The Effectiveness of Ecklonia Cava Kjellman Extract in Improving Menopausal Syndrome in Osteoporosis and Depression
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of E. cava Extract and Major Constituents
2.2. Cell Culture and Reagents
2.3. Cell Viability
2.4. ALP Assay
2.5. Reverse-Transcription and Real-Time PCR
2.6. Experimental Animals and Treatments
2.7. 5-HT Uptake Assay
2.8. Serum Serotonin Analysis
2.9. BMD Analysis
2.10. Statistical Analysis
3. Results
3.1. EC Improved Animal Condition
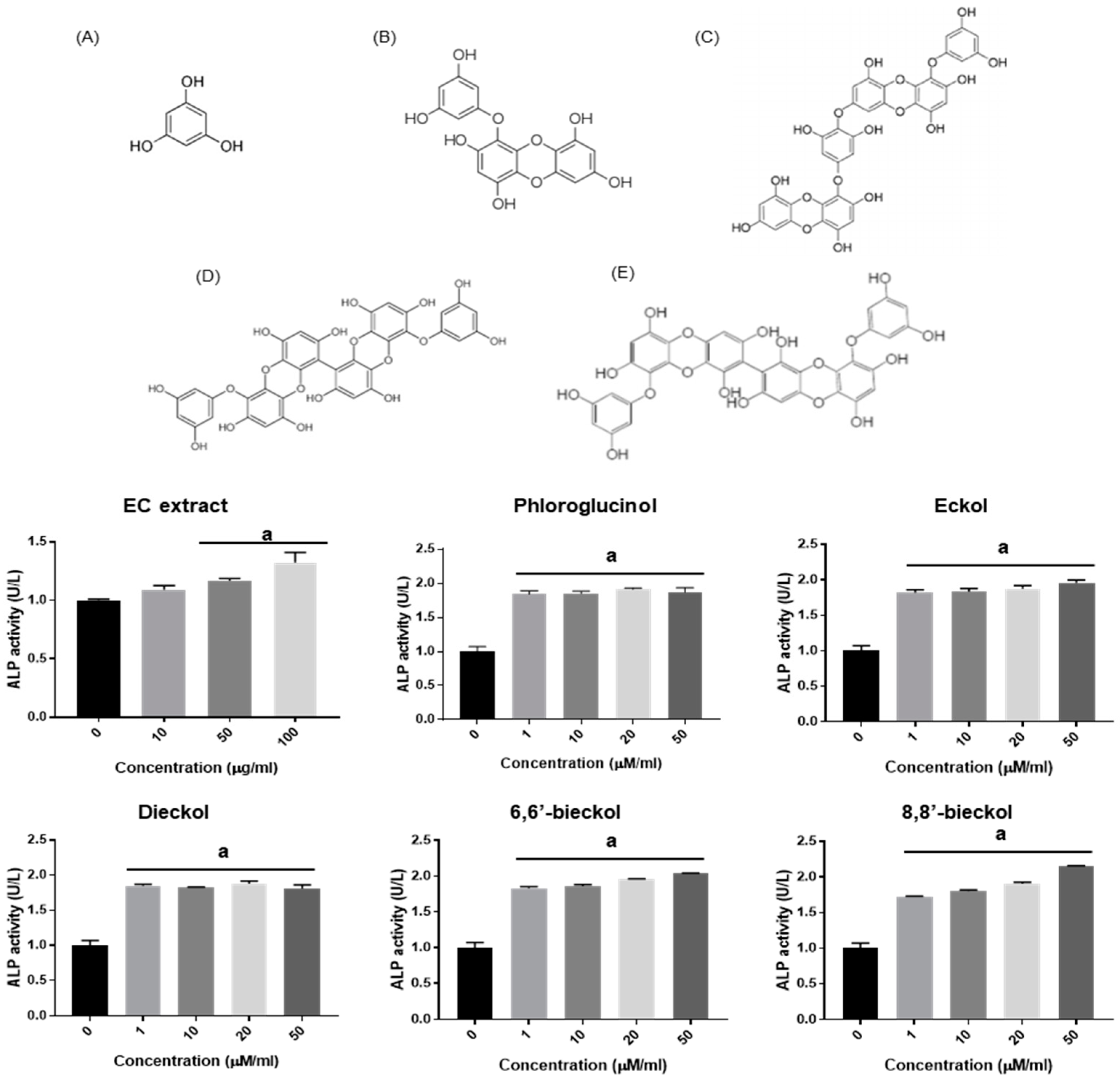
3.2. EC Enhances MC3T3-E1 Osteoblast Differentiation
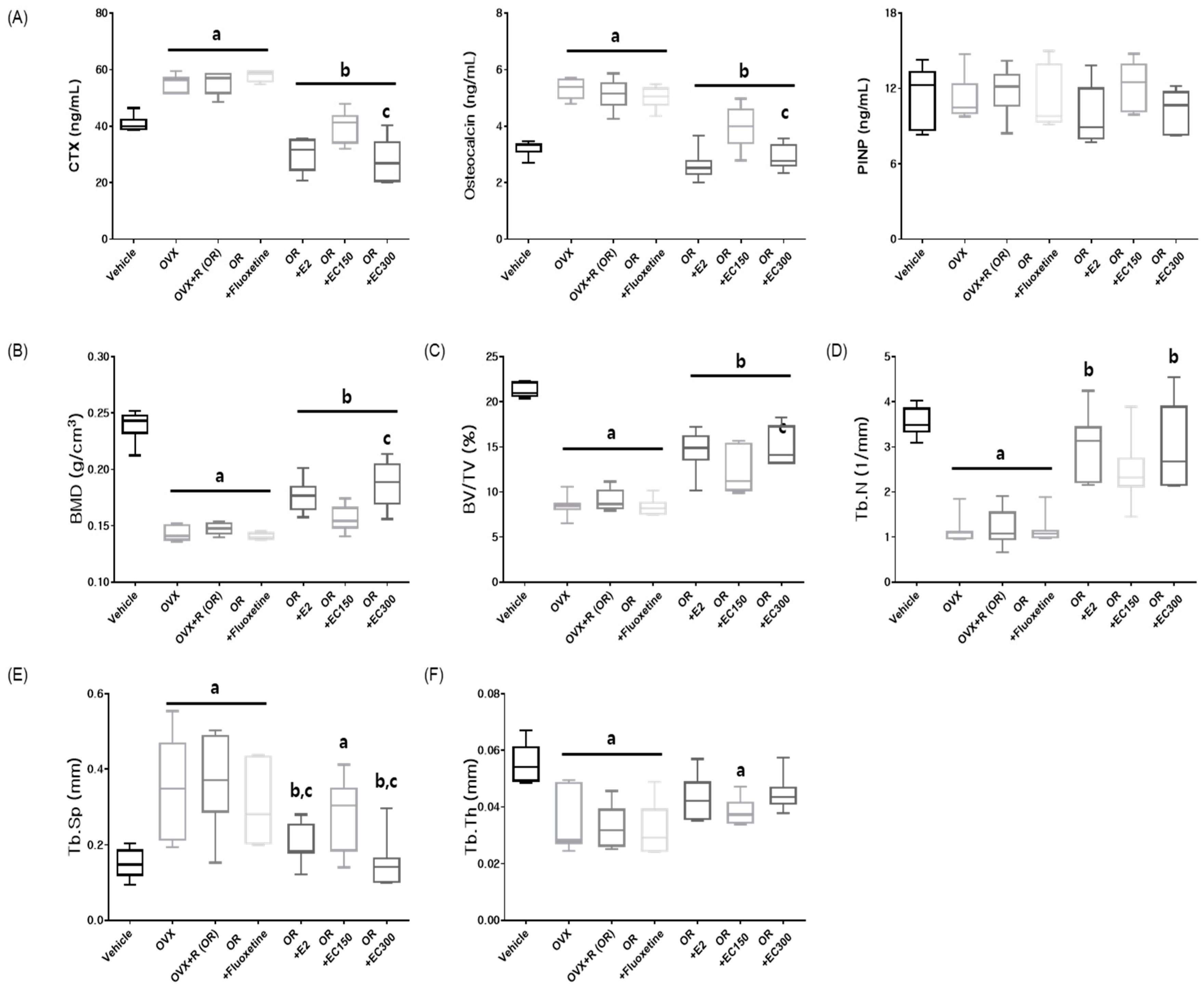
3.3. Effect of EC on In Vivo Bone Formation
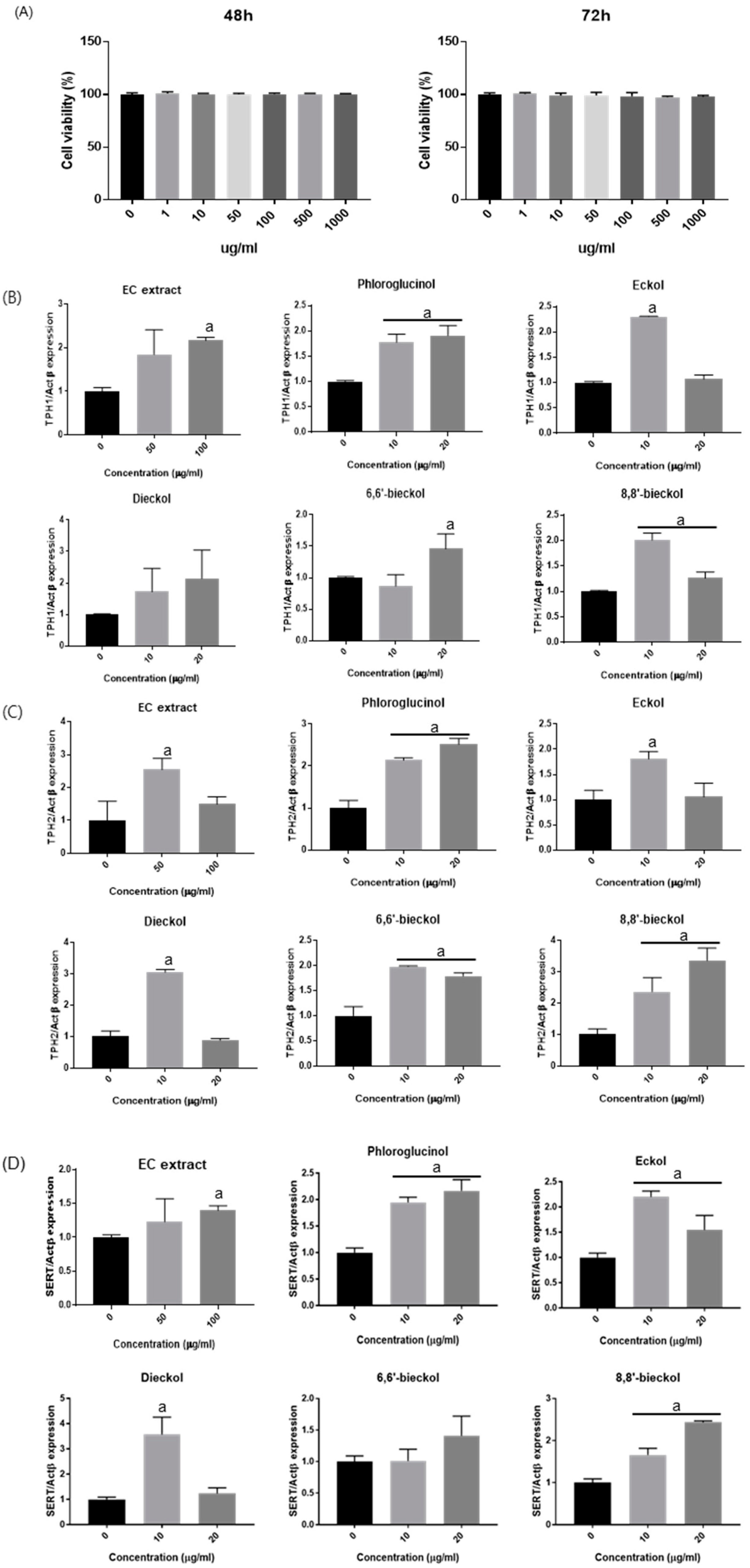
3.4. Effects of EC on TPH-1, TPH-2, and SERT mRNA Expression
3.5. Effects of EC on 5-HT Uptake by RBL-2H3 Cells
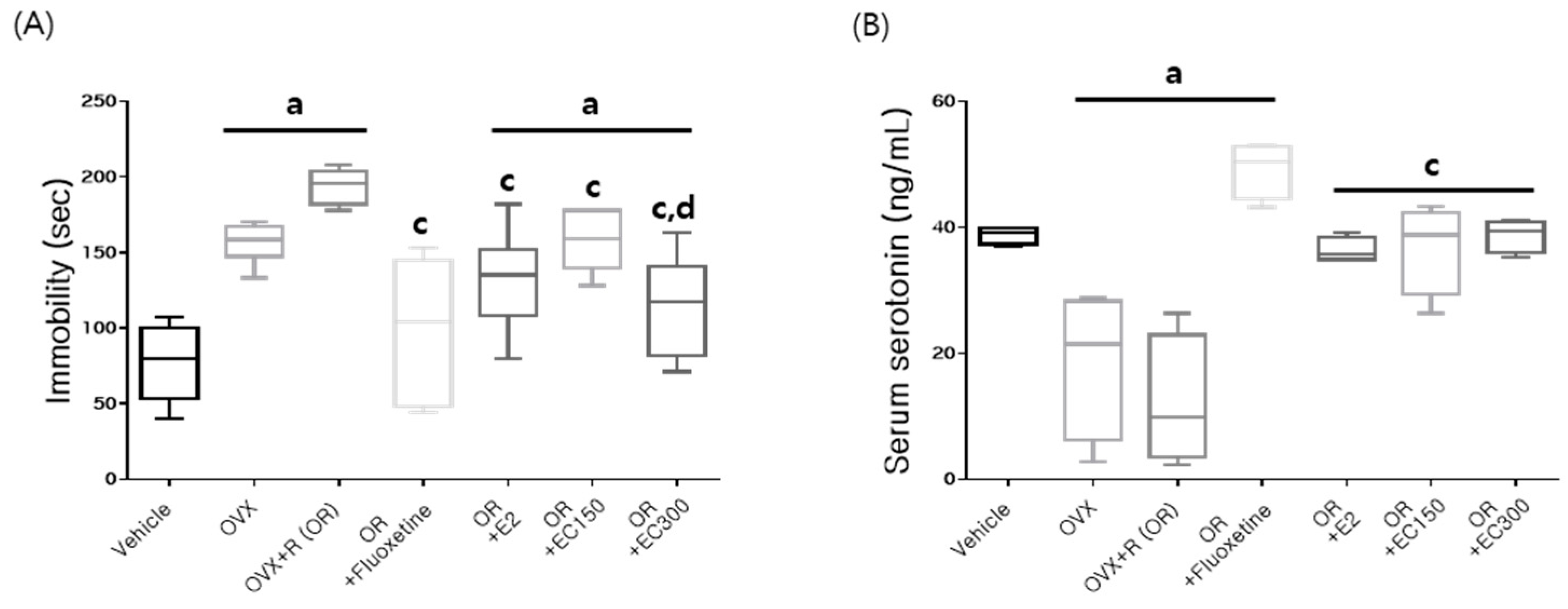
3.6. Serum Serotonin Level and Immobility Time
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lobo, R.A. Serono Symposia USA. In Perimenopause; Springer: New York, NY, USA, 1997; p. 348. [Google Scholar]
- Novi, J.M.; Ross, H.L. Perimenopause; Informa Healthcare: New York, NY, USA, 2009; p. 189. [Google Scholar]
- Chojnacki, C.; Walecka-Kapica, E.; Klupinska, G.; Pawlowicz, M.; Blonska, A.; Chojnacki, J. Effects of fluoxetine and melatonin on mood, sleep quality and body mass index in postmenopausal women. J. Physiol. Pharmacol. 2015, 66, 665–671. [Google Scholar]
- Biegon, A.; Alia-Klein, N.; Alexoff, D.L.; Fowler, J.S.; Kim, S.W.; Logan, J.; Pareto, D.; Preston-Campbell, R.; Wang, G.J.; Hildebrandt, T. Relationship of estrogen synthesis capacity in the brain with obesity and self-control in men and women. Proc. Natl. Acad. Sci. USA 2020, 117, 22962–22966. [Google Scholar] [CrossRef] [PubMed]
- Pepine, C.J.; Nichols, W.W.; Pauly, D.F. Estrogen and different aspects of vascular disease in women and men. Circ. Res. 2006, 99, 459–461. [Google Scholar] [CrossRef] [PubMed]
- Cauley, J.A. Estrogen and bone health in men and women. Steroids 2015, 99, 11–15. [Google Scholar] [CrossRef]
- Mullis, P.E.; Yoshimura, N.; Kuhlmann, B.; Lippuner, K.; Jaeger, P.; Harada, H. Aromatase deficiency in a female who is compound heterozygote for two new point mutations in the P450arom gene: Impact of estrogens on hypergonadotropic hypogonadism, multicystic ovaries, and bone densitometry in childhood. J. Clin. Endocrinol. Metab. 1997, 82, 1739–1745. [Google Scholar] [CrossRef]
- Joffe, R.T. Hormone treatment of depression. Dialogues Clin. Neurosci. 2011, 13, 127–138. [Google Scholar] [PubMed]
- Amin, Z.; Canli, T.; Epperson, C.N. Effect of estrogen-serotonin interactions on mood and cognition. Behav. Cogn. Neurosci. Rev. 2005, 4, 43–58. [Google Scholar] [CrossRef]
- Blum, I.; Vered, Y.; Lifshitz, A.; Harel, D.; Blum, M.; Nordenberg, Y.; Harsat, A.; Sulkes, J.; Gabbay, U.; Graff, E. The effect of estrogen replacement therapy on plasma serotonin and catecholamines of postmenopausal women. Isr. J. Med. Sci. 1996, 32, 1158–1162. [Google Scholar]
- Sanchez, R.L.; Reddy, A.P.; Centeno, M.L.; Henderson, J.A.; Bethea, C.L. A second tryptophan hydroxylase isoform, TPH-2 mRNA, is increased by ovarian steroids in the raphe region of macaques. Brain Res. Mol. Brain Res. 2005, 135, 194–203. [Google Scholar] [CrossRef]
- Invernizzi, R.W. Role of TPH-2 in brain function: News from behavioral and pharmacologic studies. J. Neurosci. Res. 2007, 85, 3030–3035. [Google Scholar] [CrossRef]
- Lopez-Narvaez, M.L.; Tovilla-Zarate, C.A.; Gonzalez-Castro, T.B.; Juarez-Rojop, I.; Pool-Garcia, S.; Genis, A.; Ble-Castillo, J.L.; Fresan, A. Association analysis of TPH-1 and TPH-2 genes with suicidal behavior in patients with attempted suicide in Mexican population. Compr. Psychiatry 2015, 61, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.Y.; Choi, H.; Jun, H.S. The Effect of Phloroglucinol, A Component of Ecklonia cava Extract, on Hepatic Glucose Production. Mar. Drugs 2017, 15, 106. [Google Scholar] [CrossRef]
- Kang, J.I.; Kim, S.C.; Kim, M.K.; Boo, H.J.; Jeon, Y.J.; Koh, Y.S.; Yoo, E.S.; Kang, S.M.; Kang, H.K. Effect of Dieckol, a component of Ecklonia cava, on the promotion of hair growth. Int. J. Mol. Sci. 2012, 13, 6407–6423. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.J.; Koo, D.H.; Kang, G.J.; Han, S.C.; Lee, B.W.; Koh, Y.S.; Hyun, J.W.; Lee, N.H.; Ko, M.H.; Kang, H.K.; et al. Dieckol, a Component of Ecklonia cava, Suppresses the Production of MDC/CCL22 via Down-Regulating STAT1 Pathway in Interferon-gamma Stimulated HaCaT Human Keratinocytes. Biomol. Ther. 2015, 23, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Park, E.Y.; Choi, H.; Yoon, J.Y.; Lee, I.Y.; Seo, Y.; Moon, H.S.; Hwang, J.H.; Jun, H.S. Polyphenol-Rich Fraction of Ecklonia cava Improves Nonalcoholic Fatty Liver Disease in High Fat Diet-Fed Mice. Mar. Drugs 2015, 13, 6866–6883. [Google Scholar] [CrossRef]
- Yang, H.; Lee, S.Y.; Lee, S.R.; Pyun, B.J.; Kim, H.J.; Lee, Y.H.; Kwon, S.W.; Suh, D.H.; Lee, C.H.; Hong, E.J.; et al. Therapeutic Effect of Ecklonia cava Extract in Letrozole-Induced Polycystic Ovary Syndrome Rats. Front. Pharmacol. 2018, 9, 1325. [Google Scholar] [CrossRef]
- Zhu, C.B.; Lindler, K.M.; Owens, A.W.; Daws, L.C.; Blakely, R.D.; Hewlett, W.A. Interleukin-1 receptor activation by systemic lipopolysaccharide induces behavioral despair linked to MAPK regulation of CNS serotonin transporters. Neuropsychopharmacology 2010, 35, 2510–2520. [Google Scholar] [CrossRef]
- Matsubara, K.; Harada, H.; Ando, N.; Watada, S.; Obara, H.; Matsumoto, K.; Kitagawa, Y. Estrogen deficiency attenuates neovascularization in a murine model of hindlimb ischemia. J. Surg. Res. 2012, 178, 1022–1028. [Google Scholar] [CrossRef]
- Weitzmann, M.N.; Pacifici, R. Estrogen deficiency and bone loss: An inflammatory tale. J. Clin. Investig. 2006, 116, 1186–1194. [Google Scholar] [CrossRef]
- Szulc, P.; Naylor, K.; Hoyle, N.R.; Eastell, R. Leary for the National Bone Health Alliance Bone Turnover Marker Project. Use of CTX-I and PINP as bone turnover markers: National Bone Health Alliance recommendations to standardize sample handling and patient preparation to reduce pre-analytical variability. Osteoporos. Int. 2017, 28, 2541–2556. [Google Scholar] [CrossRef]
- Garnero, P.; Grimaux, M.; Seguin, P.; Delmas, P.D. Characterization of immunoreactive forms of human osteocalcin generated in vivo and in vitro. J. Bone Miner. Res. 1994, 9, 255–264. [Google Scholar] [CrossRef]
- Jennings, K.A.; Sheward, W.J.; Harmar, A.J.; Sharp, T. Evidence that genetic variation in 5-HT transporter expression is linked to changes in 5-HT2A receptor function. Neuropharmacology 2008, 54, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Neumaier, J.F.; Root, D.C.; Hamblin, M.W. Chronic fluoxetine reduces serotonin transporter mRNA and 5-HT1B mRNA in a sequential manner in the rat dorsal raphe nucleus. Neuropsychopharmacology 1996, 15, 515–522. [Google Scholar] [CrossRef]
- Pineyro, G.; Blier, P.; Dennis, T.; de Montigny, C. Desensitization of the neuronal 5-HT carrier following its long-term blockade. J. Neurosci. 1994, 14, 3036–3047. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, S.; Afridi, B.; Aman, Z. Phloroglucinol for acceleration of labour: Double blind, randomized controlled trial. J. Pak. Med. Assoc. 2005, 55, 270–273. [Google Scholar]
- Lee, J.W.; Seok, J.K.; Boo, Y.C. Ecklonia cava Extract and Dieckol Attenuate Cellular Lipid Peroxidation in Keratinocytes Exposed to PM10. Evid. Based Complement. Altern. Med. 2018, 2018, 8248323. [Google Scholar] [CrossRef]
- Choi, H.S.; Jeon, H.J.; Lee, O.H.; Lee, B.Y. Dieckol, a major phlorotannin in Ecklonia cava, suppresses lipid accumulation in the adipocytes of high-fat diet-fed zebrafish and mice: Inhibition of early adipogenesis via cell-cycle arrest and AMPKalpha activation. Mol. Nutr. Food Res. 2015, 59, 1458–1471. [Google Scholar] [CrossRef]
- Son, M.; Oh, S.; Lee, H.S.; Ryu, B.; Jiang, Y.; Jang, J.T.; Jeon, Y.J.; Byun, K. Pyrogallol-Phloroglucinol-6,6′-Bieckol from Ecklonia cava Improved Blood Circulation in Diet-Induced Obese and Diet-Induced Hypertension Mouse Models. Mar. Drugs 2019, 17, 272. [Google Scholar] [CrossRef]
- Son, M.; Oh, S.; Choi, C.H.; Park, K.Y.; Son, K.H.; Byun, K. Pyrogallol-Phloroglucinol-6,6-Bieckol from Ecklonia cava Attenuates Tubular Epithelial Cell (TCMK-1) Death in Hypoxia/Reoxygenation Injury. Mar. Drugs 2019, 17, 602. [Google Scholar] [CrossRef]
- Park, M.H.; Heo, S.J.; Kim, K.N.; Ahn, G.; Park, P.J.; Moon, S.H.; Jeon, B.T.; Lee, S.H. 6,6′-Bieckol protects insulinoma cells against high glucose-induced glucotoxicity by reducing oxidative stress and apoptosis. Fitoterapia 2015, 106, 135–140. [Google Scholar] [CrossRef]
- Wijesekara, I.; Yoon, N.Y.; Kim, S.K. Phlorotannins from Ecklonia cava (Phaeophyceae): Biological activities and potential health benefits. Biofactors 2010, 36, 408–414. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Kim, H.J.; Lee, H.W. The Effectiveness of Ecklonia Cava Kjellman Extract in Improving Menopausal Syndrome in Osteoporosis and Depression. Appl. Sci. 2021, 11, 5315. https://doi.org/10.3390/app11125315
Yang H, Kim HJ, Lee HW. The Effectiveness of Ecklonia Cava Kjellman Extract in Improving Menopausal Syndrome in Osteoporosis and Depression. Applied Sciences. 2021; 11(12):5315. https://doi.org/10.3390/app11125315
Chicago/Turabian StyleYang, Hyun, Hye Jin Kim, and Hye Won Lee. 2021. "The Effectiveness of Ecklonia Cava Kjellman Extract in Improving Menopausal Syndrome in Osteoporosis and Depression" Applied Sciences 11, no. 12: 5315. https://doi.org/10.3390/app11125315
APA StyleYang, H., Kim, H. J., & Lee, H. W. (2021). The Effectiveness of Ecklonia Cava Kjellman Extract in Improving Menopausal Syndrome in Osteoporosis and Depression. Applied Sciences, 11(12), 5315. https://doi.org/10.3390/app11125315






