Loliolide from Artemisia princeps Suppresses Adipogenesis in Human Bone Marrow-Derived Mesenchymal Stromal Cells via Activation of AMPK and Wnt/β-catenin Pathways
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Loliolide
2.2. hBM-MSC Culture and Adipogenesis
2.3. MTT Assay for Cell Viability
2.4. Staining of Intracellular Lipid Accumulation
2.5. Quantitative Reverse Transcription PCR
2.6. Western Blot Analysis
2.7. Immunofluorescence Staining
2.8. Statistical Analysis
3. Results
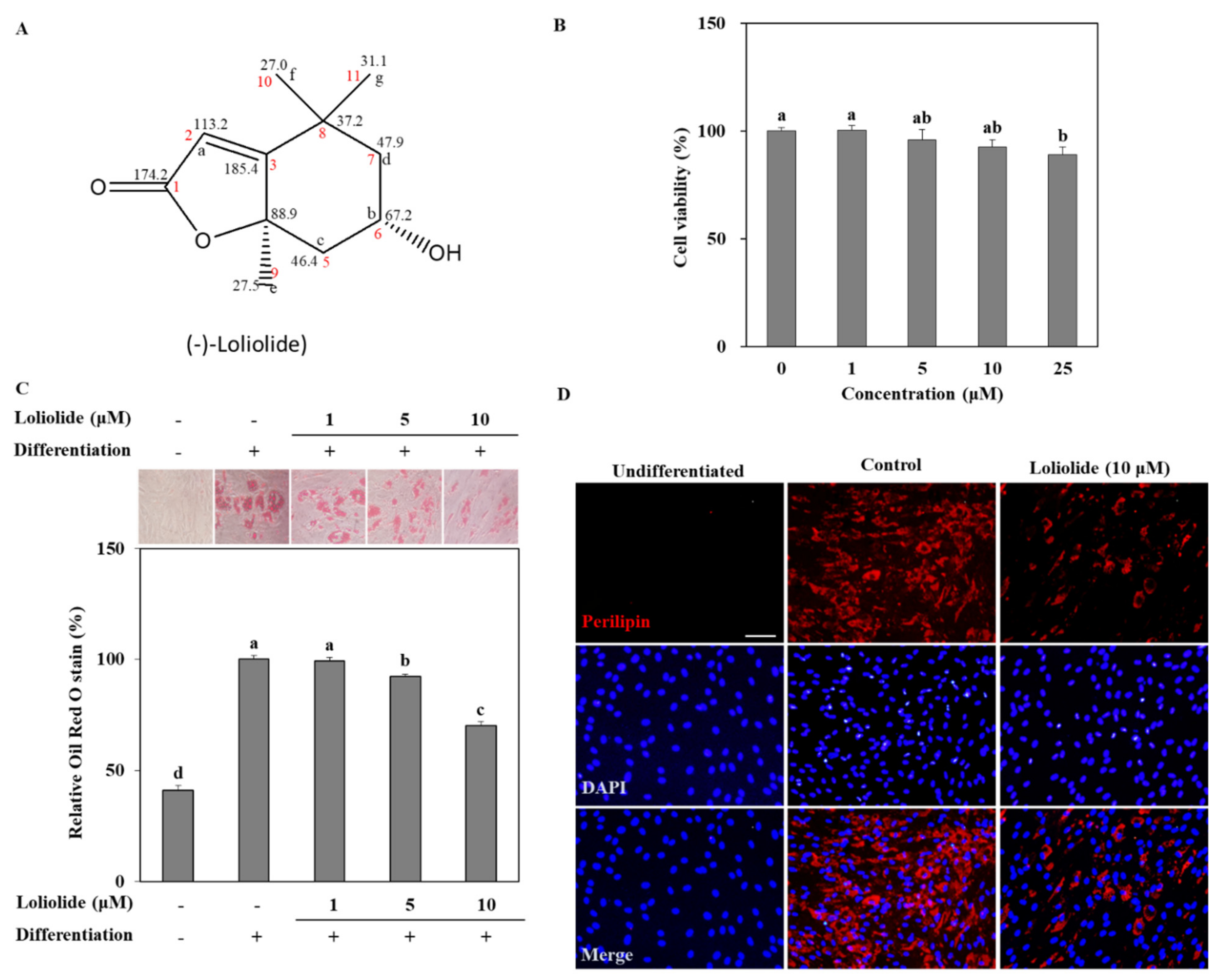
3.1. Lipid Accumulation by Differentiated hBM-MSCs
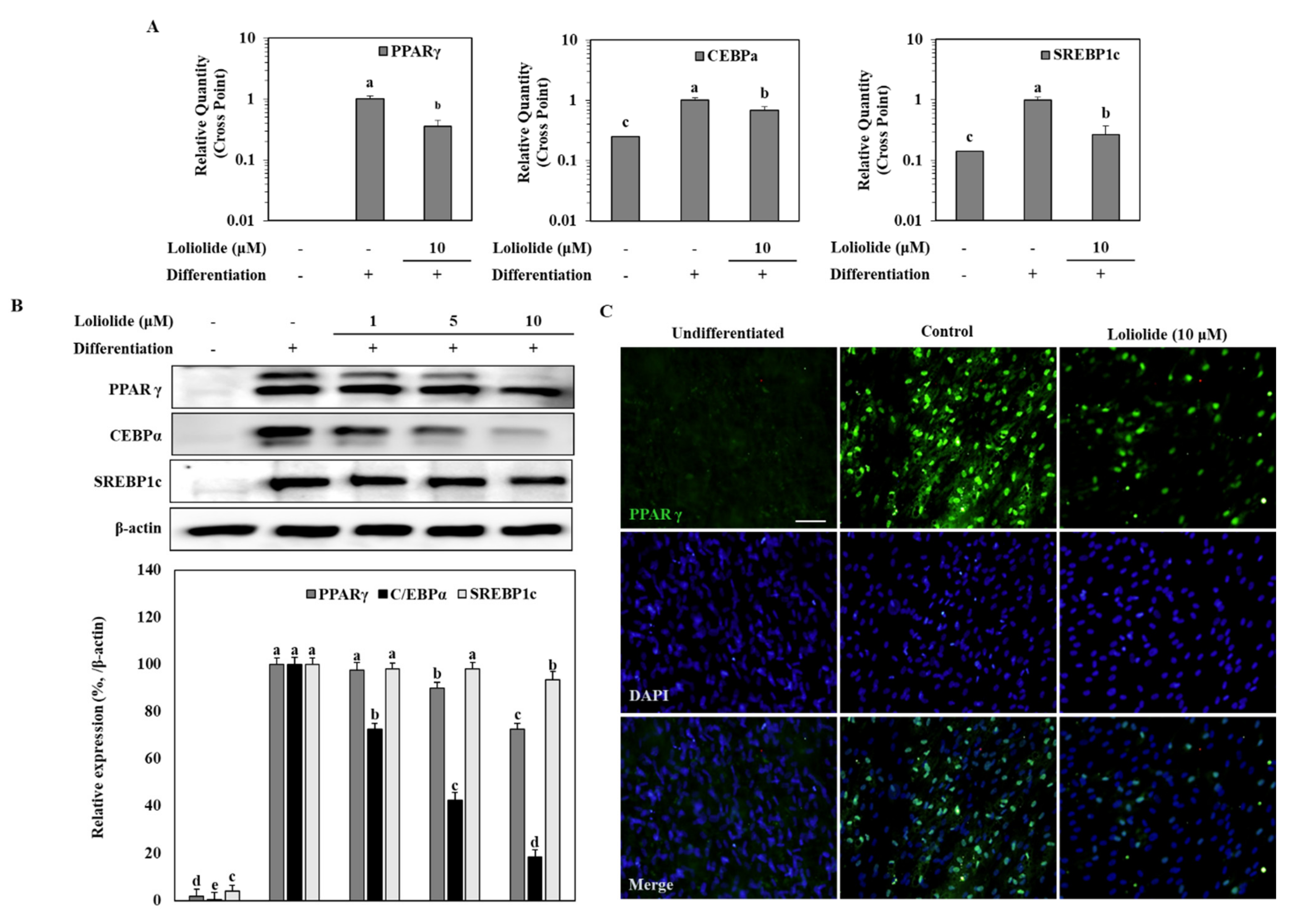
3.2. The mRNA and Protein Levels of Adipogenic Markers during Adipogenic Differentiation of hBM-MSCs
3.3. Activation of MAPK and AMPK Signaling Pathway during Adipogenic Differentiation of hBM-MSCs
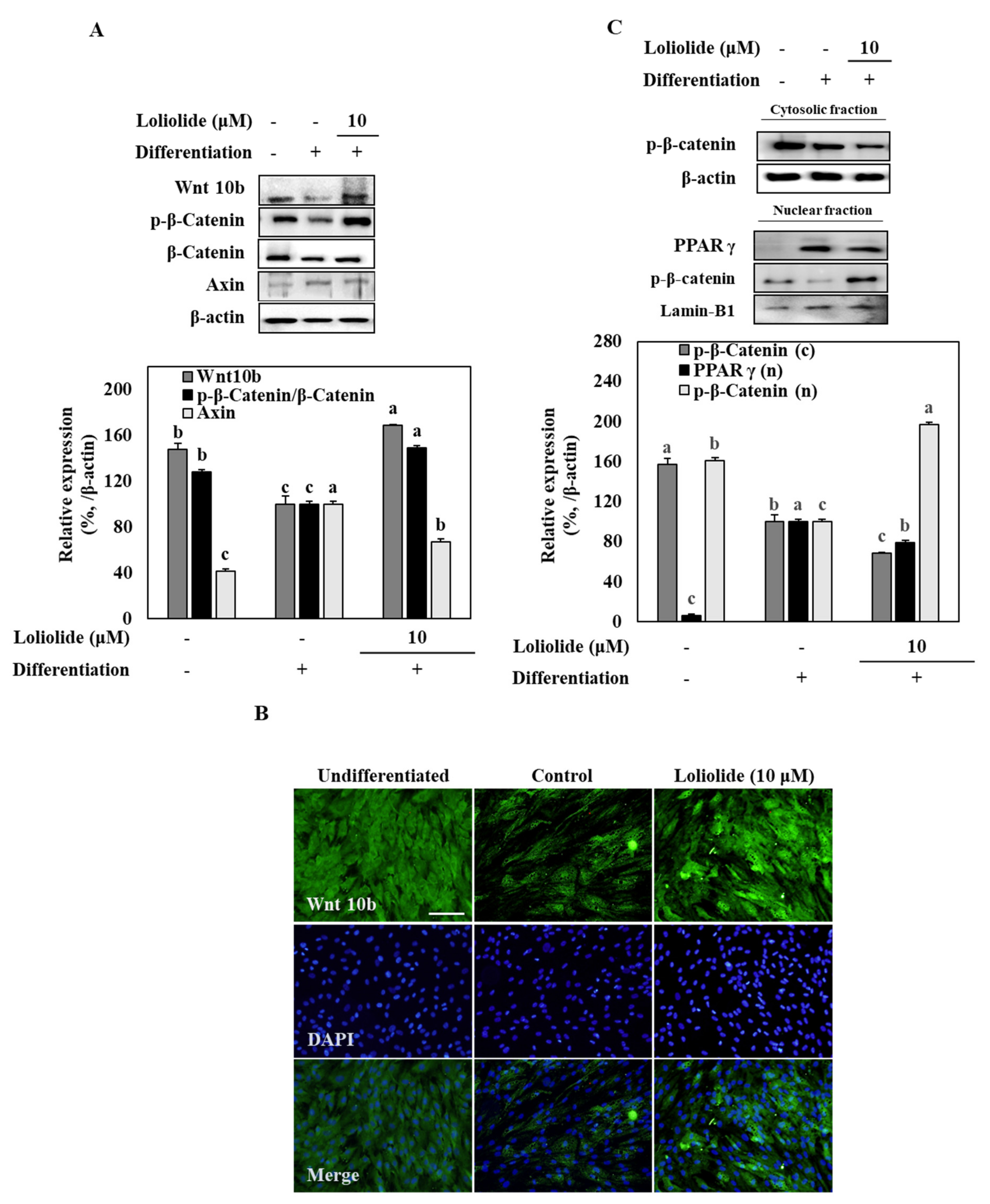
3.4. Wnt/β-Catenin Signaling during Adipogenic Differentiation of hBM-MSCs
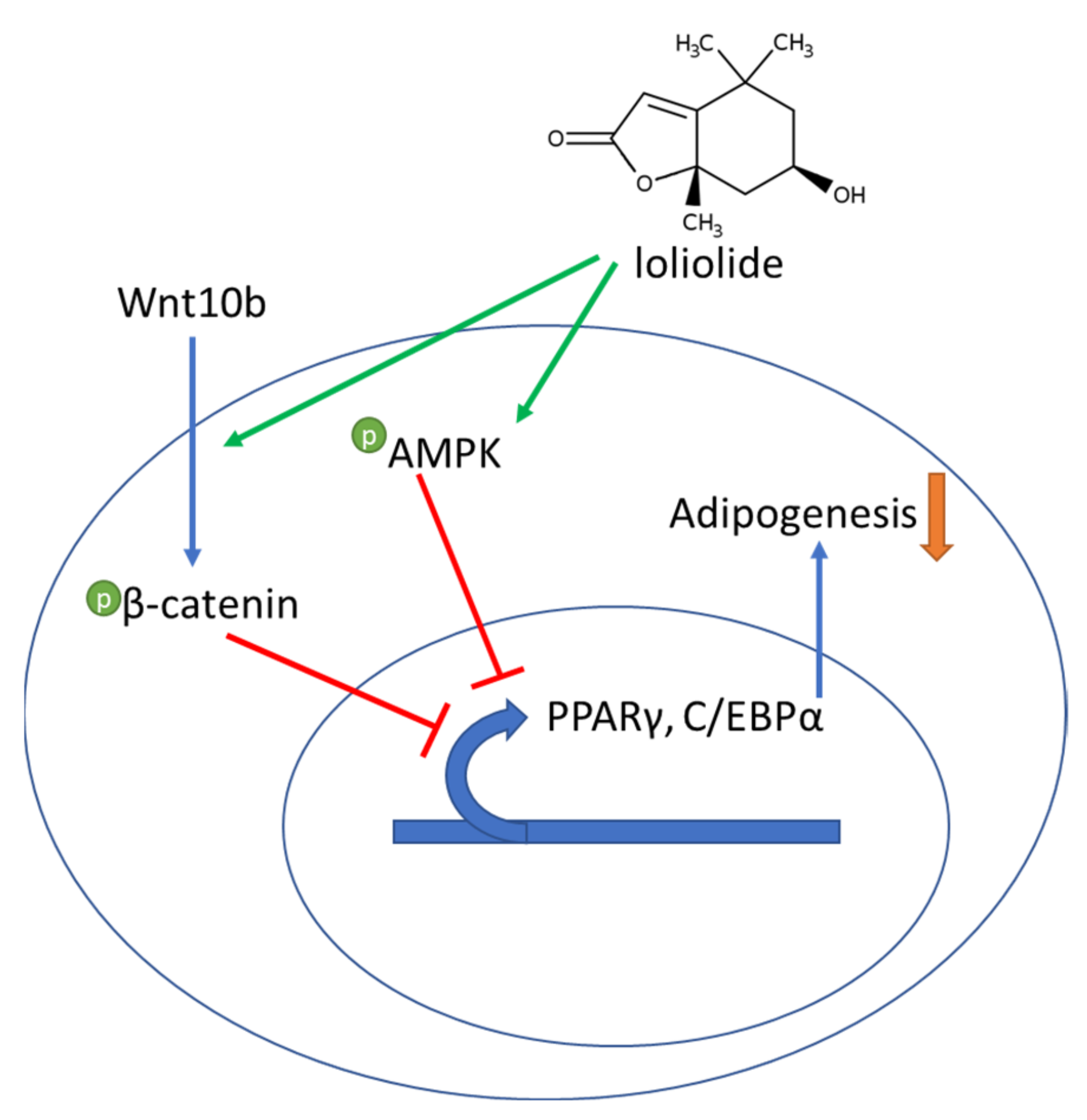
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hill, J.H.; Solt, C.; Foster, M.T. Obesity associated disease risk: The role of inherent differences and location of adipose depots. Horm. Mol. Biol. Clin. Investig. 2018, 33, 20180012. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; LeRoith, D.; Gallagher, E.J. Diabetes, obesity, and breast cancer. Endocrinology 2018, 159, 3801–3812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srivastava, G.; Apovian, C. Future pharmacotherapy for obesity: New anti-obesity drugs on the horizon. Curr. Obes. Rep. 2018, 7, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Bahmad, H.F.; Daouk, R.; Azar, J.; Sapudom, J.; Teo, J.C.M.; Abou-Kheir, W.; Al-Sayegh, M. Modeling adipogenesis: Current and future perspective. Cells 2020, 9, 2326. [Google Scholar] [CrossRef] [PubMed]
- Bacakova, L.; Zarubova, J.; Travnickova, M.; Musilkova, J.; Pajorova, J.; Slepicka, P.; Kasalkova, N.S.; Svorcik, V.; Kolska, Z.; Motarjemi, H.; et al. Stem cells: Their source, potency and use in regenerative therapies with focus on adipose-derived stem cells—A review. Biotechnol. Adv. 2018, 36, 1111–1126. [Google Scholar] [CrossRef]
- Fassio, A.; Idolazzi, L.; Rossini, M.; Gatti, D.; Adami, G.; Giollo, A.; Viapiana, O. The obesity paradox and osteoporosis. Eat. Weight Disord. Stud. Anorexia, Bulim. Obes. 2018, 23, 293–302. [Google Scholar] [CrossRef]
- Da Silva, S.V.; Renovato-Martins, M.; Ribeiro-Pereira, C.; Citelli, M.; Barja-Fidalgo, C. Obesity modifies bone marrow microenvironment and directs bone marrow mesenchymal cells to adipogenesis. Obesity 2016, 24, 2522–2532. [Google Scholar] [CrossRef] [PubMed]
- Hardouin, P.; Rharass, T.; Lucas, S. Bone marrow adipose tissue: To be or not to be a typical adipose tissue? Front. Endocrinol. 2016, 7, 85. [Google Scholar] [CrossRef]
- Lee, J.; Abdeen, A.A.; Tang, X.; Saif, T.A.; Kilian, K.A. Matrix directed adipogenesis and neurogenesis of mesenchymal stem cells derived from adipose tissue and bone marrow. Acta Biomater. 2016, 42, 46–55. [Google Scholar] [CrossRef] [Green Version]
- Shao, X.; Wang, M.; Wei, X.; Deng, S.; Fu, N.; Peng, Q.; Jiang, Y.; Ye, L.; Xie, J.; Lin, Y. Peroxisome proliferator-activated receptor-γ: Master regulator of adipogenesis and obesity. Curr. Stem Cell Res. Ther. 2016, 11, 282–289. [Google Scholar] [CrossRef]
- James, A.W. Review of signaling pathways governing MSC osteogenic and adipogenic differentiation. Scientifica 2013, 2013, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Takada, I.; Kouzmenko, A.P.; Kato, S. Wnt and PPARγ signaling in osteoblastogenesis and adipogenesis. Nat. Rev. Rheumatol. 2009, 5, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Nuttall, M. Controlling the balance between osteoblastogenesis and adipogenesis and the consequent therapeutic implications. Curr. Opin. Pharmacol. 2004, 4, 290–294. [Google Scholar] [CrossRef]
- Chen, J.-R.; Lazarenko, O.P.; Wu, X.; Tong, Y.; Blackburn, M.L.; Shankar, K.; Badger, T.M.; Ronis, M.J.J. Obesity reduces bone density associated with activation of PPARγ and suppression of Wnt/β-catenin in rapidly growing male rats. PLoS ONE 2010, 5, e13704. [Google Scholar] [CrossRef] [PubMed]
- Grabarczyk, M.; Wińska, K.; Mączka, W.; Potaniec, B.; Anioł, M. Loliolide - the most ubiquitous lactone. Folia Biol. Oecologica 2015, 11, 1–8. [Google Scholar] [CrossRef]
- Yang, X.; Kang, M.C.; Lee, K.W.; Kang, S.M.; Lee, W.W.; Jeon, Y.J. Antioxidant activity and cell protective effect of loliolide isolated from Sargassum ringgoldianum subsp. Coreanum. ALGAE 2011, 26, 201–208. [Google Scholar] [CrossRef]
- Chung, C.Y.; Liu, C.H.; Burnouf, T.; Wang, G.H.; Chang, S.P.; Jassey, A.; Tai, C.J.; Tai, C.J.; Huang, C.J.; Richardson, C.D.; et al. Activity-based and fraction-guided analysis of Phyllanthus urinaria identifies loliolide as a potent inhibitor of hepatitis C virus entry. Antivir. Res. 2016, 130, 58–68. [Google Scholar] [CrossRef]
- Lee, H.-G.; Kim, H.-S.; Je, J.-G.; Hwang, J.; Sanjeewa, K.K.A.; Lee, D.-S.; Song, K.-M.; Choi, Y.-S.; Kang, M.-C.; Jeon, Y.-J. Lipid inhibitory effect of (−)-loliolide isolated from Sargassum horneri in 3T3-L1 adipocytes: Inhibitory mechanism of adipose-specific proteins. Mar. Drugs 2021, 19, 96. [Google Scholar] [CrossRef]
- Kim, S.Y.; Lee, J.Y.; Jhin, C.; Shin, J.M.; Kim, M.; Ahn, H.R.; Yoo, G.; Son, Y.J.; Jung, S.H.; Nho, C.W. Reduction of hepatic lipogenesis by loliolide and pinoresinol from Lysimachia vulgaris via degrading liver x receptors. J. Agric. Food Chem. 2019, 67, 12419–12427. [Google Scholar] [CrossRef]
- Kim, H. Isolation and Structure Determination of Matrix Metalloproteinase Inhibitors and Antioxidants from Ligustrum japonicum fructus, Zostera asiatica and Artemisia princeps. Ph.D. Thesis, Korea Maritime and Ocean University, Busan, Korea, 2019. [Google Scholar]
- Park, K.E.; Kim, Y.A.; Jung, H.A.; Lee, H.J.; Ahn, J.W.; Lee, B.J.; Seo, Y. Three norisoprenoids from the brown alga Sargassum thunbergii. J. Korean Chem. Soc. 2004, 48, 394–398. [Google Scholar] [CrossRef] [Green Version]
- Karadeniz, F.; Oh, J.H.; Lee, J.I.; Kim, H.; Seo, Y.; Kong, C.S. 6-acetyl-2,2-dimethylchroman-4-one isolated from Artemisia princeps suppresses adipogenic differentiation of human bone marrow-derived mesenchymal stromal cells via activation of AMPK. J. Med. Food 2020, 23, 250–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menssen, A.; Haupl, T.; Sittinger, M.; Delorme, B.; Charbord, P.; Ringe, J. Differential gene expression profiling of human bone marrow-derived mesenchymal stem cells during adipogenic development. BMC Genom. 2011, 12, 461. [Google Scholar] [CrossRef] [Green Version]
- Bost, F.; Aouadi, M.; Caron, L.; Binetruy, B. The role of MAPKs in adipocyte differentiation and obesity. Biochimie 2005, 87, 51–56. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, B.; Serpell, C.J.; Fong, I.L.; Wong, E.H. Molecular mechanisms of adipogenesis: The anti-adipogenic role of AMP-activated protein kinase. Front. Mol. Biosci. 2020, 7, 76. [Google Scholar] [CrossRef] [PubMed]
- Romanov, Y.A.; Darevskaya, A.N.; Merzlikina, N.V.; Buravkova, L.B. Mesenchymal stem cells from human bone marrow and adipose tissue: Isolation, characterization, and differentiation potentialities. Bull. Exp. Biol. Med. 2005, 140, 138–143. [Google Scholar] [CrossRef]
- Zhao, L.J.; Jiang, H.; Papasian, C.J.; Maulik, D.; Drees, B.; Hamilton, J.; Deng, H.W. Correlation of obesity and osteoporosis: Effect of fat mass on the determination of osteoporosis. J. Bone Miner. Res. 2007, 23, 17–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, Y.; Luo, N.; Klein, R.L.; Garvey, W.T. Adiponectin promotes adipocyte differentiation, insulin sensitivity, and lipid accumulation. J. Lipid Res. 2005, 46, 1369–1379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lefterova, M.I.; Zhang, Y.; Steger, D.J.; Schupp, M.; Schug, J.; Cristancho, A.; Feng, D.; Zhuo, D.; Stoeckert, C.J.; Liu, X.S.; et al. PPAR and C/EBP factors orchestrate adipocyte biology via adjacent binding on a genome-wide scale. Genes Dev. 2008, 22, 2941–2952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Payne, V.A.; Au, W.-S.; Lowe, C.E.; Rahman, S.M.; Friedman, J.E.; O’Rahilly, S.; Rochford, J.J. C/EBP transcription factors regulate SREBP1c gene expression during adipogenesis. Biochem. J. 2010, 425, 215–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, D.; Ma, F.; Ouyang, S.; Liu, Z.; Li, Y.; Wu, J. Effects of macrophages and CXCR2 on adipogenic differentiation of bone marrow mesenchymal stem cells. J. Cell. Physiol. 2018, 534, 9475–9485. [Google Scholar] [CrossRef]
- Tanabe, Y. Inhibition of adipocyte differentiation by mechanical stretching through ERK-mediated downregulation of PPAR2. J. Cell Sci. 2004, 117, 3605–3614. [Google Scholar] [CrossRef] [Green Version]
- Fogarty, S.; Hardie, D.G. Development of protein kinase activators: AMPK as a target in metabolic disorders and cancer. Biochim. Biophys. Acta Proteins Proteom. 2010, 1804, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.H.; Kim, S.G. AMPK-dependent metabolic regulation by PPAR agonists. PPAR Res. 2010, 2010, 549101. [Google Scholar] [CrossRef]
- Prestwich, T.C.; MacDougald, O.A. Wnt/β-catenin signaling in adipogenesis and metabolism. Curr. Opin. Cell Biol. 2007, 19, 612–617. [Google Scholar] [CrossRef] [Green Version]
- Ling, L.; Nurcombe, V.; Cool, S.M. Wnt signaling controls the fate of mesenchymal stem cells. Gene 2009, 433, 1–7. [Google Scholar] [CrossRef]
- Xu, C.; Wang, J.; Zhu, T.; Shen, Y.; Tang, X.; Fang, L.; Xu, Y. Cross-talking between PPAR and WNT signaling and its regulation in mesenchymal stem cell differentiation. Curr. Stem Cell Res. Ther. 2016, 11, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Cawthorn, W.P.; Bree, A.J.; Yao, Y.; Du, B.; Hemati, N.; Martinez-Santibañez, G.; MacDougald, O.A. Wnt6, Wnt10a and Wnt10b inhibit adipogenesis and stimulate osteoblastogenesis through a β-catenin-dependent mechanism. Bone 2012, 50, 477–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, L.; Song, C.; Ni, M.; Meng, F.; Xie, H.; Li, G. Cellular retinol-binding protein 1 (CRBP-1) regulates osteogenenesis and adipogenesis of mesenchymal stem cells through inhibiting RXRα-induced β-catenin degradation. Int. J. Biochem. Cell Biol. 2012, 44, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Ma, X.; Wang, N.; Jia, M.; Bi, L.; Wang, Y.; Li, M.; Zhang, H.; Xue, X.; Hou, Z.; et al. Activation of GLP-1 receptor promotes bone marrow stromal cell osteogenic differentiation through β-catenin. Stem Cell Rep. 2016, 6, 579–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.; Bae, S.; Kim, K.; Kim, W.; Chung, S.I.; Yoon, Y. β-catenin mediates the anti-adipogenic effect of baicalin. Biochem. Biophys. Res. Commun. 2010, 398, 741–746. [Google Scholar] [CrossRef]
- Lee, Y.R.; Bae, S.; Kim, J.Y.; Lee, J.; Cho, D.H.; Kim, H.S.; An, I.S.; An, S. Monoterpenoid loliolide regulates hair follicle inductivity of human dermal papilla cells by activating the Akt/ ¥-Catenin signaling pathway. J. Microbiol. Biotechnol. 2019, 29, 1830–1840. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Wang, L.; Fernando, I.P.S.; Je, J.-G.; Ko, S.-C.; Kang, M.C.; Lee, J.M.; Yim, M.J.; Jeon, Y.J.; Lee, D.S. Antioxidant efficacy of (−)-loliolide isolated from Sargassum horneri against AAPH-induced oxidative damage in Vero cells and zebrafish models in vivo. J. Appl. Phycol. 2020, 32, 3341–3348. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, J.H.; Karadeniz, F.; Jang, M.-S.; Kim, H.; Seo, Y.; Kong, C.-S. Loliolide from Artemisia princeps Suppresses Adipogenesis in Human Bone Marrow-Derived Mesenchymal Stromal Cells via Activation of AMPK and Wnt/β-catenin Pathways. Appl. Sci. 2021, 11, 5435. https://doi.org/10.3390/app11125435
Oh JH, Karadeniz F, Jang M-S, Kim H, Seo Y, Kong C-S. Loliolide from Artemisia princeps Suppresses Adipogenesis in Human Bone Marrow-Derived Mesenchymal Stromal Cells via Activation of AMPK and Wnt/β-catenin Pathways. Applied Sciences. 2021; 11(12):5435. https://doi.org/10.3390/app11125435
Chicago/Turabian StyleOh, Jung Hwan, Fatih Karadeniz, Mi-Soon Jang, Hojun Kim, Youngwan Seo, and Chang-Suk Kong. 2021. "Loliolide from Artemisia princeps Suppresses Adipogenesis in Human Bone Marrow-Derived Mesenchymal Stromal Cells via Activation of AMPK and Wnt/β-catenin Pathways" Applied Sciences 11, no. 12: 5435. https://doi.org/10.3390/app11125435
APA StyleOh, J. H., Karadeniz, F., Jang, M.-S., Kim, H., Seo, Y., & Kong, C.-S. (2021). Loliolide from Artemisia princeps Suppresses Adipogenesis in Human Bone Marrow-Derived Mesenchymal Stromal Cells via Activation of AMPK and Wnt/β-catenin Pathways. Applied Sciences, 11(12), 5435. https://doi.org/10.3390/app11125435





