Abstract
Phaseolus vulgaris is the most legume cultivated in the world; in Mexico, it is considered the second most important crop after corn. The aim of this research was to determine the characteristics of Xanthomonas campestris strain “Xcf1-APJR” isolated from the leaves of bean crops, and determine the antimicrobial activity of cinnabarin on this strain. Bacterial cultures were obtained from leaves with necrotic leaf spot symptoms of bean plant variety “Flor de Mayo M38” in Puebla, Mexico. The antimicrobial activity of cinnabarin was tested at 7, 14 and 21 days on X. campestris pv. campestris. The Xcf1-APJR strain showed 100% identity with X. campestris pv. campestris as a causal agent of necrotic leaf spot. Treatment with a potato dextrose medium with a dehydrated sugar cane (PDA+C) showed a higher orange pigmentation than the other treatments after 7, 14 and 21 days of incubation and a higher concentration of cinnabarin (54.33 InU/g) with in vitro antimicrobial activity against X. campestris pv. campestris.
1. Introduction
Beans (Phaseolus vulgaris L.) are the most important legume harvested around the world [1]. The global production of beans in 2019 was 28,974,968 tons, with Myanmar being the main producer, with 5,849,622 tons, followed by India with 5,310,000 tons and Brazil with 2,906,508 tons. Mexico occupies the eighth place, with a production of 879,404 tons [2]. Within Mexico, Puebla State contributes 28,300 tons over 44,541 hectares [3].
Bacterial blight is the most common disease affecting bean crops. This disease is caused by Xanthomonas and causes significant losses globally [4]. X. campestris pv. phaseoli (Smith), has a high incidence in bean crops and is the fourth most important phytopathogen of this crop. The losses caused by this bacterium vary between 10% and 40% of yield according to the cultivation cycle, climatic conditions, bean variety and bacterial proliferation [5].
In Mexico, bacterial blight can be controlled using chemicals and biological alternatives [6,7]. Agrochemicals help to obtain a higher production, but their excessive use is not recommended because of known side effects. Therefore, the use of new alternatives for disease management may be contemplated [8]. In this context, biological control is considered one of most efficient and ecologically acceptable practices for the development of agroecology [9].
Fungi possess a broad spectrum of antibiotics, enzymes and dyes. Genus Pycnoporus is representative of homobasidiomycetes, which has a great lignocellulosic potential. The species of the genus Pycnoporus produce pigments and have biotechnological potential because of their lignocellulytic capacity and because they produce laccases, tyrosinases, cellobiose dehydrogenases, quinases, invertases and xylases. There is a growing search for antiviral, antioxidant, antifungal and antibacterial compounds in secondary metabolites of this fungus. The red-orange pigments of basidiomata are principally derived from oxidation of 3-hydroxyanthranilic, such as cinnabarinic acid and cinnabarin [10].
Cinnabarin is an orange pigment, which belongs to the phenoxazine group, with chemical formula 2-amino-9-formylphenoxazone-1-carboxylic acid (CH2OH-COOH-NH2). It can be produced in vitro in solid or liquid media or in vivo with the production of basidiocarps [10]. This anthraquinone exhibits antimicrobial activity against Bacillus cereus, Enterococcus faecalis, Enteroccocus faecium, Escherichia coli, Klebsiela pneumoniae, Lissteria measureneroides, Lactobacillus plantarum, Pseudomonas aeruginosa, Salmonella sp. and Staphylococcus aureus [11]. The production of this antibiotic occurs at days 18–22 of fungal growth. Synthesis of cinnabarin is increased at a slightly alkaline pH (6.0) and 28 °C [12].
In the summer of 2019, necrotic V-shaped lesions and necrotic leaf spots were observed on margins of the leaves, surrounded by yellowing, in bean crop var. “Flor de Mayo M38” from San Diego Ecatepec-Amozoc, Puebla, Mexico. These symptoms were observed in 40% of the crops. Therefore, the objectives of this work were to identify the causal agent of this disease and to determine the in vitro antimicrobial activity of cinnabarin on the causal agent found.
2. Materials and Methods
2.1. Isolation of Causal Agent of Bacterial Blight
The sampling was carried out on 50 plants of Phaseolus vulgaris (var. “Flor de Mayo M38”) showing symptoms of Xanthomonas sp. (bacterial blight) in San Diego Ecatepec, Amozoc, Puebla, Mexico (19°02′46.6″ N 98°05′15.6″ W). Necrotic leaves with irregular leaf spots, surrounded by yellowing and chlorosis, were collected during the months of May to September 2019. The geographical area in which the samples were obtained presented weather (cwb) with an average annual rainfall of 1500 mm and an altitude of 2320 masl [13]. The samples were kept in plastic bags and transported at 4 °C to the laboratory.
Small pieces of tissue (1 cm2) were disinfected with 1% sodium hypochlorite for 3 min and washed with sterile distilled water. The isolation was done by means of direct seeding of the affected tissue in innutritious agar (peptone 5 g/L, meat extract 4 g/L, agar 15 g/L), seeding four pieces per Petri dish (90 × 15 mm). These were sealed and incubated to 36 °C for 72 h. Subsequently, bacterial transfers were made to obtain pure colonies and were subjected to Gram staining [6]. Only the Gram (-) isolates were purified in YDC medium (agar, yeast extract, dextrose and calcium carbonate) and BK (Middlebrook) at 36 °C for 48 h. Preliminary identification was carried out using the morphological characteristics of the colonies and biochemical characterization [14]. The samples were preserved in 20% glycerol.
2.2. Molecular Identification
The most common bacterial strain was designated as Xcf1-APJR. The extraction of DNA was performed with the Zymocleantm Gel-DNA Recovery Kit (Zymo Research, Irvine, CA, USA) following the manufacturer’s instructions. The DNA was resuspended in 100 μL HPLC water, to be immediately quantified by spectrophotometry (Nanodrop 2000 C, Thermo Scientific, Waltham, MA, USA) at A260/280 and A230/260 nm. Afterward, the genetic material was diluted to a final concentration of 20 ng mL−1 and used as a template for PCR reactions. The sequence used the universal primers 518F (5′CCAGCAGCCGCGGTAATACG3′) and 800R (5′TACCAGGGTATCTAATCC-3′) to amplify the gen 16S rDNA partial [15].
The PCR test was prepared for a volume of 25 μL−1, using 5 μL−1 of buffer 5×, 2.5 μL−1 of DNTPs, 2.0 μL−1 of Primers 518F, 2.0 μL−1 of Primers 800R, 0.5 μL−1 of Taq polymerase (5U), 3 μL−1 of DNA and 10 μL−1 of sterile deionized water. Amplification was carried out in a Peltier DNA thermos (PTC-200, BIO-RAD). The PCR conditions were as follows: initial denaturation at 95 °C for 2 min, followed by 35 cycles at 95 °C for 2 min, 59 °C for 1 min, and 72 °C for 1.5 min. Final elongation was at 72 °C for 5 min. The PCR products were verified on 2% agarose gel electrophoresis (Seakem, Invitrogen, Carlsbad, CA, USA) and stained with 10,000 × Gel-Red (Biotium, Fremont, CA, USA). Finally, PCR products were cleaned with ExoSAP-IT (Affymetrix, Santa Clara, CA, USA). The fraction was individually sequenced using the BigDye Terminator v3.1 Cycle Sequencing Kit (AppliedBiosystems, Carlsbad, CA, USA) on a 3130 Genetic Analyzer Sequencer (Applied Biosystems, Carlsbad, CA, USA) in facilities at the Mexico Postgraduate College. Sequences were assembled and edited using SeqMan (DNAStar, Madison, WI, USA) and compared with nonredundant sequences in GenBank™ using the Blast algorithm.
The identification of the pathovar of the Xcf1-APJR strain was carried out using serological characteristics, through a complete commercial kit (LOEWE Biochemica GmbH, Sauerlach, Germany). Double sandwich enzyme-linked immunosorbent assays (DAS-ELISA) of antibodies specific for X. campestris pv. campestris were carried out in triplicate according to the manufacturer’s instructions. Absorbance results at 405 nm were read 4 h after addition of the substrate, using an automatic ELISA reader for serological plates (Universal Microplate Reader ELx800 BioTek Instruments, Winooski, VT, USA). Readings were normalized to R-values (OD-sample/OD-negative control). R = 2.0 was used as a threshold to distinguish a positive response from a negative response for the three samples tested, with an average of two replicates (two wells) for this study [16].
2.3. In Vitro Pathogenicity Tests
The pathogenicity test was carried out on 50 healthy bean plants of “Flor de Mayo M38” variety. Bacterial growth of 48 h in YDC medium was used at 28 °C to prepare the bacterial suspension (108 CFU mL−1). The first two lower leaves of 30-day-old plants were inoculated by sprinkling. Ten plants sprayed with sterile distilled water were used as a negative control; they were kept for 20 days in a greenhouse at 18–26 °C and a relative humidity of 60%.
After 14 days post-inoculation, the plants showing symptoms of disease were used to the re-isolate the causal agent of bacterial blight. The morphology, partial sequence, and polyclonal-specific identification of Xcf1-APJR strain were carried out as described in Section 2.2 in order to complete Koch’s postulates [17].
2.4. Production and Synthesis of Cinnabarin from Pycnoporus sanguineus
To produce cinnabarin, the CP-MaPs strain was grown in potato dextrose agar medium with dehydrated papaya (PDA+P), potato dextrose agar medium with glucose (PDA+G) and potato dextrose agar medium with dehydrated sugar cane (PDA+C). These culture media were prepared in a ratio of 3:1 (v/v). In addition, potato dextrose agar (PDA) culture medium was established as a control group. Sterile cellophane membranes (4.25 cm diameter) were placed on top of each culture medium. Later, 5 mm agar fragments were inoculated with 10-day-old P. sanguineus mycelium and incubated in the dark at 28 ± 2 °C for 7, 14 and 21 days. Radial growth was measured every 12 h to estimate growth velocity (cm), which was calculated as previously reported (cm d−1) [18]. The experiments were carried out in triplicate.
After the incubation times, the cellophane membranes containing mycelium were removed to calculate the fungal biomass by weight difference, using a convection air oven at 105 °C for 2 h. The dry weight was expressed in mg box−1 [19]. The dried mycelium was triturated with 10 mL of ethyl acetate to extract cinnabarin in a 500 mL Erlenmeyer flask to a final volume of 50 mL. Subsequently, the flask was placed under continuous shaking at 1000 rpm for 24 h. The ethyl acetate fraction was filtered using Whatman No. 4 paper and subsequently concentrated on a Büchi rotary evaporator (461) at 75 °C [20]. Finally, the crude extract was weighed and stored in amber bottles.
2.5. Purification of Cinnabarin
The quantification of cinnabarin in the crude extract was carried out using the agar diffusion method described by Smânia et al. [21]. In addition, the dissolved biomass of each treatment was calculated based on Equation (1) [22], as follows:
Biomass Dissolved (mg mL−1) = Initial weight − Final weight
To determine the efficiency of the extracts, Equation (2) was used:
Efficiency (%) = (BD/Initial sample weight (mg mL−1)) × 100
Finally, cinnabarin synthesis was indirectly determined by the agar diffusion method, where in cinnabarin concentration in each treatment was estimated by the formula Y = AX + B; where (Y) corresponds to the diameter of the zone of inhibition (mm), (AX) is equivalent to the logarithm of the amount of cinnabarin concentrate (InU/g) in ethyl acetate extract that produced an inhibition equivalent to 1 mg of cinnabarin [20] and (B) represents different treatments. The experiments were carried out in triplicate.
2.6. Antimicrobial Activity
For the antimicrobial activity test, the agar disc diffusion technique was used, with 7 mm diameter discs of sterilized filter paper [23]. These discs were impregnated with a 5 μL aliquot of previously prepared cinnabarin. The impregnated discs were air dried in a sterile environment.
The inoculum of X. campestris pv campestris strain Xcf1-APJR was prepared in 10 mL sterile yeast broth (YS) incubated at 36 ± 2 °C for 24 h. Subsequently the inoculum was adjusted using the McFarland scale (1.5 × 108 CFU mL−1) [24].
Finally, 0.1 mL of the inoculum was spread at a concentration of 1 × 108 CFU mL−1 in Petri dishes containing Mueller Hinton Agar (20 g of MH and 17 g of agar) in triplicate. The cinnabarin-impregnated discs were placed inside Petri dishes, and a negative control disc (sterile water solution) and a positive control disc (streptomycin 225 mg L−1) were included and incubated at 36 ± 2 °C for 48 h.
The diameter of the inhibition zone was measured with a digital Vernier (CD-6 Mitutoyo) and expressed in millimeters. The percentage of inhibition caused by cinnabarin was analyzed using Equations (3) and (4) [25], classifying the pure extract of cinnabarin according to Cormican and Pfaller [26] (Equations (3) and (4)):
Growth (%) = (Inhibition Zone (mm)/Negative Control (mm)) × 100
Inhibition of Growth (%) = 100 − Growth (%)
2.7. Statistical Analysis
A randomized experimental design was used in triplicate with a 4 × 3 × 1 factorial arrangement, where the variable factors were culture medium with four levels (PDA, PDA+G, PDA+C, PDA+P), incubation time (7, 14 and 21 days) and a the Xcf1-APJR strain. The data were subjected to the Bartlett homogeneity test. The inhibition of bacterial growth and the efficiency of the extract were expressed as percentages and were transformed with √x + 1 angular arccosine. The growth speed, dissolved biomass, area of inhibition and synthesis of cinnabarin were analyzed by ANOVA and the Tukey-Kramer-HSD mean comparison test with a significance level of 0.05, using the software SPSS Statistics version 17 for Windows.
3. Results
3.1. Isolation, Characterization and Identification of the Causative Agent of Bacterial Blight
Ten representative isolates of 50 different plants developed yellow bacterial colonies in YDC medium, with convex elevations containing whole edges and mucoid appearance; the isolated “Xcf1-APJR” was the most representative of all the samples analyzed (Figure 1a).
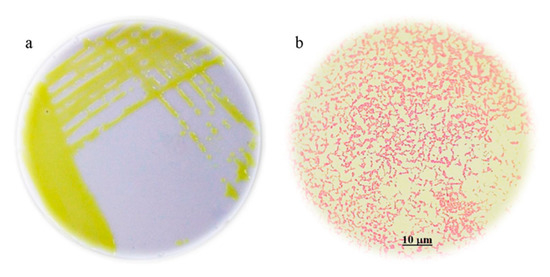
Figure 1.
Characterization of the Xcf1-APJR strain: (a) Morphology of yellow colonies, with a smooth, luminous and butyrous appearance in medium culture YDC with 10 days of growth; (b) negative gram bacilli (60×).
Xcf1-APJR strain grew at 36 ± 2 °C, and the production of oxygen after inoculation with hydrogen peroxide clearly indicated a catalase-positive result, whereas the Kovac’s oxidase test was negative. Furthermore, the bacterium did not reduce nitrates to nitrite and was unable to grow in casamino acid-peptone-glucose (CPG) medium with 0.1% tetrazol trifenyl chloride. The KOH solubility test and gram staining confirmed its gram-negative condition (Figure 1b).
The amplification of 16S rDNA gene region showed a product of 1,418 bp, which presented 100% identity with X. campestris pv. campestris (ID: NZ_AP019684.1) in the Gen Bank nucleotide database of the National Center for Biotechnology Information. This sequence was deposited in the same database, with the accession number MT645246; it is attached as supplementary information (Supplementary Sequence S1).
Finally, the indirect DAS-ELISA test, performed on a microtiter plate, confirmed the identity of the Xcf1-APJR strain by positively reacting with the specific antibodies for X. campestris pv campestris. Absorbance values for OD (n = 6) of A0.420 were obtained, while absorbance of OD-negative control (n = 6) was A0.172. The result for normalization of R = 2.44 ± 1.02 for a bacterial suspension of 2.5 × 107 CFU/mL.
3.2. Pathogenicity Tests
Koch’s postulates confirmed that Xcf1-APJR strain produced typical symptoms of bacterial blight at five days after inoculation. In addition, hypersensitivity was observed at 24 h. Necrotic spots 10 mm in diameter surrounded by a chlorotic halo were observed (Figure 2a). Brown lesions on young leaves with watery appearance and evidence of necrosis were also recorded. Wilted seedlings showed necrosis at 14 days after post-inoculation, as shown in Figure 2b. No symptoms were observed in the control group.
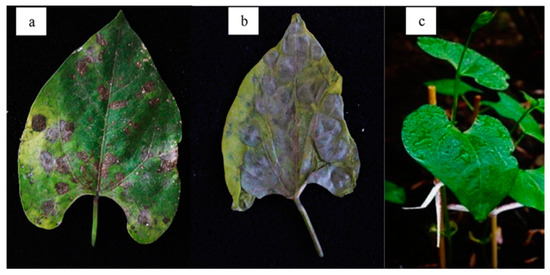
Figure 2.
Pathogenicity tests with strain Xcf1-APJR: (a) Leaf of P. vulgaris with necrotic foliar stain and chlorotic halo at 7 days after inoculation; (b) wilting and total necrosis of foliar tissue at 14 days; (c) control group without symptoms.
Koch’s postulates were confirmed by the re-insulation of the Xcf2-APJR strain, which presented the same colony morphology described above. Using PCR of repetitive sequence and with the access number (MT645261) in the gene bank, it was possible to confirm the identity of re-isolation of the original strain (Supplementary Sequence S1).
3.3. Evaluation of Synthesis of Cinnabarin from Pycnoporus sanguineus
In all treatments, the hyphal growth of the CP-MaPs strain covered Petri dishes at 7 days after sowing. The texture of the colonies varied from cotton to velvety, presenting aerial mycelium in Figure 3(a1). The color of the strain changed from white to an intense orange at 7, 14 and 21 days after sowing (Figure 3). Interestingly, the diameter of the colonies had statistically significant differences (p < 0.001). PDA+C treatment presented the best growth rate of 0.4666 ± 1.33 cm d−1 and the highest pigmentation at 21 days (see Figure 3(d3)) as well as the greatest amount of fungal biomass (54.60 ± 10.2 mg box−1). PDA+P presented a growth rate of 0.4333 ± 0.43 cm d−1 and a biomass of 49.45 ± 11.9 mg box−1, while PDA treatment was the least effective (Table 1).
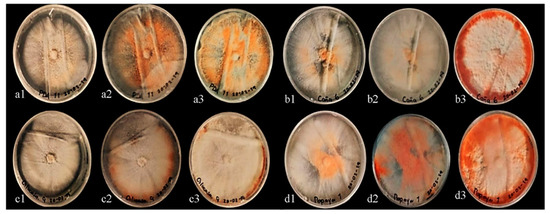
Figure 3.
Development of strain CP-MaPs (P. sanguineus) and production of biomass: (a1–a3) = Control; (b1–b3) = PDA+C; (c1–c3) = PDA+G; (d1–d3) = PDA+P; (a1,b1,c1,d1) = incubation at 7 days; (a2,b2,c2,d2) = incubation at 14 days; (a3,b3,c3,d3) = incubation at 21 days.

Table 1.
Radial growth, specific growth rate and biomass obtained at 7 days of incubation for P. sanguineus.
After the evaluation period (7, 14 and 21 days), it was found that the production of cinnabarin showed statistically significant differences (p < 0.001) among culture media (Table 2). The efficiency of the extract, understood as the relationship between the dry weight of the extract and the dry weight of the biomass, was not the same for the different treatments (PDA+G had a better performance, with 8.54% and the largest amount of dissolved biomass (0.013179 g)). However, fungal growth was not related to the increase of cinnabarin.

Table 2.
Biomass dissolved, efficiency of the extract and zone of inhibition of effect of cinnabarin on X. campestris pv. campestris.
The Medium PDA+C presented the largest inhibition area in 21 days, with 16.58 ± 1.26 mm, followed by the medium PDA+P (15.62 ± 0.91 mm). The PDA treatment presented the lowest area of inhibition at 7, 14 and 21 days.
According to ANOVA, the addition of dehydrated papaya, dehydrated sugarcane and glucose to the culture medium PDA (3:1 v/v) affected the synthesis of cinnabarin (F = 79.07; p < 0.001). In this context, the mycelium of P. sanguineus from the PDA+C treatment showed an orange coloration that was clearly stronger than the other treatments at 21 days of growth.
The PDA+C treatment had the highest yield of cinnabarin (54.33 InU/g), with statistically significant differences (p < 0.0001), followed by the PDA+P treatment at 51.13 (InU/g). The PDA (Control) treatment presented the lowest cinnabarin yield at 7, 14 and 21 days, with 26.10, 30.51 and 36.15 InU/g, respectively (Figure 4).
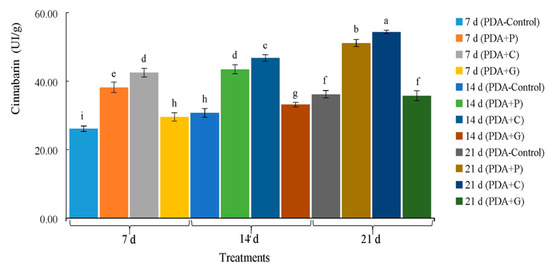
Figure 4.
Synthesis of cinnabarin from P. sanguineus in different media at 7, 14 and 21 days. InU = G Inhibitory unit = Quantity (mg) that gives an inhibition equivalent to 1 mg of cinnabarin by diffusion assay (Smânia et al., 1997). Media followed by the same letter does not present statistically significant differences (p ≤ 0.05) by Tukey’s test.
3.4. Antimicrobial Activity In Vitro
Antimicrobial activity of cinnabarin in different media assayed is shown in Table 2 and Figure 5. Statistically significant differences (p < 0.001) were found among inhibition zones for the growth of X. campestris pv. campestris. The medium PDA+C produced 16.58 ± 1.26 mm, whereas the PDA Control generated 8.13 ± 0.11 mm after 48 h.
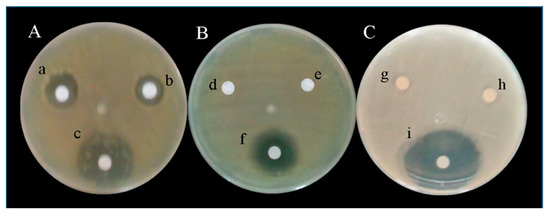
Figure 5.
(A) Inhibition zone (mm) of X. campestris pv. campestris by the effect of cinnabarin in PDA+C at 7 (a), 14 (b) and 21 (c) days; (B) negative controls (d and e) and PDA+P at 21 days (f); (C) negative controls (g and h) and positive control (i) at 48 h.
The results of ANOVA revealed that the cinnabarin produced by P. sanguineus generated a statistically significant inhibition on the growth of X. campestris pv. campestris. The medium PDA+C presented the highest percentage of inhibition (73.69%) at 48 h (Figure 6), followed by PDA+P treatment with 69.43%, and PDA treatment with 36.14%, at day 7.
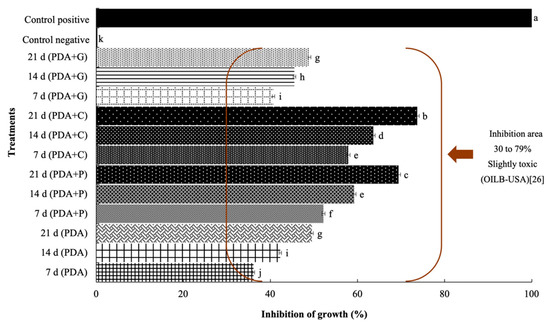
Figure 6.
Percentage of inhibition for the growth of X. campestris pv. campestris due to the effect of cinnabarin from P. sanguineus in different media at 7, 14 and 21 days.
4. Discussion
According to the morphological characteristics corresponding to the genus Xanthomona, it was confirmed that Xcf1-APJR strain is within this bacterial genus. [27]. Schaad et al. [27] indicated that Xanthomona, is represented by mocoid colonies, which are convex and bright in NGA. The growth of the isolate in YDC confirmed the presence of the yellow colonies of Xanthomonas. [28]
The sequence of rDNA 16S gene region of the Xcf1-APJR strain showed 100% identity with X. campestris pv. campestris (ID: NZ_AP019684) [29,30]. Likewise, the serological method endorsed the identification of strain Xcf1-APJR as X. campestris pv. campestris, [27]. The pathogenicity test revealed similar results to those already reported by Duveiller et al. [28] for X. campestris. The typical symptoms emerged five days post-inoculation, and hypersensitivity was observed 24 h after inoculation.
The wet necrotic spots surrounded by a chlorotic halo in the leaves showed the beginning of necrosis at the tissue level, which presented in the same incubation period reported by Botero et al. [31]. The necrotic foliar stain with chlorotic halo, on the margins of the leaf, was also reported by Pinto et al. [32], who observed this symptom on mature leaves of Passiflora edulis at five days post-inoculation.
After the assessment of pathogenicity tests with the re-isolated Xcf2-APJR strain, Koch’s confirmed this bacterium as the causal agent of bacterial blight. Popović et al. [33] first reported on X. campestris pv. campestris as the causal agent of black rot in rapeseed (Brassica napus) in Serbia. As far as we know, this is the first report of this pathogen causing black leaf rot in P. vulgaris in variety “Flor de Mayo M38” in Puebla, Mexico.
The growth of the CP-MaPs strain was completed at day 7 in all the treatments. This result differed from that reported by Baumer et al. [34]. The morphology of the colonies coincided with that reported by Papinutti [35] for P. sanguineus, which showed aerated and velvety orange mycelium at 29 days post-inoculation.
Baumer et al. [34] reported growth rates of 5.6 and 7.14 mm/day for wild strains of P. sanguineus (MIP20001 and MIP95002, respectively) isolated from Santa Catarina, Brazil. Acosta and Villegas [36] reported a growth rate of 9.9 mm/day, which was greater than that reported in the present investigation.
It has been shown that the production of biomass is not related to the production of secondary metabolites with antimicrobial activity [34]. The secondary metabolites produced by the genus Pycnoporus depend on the species and culture conditions assayed [10,34]. After the evaluation period (21 days), it was observed that dissolved biomass, efficiency of the extract and zone of inhibition were important to evaluate the concentration of cinnabarin in the different media.
The production of natural pigments by fungi has recently gained ground in the field of biotechnology. The variety of secondary metabolites produced by fungi could be associated with their incapacity to synthesize macromolecules from carbon dioxide or light energy, since they do not contain chlorophyll. Therefore, their survival depends on environmental conditions and the substrate where they develop [37]. Cruz-Muños [38] obtained 0.8501 g of dissolved biomass, which was higher than reported herein.
The results did not show a clear tendency, since the best conditions for the CP-MaPs strain of P. sanguineus were media enriched with dehydrated papaya, dehydrated sugarcane and glucose. According to Smânia et al. [22], the strain of P. sanguineus MIP 89007 had a higher concentration of pigments at 20 days of incubation.
The addition of dehydrated papaya, dehydrated sugar cane and glucose (3:1 v/v) to the culture medium affected the synthesis of cinnabarin. The resulting mycelium of the PDA+C treatment showed an orange coloration that was clearly more prominent than observed in other treatments. This in vitro production of cinnabarin may be a response to the pressure of abiotic factors such as nutrient availability, temperature and space. The variation of nutrients and incubation conditions are used as a strategy to enhance the synthesis of secondary metabolites. It has been reported that the best growth conditions are not directly related to the production of cinnabarin [39].
The evaluation of the antimicrobial activity of cinnabarin from P. sanguineus on X. campestris (Xcf1-APJR strain) represents a new source for the obtainment of new molecules able to control bacterial blight [40]. Little is known about the antimicrobial activity of P. sanguineus extracts against phytopathogenic bacteria. However, Toillier et al. [41] reported that the extract of P. sanguineus exerted around 20% growth inhibition on X. axonopodis pv. phaseoli. This result was less effective than that obtained in the present investigation.
The antimicrobial activity of P. sanguineus was documented by Bose [42], who isolated polyporine, an active compound against Gram negative bacteria. Studies performed by Smânia et al. [22] demonstrated that cinnabarin produced by P. sanguineus had activity against Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Salmonella typhi and Staphylococcus aureus. According to this work, cinnabarin was more effective for the control of Gram (+) than for Gram (-). According to Rosa et al. [43] extracts from P. sanguineus strain CCB277 inhibited the growth of C. krusei, L. monocytogenes and S. aureus.
Obtaining, isolating and purifying compounds with antimicrobial activity from the fermentation of filamentous fungi is a widely used methodology under various conditions and objectives. Likewise, antimicrobial activity tests reflect the genetic capacity and vulnerability of the tested organism; however, they are considered to evaluate the activity of the compound under field conditions [44]. Leon [45] reported a reduction in the severity of the angular spots in beans after spraying the foliar surface with filtrates of P. sanguineus. In addition, there is an advantage when cultivating P. sanguineus in Petri dishes, since the time of obtaining cinnabarin varies between 7 and 21 days, compared to the fungus growth cycle in solid substrate, which lasts 242 days [46].
5. Conclusions
X. campestris pv. campestris (ID: MT645246 and MT645261) was identified as the causal agent of bacterial blight in bean crop variety “Flor de Mayo M38” from San Diego Ecatepec, Amozoc, Puebla, Mexico. The growth of P. sanguineus in different media showed that the treatments enriched with dehydrated papaya, dehydrated sugarcane and glucose (3:1 v/v) presented a better production of cinnabarin after 7, 14 and 21 days of incubation. Cinnabarin had antibacterial activity against X. campestris pv. campestris in vitro.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/app11125391/s1, Sequence S1: Gen 16S rDNA partial sequencing, using universals primers 518F and 800R for Xanthomonas campestris pv. campestris (Xcf1-APJR and Xcf2-APJR strains) previously deposited at nucleotide databank of National Center for Biotechnology Information (ID: MT645246 and MT645261).
Author Contributions
Conceptualization, O.R.-A., A.P.J.y.R. and A.R.; methodology, N.V.-R., C.P.L. and O.R.-A.; software, M.A.V.d.I. and O.R.-A.; validation, N.V.-R., A.R. and O.R.-A.; formal analysis, C.P.L., M.A.V.d.I. and O.R.-A.; resources, O.R.-A. and A.R.; Original draft preparation, A.R., M.A.V.d.I. and O.R.-A.; writing—review and editing, A.R. and O.R.-A.; visualization, O.R.-A., M.A.V.d.I. and C.P.L.; supervision, N.V.-R.; project administration, O.R.-A.; funding acquisition, O.R.-A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the program PRODEP 2021 of the Secretaría de Educación Pública de México (SEP).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful to Consejo Nacional de Ciencia y Tecnología (CONACyT) and the Maestría en Manejo Sostenible de Agroecosistemas at the Benémerita Universidad Autónoma of Puebla, México.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nassary, E.K.; Baijukya, F.; Ndakidemi, P.A. Assessing the Productivity of Common Bean in Intercrop with Maize across Agro-Ecological Zones of Smallholder Farms in the Northern Highlands of Tanzania. Agriculture 2020, 10, 117. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO). Agriculture Production and Trade Statistics; Food and Agriculture Organization of the United Nations: Rome, Italy, 2021; Available online: http://www.fao.org/faostat/en/ (accessed on 28 May 2021).
- SIAP-SAGARPA. Servicio de Información Agroalimentaria y Pesquera. Secretaria de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación. 2021. Available online: www.siap.gob.mx (accessed on 30 May 2021).
- Tugume, J.K.; Osundwa, C.; Tusiime, G.; Mukankusi, C.M.; Ssekamate, A.M.; Wasswa, P.; Buruchar, R. Pathogenicity and virulence of Uganda isolates of common bacterial blight disease pathogen (Xanthomonas spp.). Afr. Crop Sci. J. 2020, 28, 213–226. [Google Scholar] [CrossRef]
- Corzo, M.; Quiñones, M.; Martinez-Coca, B.; Rivero, D.; Zamora-Gutierrez, L.; Martinez-Zubiaur, Y.; Plasencia-Marquez, O.; Pauls, K.P. Current situation of Xanthomonas spp., and Phaseolus vulgaris L. pathosystem in the west region of Cuba. Phytopathology 2019, 109. [Google Scholar] [CrossRef]
- Francisco, F.N.; Gallegos, M.G.; Ochoa, F.Y.; Hernández, C.F.; Benavides, M.A.; Castillo, R.F. Aspectos fundamentales del tizon común bacteriano (Xanthomonas axonopodis pv. phaseoli Smith): Características, patogenicidad y control. Revista Mexicana de Fitopatología 2013, 31, 147–160. [Google Scholar]
- Aguilar, C.; Mano, M.; Eulalio, A. Micro-RNAs at the host-bacteria interface: Host defense or bacterial offense. Trends Microbiol. 2019, 27, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Zavaleta, M.E. Alternativas de manejo de las enfermedades de las plantas. Terra Latinoamericana 1999, 17, 201–207. [Google Scholar]
- Rossbacher, S.; Vorburger, C. Prior adaptation of parasitoids improves biological control of symbiont-protected pests. Ecol. Appl. 2020, 13, 1868–1876. [Google Scholar] [CrossRef]
- Cruz, M.R.; Piña-Guzmán, A.B.; Yáñez-Fernández, J.; Valencia, D.G.; Bautista-Baños, S.; Villanueva, A.R. Producción de pigmentos de Pycnoporus sanguineus en medio de cultivo sólido. Agrociencia 2015, 49, 347–359. [Google Scholar]
- Zhang, Y.; Cao, J.; Wang, J.; Cheng, Y.; Zhou, M.; Wang, W.; Geng, M.; Xu, D.; Xu, Z. Comparative genomicsuncovers genetic diversity and synthetic biology of secondary metabolite production of the genus Trametes. Res. Sq. 2019, 1–25. [Google Scholar] [CrossRef]
- Téllez-Téllez, M.; Villegas, E.; Rodríguez, A.; Acosta-Urdapilleta, M.L.; O’Donovan, A.; Díaz-Godínez, G. Mycosphere Essay Fungi of Pycnoporus: Morphological and molecular identification, worldwide distribution and biotechnological potential. Mycosphere 2016, 7, 1500–1525. [Google Scholar] [CrossRef]
- García, E. Modificaciones al Sistema de Clasificación Climática de Köppen; Instituto de Geografía, Universidad Autónoma de México: Mexico City, Mexico, 2004; 170p. [Google Scholar]
- Escalona, Y.; Sanabria, M.; Rodriguez, D.; Ricón, N. Prevalencia de patógenos bacterianos en cultivos hortícolas a cielo abierto del municipio Jiménez, Estado Lara y evaluación de control alternativo mediante uso de extracto etanólico de Lippia origanoides. Revista de la Universidad de Zulia 2019, 10, 58–81. [Google Scholar]
- Rashidah, R.A.; Hasan, N.; Ramachandran, K. Screening of endophytic bacteria as biocontrol agents against bacteria leaf blight (Xanthomonas oryzae). Hayati J. Biosci. 2020, 27, 215–220. [Google Scholar]
- Popovic, T.; Balaz, J.; Ignjatov, M.; Mitrovic, P.; Gavrilovic, V.; Josic, D. Identification and Genetic Characterisation of Xanthomonas campestris pv. campestris as an Oilseed rape Pathogen in Serbia. J. Plant Pathol. 2014, 96, 553–560. [Google Scholar]
- Nico, A.I.; Alippi, A.M.; Dal-Bo, E.; Ronco, L.B. Interacción de Pseudomonas corrugata y Pseudomonas viridiflava y diferentes genotipos de tomate. Revista de la Facultad de Agronomía 2006, 6, 37–45. [Google Scholar] [CrossRef]
- Zeravakis, G.; Philippoussis, A.; Ioannidou, S.; Diamantopoulou, P. Mycelium growth kinetics and optimal temperature conditions for the cultivation of edible mushroom species on lignocellulosic substrates. Folia Microbiológica 2001, 46, e231. [Google Scholar] [CrossRef] [PubMed]
- Muy-Rangel, M.D.; Osuna-Valle, J.R.; García-Estrada, R.S.; Martín-Hernández, C.; Quintana-Obregón, E.A. Actividad antifúngica in vitro del aceite esencial de ajo (Allium sativum L.) contra Alternaria tenuissima. Revista Mexicana de Fitopatología 2018, 36, 162–171. [Google Scholar] [CrossRef]
- Smânia, E.F.; Smaünia, A.; Loguercio-Leite, C. Optimal parameters for cinnabarin synthesis by Pycnoporus sanguineus. J. Chem. Technol. Biotechnol. 1997, 70, 57–59. [Google Scholar] [CrossRef]
- Smânia, J.A.; Dellemonache, F.; Smânia, E.F.A.; Gil, M.L.; Benchetrit, L.C.; Cruz, F.S. Antibacterial activity of a substance produced by the fungus, Pycnoporus sanguineus (Fr.). Murr. J. Ethnopharmacol. 1995, 45, 177–181. [Google Scholar] [CrossRef]
- Smânia, E.F.A.; Smânia, J.A.; Loguercio-Leite, C. Cinnabarin synthesis by Pycnoporus sanguineus strains and antimicrobial activity against bacteria from food products. Revista de Microbiologia 1998, 29, 317–320. [Google Scholar] [CrossRef]
- Moulari, B.; Pellequer, Y.; Lboutounne, H.; Girad, C.; Chaumont, J.; Millet, J.; Muyar, F. Isolation and in vitro antibacterial activity of astilbin, the bioactive flavonone from the leaves of Harungana madagascariensis Lam. ex Poir. (Hypericaceae). Ethnopharmacology 2006, 106, 272–278. [Google Scholar] [CrossRef]
- Norma Oficial Mexicana (NOM-181-SSA1). Salud Ambiental. Agua para uso y Consumo Humano. Requisitos Sanitarios que Deben Cumplir las Sustancias Germicidas para Tratamiento de Agua, de Tipo Doméstico. 1998. Available online: http://legismex.mty.itesm.mx/normas/ssa1/ssa1181.pdf (accessed on 2 April 2021).
- Moreno-Limón, S.; González-Solís, L.N.; Salcedo-Martínez, S.M.; Cárdenas-Avila, M.L.; Perales-Ramírez, A. Efecto antifúngico de extractos de gobernadora (Larrea tridentata L.) sobre la inhibición in vitro de Aspergillus flavus y Penicillium sp. Polibotánica 2011, 32, 193–205. [Google Scholar]
- Cormican, M.G.; Pfaller, M.A. Standardization of antifungal susceptibility testing. J. Antimicrob. Chemother. 1996, 38, 561–578. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schaad, N.W.; Jones, J.B.; Chun, W. Laboratory Guide for Identification of Plant Pathogenic Bacteria. Biologia Plantarum 2001, 44, e546. [Google Scholar] [CrossRef]
- Lia, R.S.; Ali, M.R.; Jahan, M.S.; Akter, A.; Sumi, M.S.E.; Hasan, M.F.; Acharjee, U.K.; Islam, M.A.; Sikdar, B. Detection of Xanthomonas campestris pv. cucurbitae from bacterial leaf spot disease of cucumber and evaluation of its biological control. Adv. Biores. 2018, 9, 41–46. [Google Scholar] [CrossRef]
- Takeuchi, K.; Mitsuharaa, I. Complete Genome Sequences of Two Strains of Xanthomonas campestris pv. campestris Isolated in Japan Kasumi. Microbiol. Resour. Announc. 2020, 9, e01239-19. [Google Scholar] [CrossRef]
- Nei, M.; Kumar, S. Molecular and Evolution Phylogenetics; Oxford Univerity Press, Inc.: Oxford, UK, 2000; 333p. [Google Scholar]
- Botero, M.J.; Ramírez, M.C.; Castaño-Zapata, J. Determinación del periodo de incubación de Xanthomonas campestris pv. passiflorae, agente causante de la bacteriosis del maracuyá (Passiflora edulis var. flavicarpa Degener). Boletín Fitotecnia 2006, 107, 1–5. [Google Scholar]
- Pinto, F.; Oliveira, F.; Cardoso, J.; Vidal, J. Principais doenças do maracujazeiro na região nordeste e seu controle. Comunicado Técnico 2003, 86, 1–12. [Google Scholar]
- Popović, T.; Mitrović, P.; Jelušić, A.; Dimkić, I.; Marjanović-Jeromela, A.; Nikolić, I.; Stanković, S. Genetic diversity and virulence of Xanthomonas campestris pv. campestris isolates from Brassica napus and six Brassica oleracea crops in Serbia. Plant Pathol. 2019, 68, 1448–1457. [Google Scholar] [CrossRef]
- Baumer, J.D.; Mas-Diego, S.; Pacheco, S.M.V.; Morgado, A.F.M.; Furigo, A.F. Comparative study of mycelial growth and production of cinnabarin by different strains of Pycnoporus sanguineus. Biofar Rev. Biol. Farm. 2008, 2, 1–5. [Google Scholar]
- Papinutti, L. Pycnoporus sanguineus. Fichas Micológicas. Revista Boletín Biológica 2013, 7, 31–32. [Google Scholar]
- Acosta, L.; Medardo, F.; Villegas, A. Cultivo de Pycnoporus sanguineus en aserrín de pino, encino y cedro. In Hongos Comestibles y Medicinales en Iberoamérica: Investigación y Desarrollo en un Entorno Multicultural; Sánchez, J., Mata, G., Eds.; Instituto de Ecología: Tapachula, Mexico, 2013; pp. 329–338. ISBN 978-607-7637-73-8. [Google Scholar]
- Rosales-Lopez, C. Other important use of mushrooms. Tecnol Marcha 2019, 32, 82–90. [Google Scholar] [CrossRef]
- Cruz-Muñoz, R. Producción de Extractos de Pycnoporus sanguineus con Actividad Antimicrobiana en Hongos y Bacterias Fitopatógenas. Tesis de Maestría, Instituto Politécnico Nacional, Mexico City, Mexico, 2012; 109p. Available online: http://repositoriodigital.ipn.mx/handle/123456789/15754 (accessed on 14 October 2020).
- Keller, N.P.; Turner, G.; Bennett, J.W. Fungal secondary metabolism-from biochemistry to genomics. Nat. Rev. Microbiol. 2005, 3, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.J.; Kim, S.W.; Xu, C.P.; Choi, J.W.; Yun, J.W. Morphological and rheological properties of the three different species of basidiomycetes Phellinus in submerged cultures. Appl. Microbiol. Biotechnol. 2004, 96, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Toillier, S.L.; Iurkiv, L.; Meinerz, C.C.; Baldo, M.; Viecelli, C.A.; Kuhn, O.J.; Schwan-Estrada, K.R.F.; Stangarlin, J.R. Controle de crestamento bacteriano común (Xanthomonas axonopodis pv. phaseoli) e alteracoes bioquímicas em feijoeiro induzidas por Pycnoporus sanguineu. Arq. Inst. Biol. 2010, 77, 99–110. [Google Scholar] [CrossRef]
- Bose, S.R. Antibiotics in a Polyporus (Polystictus sanguineus). Nature 1946, 158, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Rosa, L.H.; Machado, K.M.G.; Jacob, C.C.; Capelari, M.; Rosa, C.A.; Zani, C.L. Screening of Brazilian basidiomycetes for antimicrobial activity. Memórias do Instituto Oswaldo Cruz 2003, 98, 967–974. [Google Scholar] [CrossRef]
- Sanchez-Perez, J.A.; Béjar-Castillo, V.; Villanueva-Cotrina, F.; Guevara-Granados, J.M. Actividad antimicrobiana de metabolitos secundarios de Aspergillus fumigatus sensu stricto sobre cepas clínicas de Staphylococcus aureus y Streptococcus pneumoniae. Anales de la Facultad de Medicina 2020, 81, 180–185. [Google Scholar] [CrossRef]
- León, S.I. La antracnosis y la mancha angular del frijol común (Phaseolus vulgaris L.). Temas de Ciencia y Tecnología 2009, 45–59. Available online: https://www.utm.mx/edi_anteriores/Temas39/2NOTAS%2039-3.pdf (accessed on 14 December 2020).
- Acosta-Urdapilleta, L.; Paz, G.A.; Rodríguez, A.; Adame, M.; Salgado, D.; Salgado, J.; Montiel-Peña, M.; Medrano-Vega, F.; Villegas-Villarrea, E.C. Pycnoporus sanguineus, un hongo con potencial biotecnológico. In Hacia un Desarrollo Sostenible del Sistema de Producción Consumo de los Hongos Comestibles y Medicinales en Latinoamérica: Avances y Perspectivas en el Siglo XXI; Martínez-Carrera, D., Curvetto, N., Sobal, M., Morales, P., Mora, V.-M., Eds.; Red Latinoamericana de Hongos Comestibles y Medicinales-COLPOS-UNS-CONACYT-AMC-UAEM-UPAEP-IMINAP: Puebla, México, 2010; 648p. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).