The Role of Prediabetes as a Predictive Factor for the Outcomes in Patients with STEMI. Which Is the Right Range of Glycated Hemoglobin to Adopt in This Setting?
Abstract
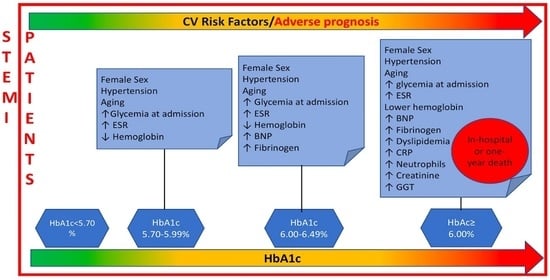
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Population Characteristics and Data Acquisition
2.2. Measurements and Follow-Up
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Correlates of HbA1c as Qualitative Variable
3.3. Correlates of HbA1c as Continuous Variable
3.4. Follow-Up
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| preT2D | Prediabetes |
| T2D | Type 2 diabetes |
| CV | Cardiovascular |
| STEMI | Acute ST-elevation Myocardial Infarction |
| HbA1c | Glycated Hemoglobin |
| ADA | American Diabetes Association |
| WHO | World Health Organization |
| FPB | Fasting Blood Glucose |
| OGTT | Oral glucose Test Tolerance |
| EF | Ejection Fraction |
| LV | Left Ventricle |
| LVEF | Left Ventricular ejection Fraction |
| CRP | C Reactive Protein |
| ESR | Erythrocyte Sedimentation Rate |
| BNP | Brain Natriuretic Peptide |
| GGT | Gamma Glutamyl Transferase |
| BMI | Body Mass Index |
| AMI | Acute myocardial infarction |
References
- Otten, R.; Kline-Rogers, E. Impact of pre-diabetic state on clinical outcomes in patients with acute coronary syndrome. Heart 2005, 91, 1466–1468. [Google Scholar] [CrossRef][Green Version]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2014, 37 (Suppl. 1), S81–S90. [Google Scholar] [CrossRef] [PubMed]
- Angeli, F.; Reboldi, G. Hyperglycemia in acute coronary syndromes: From mechanisms to prognostic implications. Ther. Adv. Cardiovasc. Dis. 2015, 9, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Bergman, M.; Abdul-Ghani, M. Review of methods for detecting glycemic disorders. Diabetes Res. Clin. Pract. 2020, 165, 108233. [Google Scholar] [CrossRef]
- Third universal definition of myocardial infarction. J. Am. Coll. Cardiol. 2012, 60, 1581–1598. [CrossRef] [PubMed]
- Lang, R.M.; Bierig, M.; Devereux, R.B.; Flachskampf, F.A.; Foster, E.; Pellikka, P.A.; Picard, M.H.; Roman, M.J.; Seward, J.; Shanewise, J.; et al. Recommendations for chamber quantification. Eur. J. Echocardiogr. 2006, 7, 79–108. [Google Scholar] [CrossRef]
- World Health Organization; IDF. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycaemia: Report of a WHO/IDF Consultation; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Soler, N.G.; Frank, S. Value of glycosylated hemoglobin measurements after acute myocardial infarction. JAMA 1981, 246, 1690–1693. [Google Scholar] [CrossRef]
- Selvin, E.; Steffes, M.W.; Zhu, H.; Matsushita, K.; Wagenknecht, L.; Pankow, J.; Coresh, J.; Brancati, F.L. Glycated Hemoglobin, Diabetes, and Cardiovascular Risk in Nondiabetic Adults. N. Engl. J. Med. 2010, 362, 800–811. [Google Scholar] [CrossRef] [PubMed]
- Giraldez, R.R.; Clare, R.M. Prevalence and clinical outcomes of undiagnosed diabetes mellitus and prediabetes among patients with high-risk non-ST-segment elevation acute coronary syndrome. Am. Heart J. 2013, 165, 918–925. [Google Scholar] [CrossRef] [PubMed]
- The Diabetes Prevention Program. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: The Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol. 2015, 3, 866–875. [Google Scholar] [CrossRef]
- Tabák, A.G.; Herder, C. Prediabetes: A high-risk state for diabetes development. Lancet 2012, 379, 2279–2290. [Google Scholar] [CrossRef]
- Cheung, B.M.; Li, C. Diabetes and hypertension: Is there a common metabolic pathway? Curr. Atheroscler. Rep. 2012, 14, 160–166. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Ferrannini, E. Insulin resistance: A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care 1991, 14, 173–194. [Google Scholar] [CrossRef]
- Shahim, B.; Gyberg, V. Undetected dysglycaemia common in primary care patients treated for hypertension and/or dyslipidaemia: On the need for a screening strategy in clinical practice. A report from EUROASPIRE IV a registry from the EuroObservational Research Programme of the European Society of Cardiology. Cardiovasc. Diabetol. 2018, 17, 21. [Google Scholar] [PubMed]
- Farhan, S.; Redfors, B. Impact of Pre-Diabetes on Coronary Plaque Composition and Clinical Outcome in Patients with Acute Coronary Syndromes: An Analysis from the PROSPECT Study. JACC Cardiovasc. Imaging 2019, 12, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Haffner, S.M.; Lehto, S. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N. Engl. J. Med. 1998, 339, 229–234. [Google Scholar] [CrossRef]
- Lenzen, M.; Ryden, L.; Öhrvik, J.; Bartnik, M.; Malmberg, K.; Reimer, W.S.O.; Simoons, M.L.; on Behalf of the Euro Heart Survey Investigators. Diabetes known or newly detected, but not impaired glucose regulation, has a negative influence on 1-year outcome in patients with coronary artery disease: A report from the Euro Heart Survey on diabetes and the heart. Eur. Heart J. 2006, 27, 2969–2974. [Google Scholar] [CrossRef] [PubMed]
- Kuusisto, J.; Mykkanen, L. NIDDM and its metabolic control predict coronary heart disease in elderly subjects. Diabetes 1994, 43, 960–967. [Google Scholar] [CrossRef]
- Lindsey, J.B.; House, J.A.; Kennedy, K.F.; Marso, S.P. Diabetes Duration Is Associated with Increased Thin-Cap Fibroatheroma Detected by Intravascular Ultrasound With Virtual Histology. Circ. Cardiovasc. Interv. 2009, 2, 543–548. [Google Scholar] [CrossRef]
- Natarajan, S.; Liao, Y.; Sinha, D.; Cao, G.; McGee, D.L.; Lipsitz, S.R. Sex Differences in the Effect of Diabetes Duration on Coronary Heart Disease Mortality. Arch. Intern. Med. 2005, 165, 430–435. [Google Scholar] [CrossRef]
- Stolar, M. Glycemic control and complications in type 2 diabetes mellitus. Am. J. Med. 2010, 123, S3–S11. [Google Scholar] [CrossRef] [PubMed]
- Dankner, R.; Danoff, A. Can ‘personalized diagnostics’ promote earlier intervention for dysglycaemia? Hypothesis ready for testing. Diabetes Metab. Res. Rev. 2010, 26, 7–9. [Google Scholar] [CrossRef] [PubMed]



| HbA1c <5.7% | HbA1c 5.7–5.99% | HbA1c 6–6.49% | HbA1c >6.49% | ||
|---|---|---|---|---|---|
| Total population | 875 (52) | 290 (17) | 196 (12) | 320 (19) | |
| Females | 183 (21) | 99 (34) | 71 (36) | 111 (35) | |
| Males | 692 (79) | 191 (66) | 120 (64) | 209 (65) | |
| Age | |||||
| <66 years (50th percentile) | 485 (55) | 120 (41) | 68 (35) | 117 (37) | |
| 66–77 years (75th percentile) | 221 (25) | 76 (26) | 57 (29) | 97 (30) | |
| >77 years | 167 (20) | 94 (32) | 71 (36) | 106 (33) | |
| CV risk factors | |||||
| Hypertension | 431 (50) | 177 (61) | 129 (66) | 220 (69) | |
| Dyslipidemia | 320 (37) | 121 (42) | 79 (40) | 156 (49) | |
| Current/ex smoking habit | 419 (48) | 111 (38) | 78 (40) | 108 (34) | |
| Ejection fraction (%) | 46 ± 9 | 44 ± 9 | 44 ± 9 | 44 ± 9 | |
| Body mass index (kg/m2) | 26 ± 4 | 27 ± 4 | 27 ± 5 | 29 ± 5 | |
| Laboratory parameters | |||||
| Creatinine (mg/dL) | 1 ± 0.6 | 1.1 ± 0.6 | 1.2 ± 0.7 | 1.4 ± 1.1 | |
| Glycemia (mg/dL) | 111 ± 29 | 125 ± 37 | 141 ± 48 | 195 ± 85 | |
| Brain natriuretic peptide (pg/mL) | 211 ± 353 | 270 ± 447 | 353 ± 665 | 350 ± 514 | |
| Fibrinogen (mg/dL) | 327 ± 110 | 333 ± 110 | 354 ± 111 | 358 ± 115 | |
| Hemoglobin (g/dL) | 14 ± 2 | 13 ± 2 | 13 ± 2 | 13 ± 2 | |
| C reactive protein (mg/dL) | 2 ± 4 | 2.1 ± 4 | 2.5 ± 5 | 2.7 ± 4 | |
| Gamma glutamyltransferase (UI/L) | 31 ± 36 | 31 ± 27 | 33 ± 36 | 36 ± 32 | |
| Monocytes (109/L) | 0.7 ± 0.4 | 0.7 ± 0.4 | 0.7 ± 0.5 | 0.7 ± 0.4 | |
| Neutrophils (109/L) | 8.6 ± 3.6 | 8.8 ± 3.7 | 9.2 ± 3.7 | 9.6 ± 4.2 | |
| Erythrocyte sedimentation rate (mm/h) | 19 ± 18 | 25 ± 21 | 25 ± 20 | 27 ± 24 |
| HbA1c% | p | ||
|---|---|---|---|
| Total population | 5.9 ± 1.1 | ||
| Females | 6.1 ± 1.2 | ||
| Males | 5.9 ± 1.1 | <0.001 | |
| Age | |||
| <66 years (50th percentile) | 5.9 ± 1.1 | ||
| 66–77 years (75th percentile) | 6 ± 1.1 | ||
| >77 years | 6.1 ± 0.9 | <0.001 | |
| CV risk factors | |||
| No-hypertension | 5.8 ± 1 | ||
| Hypertension | 6.1 ± 1.2 | <0.001 | |
| No-dyslipidemia | 5.9 ± 1.1 | ||
| Dyslipidemia | 6.1 ± 1.1 | ≤0.01 | |
| No-smoking habit | 6 ± 1.1 | ||
| Current/ex smoking habit | 5.8 ± 1 | <0.001 | |
| Ejection fraction (%) | r = −0.1 | ≤0.01 | |
| Body mass index (kg/m2) | r = 0.2 | <0.001 | |
| Laboratory parameters | |||
| Creatinine (mg/dL) | r = 0.1 | <0.001 | |
| Glycemia (mg/dL) | r = 0.6 | <0.001 | |
| Brain natriuretic peptide (pg/mL) | r = 0.1 | <0.001 | |
| Fibrinogen (mg/dL) | r = 0.1 | <0.001 | |
| Hemoglobin (g/dL) | r = −0.1 | <0.01 | |
| C reactive protein (mg/dL) | r = 0.2 | <0.001 | |
| Gamma glutamyltransferase (UI/L) | r = 0.1 | <0.001 | |
| Monocytes (109/L) | r = 0.02 | ns | |
| Neutrophils (109/L) | r = 0.1 | <0.001 | |
| Erythrocyte sedimentation rate (mm/h) | r = 0.1 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatzianagnostou, K.; Guiducci, L.; Paradossi, U.; De Caterina, A.R.; Mazzone, A.; Berti, S.; Vassalle, C. The Role of Prediabetes as a Predictive Factor for the Outcomes in Patients with STEMI. Which Is the Right Range of Glycated Hemoglobin to Adopt in This Setting? Appl. Sci. 2021, 11, 5518. https://doi.org/10.3390/app11125518
Chatzianagnostou K, Guiducci L, Paradossi U, De Caterina AR, Mazzone A, Berti S, Vassalle C. The Role of Prediabetes as a Predictive Factor for the Outcomes in Patients with STEMI. Which Is the Right Range of Glycated Hemoglobin to Adopt in This Setting? Applied Sciences. 2021; 11(12):5518. https://doi.org/10.3390/app11125518
Chicago/Turabian StyleChatzianagnostou, Kyriazoula, Letizia Guiducci, Umberto Paradossi, Alberto Ranieri De Caterina, Annamaria Mazzone, Sergio Berti, and Cristina Vassalle. 2021. "The Role of Prediabetes as a Predictive Factor for the Outcomes in Patients with STEMI. Which Is the Right Range of Glycated Hemoglobin to Adopt in This Setting?" Applied Sciences 11, no. 12: 5518. https://doi.org/10.3390/app11125518
APA StyleChatzianagnostou, K., Guiducci, L., Paradossi, U., De Caterina, A. R., Mazzone, A., Berti, S., & Vassalle, C. (2021). The Role of Prediabetes as a Predictive Factor for the Outcomes in Patients with STEMI. Which Is the Right Range of Glycated Hemoglobin to Adopt in This Setting? Applied Sciences, 11(12), 5518. https://doi.org/10.3390/app11125518