Ginsenoside Rh1 Exerts Neuroprotective Effects by Activating the PI3K/Akt Pathway in Amyloid-β Induced SH-SY5Y Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture and Measurement of Cell Viability
2.3. FDA/PI and Hoechst Staining
2.4. Reactive Oxygen Species (ROS) Assay
2.5. Immunoblot Analysis
2.6. Statistical Analyses
3. Results
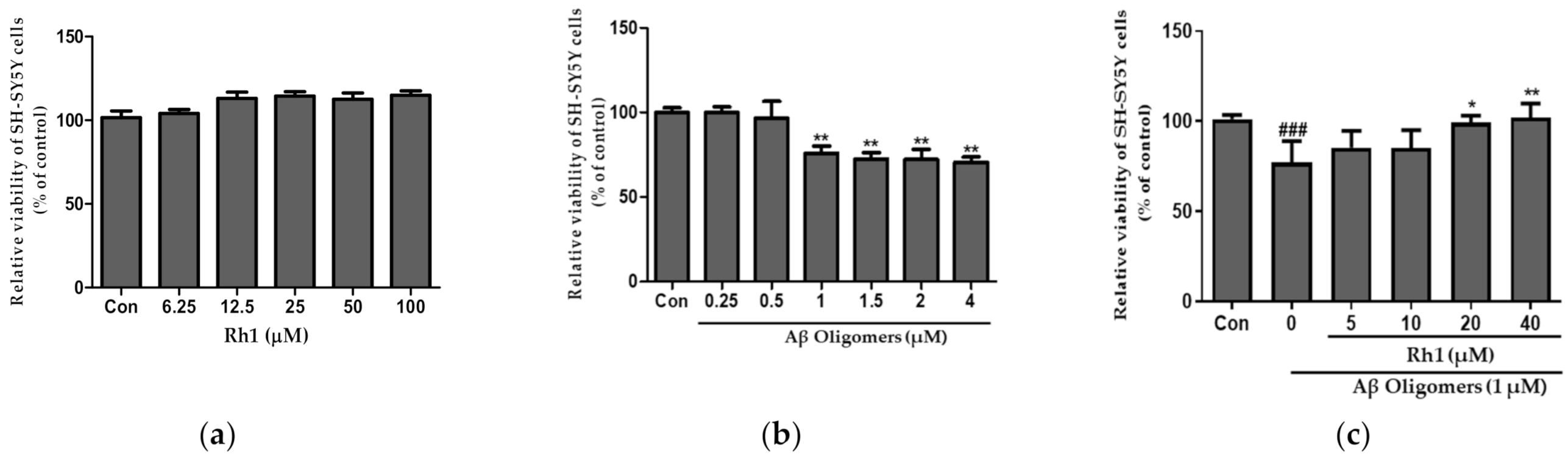
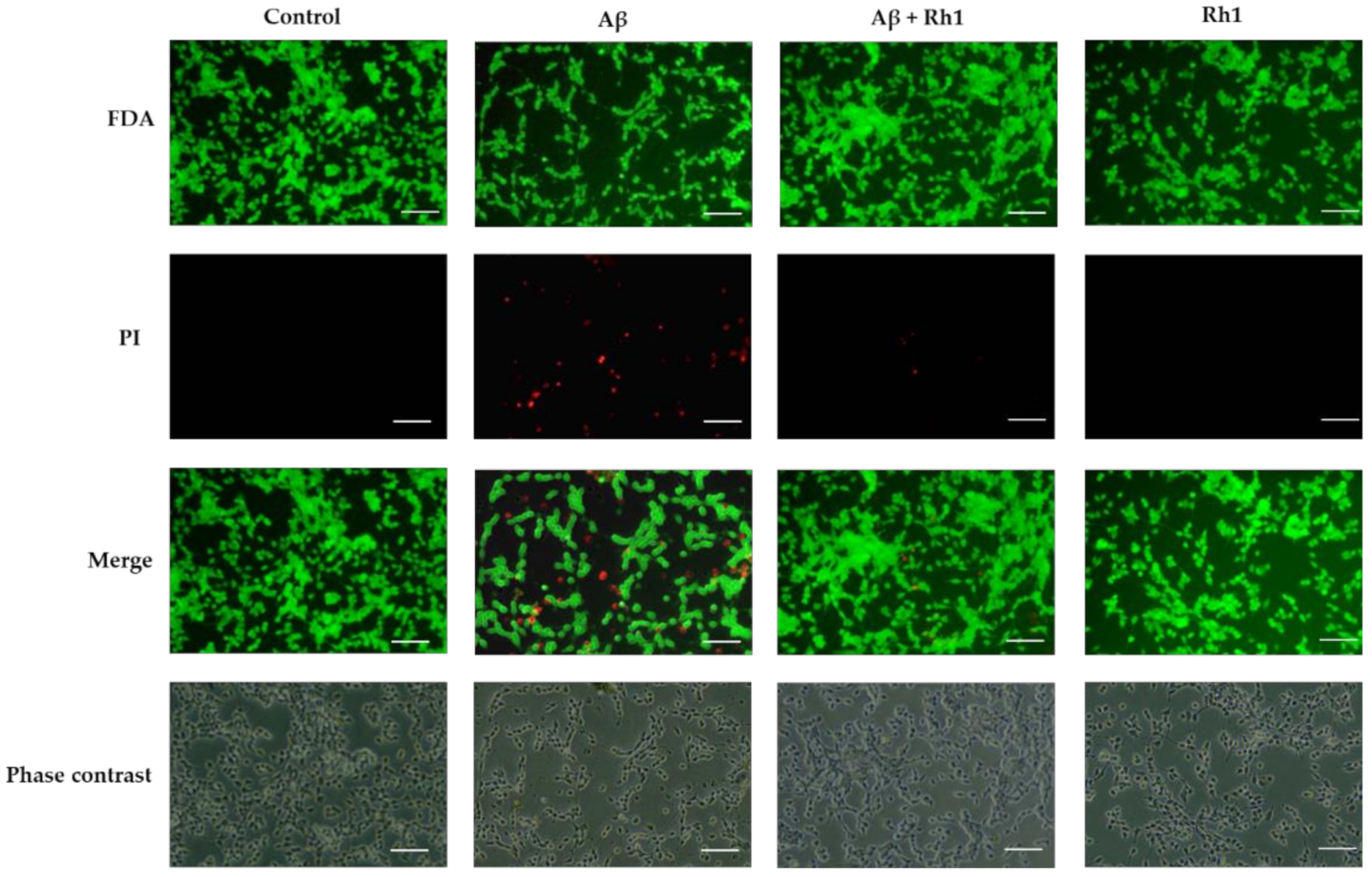
3.1. Ginsenoside Rh1 Attenuates Aβ Oligomers Neurotoxicity in SH-SY5Y Cells
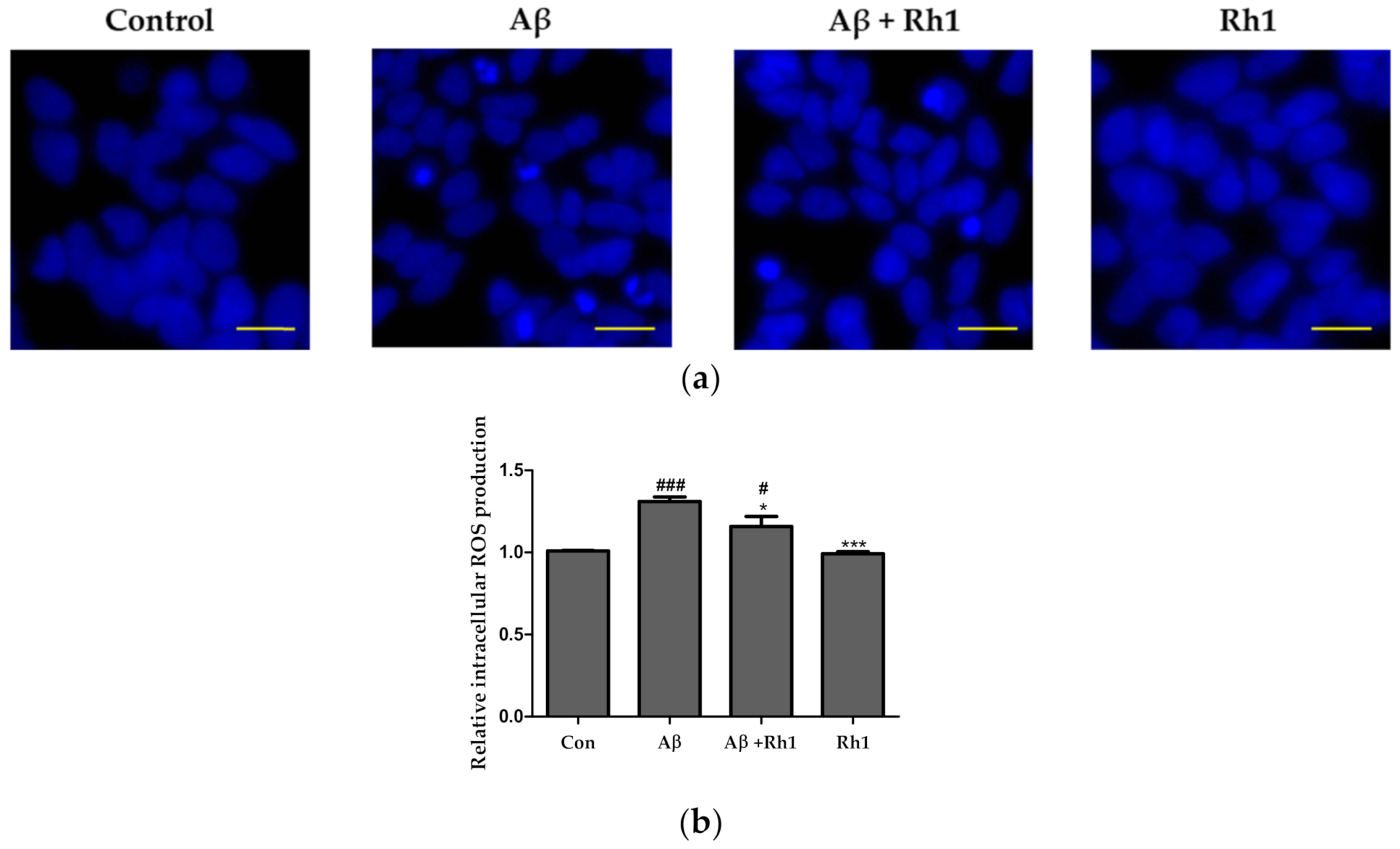
3.2. Ginsenoside Rh1 Attenuates Intracellular ROS of Aβ Oligomers in SH-SY5Y Cells
3.3. Inhibition of Akt and GSK Pathway in SH-SY5Y Cells
3.4. Cytoprotective Effects of Ginsenoside Rh1 Are Mediated through PI3K/Akt/GSK-3 Pathway
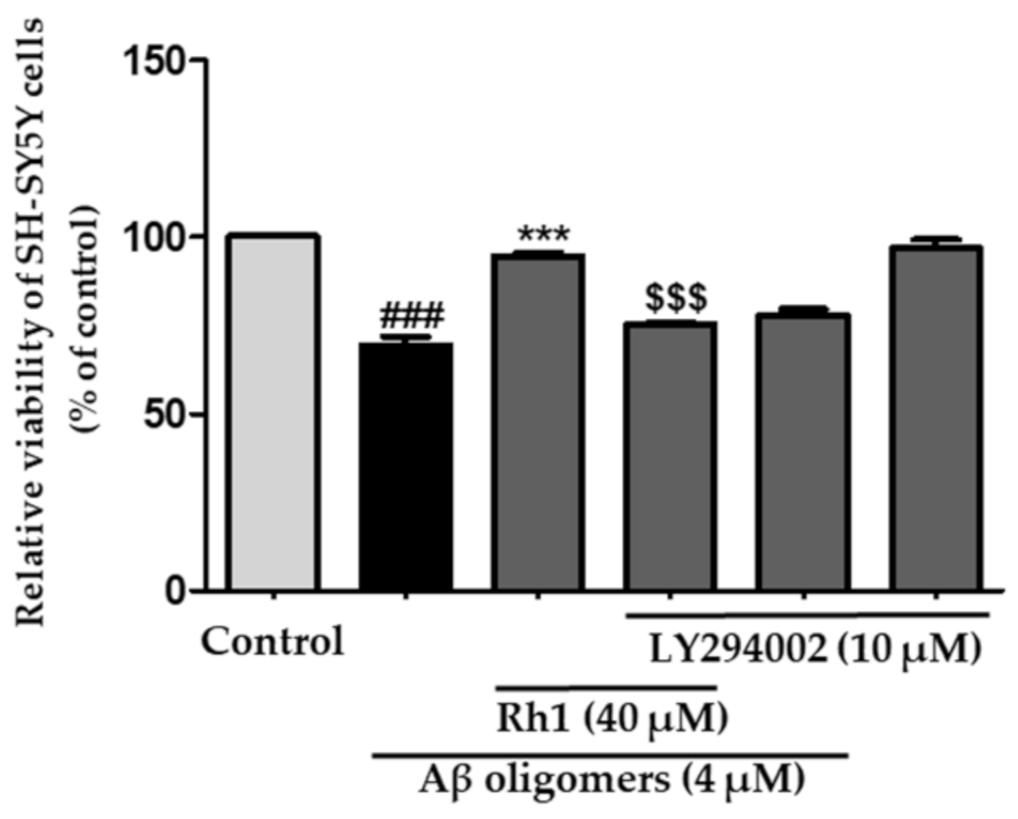
3.5. Ginsenoside Rh1 Inhibits Cell Death through the Activation of PI3K/Akt Induced by Aβ Oligomers in SH-SY5Y Cells
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duong, S.; Patel, T.; Chang, F. Dementia: What pharmacists need to know. Can. Pharm. J. 2017, 150, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, S.; Carrillo, M.C.; Farias, S.T.; Goldberg, T.E.; Hendrix, J.A.; Jaeger, J.; Knopman, D.S.; Langbaum, J.B.; Park, D.C.; Ropacki, M.T.; et al. Measuring cognition and function in the preclinical stage of Alzheimer’s disease. Alzheimer Dement. Transl. Res. Clin. Interv. 2018, 4, 64–75. [Google Scholar] [CrossRef]
- Hsiao, Y.-H.; Chang, C.-H.; Gean, P.-W. Impact of social relationships on Alzheimer’s memory impairment: Mechanistic studies. J. Biomed. Sci. 2018, 25, 3. [Google Scholar] [CrossRef] [PubMed]
- Graham, W.V.; Bonito-Oliva, A.; Sakmar, T.P. Update on Alzheimer’s Disease Therapy and Prevention Strategies. Annu. Rev. Med. 2017, 68, 413–430. [Google Scholar] [CrossRef]
- Selkoe, D.J. Normal and abnormal biology of the beta-amyloid precursor protein. Annu. Rev. Neurosci. 1994, 17, 489–517. [Google Scholar] [CrossRef]
- Prince, M.; Comas-Herrera, A.; Knapp, M.; Guerchet, M.; Karagiannidou, M. World Alzheimer Report 2016: Improving Healthcare for People Living with Dementia: Coverage, Quality and Costs Now and in the Future; Alzheimer’s Disease International: London, UK, 2016. [Google Scholar]
- Niikura, T.; Tajima, H.; Kita, Y. Neuronal cell death in Alzheimer’s disease and a neuroprotective factor, humanin. Curr. Neuropharmacol. 2006, 4, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Park, H.H.; Koh, S.H.; Choi, N.Y.; Yu, H.J.; Park, J.; Lee, Y.J.; Lee, K.Y. Coenzyme Q10 protects against amyloid beta-induced neuronal cell death by inhibiting oxidative stress and activating the P13K pathway. Neurotoxicology 2012, 33, 85–90. [Google Scholar] [CrossRef]
- Choi, H.; Park, H.H.; Lee, K.Y.; Choi, N.Y.; Yu, H.J.; Lee, Y.J.; Park, J.; Huh, Y.M.; Lee, S.H.; Koh, S.H. Coenzyme Q10 restores amyloid beta-inhibited proliferation of neural stem cells by activating the PI3K pathway. Stem Cells Dev. 2013, 22, 2112–2120. [Google Scholar] [CrossRef]
- Jope, R.S.; Johnson, G.V. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem. Sci. 2004, 29, 95–102. [Google Scholar] [CrossRef]
- Beurel, E.; Grieco, S.F.; Jope, R.S. Glycogen synthase kinase-3 (GSK3): Regulation, actions, and diseases. Pharmacol. Ther. 2015, 148, 114–131. [Google Scholar] [CrossRef]
- Doble, B.W.; Woodgett, J.R. GSK-3: Tricks of the trade for a multi-tasking kinase. J. Cell Sci. 2003, 116, 1175–1186. [Google Scholar] [CrossRef]
- Takashima, A. GSK-3 is essential in the pathogenesis of Alzheimer’s disease. J. Alzheimers Dis. 2006, 9, 309–317. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, Y.; Wang, X.; Evers, B.M. Glycogen synthase kinase-3 is a negative regulator of extracellular signal-regulated kinase. Oncogene 2006, 25, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Kheiri, G.; Dolatshahi, M.; Rahmani, F.; Rezaei, N. Role of p38/MAPKs in Alzheimer’s disease: Implications for amyloid beta toxicity targeted therapy. Rev. Neurosci. 2018, 30, 9–30. [Google Scholar] [CrossRef]
- Hoover, B.R.; Reed, M.N.; Su, J.; Penrod, R.D.; Kotilinek, L.A.; Grant, M.K.; Pitstick, R.; Carlson, G.A.; Lanier, L.M.; Yuan, L.-L. Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron 2010, 68, 1067–1081. [Google Scholar] [CrossRef]
- Bielska, A.A.; Zondlo, N.J. Hyperphosphorylation of tau induces local polyproline II helix. Biochemistry 2006, 45, 5527–5537. [Google Scholar] [CrossRef] [PubMed]
- Park, E.K.; Choo, M.K.; Han, M.J.; Kim, D.H. Ginsenoside Rh1 Possesses Antiallergic and Anti-Inflammatory Activities. Int. Arch. Allergy Immunol. 2004, 133, 113–120. [Google Scholar] [CrossRef]
- Hyun, S.H.; Kim, S.W.; Seo, H.W.; Youn, S.H.; Kyung, J.S.; Lee, Y.Y.; Gyo, I.; Park, C.-K.; Han, C.-K. Physiological and pharmacological features of the non-saponin components in Korean red ginseng. J. Ginseng Res. 2020. [Google Scholar] [CrossRef]
- Park, M.; Yoo, J.-H.; Lee, Y.-S.; Park, E.-J.; Lee, H.-J. Ameliorative effects of black ginseng on nonalcoholic fatty liver disease in free fatty acid-induced HepG2 cells and high-fat/high-fructose diet-fed mice. J. Ginseng Res. 2020, 44, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Attele, A.S.; Wu, J.A.; Yuan, C.-S. Ginseng pharmacology: Multiple constituents and multiple actions. Biochem. Pharmacol. 1999, 58, 1685–1693. [Google Scholar] [CrossRef]
- Wang, Y.-Z.; Chen, J.; Chu, S.-F.; Wang, Y.-S.; Wang, X.-Y.; Chen, N.-H.; Zhang, J.-T. Improvement of Memory in Mice and Increase of Hippocampal Excitability in Rats by Ginsenoside Rg1′s Metabolites Ginsenoside Rh1 and Protopanaxatriol. J. Pharmacol. Sci. 2009, 109, 504–510. [Google Scholar] [CrossRef]
- Cheng, Y.; Shen, L.-H.; Zhang, J.-T. Anti-amnestic and anti-aging effects of ginsenoside Rg1 and Rb1 and its mechanism of action. Acta Pharmacol. Sin. 2005, 26, 143–149. [Google Scholar] [CrossRef]
- Yun, T.K.; Lee, Y.S.; Lee, Y.H.; Kim, S.I.; Yun, H.Y. Anticarcinogenic effect of Panax ginseng C.A. Meyer and identification of active compounds. J. Korean Med Sci. 2001, 16, S6–S18. [Google Scholar] [CrossRef]
- Kim, B.H.; Lee, Y.G.; Park, T.Y.; Kim, H.B.; Rhee, M.H.; Cho, J.Y. Ginsenoside Rp1, a ginsenoside derivative, blocks lipopolysaccharide-induced interleukin-1beta production via suppression of the NF-kappaB pathway. Planta Med. 2009, 75, 321–326. [Google Scholar] [CrossRef]
- Xie, J.-t.; Wang, C.-z.; Wang, A.-b.; Wu, J.; Basila, D.; Yuan, C.-s. Antihyperglycemic effects of total ginsenosides from leaves and stem of Panax ginseng. Acta Pharmacol. Sin. 2005, 26, 1104–1110. [Google Scholar] [CrossRef]
- Tanaka, O.; Nagai, M.; Shibata, S. Chemical Studies on the Oriental Plant Drugs. XVI. The Stereochemistry of Protopanaxadiol, a Genuine Sapogenin of Ginseng. Chem. Pharm. Bull. 1966, 14, 1150–1156. [Google Scholar] [CrossRef]
- Lee, S.; Lee, M.; Ko, Y.; Choi, H.; Jeong, J.; Choi, K.-M.; Cha, J.-D.; Hwang, S.-M.; Jung, H.; Park, J.; et al. Effects of Extracts of Unripe Black Raspberry and Red Ginseng on Cholesterol Synthesis. Korean J. Food Sci. Technol. 2013, 45. [Google Scholar] [CrossRef]
- Jung, J.-S.; Shin, J.A.; Park, E.-M.; Lee, J.-E.; Kang, Y.-S.; Min, S.-W.; Kim, D.-H.; Hyun, J.-W.; Shin, C.-Y.; Kim, H.-S. Anti-inflammatory mechanism of ginsenoside Rh1 in lipopolysaccharide-stimulated microglia: Critical role of the protein kinase A pathway and hemeoxygenase-1 expression. J. Neurochem. 2010, 115, 1668–1680. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Jin, Y.; Lim, W.; Ji, S.; Choi, S.; Jang, S.; Lee, S. A ginsenoside-Rh1, a component of ginseng saponin, activates estrogen receptor in human breast carcinoma MCF-7 cells. J. Steroid Biochem. Mol. Biol. 2003, 84, 463–468. [Google Scholar] [CrossRef]
- Kim, D.-H. Gut microbiota-mediated pharmacokinetics of ginseng saponins. J. Ginseng Res. 2018, 42, 255–263. [Google Scholar] [CrossRef]
- Zheng, M.-M.; Xu, F.-X.; Li, Y.-J.; Xi, X.-Z.; Cui, X.-W.; Han, C.-C.; Zhang, X.-L. Study on Transformation of Ginsenosides in Different Methods. Biomed. Res. Int. 2017, 2017, 8601027. [Google Scholar] [CrossRef] [PubMed]
- Lyu, X.; Xu, X.; Song, A.; Guo, J.; Zhang, Y.; Zhang, Y. Ginsenoside Rh1 inhibits colorectal cancer cell migration and invasion in vitro and tumor growth in vivo. Oncol. Lett. 2019, 18, 4160–4166. [Google Scholar] [CrossRef] [PubMed]
- Mathiyalagan, R.; Wang, C.; Kim, Y.J.; Castro-Aceituno, V.; Ahn, S.; Subramaniyam, S.; Simu, S.Y.; Jiménez-Pérez, Z.E.; Yang, D.C.; Jung, S.-K. Preparation of Polyethylene Glycol-Ginsenoside Rh1 and Rh2 Conjugates and Their Efficacy against Lung Cancer and Inflammation. Molecules 2019, 24, 4367. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Cho, S.-H.; Kim, J.-E.; Lee, C.; Lee, J.-H.; Baek, M.-C.; Song, G.-Y.; Bae, J.-S. Suppressive Effects of Ginsenoside Rh1 on HMGB1-Mediated Septic Responses. Am. J. Chin. Med. 2019, 47, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Kim, K.-A.; Dong Hyun, K. Ginsenoside Rh1 Ameliorates High Fat Diet-Induced Obesity in Mice by Inhibiting Adipocyte Differentiation. Biol. Pharm. Bull. 2013, 36, 102–107. [Google Scholar] [CrossRef]
- Lin, J.; Yu, J.; Zhao, J.; Zhang, K.; Zheng, J.; Wang, J.; Huang, C.; Zhang, J.; Yan, X.; Gerwick, W.H.; et al. Fucoxanthin, a Marine Carotenoid, Attenuates β-Amyloid Oligomer-Induced Neurotoxicity Possibly via Regulating the PI3K/Akt and the ERK Pathways in SH-SY5Y Cells. Oxidative Med. Cell. Longev. 2017, 2017, 6792543. [Google Scholar] [CrossRef]
- Chen, X.; Li, H.; Yang, Q.; Lan, X.; Wang, J.; Cao, Z.; Shi, X.; Li, J.; Kan, M.; Qu, X.; et al. Ginsenoside compound K ameliorates Alzheimer’s disease in HT22 cells by adjusting energy metabolism. Mol. Biol. Rep. 2019, 46, 5323–5332. [Google Scholar] [CrossRef]
- Du, Y.; Fu, M.; Wang, Y.T.; Dong, Z. Neuroprotective Effects of Ginsenoside Rf on Amyloid-β-Induced Neurotoxicity in vitro and in vivo. J. Alzheimers Dis. 2018, 64, 309–322. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Li, W.; Wang, Z.; Guo, P.; Li, L.; Li, N. Metabolic profiling of the effects of ginsenoside Re in an Alzheimer’s disease mouse model. Behav. Brain Res. 2018, 337, 160–172. [Google Scholar] [CrossRef]
- Zheng, Y.; Gao, X.; Chen, Q.; Yan, P.; Liu, R.; Liang, H.; Zhang, M. Effect of Ginsenosides on Prevention of Alzheimer’s Disease. IOP Conf. Ser. Mater. Sci. Eng. 2019, 612, 022004. [Google Scholar] [CrossRef]
- Zhao, J.; Lu, S.; Yu, H.; Duan, S.; Zhao, J. Baicalin and ginsenoside Rb1 promote the proliferation and differentiation of neural stem cells in Alzheimer’s disease model rats. Brain Res. 2018, 1678, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ren, Z.; Zhang, J.; Chuang, C.C.; Kandaswamy, E.; Zhou, T.; Zuo, L. Role of ROS and Nutritional Antioxidants in Human Diseases. Front. Physiol. 2018, 9, 477. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [PubMed]
- Viola, K.L.; Klein, W.L. Amyloid β oligomers in Alzheimer’s disease pathogenesis, treatment, and diagnosis. Acta Neuropathol. 2015, 129, 183–206. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, B. Oxidative stress and the pathogenesis of Alzheimer’s disease. Oxidative Med. Cell. Longev. 2013, 2013, 316523. [Google Scholar] [CrossRef]
- Zhu, X.; Perry, G.; Moreira, P.I.; Aliev, G.; Cash, A.D.; Hirai, K.; Smith, M.A. Mitochondrial abnormalities and oxidative imbalance in Alzheimer disease. J. Alzheimers Dis. 2006, 9, 147–153. [Google Scholar] [CrossRef]
- Lee, S.Y.; Jeong, J.J.; Eun, S.H.; Kim, D.H. Anti-inflammatory effects of ginsenoside Rg1 and its metabolites ginsenoside Rh1 and 20(S)-protopanaxatriol in mice with TNBS-induced colitis. Eur. J. Pharmacol. 2015, 762, 333–343. [Google Scholar] [CrossRef]
- Tam, D.N.H.; Truong, D.H.; Nguyen, T.T.H.; Quynh, L.N.; Tran, L.; Nguyen, H.D.; Shamandy, B.E.; Le, T.M.H.; Tran, D.K.; Sayed, D.; et al. Ginsenoside Rh1: A Systematic Review of Its Pharmacological Properties. Planta Med. 2018, 84, 139–152. [Google Scholar] [CrossRef]
- Jung, J.-S.; Lee, S.-Y.; Kim, D.-H.; Kim, H.-S. Protopanaxatriol Ginsenoside Rh1 Upregulates Phase II Antioxidant Enzyme Gene Expression in Rat Primary Astrocytes: Involvement of MAP Kinases and Nrf2/ARE Signaling. Biomol. Ther. 2016, 24, 33–39. [Google Scholar] [CrossRef]
- Jin, Y.; Baek, N.; Back, S.; Myung, C.-S.; Heo, K.-S. Inhibitory Effect of Ginsenosides Rh1 and Rg2 on Oxidative Stress in LPS-Stimulated RAW 264.7 Cells. J. Bacteriol. Virol. 2018, 48, 156–165. [Google Scholar] [CrossRef][Green Version]
- Jung, J.S.; Kim, D.H.; Kim, H.S. Ginsenoside Rh1 suppresses inducible nitric oxide synthase gene expression in IFN-gamma-stimulated microglia via modulation of JAK/STAT and ERK signaling pathways. Biochem. Biophys. Res. Commun. 2010, 397, 323–328. [Google Scholar] [CrossRef]
- Hou, J.; Xue, J.; Lee, M.; Yu, J.; Sung, C. Long-term administration of ginsenoside Rh1 enhances learning and memory by promoting cell survival in the mouse hippocampus. Int. J. Mol. Med. 2014, 33, 234–240. [Google Scholar] [CrossRef]
- Matsuda, S.; Nakagawa, Y.; Tsuji, A.; Kitagishi, Y.; Nakanishi, A.; Murai, T. Implications of PI3K/AKT/PTEN Signaling on Superoxide Dismutases Expression and in the Pathogenesis of Alzheimer’s Disease. Diseases 2018, 6, 28. [Google Scholar] [CrossRef]
- Blasko, I.; Wagner, M.; Whitaker, N.; Grubeck-Loebenstein, B.; Jansen-Dürr, P. The amyloid β peptide Aβ (25–35) induces apoptosis independent of p53. FEBS Lett. 2000, 470, 221–225. [Google Scholar] [CrossRef]
- Copani, A.; Condorelli, F.; Caruso, A.; Vancheri, C.; Sala, A.; Stella, A.M.G.; Canonico, P.L.; Nicoletti, F.; Sortino, M.A. Mitotic signaling by β-amyloid causes neuronal death. FASEB J. 1999, 13, 2225–2234. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chu, Y.; Zhang, L.; Yin, L.; Li, L. Ginsenoside Rg1 prevents SK-N-SH neuroblastoma cell apoptosis induced by supernatant from Aβ1–40-stimulated THP-1 monocytes. Brain Res. Bull. 2012, 88, 501–506. [Google Scholar] [CrossRef]
- Lu, C.; Shi, Z.; Dong, L.; Lv, J.; Xu, P.; Li, Y.; Qu, L.; Liu, X. Exploring the Effect of Ginsenoside Rh1 in a Sleep Deprivation-Induced Mouse Memory Impairment Model. Phytother. Res. 2017, 31, 763–770. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, M.; Kim, S.-H.; Lee, H.-J. Ginsenoside Rh1 Exerts Neuroprotective Effects by Activating the PI3K/Akt Pathway in Amyloid-β Induced SH-SY5Y Cells. Appl. Sci. 2021, 11, 5654. https://doi.org/10.3390/app11125654
Park M, Kim S-H, Lee H-J. Ginsenoside Rh1 Exerts Neuroprotective Effects by Activating the PI3K/Akt Pathway in Amyloid-β Induced SH-SY5Y Cells. Applied Sciences. 2021; 11(12):5654. https://doi.org/10.3390/app11125654
Chicago/Turabian StylePark, Miey, So-Hyeun Kim, and Hae-Jeung Lee. 2021. "Ginsenoside Rh1 Exerts Neuroprotective Effects by Activating the PI3K/Akt Pathway in Amyloid-β Induced SH-SY5Y Cells" Applied Sciences 11, no. 12: 5654. https://doi.org/10.3390/app11125654
APA StylePark, M., Kim, S.-H., & Lee, H.-J. (2021). Ginsenoside Rh1 Exerts Neuroprotective Effects by Activating the PI3K/Akt Pathway in Amyloid-β Induced SH-SY5Y Cells. Applied Sciences, 11(12), 5654. https://doi.org/10.3390/app11125654






