Non-Thermal Atmospheric Pressure Argon-Sourced Plasma Flux Promotes Wound Healing of Burn Wounds and Burn Wounds with Infection in Mice through the Anti-Inflammatory Macrophages
Abstract
1. Introduction
2. Materials and Methods
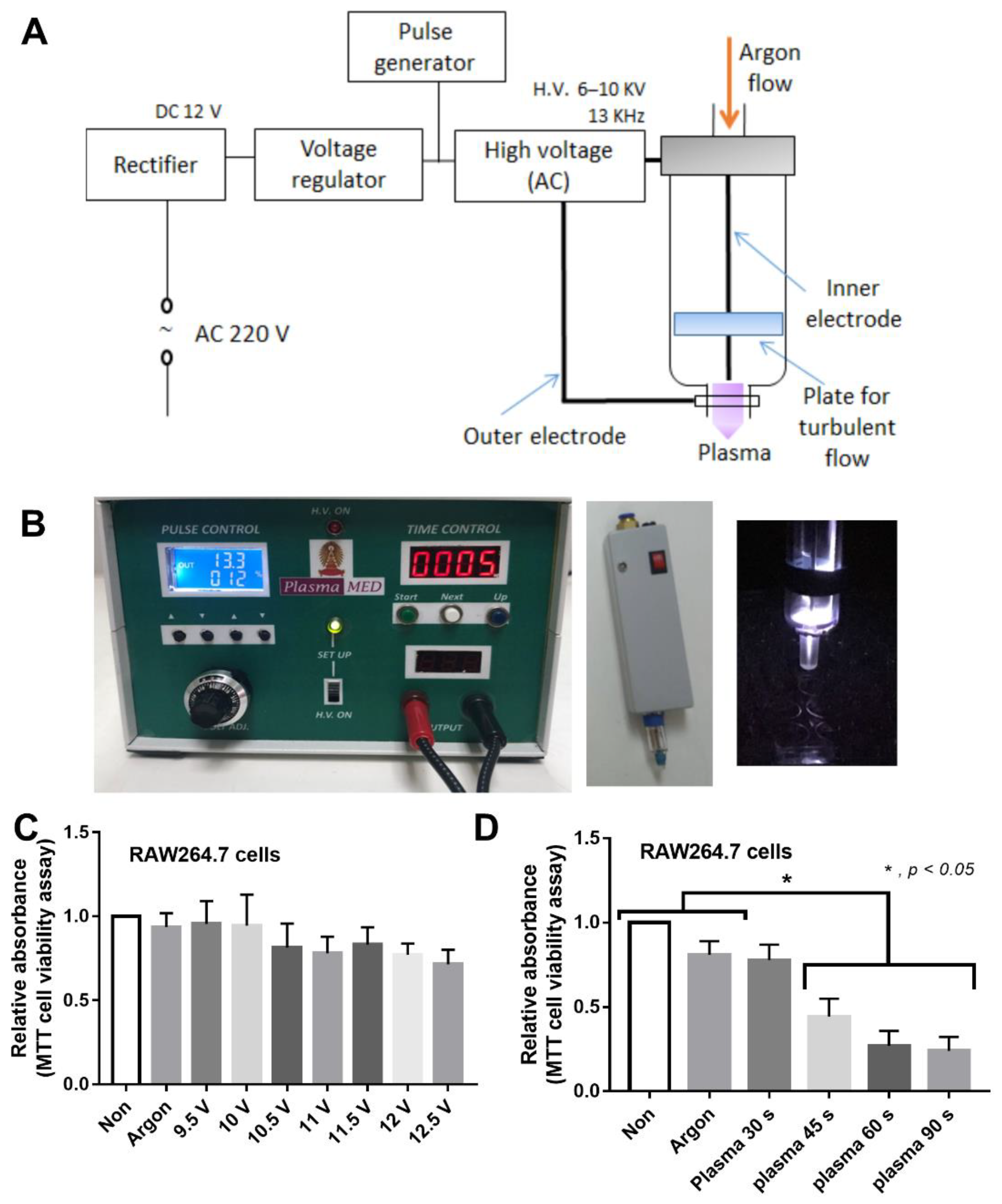
2.1. Animal Model and Plasma Flux Generator
2.2. Wound Injury Score and Gut Permeability Determination
2.3. Mouse Sample Analysis
2.4. The In Vitro Experiments on Macrophages
2.5. The In Vitro Experiments on Fibroblasts
2.6. Statistical Analysis
3. Results
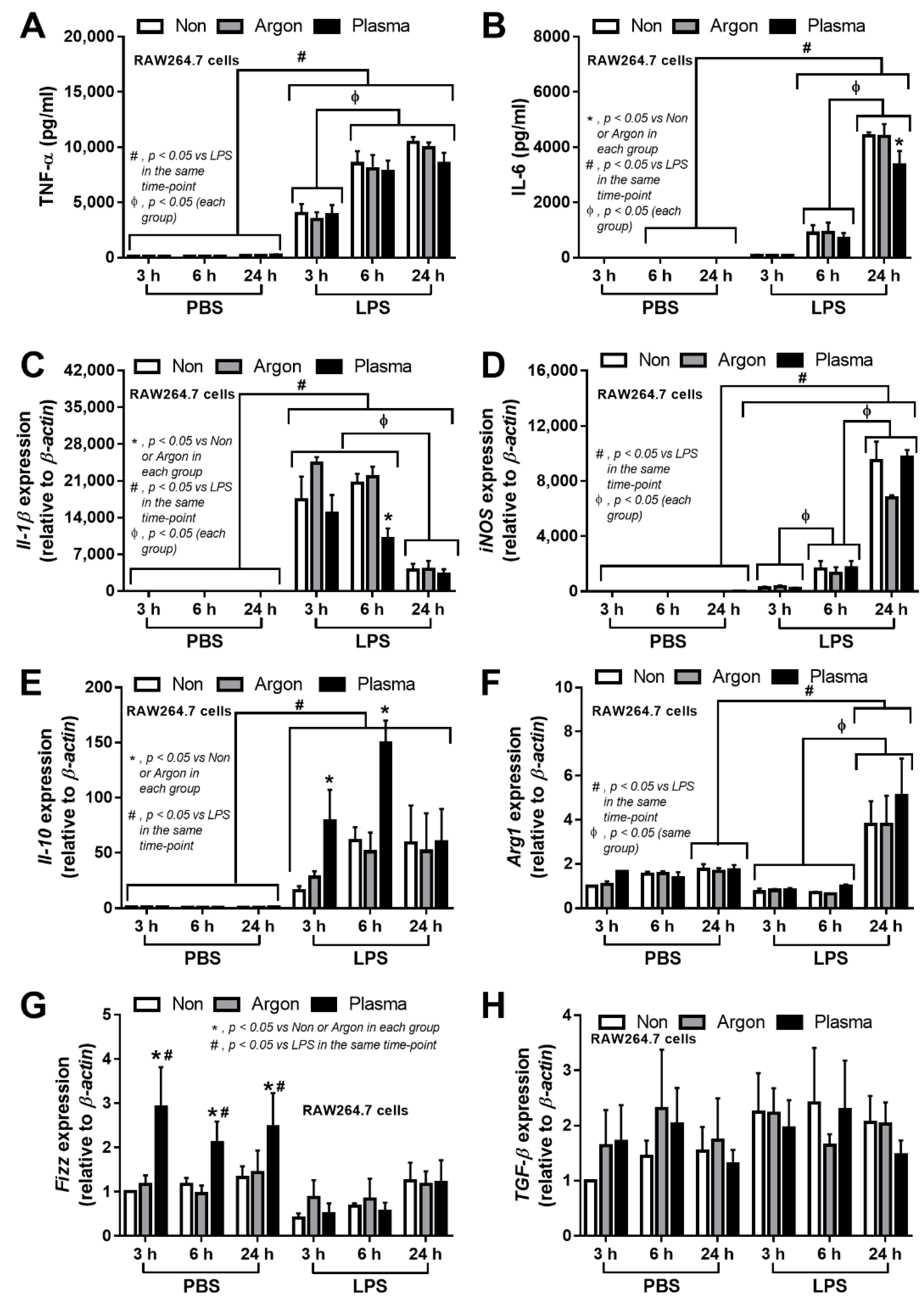
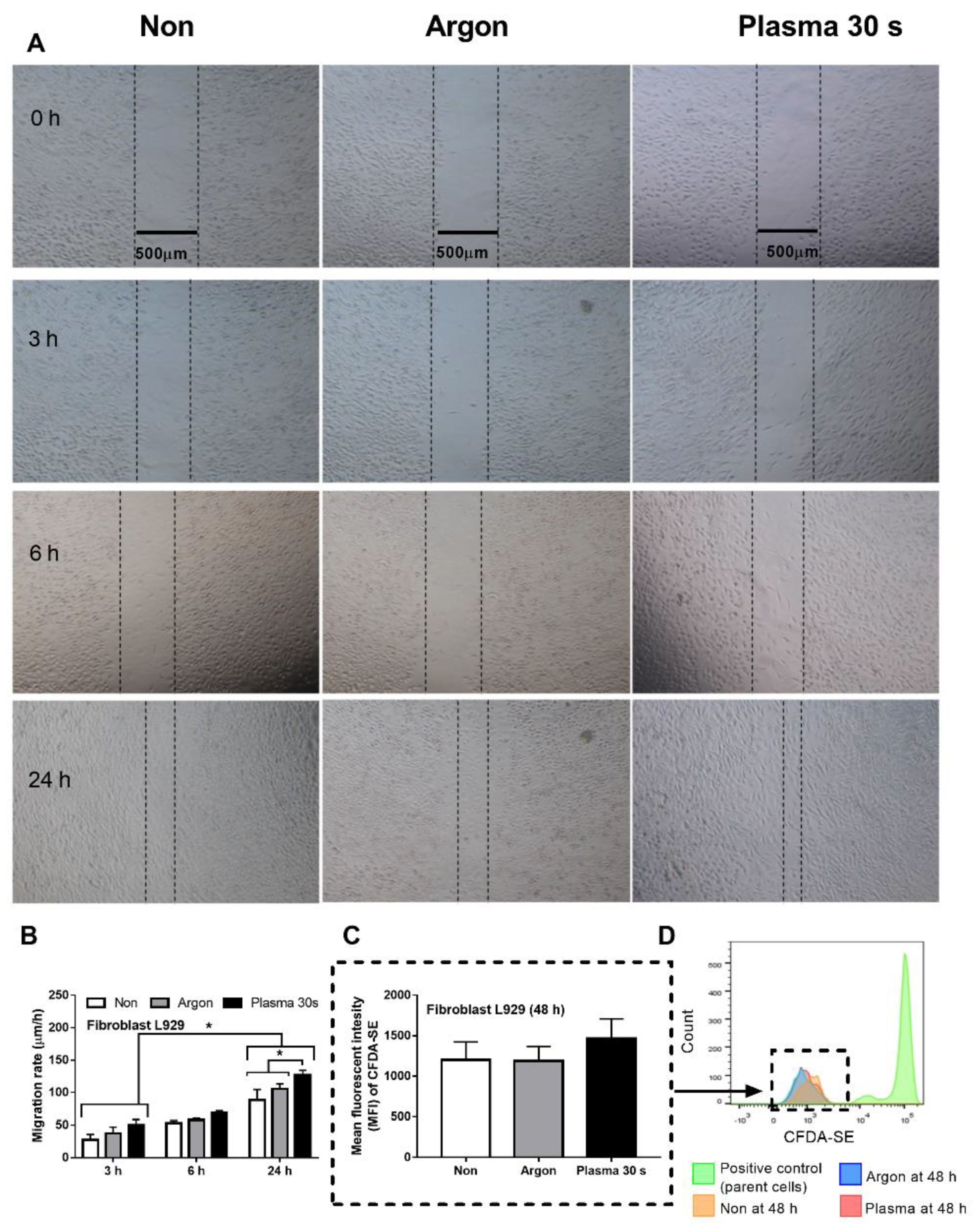
3.1. Non-Thermal Plasma Flux Induced Anti-Inflammatory Macrophages and Fibroblast Migration, but Not Fibroblast Proliferation
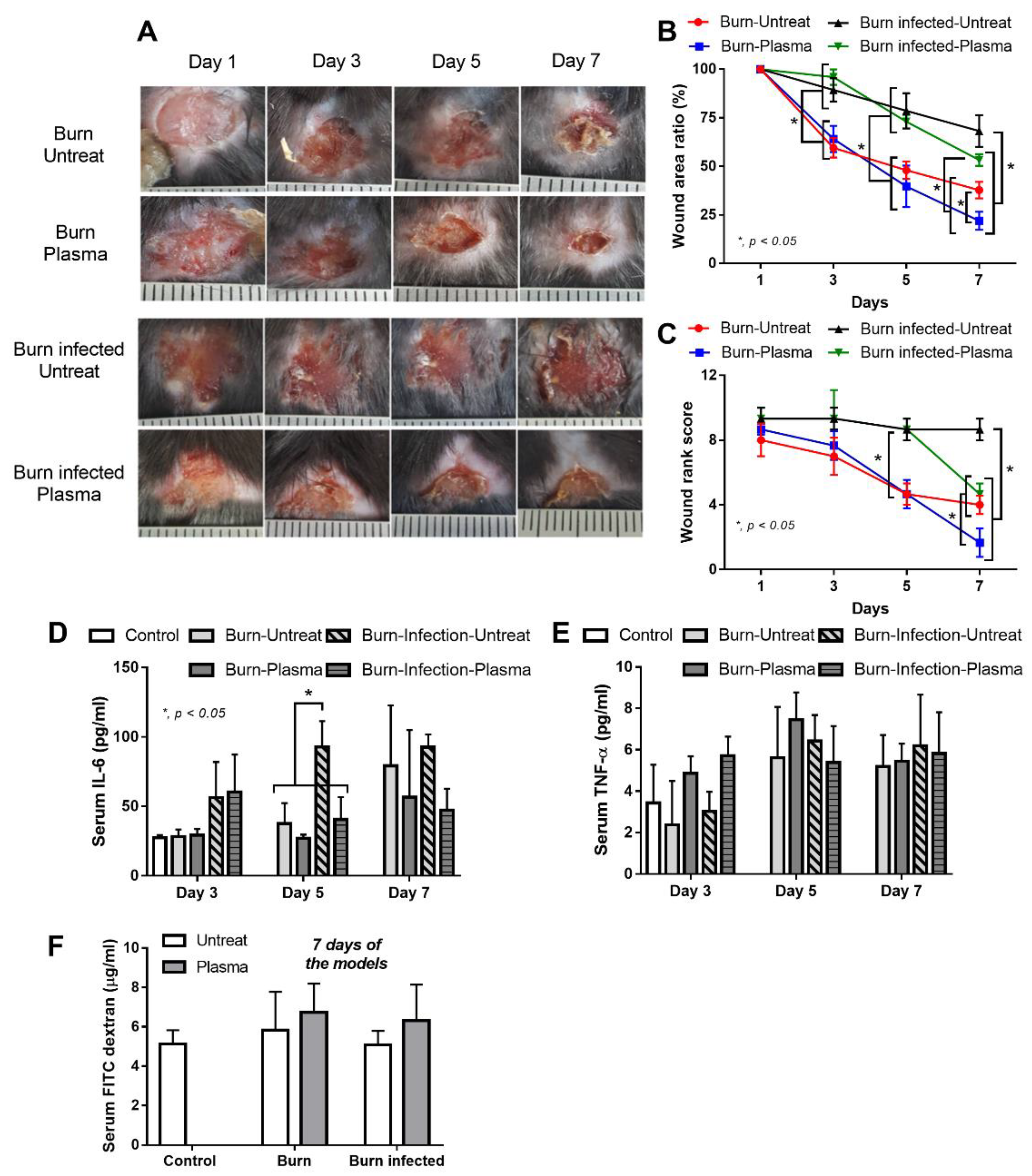
3.2. Plasma Flux Promoted Wound Healing in Burn Wounds of Mice, Regardless of Infection
4. Discussion
4.1. Non-Thermal Plasma Flux Induced Anti-Inflammatory Macrophages and Fibroblast Migration
4.2. Non-Thermal Plasma Flux Promoted Healing Process of Burn Wounds, Regardless of Infection
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Izadjoo, M.; Zack, S.; Kim, H.; Skiba, J. Medical applications of cold atmospheric plasma: State of the science. J. Wound Care 2018, 27, S4–S10. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, T.; Semmler, M.L.; Schafer, M.; Bekeschus, S.; Emmert, S.; Boeckmann, L. plasma medicine: Applications of cold atmospheric pressure plasma in dermatology. Oxidative Med. Cell. Longev. 2019, 2019, 3873928. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.U.; Jawaid, P.; Uchiyama, H.; Kondo, T. Comparison of free radicals formation induced by cold atmospheric plasma, ultrasound, and ionizing radiation. Arch. Biochem. Biophys. 2016, 605, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.J. The broad spectrum of responses to oxidants in proliferating cells: A new paradigm for oxidative stress. IUBMB Life 1999, 48, 41–47. [Google Scholar] [CrossRef]
- Jacobson, M.D. Reactive oxygen species and programmed cell death. Trends Biochem. Sci. 1996, 21, 83–86. [Google Scholar] [CrossRef]
- Fridman, A.A. Plasma Chemistry; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2008; 978p. [Google Scholar]
- Kalghatgi, S.; Kelly, C.M.; Cerchar, E.; Torabi, B.; Alekseev, O.; Fridman, A.; Friedman, G.; Azizkhan-Clifford, J. Effects of non-thermal plasma on mammalian cells. PLoS ONE 2011, 6, e16270. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Hileman, E.O.; Plunkett, W.; Keating, M.J.; Huang, P. Free radical stress in chronic lymphocytic leukemia cells and its role in cellular sensitivity to ROS-generating anticancer agents. Blood 2003, 101, 4098–4104. [Google Scholar] [CrossRef] [PubMed]
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N.T. Reactive oxygen species (ROS) and wound healing: The functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int. Wound J. 2017, 14, 89–96. [Google Scholar] [CrossRef]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef]
- Kvam, E.; Davis, B.; Mondello, F.; Garner, A.L. Nonthermal atmospheric plasma rapidly disinfects multidrug-resistant microbes by inducing cell surface damage. Antimicrob. Agents Chemother. 2012, 56, 2028–2036. [Google Scholar] [CrossRef]
- Schmidt, A.; Bekeschus, S. Redox for repair: Cold physical plasmas and Nrf2 signaling promoting wound healing. Antioxidants 2018, 7, 146. [Google Scholar] [CrossRef]
- Haertel, B.; von Woedtke, T.; Weltmann, K.D.; Lindequist, U. Non-thermal atmospheric-pressure plasma possible application in wound healing. Biomol. Ther. 2014, 22, 477–490. [Google Scholar] [CrossRef]
- Fathollah, S.; Mirpour, S.; Mansouri, P.; Dehpour, A.R.; Ghoranneviss, M.; Rahimi, N.; Safaie Naraghi, Z.; Chalangari, R.; Chalangari, K.M. Investigation on the effects of the atmospheric pressure plasma on wound healing in diabetic rats. Sci. Rep. 2016, 6, 19144. [Google Scholar] [CrossRef]
- Duchesne, C.; Banzet, S.; Lataillade, J.J.; Rousseau, A.; Frescaline, N. Cold atmospheric plasma modulates endothelial nitric oxide synthase signalling and enhances burn wound neovascularisation. J. Pathol. 2019, 249, 368–380. [Google Scholar] [CrossRef]
- Jeschke, M.G.; van Baar, M.E.; Choudhry, M.A.; Chung, K.K.; Gibran, N.S.; Logsetty, S. Burn injury. Nat. Rev. Dis. Primers 2020, 6. [Google Scholar] [CrossRef] [PubMed]
- Smolle, C.; Cambiaso-Daniel, J.; Forbes, A.A.; Wurzer, P.; Hundeshagen, G.; Branski, L.K.; Huss, F.; Kamolz, L.P. Recent trends in burn epidemiology worldwide: A systematic review. Burns 2017, 43, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Lachiewicz, A.M.; Hauck, C.G.; Weber, D.J.; Cairns, B.A.; van Duin, D. Bacterial infections after burn injuries: Impact of multidrug resistance. Clin. Infect. Dis. 2017, 65, 2130–2136. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Vrahas, M.S.; Murray, C.K.; Hamblin, M.R. Ultraviolet C irradiation: An alternative antimicrobial approach to localized infections? Expert Rev. Anti Infect. Ther. 2012, 10, 185–195. [Google Scholar] [CrossRef]
- Nicol, M.J.; Brubaker, T.R.; Honish, B.J., II; Simmons, A.N.; Kazemi, A.; Geissel, M.A.; Whalen, C.T.; Siedlecki, C.A.; Bilen, S.G.; Knecht, S.D.; et al. Antibacterial effects of low-temperature plasma generated by atmospheric-pressure plasma jet are mediated by reactive oxygen species. Sci. Rep. 2020, 10, 3066. [Google Scholar] [CrossRef]
- Schmidt, A.; Bekeschus, S.; Wende, K.; Vollmar, B.; von Woedtke, T. A cold plasma jet accelerates wound healing in a murine model of full-thickness skin wounds. Exp. Dermatol. 2017, 26, 156–162. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Kaushik, N.; Adhikari, M.; Ghimire, B.; Linh, N.N.; Mishra, Y.K.; Lee, S.J.; Choi, E.H. Preventing the solid cancer progression via release of anticancer-cytokines in co-culture with cold plasma-stimulated macrophages. Cancers 2019, 11, 842. [Google Scholar] [CrossRef] [PubMed]
- Dang, C.P.; Leelahavanichkul, A. Over-expression of miR-223 induces M2 macrophage through glycolysis alteration and attenuates LPS-induced sepsis mouse model, the cell-based therapy in sepsis. PLoS ONE 2020, 15, e0236038. [Google Scholar] [CrossRef] [PubMed]
- Taratummarat, S.; Sangphech, N.; Vu, C.T.B.; Palaga, T.; Ondee, T.; Surawut, S.; Sereemaspun, A.; Ritprajak, P.; Leelahavanichkul, A. Gold nanoparticles attenuates bacterial sepsis in cecal ligation and puncture mouse model through the induction of M2 macrophage polarization. BMC Microbiol. 2018, 18, 85. [Google Scholar] [CrossRef]
- Krzyszczyk, P.; Schloss, R.; Palmer, A.; Berthiaume, F. The role of macrophages in acute and chronic wound healing and interventions to promote pro-wound healing phenotypes. Front. Physiol. 2018, 9, 419. [Google Scholar] [CrossRef]
- Cai, E.Z.; Ang, C.H.; Raju, A.; Tan, K.B.; Hing, E.C.; Loo, Y.; Wong, Y.C.; Lee, H.; Lim, J.; Moochhala, S.M.; et al. Creation of consistent burn wounds: A rat model. Arch. Plast. Surg. 2014, 41, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Missiakas, D.; Schneewind, O. Mouse models for infectious diseases caused by Staphylococcus aureus. J. Immunol. Methods 2014, 410, 88–99. [Google Scholar] [CrossRef]
- Lu, X.; Naidis, G.V.; Laroussi, M.; Reuter, S.; Graves, D.B.; Ostrikov, K. Reactive species in non-equilibrium atmospheric-pressure plasmas: Generation, transport, and biological effects. Phys. Rep. 2016, 630, 1–84. [Google Scholar] [CrossRef]
- Jang, S.I.; Mok, J.Y.; Jeon, I.H.; Park, K.H.; Nguyen, T.T.; Park, J.S.; Hwang, H.M.; Song, M.S.; Lee, D.; Chai, K.Y. Effect of electrospun non-woven mats of dibutyryl chitin/poly(lactic acid) blends on wound healing in hairless mice. Molecules 2012, 17, 2992–3007. [Google Scholar] [CrossRef]
- Baron, P.; Traber, L.D.; Traber, D.L.; Nguyen, T.; Hollyoak, M.; Heggers, J.P.; Herndon, D.N. Gut failure and translocation following burn and sepsis. J. Surg. Res. 1994, 57, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Earley, Z.M.; Akhtar, S.; Green, S.J.; Naqib, A.; Khan, O.; Cannon, A.R.; Hammer, A.M.; Morris, N.L.; Li, X.; Eberhardt, J.M.; et al. Burn injury alters the intestinal microbiome and increases gut permeability and bacterial translocation. PLoS ONE 2015, 10, e0129996. [Google Scholar] [CrossRef]
- Panpetch, W.; Sawaswong, V.; Chanchaem, P.; Ondee, T.; Dang, C.P.; Payungporn, S.; Leelahavanichkul, A. Candida administration worsens cecal ligation and puncture-induced sepsis in obese mice through gut dysbiosis enhanced systemic inflammation, impact of pathogen-associated molecules from gut translocation and saturated fatty acid. Front. Immunol. 2020, 11, 561652. [Google Scholar] [CrossRef]
- Ondee, T.; Gillen, J.; Visitchanakun, P.; Somparn, P.; Issara-Amphorn, J.; Dang Phi, C.; Chancharoenthana, W.; Gurusamy, D.; Nita-Lazar, A.; Leelahavanichkul, A. Lipocalin-2 (Lcn-2) attenuates polymicrobial sepsis with LPS preconditioning (LPS Tolerance) in FcGRIIb deficient lupus mice. Cells 2019, 8, 1064. [Google Scholar] [CrossRef] [PubMed]
- Visitchanakun, P.; Saisorn, W.; Wongphoom, J.; Chatthanathon, P.; Somboonna, N.; Svasti, S.; Fucharoen, S.; Leelahavanichkul, A. Gut leakage enhances sepsis susceptibility in iron-overloaded beta-thalassemia mice through macrophage hyperinflammatory responses. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, G966–G979. [Google Scholar] [CrossRef]
- Issara-Amphorn, J.; Chancharoenthana, W.; Visitchanakun, P.; Leelahavanichkul, A. Syk Inhibitor attenuates polymicrobial sepsis in FcgRIIb-deficient lupus mouse model, the impact of lupus characteristics in sepsis. J. Innate Immun. 2020, 12, 461–479. [Google Scholar] [CrossRef]
- Panpetch, W.; Kullapanich, C.; Dang, C.P.; Visitchanakun, P.; Saisorn, W.; Wongphoom, J.; Wannigama, D.L.; Thim-Uam, A.; Patarakul, K.; Somboonna, N.; et al. Candida administration worsens uremia-induced gut leakage in bilateral nephrectomy mice, an impact of gut fungi and organismal molecules in uremia. Msystems 2021, 6. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, S.; Fujiwara, T.; Matsuzaki, S.; Shingaki, K.; Taniguchi, M.; Miyata, S.; Tohyama, M.; Sakai, Y.; Yano, K.; Hosokawa, K.; et al. bFGF regulates PI3-kinase-Rac1-JNK pathway and promotes fibroblast migration in wound healing. PLoS ONE 2010, 5, e12228. [Google Scholar] [CrossRef] [PubMed]
- Brun, P.; Pathak, S.; Castagliuolo, I.; Palu, G.; Brun, P.; Zuin, M.; Cavazzana, R.; Martines, E. Helium generated cold plasma finely regulates activation of human fibroblast-like primary cells. PLoS ONE 2014, 9, e104397. [Google Scholar] [CrossRef]
- Jaroonwitchawan, T.; Visitchanakun, P.; Dang, P.C.; Ritprajak, P.; Palaga, T.; Leelahavanichkul, A. Dysregulation of lipid metabolism in macrophages is responsible for severe endotoxin tolerance in FcgRIIB-deficient lupus mice. Front. Immunol. 2020, 11, 959. [Google Scholar] [CrossRef] [PubMed]
- Udompornpitak, K.; Bhunyakarnjanarat, T.; Charoensappakit, A.; Dang, C.P.; Saisorn, W.; Leelahavanichkul, A. Lipopolysaccharide-enhanced responses against aryl hydrocarbon receptor in fcgriib-deficient macrophages, a profound impact of an environmental toxin on a lupus-like mouse model. Int. J. Mol. Sci. 2021, 22, 4199. [Google Scholar] [CrossRef]
- Issara-Amphorn, J.; Surawut, S.; Worasilchai, N.; Thim-Uam, A.; Finkelman, M.; Chindamporn, A.; Palaga, T.; Hirankarn, N.; Pisitkun, P.; Leelahavanichkul, A. The synergy of endotoxin and (1-->3)-beta-D-glucan, from gut translocation, worsens sepsis severity in a lupus model of fc gamma receptor IIb-deficient mice. J. Innate Immun. 2018, 10, 189–201. [Google Scholar] [CrossRef]
- Church, D.; Elsayed, S.; Reid, O.; Winston, B.; Lindsay, R. Burn wound infections. Clin. Microbiol. Rev. 2006, 19, 403–434. [Google Scholar] [CrossRef] [PubMed]
- Herndon, D.N.; Zeigler, S.T. Bacterial translocation after thermal injury. Crit. Care Med. 1993, 21, S50–S54. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Lim, D.; Kim, D.; Jeon, J.; Oh, T. In vitro antibacterial effects of non-thermal atmospheric plasma irradiation on Staphylococcus pseudintermedius and Pseudomonas aeruginosa. Pol. J. Vet. Sci. 2020, 23, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Bainbridge, P. Wound healing and the role of fibroblasts. J. Wound Care 2013, 22, 407–408, 410–412. [Google Scholar] [CrossRef]
- Sung, S.J.; Huh, J.B.; Yun, M.J.; Chang, B.M.; Jeong, C.M.; Jeon, Y.C. Sterilization effect of atmospheric pressure non-thermal air plasma on dental instruments. J. Adv. Prosthodont. 2013, 5, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Kalghatgi, S.U.; Fridman, G.; Cooper, M.; Nagaraj, G.; Peddinghaus, M.; Balasubramanian, M.; Vasilets, V.N.; Gutsol, A.F.; Fridman, A.; Friedman, G. Mechanism of blood coagulation by nonthermal atmospheric pressure dielectric barrier discharge plasma. IEEE Trans. Plasma Sci. 2007, 35, 1559–1566. [Google Scholar] [CrossRef]
- Gilmore, B.F.; Flynn, P.B.; O’Brien, S.; Hickok, N.; Freeman, T.; Bourke, P. Cold plasmas for biofilm control: opportunities and challenges. Trends Biotechnol. 2018, 36, 627–638. [Google Scholar] [CrossRef]
- Kubinova, S.; Zaviskova, K.; Uherkova, L.; Zablotskii, V.; Churpita, O.; Lunov, O.; Dejneka, A. Non-thermal air plasma promotes the healing of acute skin wounds in rats. Sci. Rep. 2017, 7, 45183. [Google Scholar] [CrossRef]
- Wolff, C.M.; Kolb, J.F.; Weltmann, K.D.; von Woedtke, T.; Bekeschus, S. Combination treatment with cold physical plasma and pulsed electric fields augments ROS production and cytotoxicity in lymphoma. Cancers 2020, 12, 845. [Google Scholar] [CrossRef]
- Boeckmann, L.; Schafer, M.; Bernhardt, T.; Semmler, M.L.; Jung, O.; Ojak, G.; Fischer, T.; Peters, K.; Nebe, B.; Muller-Hilke, B.; et al. Cold atmospheric pressure plasma in wound healing and cancer treatment. Appl. Sci. 2020, 10, 6898. [Google Scholar] [CrossRef]
- Smolkova, B.; Frtus, A.; Uzhytchak, M.; Lunova, M.; Kubinova, S.; Dejneka, A.; Lunov, O. Critical analysis of non-thermal plasma-driven modulation of immune cells from clinical perspective. Int. J. Mol. Sci. 2020, 21, 6226. [Google Scholar] [CrossRef]
- Gloire, G.; Legrand-Poels, S.; Piette, J. NF-kappaB activation by reactive oxygen species: Fifteen years later. Biochem. Pharmacol. 2006, 72, 1493–1505. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.J.; Liu, Z.G. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Levrand, S.; Pesse, B.; Feihl, F.; Waeber, B.; Pacher, P.; Rolli, J.; Schaller, M.D.; Liaudet, L. Peroxynitrite is a potent inhibitor of NF-{kappa}B activation triggered by inflammatory stimuli in cardiac and endothelial cell lines. J. Biol. Chem. 2005, 280, 34878–34887. [Google Scholar] [CrossRef] [PubMed]
- Ogino, T.; Hosako, M.; Hiramatsu, K.; Omori, M.; Ozaki, M.; Okada, S. Oxidative modification of IkappaB by monochloramine inhibits tumor necrosis factor alpha-induced NF-kappaB activation. Biochim. Biophys. Acta 2005, 1746, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Sindrilaru, A.; Peters, T.; Wieschalka, S.; Baican, C.; Baican, A.; Peter, H.; Hainzl, A.; Schatz, S.; Qi, Y.; Schlecht, A.; et al. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J. Clin. Investig. 2011, 121, 985–997. [Google Scholar] [CrossRef]
- Bekeschus, S.; Scherwietes, L.; Freund, E.; Liedtke, K.R.; Hackbarth, C.; von Woedtke, T.; Partecke, L.I. Plasma-treated medium tunes the inflammatory profile in murine bone marrow-derived macrophages. Clin. Plasma Med. 2018, 11, 1–9. [Google Scholar] [CrossRef]
- Jonkers, J.; Van De Sande, M.; Sola, A.; Gamero, A.; Van Der Mullen, J. On the differences between ionizing helium and argon plasmas at atmospheric pressure. Plasma Sources Sci. Technol. 2002, 12, 30. [Google Scholar] [CrossRef]
- Addis, R.; Cruciani, S.; Santaniello, S.; Bellu, E.; Sarais, G.; Ventura, C.; Maioli, M.; Pintore, G. Fibroblast proliferation and migration in wound healing by phytochemicals: Evidence for a novel synergic outcome. Int. J. Med. Sci. 2020, 17, 1030–1042. [Google Scholar] [CrossRef]
- Weltmann, K.D.; von Woedtke, T. Plasma medicine-current state of research and medical application. Plasma Phys. Contr. Fusion 2017, 59. [Google Scholar] [CrossRef]
- Jeong, W.S.; Kwon, J.S.; Choi, E.H.; Kim, K.M. The effects of non-thermal atmospheric pressure plasma treated titanium surface on behaviors of oral soft tissue cells. Sci. Rep. 2018, 8, 15963. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Song, Y.S.; Lee, H.J.; Hong, J.W.; Kim, G.C. Inhibition of inflammatory reactions in 2,4-Dinitrochlorobenzene induced Nc/Nga atopic dermatitis mice by non-thermal plasma. Sci. Rep. 2016, 6, 27376. [Google Scholar] [CrossRef]
- Arturson, G. Pathophysiology of the burn wound. Ann. Chir. Gynaecol. 1980, 69, 178–190. [Google Scholar]
- Gomez, R.; Murray, C.K.; Hospenthal, D.R.; Cancio, L.C.; Renz, E.M.; Holcomb, J.B.; Wade, C.E.; Wolf, S.E. Causes of mortality by autopsy findings of combat casualties and civilian patients admitted to a burn unit. J. Am. Coll. Surg. 2009, 208, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Ballard, J.; Edelman, L.; Saffle, J.; Sheridan, R.; Kagan, R.; Bracco, D.; Cancio, L.; Cairns, B.; Baker, R.; Fillari, P.; et al. Positive fungal cultures in burn patients: A multicenter review. J. Burn Care Res. 2008, 29, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Abdullahi, A.; Amini-Nik, S.; Jeschke, M.G. Animal models in burn research. Cell Mol. Life Sci. 2014, 71, 3241–3255. [Google Scholar] [CrossRef]
- Van de Goot, F.; Krijnen, P.A.J.; Begieneman, M.P.V.; Uhich, M.M.W.; Middelkoop, E.; Niessen, H.W.M. Acute inflammation is persistent locally in burn wounds: A pivotal role for complement and C-reactive protein. J. Burn. Care Res. 2009, 30, 274–280. [Google Scholar] [CrossRef]


| Primers | ||
|---|---|---|
| β-actin | Forward | 5′-CGGTTCCGATGCCCTGAGGCTCTT-3′ |
| Reward | 5′-CGTCACACTTCATGATGGAATTGA-3′ | |
| Inducible nitric oxide synthase (iNOS) | Forward | 5′-CCCTTCCGAAGTTTCTGGCAGCAGC-3′ |
| Reward | 5′-GGCTGTCAGAGCCTCGTGGCTTTG-3′ | |
| Arginase 1 (Arg-1) | Forward | 5′-CAGAAGAATG GAAGAGTCAG-3′ |
| Reward | 5′-CAGATATGCA GGGA GTCACC-3′ | |
| Found in inflammatory zone (Fizz) | Forward | 5′-GCCAGGTCCTGGAACCTTTC-3′ |
| Reward | 5′-GGAGCAGGGAGATGCAGATGAG-3′ | |
| Interleukin-1β (IL-1β) | Forward | 5′-GAAATGCCACCTTTTGACAGTG-3′ |
| Reward | 5′-TGGATGCTCTCATCAGGACAG-3′ | |
| Interleukin-10 (IL-10) | Forward | 5′-GCTCTTACTGACTGGCATGAG-3′ |
| Reward | 5′-CGCAGCTCTAGGAGCATGTG-3′ | |
| Transforming growth factor-β (TGF-β) | Forward | 5′-CAGAGCTGCGCTTGCAGAG-3′ |
| Reward | 5′-GTCAGCAGCCGGTTACCAAG-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dang, C.P.; Weawseetong, S.; Charoensappakit, A.; Sae-Khow, K.; Thong-Aram, D.; Leelahavanichkul, A. Non-Thermal Atmospheric Pressure Argon-Sourced Plasma Flux Promotes Wound Healing of Burn Wounds and Burn Wounds with Infection in Mice through the Anti-Inflammatory Macrophages. Appl. Sci. 2021, 11, 5343. https://doi.org/10.3390/app11125343
Dang CP, Weawseetong S, Charoensappakit A, Sae-Khow K, Thong-Aram D, Leelahavanichkul A. Non-Thermal Atmospheric Pressure Argon-Sourced Plasma Flux Promotes Wound Healing of Burn Wounds and Burn Wounds with Infection in Mice through the Anti-Inflammatory Macrophages. Applied Sciences. 2021; 11(12):5343. https://doi.org/10.3390/app11125343
Chicago/Turabian StyleDang, Cong Phi, Sirapong Weawseetong, Awirut Charoensappakit, Kritsanawan Sae-Khow, Decho Thong-Aram, and Asada Leelahavanichkul. 2021. "Non-Thermal Atmospheric Pressure Argon-Sourced Plasma Flux Promotes Wound Healing of Burn Wounds and Burn Wounds with Infection in Mice through the Anti-Inflammatory Macrophages" Applied Sciences 11, no. 12: 5343. https://doi.org/10.3390/app11125343
APA StyleDang, C. P., Weawseetong, S., Charoensappakit, A., Sae-Khow, K., Thong-Aram, D., & Leelahavanichkul, A. (2021). Non-Thermal Atmospheric Pressure Argon-Sourced Plasma Flux Promotes Wound Healing of Burn Wounds and Burn Wounds with Infection in Mice through the Anti-Inflammatory Macrophages. Applied Sciences, 11(12), 5343. https://doi.org/10.3390/app11125343







