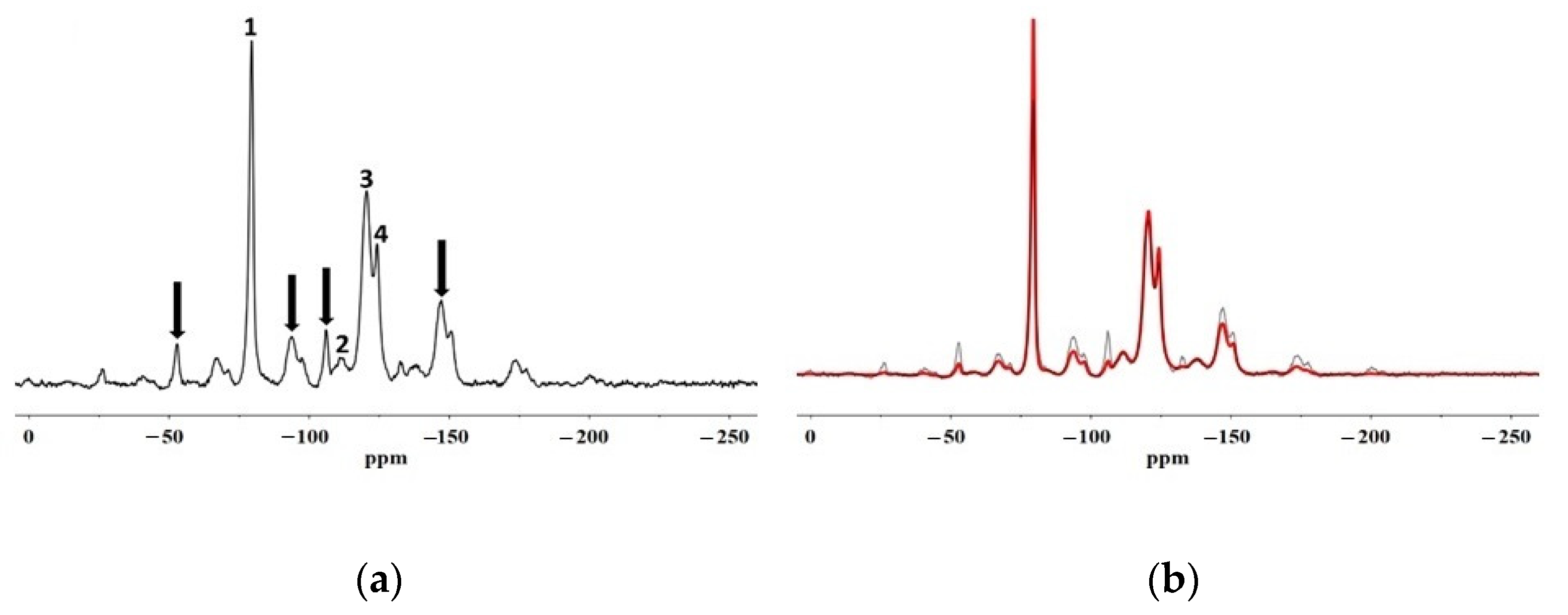
In this paper, SEM, X-ray microscopy and ss-NMR were used to correlate the microscopic properties with the protective efficacy of two polymeric formulations on Cusa’s stone. The results obtained by X-ray synchrotron microscopy coupled with X-ray fluorescence prove that the performance of both coatings is linked to their distribution within the stone, which are usually indicated only at the macroscopic level in terms of the permeability of the stone to water vapour and water repellence. In fact, the Fluo AQ shows greater affinity with the stone substrate and the formation of a continuous and homogeneous film adhering to the walls of the pores, without obstructing the empty spaces in which the water molecules can diffuse, and which confers an important hydrophobic character. On the contrary, the Fluoline HY clogs the pores, limiting the water vapour permeability. The interactions with the stone substrate were investigated by comparing the ss–NMR spectra of Fluoline HY and Fluo AQ in the form of a polymeric film with the corresponding spectra obtained from the stone samples treated with each protective agent. No changes in the chemical environment of the observed nuclei were found. The analysis of relaxation times highlighted the changes in the structural organization and in the dynamics of polymeric systems. The two protective agents in contact with the stone substrate are less mobile, probably due to a “confinement effect” also highlighted by the increase in the intensity of the side bands in the spectra obtained from the stone treated with the protective. The smaller mobility of the polymeric chains of Fluo AQ, together with its cross-linked organisation, can explain the greater water repellence imparted to the stone substrate compared to Fluoline HY, in agreement with the X-ray microscopy. The crosslinking of the polymeric system could cause the formation of a more homogeneous surface film. The study of the interactions between the protective materials used and the stone substrate is fundamental for obtaining indirect information on protective treatments and represents an alternative approach to the traditional methods used up to now. The obtained results show how X-ray synchrotron microscopy coupled with X-ray fluorescence is a powerful tool to overcome some drawbacks of classical imaging and to carry out an in-depth investigation on the spatial distribution of polymers in the stone and how ss-NMR spectroscopy is a powerful tool to interpret and predict the effectiveness of water repellence treatments for the protection of stone surfaces knowing the structural characteristics and physical properties of the polymer. Both techniques are useful for interpreting and correlating micro and macro behaviour for the description of the protective action. In the future, this approach needs to be made more thorough and could be applied to other case studies involving other kinds of protectives. The obtained results are of utmost importance for fundamental research, but also for manufacturing companies aiming to developing new commercial products, upgrading existing ones or orienting their end-user handling.