Abstract
(1) Background: Intrinsic characteristics of the implant surface and the possible presence of endotoxins may affect the bone–implant interface and cause an inflammatory response. This study aims to evaluate the possible inflammatory response induced in vitro in macrophages in contact with five different commercially available dental implants. (2) Methods: one zirconia implant NobelPearl® (Nobel Biocare) and four titanium implants, Syra® (Sweden & Martina), Prama® (Sweden & Martina), 3iT3® (Biomet 3i) and Shard® (Mech & Human), were evaluated. After 4 h of contact of murine macrophage cells J774a.1 with the implants, the total RNA was extracted, transcribed to cDNA and the gene expression of the macrophages was evaluated by quantitative PCR (qPCR) in relation to the following genes: GAPDH, YWHAZ, IL1β, IL6, TNFα, NOS2, MMP-9, MMP-8 and TIMP3. The results were statistically analyzed and compared with negative controls. (3) Results: No implant triggered a significant inflammatory response in macrophages, although 3iT3 exhibited a slight pro-inflammatory effect compared to other samples. (4) Conclusions: All the samples showed optimal outcomes without any inflammatory stimulus on the examined macrophagic cells.
1. Introduction
Following the spread of the use of titanium implants and patients’ high expectations, dental implants improved with new surface treatments are continuously delivered on the market, providing implants with different chemical compositions and topographies of their surface. Such intrinsic characteristics of the implant surface (chemical composition, wettability, topography) and the possible presence of endotoxins or other contaminants on their surface, as a result of environmental influence or manufacturing and packaging processes, affect the bone–implant interface and cause an inflammatory response, which has been traditionally defined by the evaluation of pyrogenicity. This feature has always been taken into consideration in the qualification of medical devices to prevent pyrogenic effects, and in recent years it has become particularly relevant for devices in contact with bone. In fact, there is evidence of the stimulation of osteoclastogenesis, and therefore of potential bone resorption induced by cytokines developed in the inflammatory response at the tissue–device interface [1,2].
Recent work from Albrektsson et al. [3] has described the dynamic phenomenon of osseointegration of titanium dental implants as an immune-mediated foreign body reaction (FBR), that is concomitant to the wound-healing process consequent to surgical trauma. The authors thus introduced the concept of foreign body equilibrium (FBE) applied to osseointegration of dental implants [4]. This notion relies on the fact that dental implants, although biocompatible, are not completely inert materials. Indeed, the concept of biocompatibility should not be translated into that of biological inertia. In fact, once dental implants are inserted, a cascade of biological phenomena starts at the host–implant interface, and the crosstalk between cells of the immune and the musculoskeletal systems plays a pivotal role in the process of osseointegration [2].
However, while the effect of dental implants in contact with cells such as osteoblasts and fibroblasts has been widely investigated [5,6], their influence on cells of the immune system has been neglected [7]. In particular, monocyte/macrophages play a central role in bone homeostasis; they can have a direct osteolytic role, and they are fundamental in bone/biomaterial integration around dental implants and in the bone loss pathway during FBR [4,7,8,9].
Once the implant is inserted, after plasma proteins adhesion to the implant surface and a considerable recruitment of neutrophils in the implantation site, the first cells to come into contact with the biomaterial are those derived from the monocyte/macrophage lineage [10]. The last are considered mainly responsible for the host response toward the foreign biomaterial, based on their behavior during the chronic inflammation stage of implantation.
The lymphocyte Th1 response generally promotes the polarization of circulant monocytes recruited in the peri-implant site, into “classically activated” or “pro-inflammatory” M1 macrophages (Miron et al., 2018). This type of macrophage releases proinflammatory cytokines (such as TNFα, IL-6 e IL-1β), which promote osteoclastogenesis and bone resorption, but also secrete the pro-osteogenic oncostatin-M, in addition to help in debris and dead cells cleaning [11].
On the other hand, Th2 response promotes resident macrophage polarization into “alternative activated” or “wound-healing” M2 macrophages, which release a set of anti-inflammatory/regulatory molecules, such as IL-4, IL-10 and TGF-β, that mitigates the host inflammatory reaction [12].
In physiological conditions, both macrophagic phenotypes are essential for a proper wound repair, and if a balance among them is obtained, the implantation process will result in successful tissue integration. However, possible cellular shifts at the implant interface are influenced by several factors (e.g., features of the tissues, local microenvironment, physio-pathological conditions of the patient). In the presence of M1 chronic activation, the frustrated immune response might result in a fibrous encapsulation of the implant [13]. According to Pajarinen et al. [14], the promotion of M2 macrophage polarization, pharmacologically or by biomaterial solutions in peri-implant tissues, provided with the fight of M1 polarizing factors (e.g., bacterial biofilm formation), and might restrict wear-particle-induced inflammation and osteolysis.
A recent clinical study investigated the inflammatory cell infiltrate at implants affected by peri-implantitis [15]. They found higher expression of M1 pro-inflammatory macrophages and subsequently an increased M1/M2 ratio, particularly at advanced peri-implantitis cases. In addition, a significant correlation was found between M1 expression and probing depth (PD) values [15].
A pre-clinical investigation by Abaricia et al. [16] also demonstrated that the initial inflammatory response differs between most of the clinical implants analyzed. Changes in surface roughness, chemistry and wettability seem to play a significant role in macrophagic activation and condition the inflammatory microenvironment. However, it was not possible to identify a single implant surface characteristic that determined the activation of macrophages towards the M1 or M2 phenotype. The authors suggested that a combination of these features direct macrophagic activation. [16]
In a recently published paper, we thoroughly described both the chemical composition and the surface topography of five modern commercially available dental implants with different macro- and micro-structures [17]. The present in vitro investigation was intended to measure other surface properties deemed important for the intended clinical purpose of the same five types of dental implants. In particular, the assessments took into consideration the inflammatory response of macrophages placed in contact with the implants
2. Materials and Methods
This research is reported according to the Standards for Reporting Qualitative Research (SRQR)* http://www.equator-network.org/reporting-guidelines/srqr/ (accessed on 3 January 2021). Ethics approval was not required for this in vitro study.
The implants investigated are reported in Table 1.

Table 1.
List of the dental implants tested in the present investigation.
Each sample was tested in its “clinical use” condition. In fact, all specimens to be tested were sent to the analysis laboratory (Nobel Bio Ricerche Srl, Portacomaro, AT, Italy) in their original sealed and perfectly intact package, all well before their expiration date. The samples were extracted from their original packaging immediately before the analysis, taking every effort to prevent any contamination.
For each implant type, 3 samples were used for the inflammatory response tests. One additional sample was kept in reserve should anomalous results emerge with the need for confirmation.
The basics of the method applied in our study are described in a previous paper (Morra et al., 2015).
2.1. Cell Culture
The murine macrophage cell line J774a.1 (inoculum: 6.8 × 105 cell/mL in Vf = 3 mL) was cultured in Dulbecco’s modified Eagle medium (DMEM—Gibco Invitrogen, Cergy-Pontoise, France) and incubated in direct contact with titanium or zirconia dental implants in 12-well plates for 4 h [2]. Each test sample was represented by one implant, inserted in a single well, on which the cells were seeded. Polystyrene was used as the negative control and tested in 3 replicates.
The cells were observed under optical microscopy. After 4 h, cells were rounded, translucent, very numerous and well distributed into the well. No signs of toning were found in the growth medium. The implants were then removed from their culture wells and inserted in new ones, in order to extract the total RNA only from the cells present on the implant surface. Wells containing polystyrene were directly lysed after removing the growth culture.
2.2. RNA Extraction
The extraction of total RNA was performed using the extraction kit Maxwell® RSC simplyRNA Cells (Promega, Madison, WI, USA) following the supplier’s instructions and the nucleic acid automated extractor Maxwell® RSC instrument (Promega). RNAs of extracted samples at 4 h, eluted in H2O, were frozen at −20 °C overnight, while waiting for reverse transcription the day after.
Assessment of total RNA concentration was made via the quantification kit QuantiFluor® RNA System (Promega, Madison, WI, USA) and the Quantus™ Fluorometer (Promega, Madison, WI, USA). All samples were quantified at the same time, in order to avoid any difference in the procedure among the samples.
2.3. Total RNA Reverse Transcription into cDNA
All RNA samples were diluted at the concentration of 39 ng/μL. The diluted RNA was then reverse-transcribed into cDNA via the High-Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA, USA) following the supplier’s recommendations, and the thermocycler Thermalcycler 2720 (Applied Biosystems, Foster City, CA, USA). The final concentration for each RT reaction was 19.50 ng/μL. All the samples were reverse-transcribed at the same time, in order to avoid any difference. The obtained cDNA was conserved at −20 °C, until RT-qPCR analysis.
2.4. Evaluation of Quality and Integrity of Extracted RNA
Assessment of the RNA quality, and particularly of RNA integrity, is crucial for many downstream applications, in particular RT-qPCR. In fact, the use of high-quality RNA ensures reproducible and relevant results, which may be impaired if some degrees of degradation occurred [18]. The quality and integrity of extracted RNA was evaluated for each sample using the Qubit RNA IQ Assay Kit (Invitrogen, Carlsbad, CA, USA), together with the Invitrogen™Qubit™ 4 Fluorometer. They enable the quick measurement of RNA quality on a fluorescence-based method, utilizing two dyes with two separate emission channels, one that selectively binds to degraded RNA and another that selectively binds to large and intact RNA.
All the samples showed an IQ value > 7.
2.5. Gene Expression Analysis by Real-Time RT-qPCR
Gene expression analysis was performed through quantitative PCR (qPCR), after the process of reverse transcription of total RNA to cDNA (rt-qPCR).
For each sample, the analysis was made in technical duplicate and by including a no template control (NTC) for each of the 96-well plates.
The 96-well plates preparation provided the use of the TaqMan Fast Advanced Master Mix (Thermofisher, Waltham, MA, USA).
The following genes were analyzed: GAPDH (housekeeping), YWHAZ (housekeeping), interleukins IL-1β and IL-6, tumor necrosis factor TNF-α, nitric oxide synthase NOS 2, matrix metalloproteinases MMP-8 and MMP-9, and tissue inhibitor of matrix metalloproteinases TIMP 3.
Amplification of the genes to be evaluated was carried out by using TaqMan probes of Mus Musculus (Table 2), since J774a.1 cells are derived from Mus Musculus.

Table 2.
List of the TaqMan probes employed in this experiment and their AssayID.
Once the plates were loaded and sealed with an adhesive film, they were centrifugated at 900 rpm in order to eliminate any bubbles and keep all the reaction to the bottom of the well.
The amplification was carried on using the QuantStudio 5 Real-Time PCR System. To analyze the amplified genes the comparative method ΔΔCq (or ΔΔCt) was used, which allows the quantification of the target template in a sample by comparing its expression to the expression of another gene in the same sample (defined as “reference gene” or “housekeeping gene”). The reference gene must be constitutively expressed in every sample, in every experimental condition, at the same level, without considerable differences. Since completely stable housekeeping genes have not been demonstrated in all the treatments in all cells, it is necessary to verify the proper genes every time when different treatments and different cells are used. In addition, by using several housekeeping genes, it is possible to get a higher approximation to stability.
This comparison provides a value, defined ΔCq, that, in turn, is compared to the ΔCq of the reference sample, providing the ΔΔCq value. Through a series of mathematical formulas, the ΔΔCq value is converted to a fold-change value.
Our samples were analyzed by normalizing the values against the reference sample Polystyrene 1 and against the mean of the ΔCq values of the two housekeeping genes YWHAZ and GAPDH.
The analysis was made using the online software provided on the cloud by Thermofisher.
2.6. Statistical Analysis
The statistical analysis was performed through the one-way ANOVA with replication test, followed by the post-hoc Tukey HSD test, after confirmation of normality and homogeneity of variances in samples. In the case of unequal variances, the Welch’s ANOVA was applied. The analysis was made by using both the Excel data analysis module and the statistical analysis software PAST [19]. Results were represented as the Mean ± Standard Deviation (SD) of 3 biological samples (n = 3) and were considered statistically significant at p < 0.05 (* = p < 0.05; ** = p < 0.01).
3. Results
The expressions of genes that code for interleukin IL-1β, IL-6, TNF-α, NOS 2, MMP-8, MMP-9 and TIMP-3 have been evaluated through quantitative PCR, preceded by reverse transcription (RT-qPCR) at 4 h of incubation of murine macrophage cells J774a.1 in direct contact with different implant types. The results of the analysis of gene TIMP-3 expression showed very low levels of mRNA, with amplification curves that amounted to >35 Ct. As a consequence, they were excluded from the final analysis. The outcomes of gene expression are reported in Figure 1. Figure 2 shows the statistically significant differences among the samples.
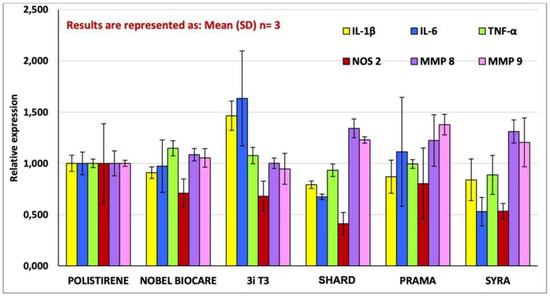
Figure 1.
The graph shows the expression of the genes that code for IL-6, TNF-α, NOS 2, MMP-8, and MMP-9 by J774-A1 macrophage cells placed in contact with the implant samples as well as with the negative control (polystyrene for cell cultures) for a duration of 4 h. The data are presented as fold expression of the gene with respect to the control, which is the ratio between gene expression in the sample under examination and gene expression in the control (i.e., if the measured value is 1, it means that the inflammatory cells in contact with the sample under examination express the same quantity of the gene as the inflammatory cells in contact with the negative control; if the value is 2, it means that they express double, and so on). Results are expressed as Mean (SD), n = 3.
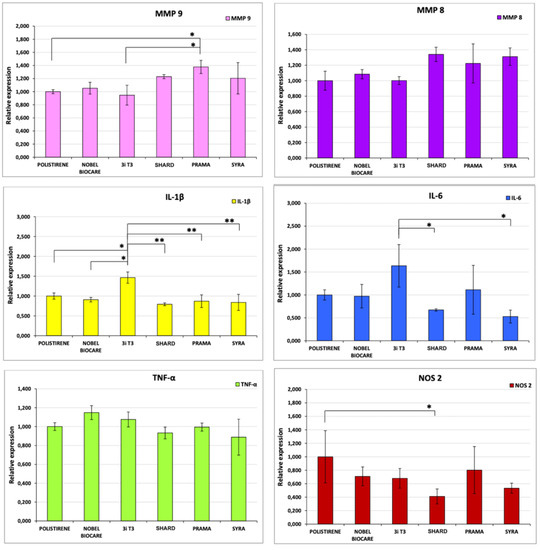
Figure 2.
Outcomes of the statistical analysis of gene expression data for each sample for each gene analyzed. Samples presenting a statistically significant difference in gene expression are indicated with *. * p < 0.05, ** p < 0.01. Results are expressed as Mean (SD), n = 3.
RTqPCR analysis results showed a non-inflammation pattern of gene expression for all the tested implants, except for a mild pro-inflammatory effect induced by 3iT3 hybrid implants. In particular, in macrophagic cells in contact with 3iT3 implants, a statistically significant increase in transcripts for IL-1β (1.47-fold) in comparison with polystyrene and Nobel Pearl implants was found with p < 0.05, and compared with the other implant types with p < 0.01.
Regarding the expression of IL-6 (Figure 2), the response was very low in all the samples analyzed. Only the 3iT3 sample promoted an expression that was roughly 1.5 times greater than that of the negative control without a statistically significant difference, while the difference (1.63-fold) was significant compared to Shard and Syra (p < 0.05), which are the two implants that induced the lower response, even lower than that of the negative control (albeit not statistically significantly).
Prama implant showed a significantly greater expression of MMP-9 (p < 0.05), while Shard had a significantly lower expression of NOS-2 (p < 0.05) compared to the negative control.
On the contrary, no statistically significant differences were observed for any of the implants in the expression of TNF-α and of MMp-8 genes in comparison with polystyrene nor between the individual implant types.
The low levels of mRNAs of IL-1β and IL-6, that are the main pro-inflammatory cytokines involved in foreign body reactions and in pathogen response, show that 3iT3 implants induced a mild inflammatory response in macrophages at 4 h of incubation, differently from the other implants. In any case, the response was very low, and all the implants tested might be considered devoid of pro-inflammatory effects.
4. Discussion
Replacement of body components must respect several functional properties, with one of the basic features being restoration of the removed organ in terms of strength and biofunctional and biomechanical properties. For this purpose, materials with complex chemical properties characterized by a controlled effect on the immune system of the body and biocompatibility have been used. The most important feature of the biomaterial is biocompatibility, which is the ability of the material to show proper reaction during contact with tissue, blood or plasma for a specific time period, either inside or outside the body. For such reasons the dental implants marketed by companies must meet stringent requirements in terms of chemical purity and strength of materials as well as their biocompatibility. The development of inflammatory reactions after insertion of the implant may result from the body’s reaction to the inserted implant and the bacterial microflora in the oral cavity.
In the present study, for the evaluation of the inflammatory response next to different implant types, a direct contact method was adopted. Inflammatory cells (macrophages) were placed in contact with the devices to be evaluated, and the effects on the expression of genes that code for typical proteins of the inflammatory processes (cytokines and chemokines) were measured.
The measurement of pro-inflammatory cytokines produced by macrophages provides the evaluation of an eventual inflammatory response of such cells in contact with the implant surface or with eventual particles released in the surrounding environment. The analysis at 4 h of incubation allowed evaluating pro-inflammatory genes during the early inflammatory response. In fact, macrophages release inflammatory mediators during a FBR and in the presence of bacterial endotoxins, which can adhere to the implant surface and promote a cascade of events that include the recall of other inflammatory cells, leading to progressive destruction of peri-implant tissues, up to bone tissue resorption and, ultimately, implant failure.
In order to carry out their functions, macrophages must recognize the pro-inflammatory stimulus, which will determine a modification of their state of quiescence (M0) to an activated state that, based on the specific requested function, can be a “classical” or an “alternative” activation [7,18,19]. This modification, from phenotypical and functional viewpoints, is defined as “macrophagic polarization” and includes different macrophage subtypes that are able to express a specific set of inflammatory cytokines, and several types of other molecules. Namely, M1 macrophages promote and lead the pro-inflammatory response and produce high levels of IL-1β, IL-6 and TNF-α, in addition to the enzyme NOS, while M2 macrophages are defined as “anti-inflammatory” because they mitigate the pro-inflammatory response during tissue repair and tissue remodeling processes, and they produce high levels of IL-4, IL-10 and TGF-β [7,15,16,18,19].
Morra et al., applying the same research methods herein used, reports that after 4 h of contact, the expression of the gene that codes for IL-6 is highly sensitive to the presence of adherent endotoxins on the implant surfaces regardless of their specific topography. Endotoxins are molecular species typical of the walls of bacteria. When our immune system senses their presence, it triggers the inflammatory reaction. Adherent endotoxins can be present on the surfaces of medical devices, including implant surfaces, as a consequence of the presence of bacteria that are no longer active following sterilization, but whose “corpses” can still trigger pyrogenic reactions through the molecular structures of their walls, promoting osteoclastogenic activity. A poor awareness of the production processes, for example the use of non-sterile washing water or the handling of the implants packaging with gloves that are not perfectly clean, can lead to the accumulation of endotoxins that adhere to the implant surfaces. In this respect, this measure can be considered an assessment of the “biological cleaning” of the implant. In the aforementioned paper from Morra et al., the 22 different types of clinically available implants evaluated exhibited wide variation of adherent endotoxin, evoking a significantly different device-induced macrophage activation. Among tested implants, nine exhibited an expression of IL-6 that was 10 times higher than that of the control, including three implants with an expression greater than 40 times, and one implant with an expression greater than 100 times. The amount of adherent endotoxin can overwhelm the contribution of other implant surface characteristics (including chemical composition and topography) in affecting bone regeneration at the bone–implant interface and could be responsible for the so-called “aseptic loosening” of orthopedic prostheses and possibly of the breakdown of osseointegration at dental implants [2,4,20].
In addition to IL-6, the contact between inflammatory cells and surfaces triggers the production of a large number of other cytokines and chemokines influencing the peri-implant biological environment. The study of the biochemical pathways that they can potentially influence is very complex.
Nitric oxide (NO) is a powerful gas molecule that acts as neurotransmitter and vasodilator; in addition, it is produced by immune cells as an effector molecule against tumoral cells and pathogens. In particular, macrophages produce high levels of NO after being stimulated by various cytokines or by endotoxins such as bacterial lipopolysaccharide (LPS) that, after binding to CD14 receptor, activates the NF-κB signaling pathway. The activation of transcription factors such as NF-κB leads to the transcription of inducible genes involved in inflammatory response, such as iNOS (NOS 2) and cyclooxygenase 2 (COX-2). iNOS is expressed by macrophages after a pro-inflammatory stimulus, and it determines the production of high levels of NO, creating an effective defense mechanism.
In our experiment using RTqPCR, no increase in NOS expression was observed; on the contrary, a general decrease in NOS was evident in implant-incubated cells in comparison to polystyrene. This decrease was statistically significant for Shard implants (p < 0.05).
After their activation, macrophages also produce MMPs, a protease that is able to degrade extracellular matrix components. MMPs are also required for the correct process of cellular migration to the site of infection. Among MMPs, MMP-8 is one of the main collagenases found in periodontal tissues affected by periodontitis and peri-implantitis. Its correlation with the entity of tissue damage and to pathology degree has been proven and has led to its identification as a marker of active inflammation. The evaluation of MMP-8 expression induced by the analyzed implants showed a mild increase in all the implant types in comparison to polystyrene, but without a statistically significant difference.
MMP-9 is another metalloprotease that is thoroughly expressed in tissues affected by periodontal disease, and, such as other MMPs, it has a regulating role in the inflammatory process thanks to its protease activity, which allows transforming a target substrate activating or inactivating it. Since MMPs can carry out destructive actions if not controlled, their expression and function are strictly regulated both at transcriptional and post-translational levels. A statistically significant mild increase (p < 0.05) in MMP-9 expression was found in Prama® implants, in comparison with 3i T3® implants and to polystyrene reference sample.
Molecules such as TIMPs act as MMP antagonists, inactivating them, and through this inhibition they play a key role in regulating an insult-induced inflammation. TIMP-3, in particular, can silence several MMPs, and it is produced by various cells, such as macrophages. At a transcriptional level, it has been shown that macrophages express low rates of TIMP-3 when they are in their state of quiescence, M0 or in M2, while mRNA levels increase after LPS stimulation and therefore with M1 polarization. The results of the analysis of gene TIMP-3 expression in the present study show very low levels of mRNA, with amplification curves that amounted to >35 Ct. As a consequence, they were excluded from the final analysis. These levels of TIMP-3 mRNA further confirm that the analyzed implants are not pro-inflammatory.
Further studies designed to evaluate protein levels of such genes could clarify and confirm the data obtained in our experiment.
5. Conclusions
In conclusion, all the implants examined can be considered to have no pro-inflammatory effects with expressions of pro-inflammatory genes that, in some cases, were even lower than that of the negative control. The modest, but significantly higher, expression of IL-1β in macrophagic cells in contact with 3i T3® compared to the negative control could correlate to the SEM and XPS results reported in our previous paper [17], which indicated a slightly lower level of surface “cleanliness”, although falling within an area of full acceptability.
The low or no pro-inflammatory response of the implants analyzed suggests that in each case a conscious and careful cleaning and decontamination process was adopted, which probably involved a final step of decontamination with plasma, one of the most effective methods for inactivating adherent endotoxins.
The limitations of the present in vitro study must be mentioned, including the small sample size and the impossibility to draw clinical indications directly on the basis of in vitro experiments. In addition, investigating the mechanisms of action of increased expression of specific pro-inflammatory genes was not within the scope of this study, and it is not possible to assume possible mechanisms of action on the basis of the results of this experiment. Further studies are needed to address this point. However, the present investigation might help understanding the mechanisms governing the host response to dental implants with different macro- and micro-structures.
The field of osteoimmunology, investigating the interactions between bone, hematopoietic and immune systems, is growing and might shed light on the biological phenomena governing osseointegration and bone maintenance next to dental implants.
Author Contributions
M.M., D.B. and P.P. conceived the ideas; F.B. and F.P. collected the data; M.M. and P.P. analyzed the data; and M.M. and F.D. led the writing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Borton, L.K.; Coleman, K.P. Material-mediated pyrogens in medical devices: Applicability of the in vitro Monocyte Activation Test. ALTEX 2018, 35, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Morra, M.; Cassinelli, C.; Bollati, D.; Cascardo, G.; Bellanda, M. Adherent endotoxin on dental implant surfaces: A reappraisal. J. Oral Implantol. 2015, 41, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Dahlin, C.; Jemt, T.; Sennerby, L.; Turri, A.; Wennerberg, A. Is marginal bone loss around oral implants the result of a provoked foreign body reaction? Clin. Implant Dent. Relat. Res. 2014, 16, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Trindade, R.; Albrektsson, T.; Tengvall, P.; Wennerberg, A. Foreign Body Reaction to Biomaterials: On Mechanisms for Buildup and Breakdown of Osseointegration. Clin. Implant Dent. Relat. Res. 2016, 18, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Conserva, E.; Lanuti, A.; Menini, M. Cell behavior related to implant surfaces with different microstructure and chemical composition: An in vitro analysis. Int. J. Oral Maxillofac. Implants 2010, 25, 1099–1107. [Google Scholar] [PubMed]
- Conserva, E.; Menini, M.; Ravera, G.; Pera, P. The role of surface implant treatments on the biological behavior of SaOS-2 osteoblast-like cells. An in vitro comparative study. Clin. Oral Implants Res. 2013, 24, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Insua, A.; Monje, A.; Wang, H.L.; Miron, R.J. Basis of bone metabolism around dental implants during osseointegration and peri-implant bone loss. J. Biomed. Mater. Res. A 2017, 105, 2075–2089. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Crotti, T.N.; McHugh, K.P.; Matsuzaki, K.; Gravallese, E.M.; Bierbaum, B.E.; Goldring, S.R. The role played by cell-substrate interactions in the pathogenesis of osteoclast-mediated peri-implant osteolysis. Arthritis Res. Ther. 2006, 8, R70. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, Y.; Sasaki, K.; Sasaki, A.; Takakubo, Y.; Hasegawa, H.; Ogino, T.; Konttinen, Y.T.; Salo, J.; Takagi, M. Enhanced osteolytic potential of monocytes/macrophages derived from bone marrow after particle stimulation. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 84, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Shah, F.A.; Thomsen, P.; Palmquist, A. Osseointegration and current interpretations of the bone-implant interface. Acta Biomater. 2019, 84, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Champagne, C.M.; Takebe, J.; Offenbacher, S.; Cooper, L.F. Macrophage cell lines produce osteoinductive signals that include bone morphogenetic protein-2. Bone 2002, 30, 26–31. [Google Scholar] [CrossRef]
- Miron, R.J.; Bosshardt, D.D. Multinucleated Giant Cells: Good Guys or Bad Guys? Tissue Eng. Part. B Rev. 2018, 24, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Hachim, D.; LoPresti, S.T.; Rege, R.D.; Umeda, Y.; Iftikhar, A.; Nolfi, A.L.; Skillen, C.D.; Brown, B.N. Distinct macrophage populations and phenotypes associated with IL-4 mediated immunomodulation at the host implant interface. Biomater. Sci. 2020, 8, 5751–5762. [Google Scholar] [CrossRef] [PubMed]
- Pajarinen, J.; Kouri, V.P.; Jamsen, E.; Li, T.F.; Mandelin, J.; Konttinen, Y.T. The response of macrophages to titanium particles is determined by macrophage polarization. Acta Biomater. 2013, 9, 9229–9240. [Google Scholar] [CrossRef] [PubMed]
- Galarraga-Vinueza, M.E.; Obreja, K.; Ramanauskaite, A.; Magini, R.; Begic, A.; Sader, R.; Schwarz, F. Macrophage polarization in peri-implantitis lesions. Clin. Oral Investig. 2020, 25, 2335–2344. [Google Scholar] [CrossRef] [PubMed]
- Abaricia, J.O.; Shah, A.H.; Ruzga, M.N.; Olivares-Navarrete, R. Surface characteristics on commercial dental implants differentially activate macrophages in vitro and in vivo. Clin. Oral Implants Res. 2021, 32, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Menini, M.; Pera, F.; Bagnasco, F.; Delucchi, F.; Morganti, E.; Canullo, L.; Pesce, P. Morphological and Chemical Characterization of Titanium and Zirconia Dental Implants with Different Macro- and Micro-Structure. Appl. Sci. 2020, 10, 7520. [Google Scholar] [CrossRef]
- Fleige, S.; Pfaffl, M.W. RNA integrity and the effect on the real-time qRT-PCR performance. Mol. Asp. Med. 2006, 27, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Hammer, Y.; Harper, D.A.T. Paleontological Data Analysis; Blackwell Publishing: Malden, MA, USA, 2005. [Google Scholar]
- Omar, O.M.; Graneli, C.; Ekstrom, K.; Karlsson, C.; Johansson, A.; Lausmaa, J.; Wexell, C.L.; Thomsen, P. The stimulation of an osteogenic response by classical monocyte activation. Biomaterials 2011, 32, 8190–8204. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).