Acid Dentin Lysates Increase Amelotin Expression in Oral Epithelial Cells and Gingival Fibroblasts
Abstract
1. Introduction
2. Methods
2.1. Acid Dentin Lysate Preparation
2.2. Cell Culture
2.3. RT-PCR
2.4. Immunostaining
2.5. Gelatin Coating
2.6. Western Blot
2.7. Statistical Analysis
3. Results
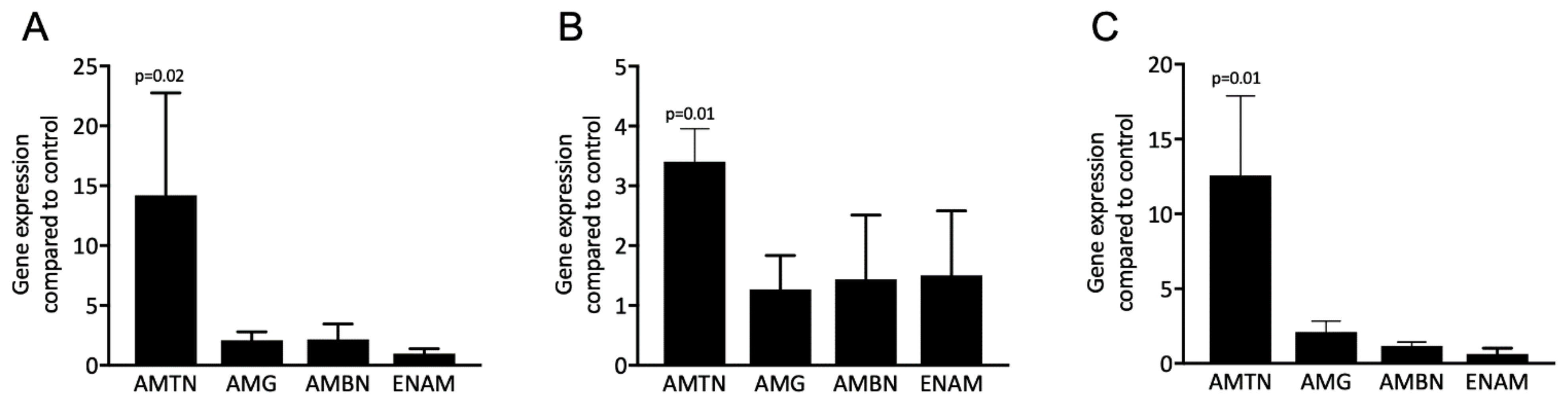
3.1. Acid Dentin Lysates Induce Expression of the AMTN but Not the Enamel Matrix Proteins
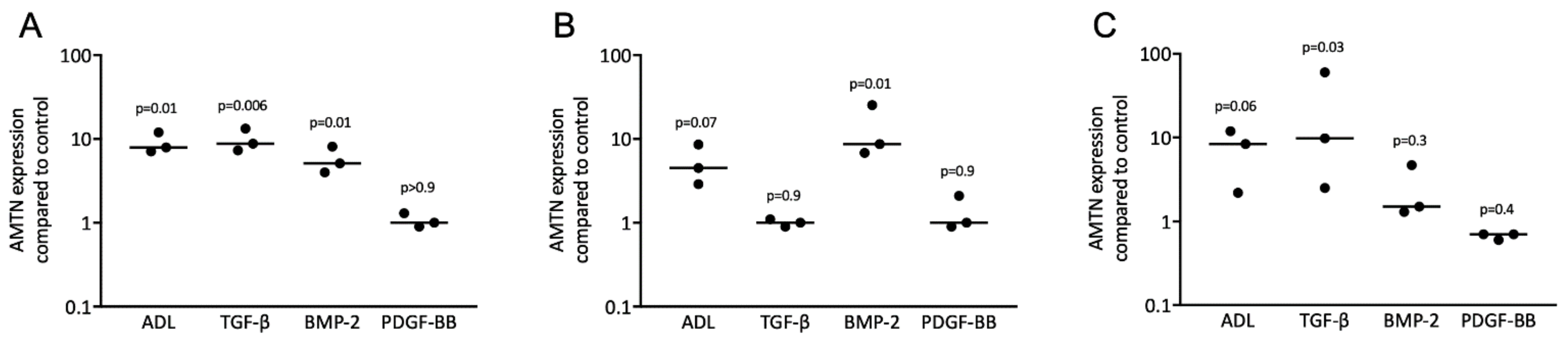
3.2. Acid Dentin Lysate-Induced Expression of AMTN Is Based on Activation of the TGF-β Receptor Type 1 Kinase
3.3. TGF-β and BMP-2 Can Induce Expression of AMTN
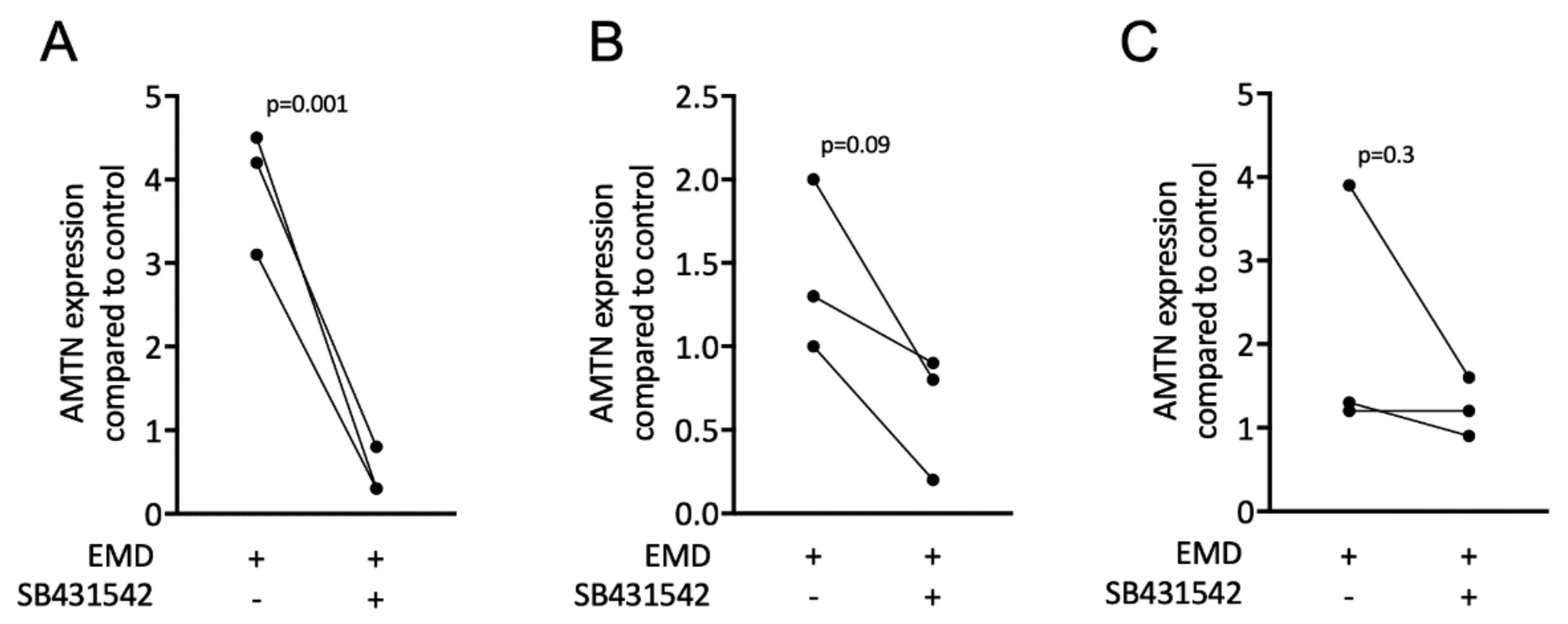
3.4. EMD Induces Expression of AMTN
3.5. ADL, TGF-β1, and BMP-2 Increase Positive Staining of AMTN in Nucleus
3.6. Smad3 Phosphorylation Is Induced by ADL and TGF-β1 in HSC2
3.7. Inhibitors of TGF-β Receptor 1 Kinase Downregulate Expression of AMTN
3.8. AMTN-Induced Activity of ADL Binds to Gelatin
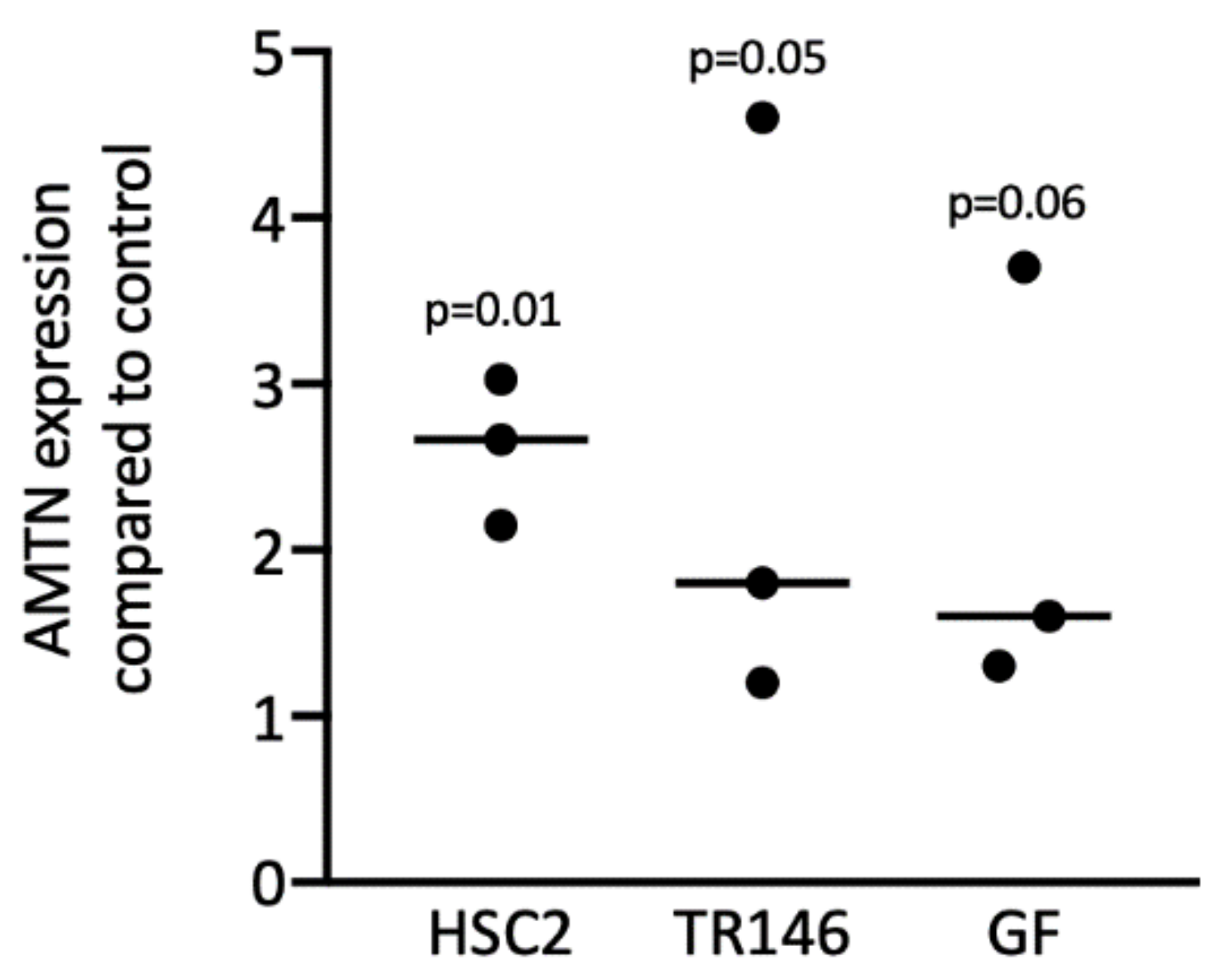
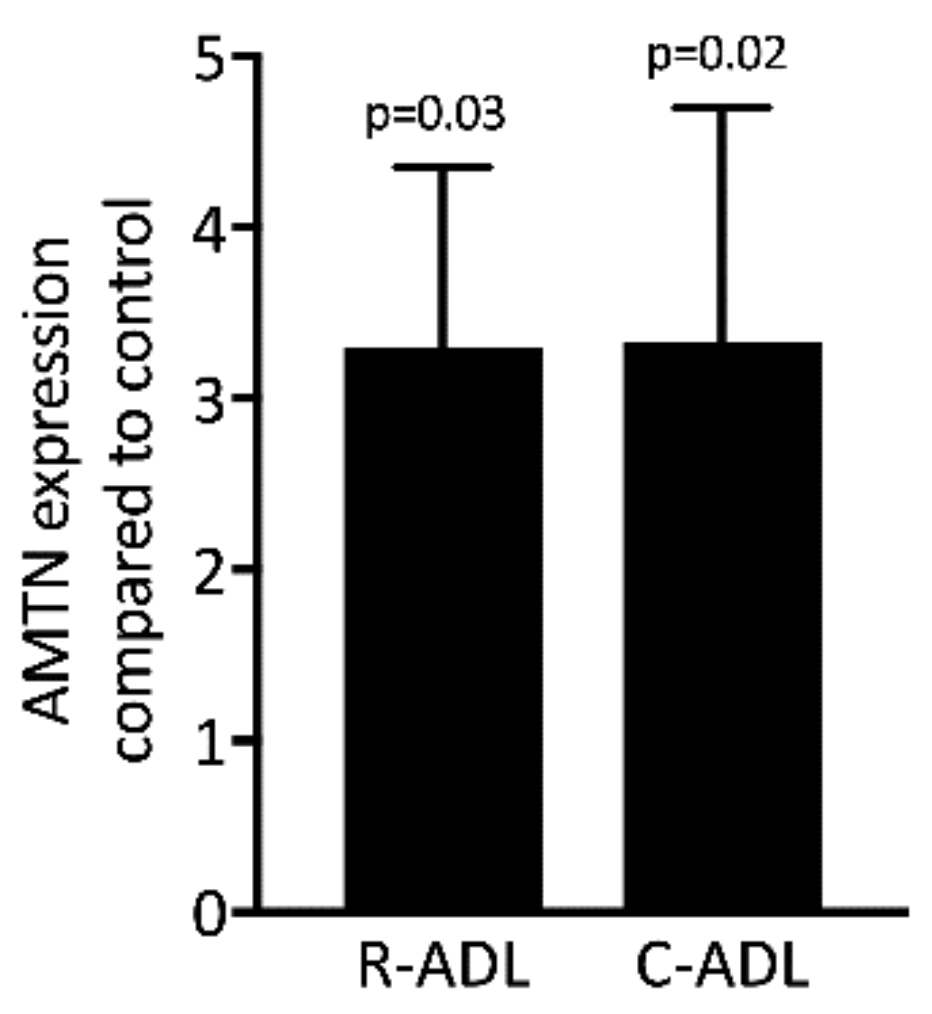
3.9. Root- and Crown-Derived ADL Induces Expression of AMTN
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sculean, A.; Gruber, R.; Bosshardt, D.D. Soft tissue wound healing around teeth and dental implants. J. Clin. Periodontol. 2014, 41, S6–S22. [Google Scholar] [CrossRef]
- Nishio, C.; Wazen, R.; Moffatt, P.; Nanci, A. Expression of odontogenic ameloblast-associated and amelotin proteins in the junctional epithelium. Periodontology 2000 2013, 63, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Moutsopoulos, N.M.; Konkel, J.E. Tissue-Specific Immunity at the Oral Mucosal Barrier. Trends Immunol. 2018, 39, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Bosshardt, D.D.; Lang, N.P. The junctional epithelium: From health to disease. J. Dent. Res. 2005, 84, 9–20. [Google Scholar] [CrossRef]
- Yajima-Himuro, S.; Oshima, M.; Yamamoto, G.; Ogawa, M.; Furuya, M.; Tanaka, J.; Nishii, K.; Mishima, K.; Tachikawa, T.; Tsuji, T.; et al. The junctional epithelium originates from the odontogenic epithelium of an erupted tooth. Sci. Rep. 2014, 4, 4867. [Google Scholar] [CrossRef]
- Fouillen, A.; Dos Santos Neves, J.; Mary, C.; Castonguay, J.D.; Moffatt, P.; Baron, C.; Nanci, A. Interactions of AMTN, ODAM and SCPPPQ1 proteins of a specialized basal lamina that attaches epithelial cells to tooth mineral. Sci. Rep. 2017, 7, 46683. [Google Scholar] [CrossRef]
- Iwasaki, K.; Bajenova, E.; Somogyi-Ganss, E.; Miller, M.; Nguyen, V.; Nourkeyhani, H.; Gao, Y.; Wendel, M.; Ganss, B. Amelotin—A Novel Secreted, Ameloblast-specific Protein. J. Dent. Res. 2005, 84, 1127–1732. [Google Scholar] [CrossRef]
- Dos Santos Neves, J.; Wazen, R.M.; Kuroda, S.; Francis Zalzal, S.; Moffatt, P.; Nanci, A. Odontogenic ameloblast-associated and amelotin are novel basal lamina components. Histochem. Cell Biol. 2012, 137, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Abbarin, N.; San Miguel, S.; Holcroft, J.; Iwasaki, K.; Ganss, B. The enamel protein amelotin is a promoter of hydroxyapatite mineralization. J. Bone Miner. Res. 2015, 30, 775–785. [Google Scholar] [CrossRef]
- Nishio, C.; Wazen, R.; Kuroda, S.; Moffatt, P.; Nanci, A. Expression pattern of odontogenic ameloblast-associated and amelotin during formation and regeneration of the junctional epithelium. Eur. Cell Mater. 2010, 20, 393–402. [Google Scholar] [CrossRef]
- Yamazaki, M.; Mezawa, M.; Noda, K.; Iwai, Y.; Matsui, S.; Takai, H.; Nakayama, Y.; Ogata, Y. Transcriptional regulation of human amelotin gene by interleukin-1beta. FEBS Open Biol. 2018, 8, 974–985. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, Y.; Takai, H.; Matsui, S.; Matsumura, H.; Zhou, L.; Kato, A.; Ganss, B.; Ogata, Y. Proinflammatory cytokines induce amelotin transcription in human gingival fibroblasts. J. Oral Sci. 2014, 56, 261–268. [Google Scholar] [CrossRef]
- Nakayama, Y.; Kobayashi, R.; Iwai, Y.; Noda, K.; Yamazaki, M.; Kurita-Ochiai, T.; Yoshimura, A.; Ganss, B.; Ogata, Y. C/EBPbeta and YY1 bind and interact with Smad3 to modulate lipopolysaccharide-induced amelotin gene transcription in mouse gingival epithelial cells. FEBS Open Biol. 2019, 9, 276–290. [Google Scholar] [CrossRef]
- Nakayama, Y.; Matsui, S.; Noda, K.; Yamazaki, M.; Iwai, Y.; Ganss, B.; Ogata, Y. TGFbeta1-induced Amelotin gene expression is downregulated by Bax expression in mouse gingival epithelial cells. J. Oral Sci. 2018, 60, 232–241. [Google Scholar] [CrossRef]
- Nakayama, Y.; Matsui, S.; Noda, K.; Yamazaki, M.; Iwai, Y.; Matsumura, H.; Izawa, T.; Tanaka, E.; Ganss, B.; Ogata, Y. Amelotin gene expression is temporarily being upregulated at the initiation of apoptosis induced by TGFbeta1 in mouse gingival epithelial cells. Apoptosis 2016, 21, 1057–1070. [Google Scholar] [CrossRef] [PubMed]
- Nashef, A.; Matthias, M.; Weiss, E.; Loos, B.G.; Jepsen, S.; van der Velde, N.; Uitterlinden, A.G.; Wellmann, J.; Berger, K.; Hoffmann, P.; et al. Translation of mouse model to human gives insights into periodontitis etiology. Sci. Rep. 2020, 10, 4892. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, Y.; Kobayashi, R.; Matsui, S.; Matsumura, H.; Iwai, Y.; Noda, K.; Yamazaki, M.; Kurita-Ochiai, T.; Yoshimura, A.; Shinomura, T.; et al. Localization and expression pattern of amelotin, odontogenic ameloblast-associated protein and follicular dendritic cell-secreted protein in the junctional epithelium of inflamed gingiva. Odontology 2017, 105, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.A.; Rosa, A.L.; Lara, V.S. Dentin matrix proteins and soluble factors: Intrinsic regulatory signals for healing and resorption of dental and periodontal tissues? Oral Dis. 2004, 10, 63–74. [Google Scholar] [CrossRef]
- Saads Carvalho, T.; Lussi, A. Chapter 9: Acidic Beverages and Foods Associated with Dental Erosion and Erosive Tooth Wear. Monogr. Oral Sci. 2020, 28, 91–98. [Google Scholar] [PubMed]
- Nasirzade, J.; Alccayhuaman, K.A.A.; Kargarpour, Z.; Kuchler, U.; Strauss, F.J.; Panahipour, L.; Kampleitner, C.; Heimel, P.; Schwarz, F.; Gruber, R. Acid Dentin Lysate Failed to Modulate Bone Formation in Rat Calvaria Defects. Biology (Basel) 2021, 10, 196. [Google Scholar] [PubMed]
- Nasirzade, J.; Kargarpour, Z.; Mitulović, G.; Strauss, F.J.; Panahipour, L.; Schwarz, F.; Gruber, R. Proteomic and genomic analysis of acid dentin lysate with focus on TGF-β signaling. Sci. Rep. 2021. in print. [Google Scholar]
- Nasirzade, J.; Kargarpour, Z.; Panahipour, L.; Schwarz, F.; Gruber, R. Cleaning Teeth Reduces the Inflammatory Response of Macrophages to Acid Dentine Lysate. Int. J. Mol. Sci. 2020, 21, 9207. [Google Scholar] [CrossRef]
- Strauss, F.J.; Stahli, A.; Beer, L.; Mitulovic, G.; Gilmozzi, V.; Haspel, N.; Schwab, G.; Gruber, R. Acid bone lysate activates TGFbeta signalling in human oral fibroblasts. Sci. Rep. 2018, 8, 16065. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Wang, Y.; Chu, Q.; Qi, C.; Gao, Y.; Gao, Y.; Xiang, L.; Zhenzhen, X.; Gao, Y. Loss of transforming growth factor-beta1 in epithelium cells affects enamel formation in mice. Arch. Oral Biol. 2018, 96, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Jinping, Z.; Qing, C.; Wenying, S.; Chunyan, Y.; Lili, X.; Yao, S.; Yumin, W.; Zhenzhen, X.; Li, Z.; Yuguang, G. Overexpression of constitutively active MAP3K7 in ameloblasts causes enamel defects of mouse teeth. Arch. Oral Biol. 2017, 84, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Sculean, A.; Cochran, D.L.; Froum, S.; Zucchelli, G.; Nemcovsky, C.; Donos, N.; Lyngstadaas, S.P.; Deschner, J.; Dard, M.; et al. Twenty years of enamel matrix derivative: The past, the present and the future. J. Clin. Periodontol. 2016, 43, 668–683. [Google Scholar] [CrossRef] [PubMed]
- Stahli, A.; Bosshardt, D.; Sculean, A.; Gruber, R. Emdogain-regulated gene expression in palatal fibroblasts requires TGF-betaRI kinase signaling. PLoS ONE 2014, 9, e105672. [Google Scholar] [CrossRef] [PubMed]
- Triplett, R.G.; Nevins, M.; Marx, R.E.; Spagnoli, D.B.; Oates, T.W.; Moy, P.K.; Boyne, P.J. Pivotal, randomized, parallel evaluation of recombinant human bone morphogenetic protein-2/absorbable collagen sponge and autogenous bone graft for maxillary sinus floor augmentation. J. Oral Maxillofac. Surg. 2009, 67, 1947–1960. [Google Scholar] [CrossRef] [PubMed]
- Ramanauskaite, A.; Sahin, D.; Sader, R.; Becker, J.; Schwarz, F. Efficacy of autogenous teeth for the reconstruction of alveolar ridge deficiencies: A systematic review. Clin. Oral Investig. 2019, 23, 4263–4287. [Google Scholar] [CrossRef]
- Sculean, A.; Nikolidakis, D.; Nikou, G.; Ivanovic, A.; Chapple, I.L.; Stavropoulos, A. Biomaterials for promoting periodontal regeneration in human intrabony defects: A systematic review. Periodontology 2000 2015, 68, 182–216. [Google Scholar] [CrossRef] [PubMed]
- Horibe, K.; Hara, M.; Nakamura, H. M2-like macrophage infiltration and transforming growth factor-beta secretion during socket healing process in mice. Arch. Oral Biol. 2021, 123, 105042. [Google Scholar] [CrossRef] [PubMed]
- Shirakata, Y.; Eliezer, M.; Nemcovsky, C.E.; Weinreb, M.; Dard, M.; Sculean, A.; Bosshardt, D.D.; Moses, O. Periodontal healing after application of enamel matrix derivative in surgical supra/infrabony periodontal defects in rats with streptozotocin-induced diabetes. J. Periodontal Res. 2014, 49, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Saito, E.; Yoshimura, Y.; Takahashi, D.; Handa, R.; Honma, Y.; Ohata, N. Attachment formation after transplantation of teeth cultured with enamel matrix derivative in dogs. J. Periodontol. 2011, 82, 1462–1468. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasirzade, J.; Kargarpour, Z.; Panahipour, L.; Gruber, R. Acid Dentin Lysates Increase Amelotin Expression in Oral Epithelial Cells and Gingival Fibroblasts. Appl. Sci. 2021, 11, 5394. https://doi.org/10.3390/app11125394
Nasirzade J, Kargarpour Z, Panahipour L, Gruber R. Acid Dentin Lysates Increase Amelotin Expression in Oral Epithelial Cells and Gingival Fibroblasts. Applied Sciences. 2021; 11(12):5394. https://doi.org/10.3390/app11125394
Chicago/Turabian StyleNasirzade, Jila, Zahra Kargarpour, Layla Panahipour, and Reinhard Gruber. 2021. "Acid Dentin Lysates Increase Amelotin Expression in Oral Epithelial Cells and Gingival Fibroblasts" Applied Sciences 11, no. 12: 5394. https://doi.org/10.3390/app11125394
APA StyleNasirzade, J., Kargarpour, Z., Panahipour, L., & Gruber, R. (2021). Acid Dentin Lysates Increase Amelotin Expression in Oral Epithelial Cells and Gingival Fibroblasts. Applied Sciences, 11(12), 5394. https://doi.org/10.3390/app11125394






