Low-Level Laser Irradiation Stimulates RANKL-Induced Osteoclastogenesis via the MAPK Pathway in RAW 264.7 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Low-Level Laser Irradiation In Vitro
2.3. Cell Proliferation Assay
2.4. TRAP Staining
2.5. Resorption Pit Assay
2.6. Western Blot Analysis
2.7. Immunofluorescent Staining
2.8. RNA Isolation and RT-qPCR
2.9. Statistical Analysis
3. Results
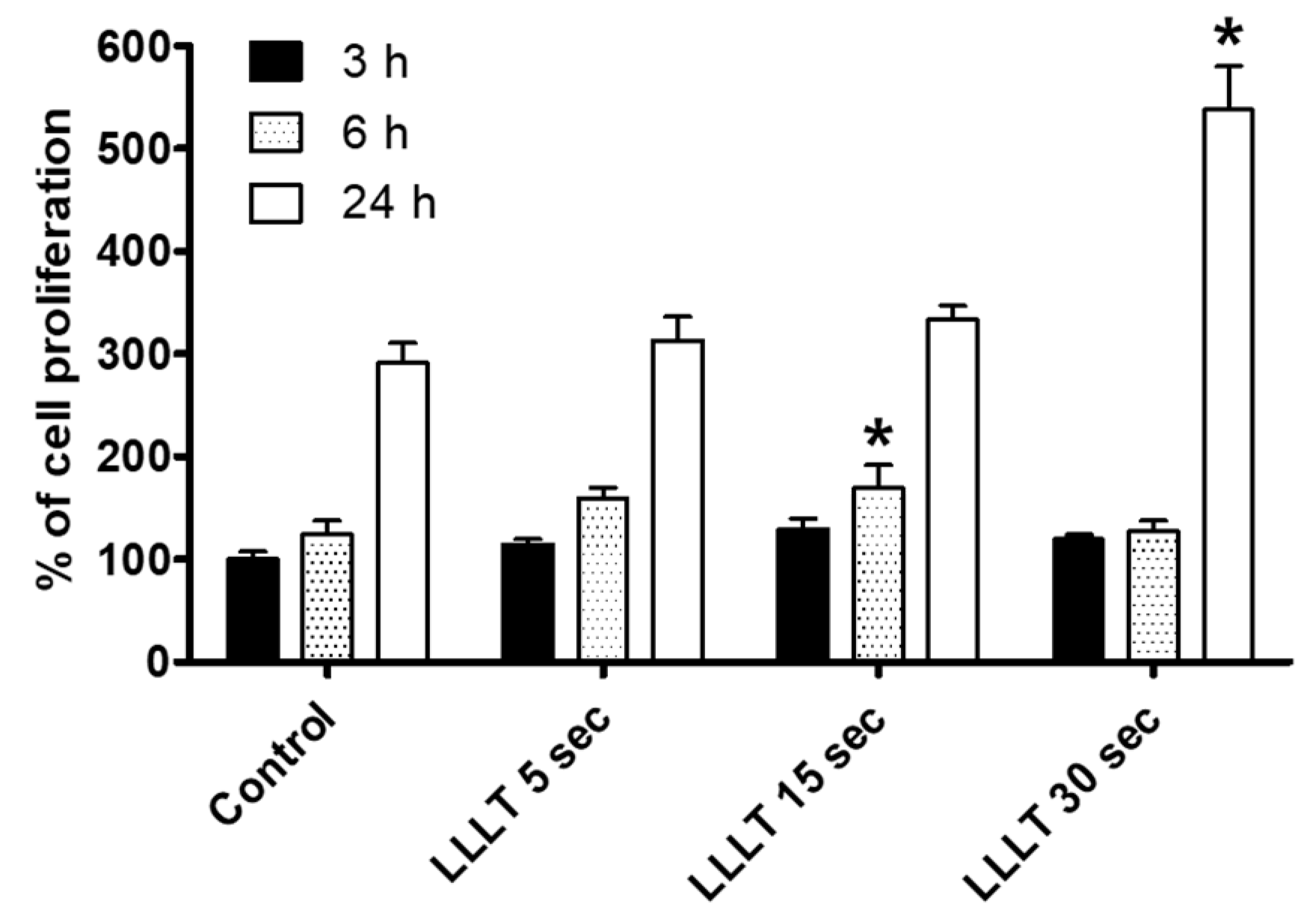
3.1. LLLT Enhances RAW 264.7 Cell Proliferation, Osteoclast Differentiation, and Bone Resorption
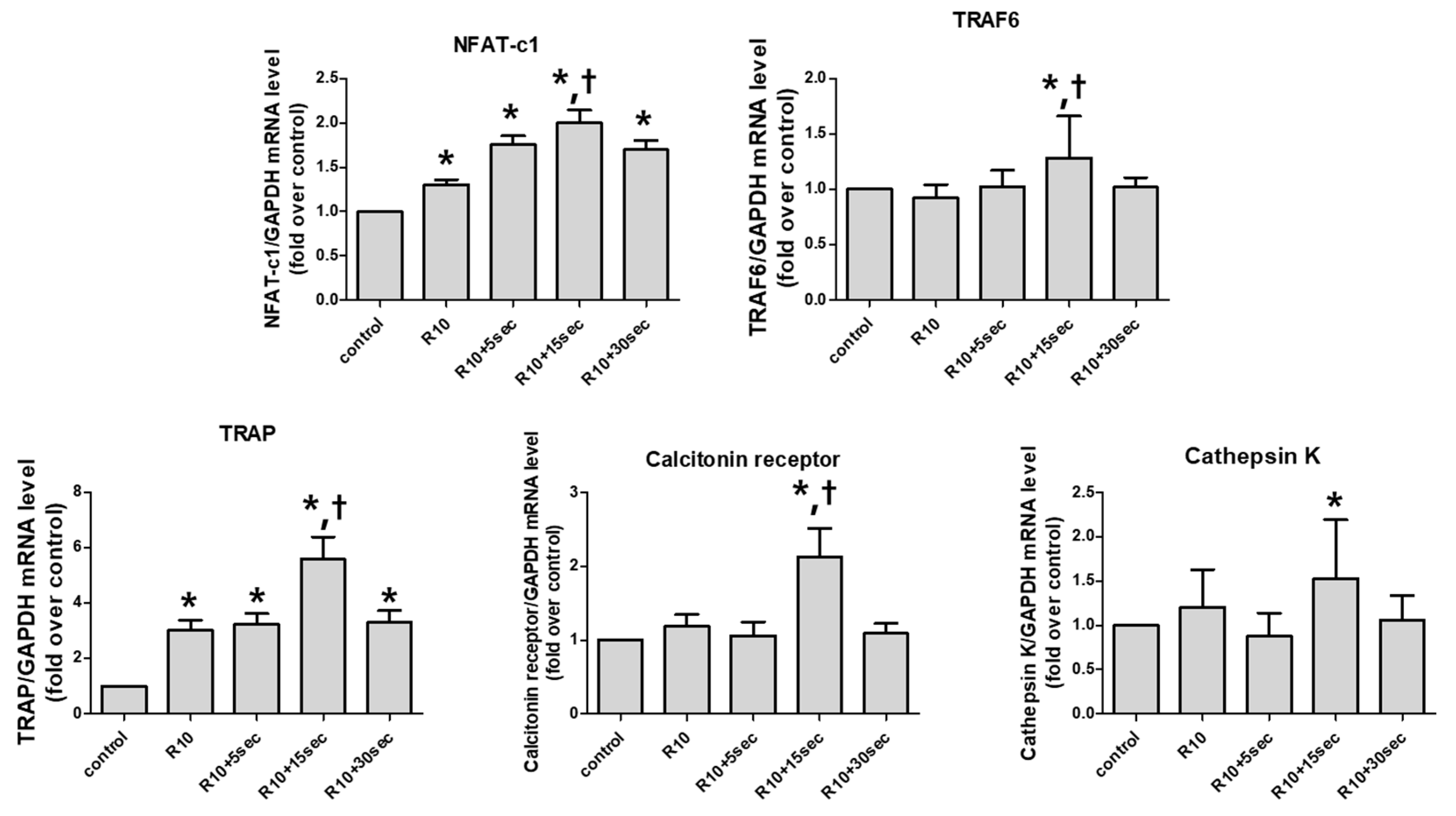
3.2. LLLT Shows a Difference in the Expression of Osteoclast-Forming Genes According to Irradiation Time
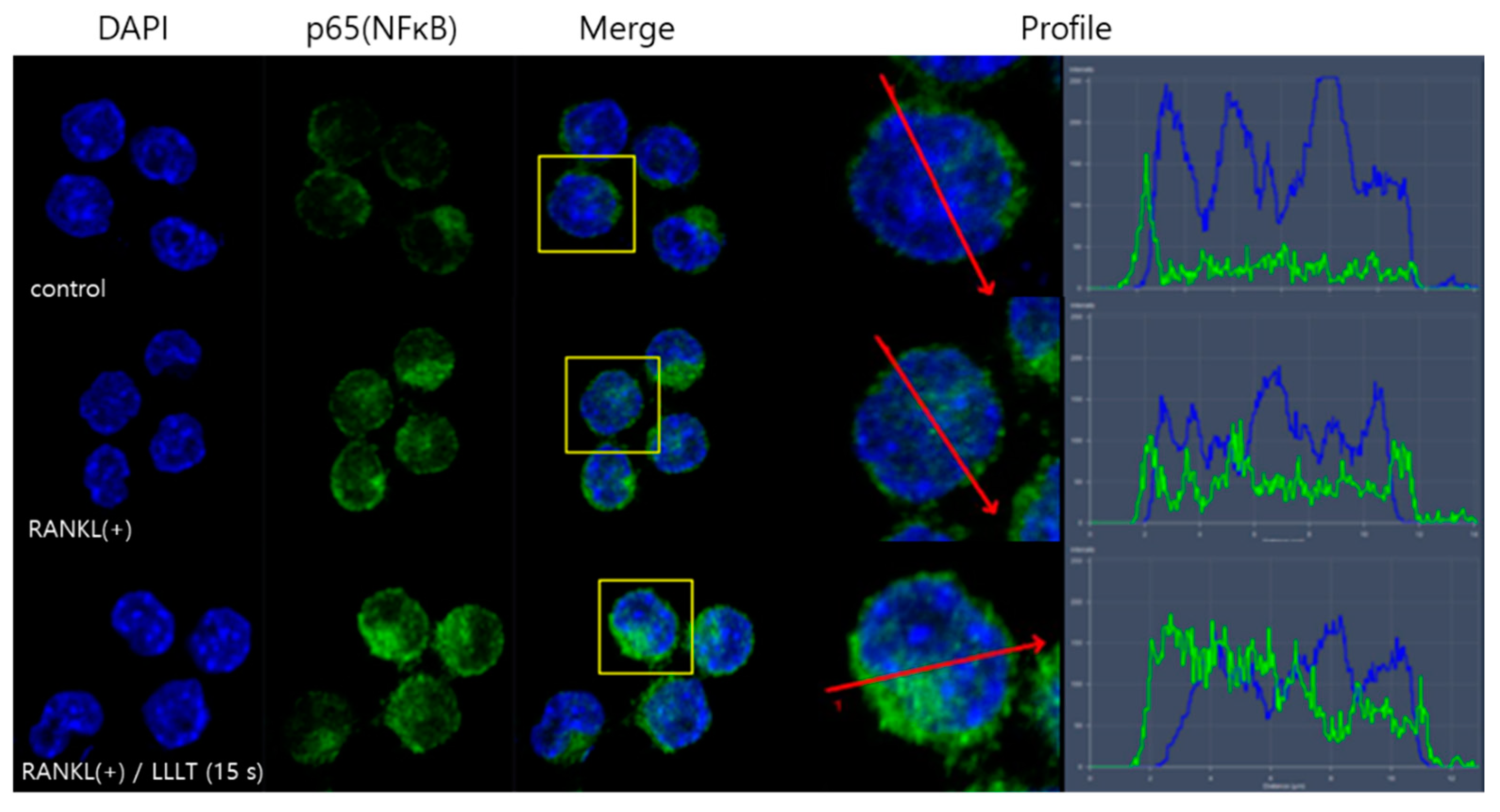
3.3. LLLT Promotes the Transfer of NF-κB from the Cytoplasm to the Nucleus
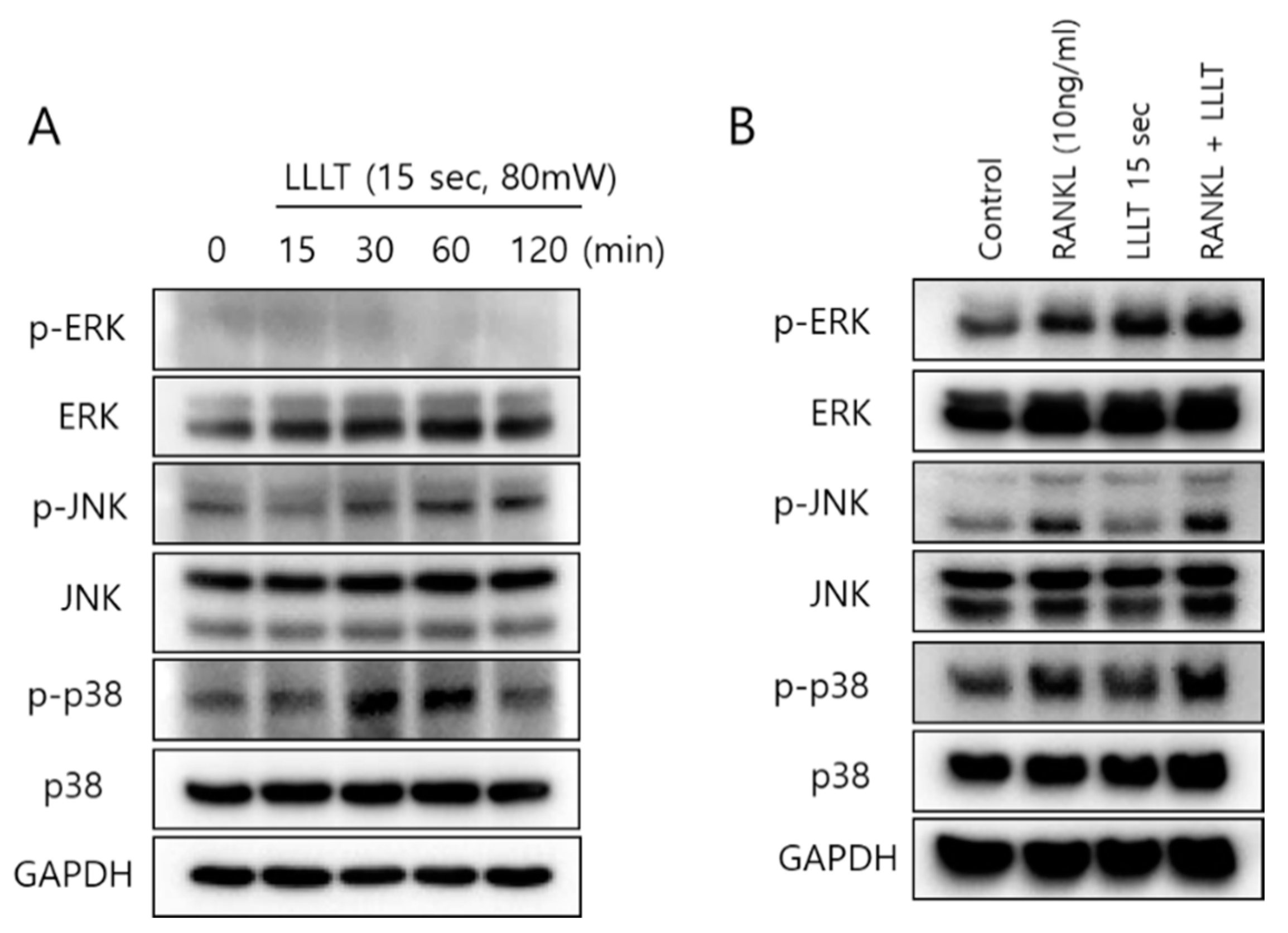
3.4. LLLT Stimulates MAPK Pathways in Osteoclast Differentiation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, H.; Chang, E.-J.; Ryu, J.; Lee, Z.H.; Lee, Y.; Kim, H.-H. Induction of c-Fos and NFATc1 during RANKL-stimulated osteoclast differentiation is mediated by the p38 signaling pathway. Biochem. Biophys. Res. Commun. 2006, 351, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Suda, T.; Takahashi, N.; Udagawa, N.; Jimi, E.; Gillespie, M.T.; Martin, T.J. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr. Rev. 1999, 20, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Teitelbaum, S.L. Bone resorption by osteoclasts. Science 2000, 289, 1504–1508. [Google Scholar] [CrossRef]
- Harris, W.H.; Heaney, R.P. Skeletal renewal and metabolic bone disease. N. Engl. J. Med. 1969, 280, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Soysa, N.S.; Neil, A.; Aoki, K.; Ohya, K. Osteoclast formation and differentiation: An overview. J. Med. Dent. Sci. 2012, 59, 65–74. [Google Scholar] [PubMed]
- Kim, J.H.; Kim, N. Regulation of NFATc1 in osteoclast differentiation. J. Bone Metab. 2014, 21, 233. [Google Scholar] [CrossRef]
- Atkins, G.J.; Kostakis, P.; Pan, B.; Farrugia, A.; Gronthos, S.; Evdokiou, A.; Harrison, K.; Findlay, D.M.; Zannettino, A.C. RANKL expression is related to the differentiation state of human osteoblasts. J. Bone Miner. Res. 2003, 18, 1088–1098. [Google Scholar] [CrossRef]
- Aihara, N.; Yamaguchi, M.; Kasai, K. Low-energy irradiation stimulates formation of osteoclast-like cells via RANK expression in vitro. Lasers Med. Sci. 2006, 21, 24–33. [Google Scholar] [CrossRef]
- Liou, S.-F.; Hsu, J.-H.; Lin, I.-L.; Ho, M.-L.; Hsu, P.-C.; Chen, L.-W.; Chen, J.; Yeh, J.-L. KMUP-1 suppresses RANKL-induced osteoclastogenesis and prevents ovariectomy-induced bone loss: Roles of MAPKs, Akt, NF-κB and calcium/calcineurin/NFATc1 pathways. PLoS ONE 2013, 8, e69468. [Google Scholar] [CrossRef]
- Lee, S.; Woo, K.; Kim, S.; Kim, H.-M.; Kwack, K.; Lee, Z.; Kim, H.-H. The phosphatidylinositol 3-kinase, p38, and extracellular signal-regulated kinase pathways are involved in osteoclast differentiation. Bone 2002, 30, 71–77. [Google Scholar] [CrossRef]
- Lee, Z.H.; Kim, H.-H. Signal transduction by receptor activator of nuclear factor kappa B in osteoclasts. Biochem. Biophys. Res. Commun. 2003, 305, 211–214. [Google Scholar] [CrossRef]
- Armstrong, A.P.; Tometsko, M.E.; Glaccum, M.; Sutherland, C.L.; Cosman, D.; Dougall, W.C. A RANK/TRAF6-dependent signal transduction pathway is essential for osteoclast cytoskeletal organization and resorptive function. J. Biol. Chem. 2002, 277, 44347–44356. [Google Scholar] [CrossRef]
- Koga, T.; Inui, M.; Inoue, K.; Kim, S.; Suematsu, A.; Kobayashi, E.; Iwata, T.; Ohnishi, H.; Matozaki, T.; Kodama, T. Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature 2004, 428, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, H. The role of NFAT in osteoclast formation. Ann. N. Y. Acad. Sci. 2007, 1116, 227–237. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, X.; Liu, Y.; He, A.; Jia, R. NFATc1: Functions in osteoclasts. Int. J. Biochem. Cell Biol. 2010, 42, 576–579. [Google Scholar] [CrossRef] [PubMed]
- Ohshiro, T.; Caldenhead, R.G. Development of low reactive-level laser therapy and its present status. J. Clin. Laser Med. Surg. 1991, 9, 267–275. [Google Scholar] [CrossRef]
- Chung, H.; Dai, T.; Sharma, S.K.; Huang, Y.-Y.; Carroll, J.D.; Hamblin, M.R. The nuts and bolts of low-level laser (light) therapy. Ann. Biomed. Eng. 2012, 40, 516–533. [Google Scholar] [CrossRef]
- Avci, P.; Gupta, A.; Sadasivam, M.; Vecchio, D.; Pam, Z.; Pam, N.; Hamblin, M.R. Low-level laser (light) therapy (LLLT) in skin: Stimulating, healing, restoring. Semin. Cutan. Med. Surg. 2013, 32, 41–52. [Google Scholar]
- Joensen, J. Biophysical and Biological Effects from Infrared Low-Level-Laser-Therapy. Ph.D. Thesis, the University of Bergen, Bergen, Norway, 2013. [Google Scholar]
- Hosseinpour, S.; Fekrazad, R.; Arany, P.R.; Ye, Q. Molecular impacts of photobiomodulation on bone regeneration: A systematic review. Prog. Biophys. Mol. Biol. 2019, 149, 147–159. [Google Scholar] [CrossRef]
- Cotler, H.B.; Chow, R.T.; Hamblin, M.R.; Carroll, J. The use of low level laser therapy (LLLT) for musculoskeletal pain. MOJ Orthop. Rheumatol. 2015, 2, 68. [Google Scholar] [CrossRef]
- Ning, T.; Zhang, K.; Heng, B.C.; Ge, Z. Diverse effects of pulsed electrical stimulation on cells-with a focus on chondrocytes and cartilage regeneration. Eur. Cells Mater. 2019, 38, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Bayram, H.; Kenar, H.; Taşar, F.; Hasırcı, V. Effect of low level laser therapy and zoledronate on the viability and ALP activity of Saos-2 cells. Int. J. Oral Maxillofac. Surg. 2013, 42, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.D.; Milward, M.R.; Cooper, P.R.; Hadis, M.; Palin, W.M. Developments in low level light therapy (LLLT) for dentistry. Dent. Mater. 2014, 30, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Eshghpour, M.; Ahrari, F.; Takallu, M. Is low-level laser therapy effective in the management of pain and swelling after mandibular third molar surgery? J. Oral Maxillofac. Surg. 2016, 74, 1322.e1–1322.e8. [Google Scholar] [CrossRef]
- Hamid, M.A. Low-level laser therapy on postoperative pain after mandibular third molar surgery. Ann. Maxillofac. Surg. 2017, 7, 207. [Google Scholar] [CrossRef] [PubMed]
- Adah, O. The Effects of Genistein, Thymoquinone, Epigallocatechin-3-gallate, 5-Fluorouracil, and Low Level Laser Therapy on FaDu Hypopharyngeal and Laryngeal Carcinoma Cells. Ph.D. Thesis, Sally McDonnell Barksdale Honors College, University of Mississippi, Oxford, MS, USA, 2016. [Google Scholar]
- Jenkins, P.A.; Carroll, J.D. How to report low-level laser therapy (LLLT)/photomedicine dose and beam parameters in clinical and laboratory studies. Photomed. Laser Surg. 2011, 29, 785–787. [Google Scholar] [CrossRef] [PubMed]
- Tam, S.Y.; Tam, V.C.; Ramkumar, S.; Khaw, M.L.; Law, H.K.; Lee, S.W. Review on the cellular mechanisms of low-level laser therapy use in oncology. Front. Oncol. 2020, 10, 1255. [Google Scholar] [CrossRef]
- Mester, E.; Jaszsagi-Nagy, E. Biological effects of laser radiation. Radiobiol. Radiother. 1971, 12, 377–385. [Google Scholar]
- Walsh, L. The current status of low level laser therapy in dentistry, Part 1. Soft tissue applications. Aust. Dent. J. 1997, 42, 247–254. [Google Scholar] [CrossRef]
- Medrado, A.R.; Pugliese, L.S.; Reis, S.R.A.; Andrade, Z.A. Influence of low level laser therapy on wound healing and its biological action upon myofibroblasts. Lasers Surg. Med. 2003, 32, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Yaakobi, T.; Maltz, L.; Oron, U. Promotion of bone repair in the cortical bone of the tibia in rats by low energy laser (He-Ne) irradiation. Calcif. Tissue Int. 1996, 59, 297–300. [Google Scholar] [CrossRef]
- Suzuki, S.S.; Garcez, A.S.; Suzuki, H.; Ervolino, E.; Moon, W.; Ribeiro, M.S. Low-level laser therapy stimulates bone metabolism and inhibits root resorption during tooth movement in a rodent model. J. Biophotonics 2016, 9, 1222–1235. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.-H.; Kim, K.-H.; Choi, N.-R.; Kim, I.-R.; Park, B.-S.; Kim, Y.-D.; Kim, U.-K.; Kim, C.-H. Effect of low-level laser therapy on bisphosphonate-treated osteoblasts. Maxillofac. Plast. Reconstr. Surg. 2016, 38, 1–8. [Google Scholar] [CrossRef]
- Li, C.; Yang, Z.; Li, Z.; Ma, Y.; Zhang, L.; Zheng, C.; Qiu, W.; Wu, X.; Wang, X.; Li, H.; et al. Maslinic acid suppresses osteoclastogenesis and prevents ovariectomy-induced bone loss by regulating RANKL-mediated NF-κB and MAPK signaling pathways. J. Bone Miner. Res. 2011, 26, 644–656. [Google Scholar] [CrossRef] [PubMed]
- Ayukawa, Y.; Yasukawa, E.; Moriyama, Y.; Ogino, Y.; Wada, H.; Atsuta, I.; Koyano, K. Local application of statin promotes bone repair through the suppression of osteoclasts and the enhancement of osteoblasts at bone-healing sites in rats. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2009, 107, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Beck Jr, G.R.; Ha, S.-W.; Camalier, C.E.; Yamaguchi, M.; Li, Y.; Lee, J.-K.; Weitzmann, M.N. Bioactive silica-based nanoparticles stimulate bone-forming osteoblasts, suppress bone-resorbing osteoclasts, and enhance bone mineral density in vivo. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Lemaire, V.; Tobin, F.L.; Greller, L.D.; Cho, C.R.; Suva, L.J. Modeling the interactions between osteoblast and osteoclast activities in bone remodeling. J. Theor. Biol. 2004, 229, 293–309. [Google Scholar] [CrossRef]
- Dörtbudak, O.; Haas, R.; Mailath-Pokorny, G. Biostimulation of bone marrow cells with a diode soft laser. Clin. Oral Implant. Res. 2000, 11, 540–545. [Google Scholar] [CrossRef]
- Coombe, A.; Ho, C.T.; Darendeliler, M.; Hunter, N.; Philips, J.; Chapple, C.; Yum, L. The effects of low level laser irradiation on osteoblastic cells. Clin. Orthod. Res. 2001, 4, 3–14. [Google Scholar] [CrossRef]
- Stein, E.; Koehn, J.; Sutter, W.; Wendtlandt, G.; Wanschitz, F.; Thurnher, D.; Baghestanian, M.; Turhani, D. Initial effects of low-level laser therapy on growth and differentiation of human osteoblast-like cells. Wien. Klin. Wochenschr. 2008, 120, 112–117. [Google Scholar] [CrossRef]
- Soudry, M.; Franquin, J.; Pourreau-Schreider, N.; Martin, P. Effect of a helium-neon laser on cellular growth: An in vitro study of human gingival fibroblasts. J. Biol. Buccale 1988, 16, 129–135. [Google Scholar]
- Schultz, R.J.; Krishnamurthy, S.; Thelmo, W.; Rodriguez, J.E.; Harvey, G. Effects of varying intensities of laser energy on articular cartilage: A preliminary study. Lasers Surg. Med. 1985, 5, 577–588. [Google Scholar] [CrossRef]
- Kana, J.S.; Hutschenreiter, G. Effect of low-power density laser radiation on healing of open skin wounds in rats. Arch. Surg. 1981, 116, 293–296. [Google Scholar] [CrossRef]
- Abergel, R.P.; Meeker, C.A.; Lam, T.S.; Dwyer, R.M.; Lesavoy, M.A.; Uitto, J. Control of connective tissue metabolism by lasers: Recent developments and future prospects. J. Am. Acad. Dermatol. 1984, 11, 1142–1150. [Google Scholar] [CrossRef]
- Pereira, L.O.; Longo, J.P.F.; Azevedo, R.B. Laser irradiation did not increase the proliferation or the differentiation of stem cells from normal and inflamed dental pulp. Arch. Oral Biol. 2012, 57, 1079–1085. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, S.; Niwa, T.; Karakida, T.; Kobayashi, K.; Yamamoto, R.; Chiba, R.; Yamakoshi, Y.; Hosoya, N. Effects of Er: YAG and diode laser irradiation on dental pulp cells and tissues. Int. J. Mol. Sci. 2018, 19, 2429. [Google Scholar] [CrossRef] [PubMed]
- Bubici, C.; Papa, S. JNK signalling in cancer: In need of new, smarter therapeutic targets. Br. J. Pharmacol. 2014, 171, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Kong, N.; Ye, L.; Han, W.; Zhou, J.; Zhang, Q.; He, C.; Pan, H. p38 and JNK MAPK pathways control the balance of apoptosis and autophagy in response to chemotherapeutic agents. Cancer Lett. 2014, 344, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, F.; Nishimura, R.; Matsubara, T.; Tanaka, S.; Inoue, J.-I.; Reddy, S.V.; Hata, K.; Yamashita, K.; Hiraga, T.; Watanabe, T. Critical roles of c-Jun signaling in regulation of NFAT family and RANKL-regulated osteoclast differentiation. J. Clin. Investig. 2004, 114, 475–484. [Google Scholar] [CrossRef]
- Yeon, J.-T.; Choi, S.-W.; Park, K.-I.; Choi, M.-K.; Kim, J.-J.; Youn, B.-S.; Lee, M.-S.; Oh, J.-M. Glutaredoxin2 isoform b (Glrx2b) promotes RANKL-induced osteoclastogenesis through activation of the p38-MAPK signaling pathway. BMB Rep. 2012, 45, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Aleksic, V.; Aoki, A.; Iwasaki, K.; Takasaki, A.A.; Wang, C.-Y.; Abiko, Y.; Ishikawa, I.; Izumi, Y. Low-level Er: YAG laser irradiation enhances osteoblast proliferation through activation of MAPK/ERK. Lasers Med. Sci. 2010, 25, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Sudo, T.; Osada, H.; Saito, T.; Matsumoto, M.; Tsujimoto, M. Involvement of p38 mitogen-activated protein kinase signaling pathway in osteoclastogenesis mediated by receptor activator of NF-κB ligand (RANKL). J. Biol. Chem. 2000, 275, 31155–31161. [Google Scholar]
- Meng, J.; Hong, J.; Zhao, C.; Zhou, C.; Hu, B.; Yang, Y.; Jiang, G.; Li, S.; Shi, Z.; Cai, X. Low-intensity pulsed ultrasound inhibits RANKL-induced osteoclast formation via modulating ERK-c-Fos-NFATc1 signaling cascades. Am. J. Transl. Res. 2018, 10, 2901. [Google Scholar]
- Jonat, C.; Rahmsdorf, H.J.; Park, K.-K.; Cato, A.C.; Gebel, S.; Ponta, H.; Herrlich, P. Antitumor promotion and antiinflammation: Down-modulation of AP-1 (Fos/Jun) activity by glucocorticoid hormone. Cell 1990, 62, 1189–1204. [Google Scholar] [CrossRef]
- Hu, E.; Mueller, E.; Oliviero, S.; Papaioannou, V.; Johnson, R.; Spiegelman, B. Targeted disruption of the c-fos gene demonstrates c-fos-dependent and-independent pathways for gene expression stimulated by growth factors or oncogenes. EMBO J. 1994, 13, 3094–3103. [Google Scholar] [CrossRef]
- Lambertini, E.; Penolazzi, L.; Tavanti, E.; Pocaterra, B.; Schincaglia, G.P.; Torreggiani, E.; Franceschetti, T.; Vecchiattini, R.; Gambari, R.; Piva, R. Modulation of expression of specific transcription factors involved in the bone microenvironment. Minerva Biotec 2008, 20, 69–77. [Google Scholar]
- Basso, F.G.; Oliveira, C.F.; Kurachi, C.; Hebling, J.; de Souza Costa, C.A. Biostimulatory effect of low-level laser therapy on keratinocytes in vitro. Lasers Med. Sci. 2013, 28, 367–374. [Google Scholar] [CrossRef]
- Zhang, L.; Xing, D.; Gao, X.; Wu, S. Low-power laser irradiation promotes cell proliferation by activating PI3K/Akt pathway. J. Cell. Physiol. 2009, 219, 553–562. [Google Scholar] [CrossRef]
- Renno, A.; McDonnell, P.; Parizotto, N.; Laakso, E.-L. The effects of laser irradiation on osteoblast and osteosarcoma cell proliferation and differentiation in vitro. Photomed. Laser Surg. 2007, 25, 275–280. [Google Scholar] [CrossRef]
- Fukuhara, E.; Goto, T.; Matayoshi, T.; Kobayashi, S.; Takahashi, T. Optimal Low-Energy Laser Irradiation Causes Temporal G 2/M Arrest on Rat Calvarial Osteoblasts. Calcif. Tissue Int. 2006, 79, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Arisu, H.D.; Türköz, E.; Bala, O. Effects of Nd: Yag laser irradiation on osteoblast cell cultures. Lasers Med. Sci. 2006, 21, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Bouvet-Gerbettaz, S.; Merigo, E.; Rocca, J.P.; Carle, G.F.; Rochet, N. Effects of low-level laser therapy on proliferation and differentiation of murine bone marrow cells into osteoblasts and osteoclasts. Lasers Surg. Med. 2009, 41, 291–297. [Google Scholar] [CrossRef] [PubMed]


| Groups | LLLT Exposure Time | Total Energy Density | RANKL Treatment (10 ng/mL) |
|---|---|---|---|
| Group 1 | 0 s | - | - |
| Group 2 | 0 s | - | + |
| Group 3 | 5 s | 0.4 J/cm2 | + |
| Group 4 | 15 s | 1.2 J/cm2 | + |
| Group 5 | 30 s | 2.4 J/cm2 | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, J.-M.; Park, B.-S.; Shin, S.-H.; Kim, I.-R. Low-Level Laser Irradiation Stimulates RANKL-Induced Osteoclastogenesis via the MAPK Pathway in RAW 264.7 Cells. Appl. Sci. 2021, 11, 5360. https://doi.org/10.3390/app11125360
Song J-M, Park B-S, Shin S-H, Kim I-R. Low-Level Laser Irradiation Stimulates RANKL-Induced Osteoclastogenesis via the MAPK Pathway in RAW 264.7 Cells. Applied Sciences. 2021; 11(12):5360. https://doi.org/10.3390/app11125360
Chicago/Turabian StyleSong, Jae-Min, Bong-Soo Park, Sang-Hun Shin, and In-Ryoung Kim. 2021. "Low-Level Laser Irradiation Stimulates RANKL-Induced Osteoclastogenesis via the MAPK Pathway in RAW 264.7 Cells" Applied Sciences 11, no. 12: 5360. https://doi.org/10.3390/app11125360
APA StyleSong, J.-M., Park, B.-S., Shin, S.-H., & Kim, I.-R. (2021). Low-Level Laser Irradiation Stimulates RANKL-Induced Osteoclastogenesis via the MAPK Pathway in RAW 264.7 Cells. Applied Sciences, 11(12), 5360. https://doi.org/10.3390/app11125360






