Can Sustainable Packaging Help to Reduce Food Waste? A Status Quo Focusing Plant-Derived Polymers and Additives
Abstract
Featured Application
Abstract
1. Introduction
2. Challenges in Food Supply Chains Related to Packaging Characteristics
2.1. Current Packaging Characteristics
2.2. Food Waste and the Meaning of Packaging
3. Plastics Used for Food Packaging
3.1. Classification of Plastics
3.2. Selected Biodegradable Synthetically Manufactured Polymers
3.2.1. Biomass-derived Chemically Manufactured Polymers
3.2.2. Polymers Produced by Microorganisms
3.3. Selected Plant-derived Polymers
3.3.1. Lignocellulosic Biomass and Lignin
3.3.2. Protein-Based Polymers
3.3.3. Polysaccharides
Starch
Cellulose and Derivatives
Alginates
Chitosan
4. Natural Additives in the Context of Active Food Packaging
- Addition of absorbers and scavengers of gases, off flavors, moisture, taints, UV light;
- Removal of catalyzing undesired food components;
- Addition of emitters/generators of gases and flavors;
- Release of antioxidant and/or antimicrobial compounds;
- Temperature controlled systems (insulting materials; self-heating or cooling).
4.1. Plant Essential Oils
4.2. Plant Extracts of Various Biomasses
4.3. Encapsulated Plant Essential Oils
5. Adoption Potential of Bio-Based (Active) Packaging along the Value Chain
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
List of Abbreviations
| APET | Amorphous poly(ethylene terephthalate) |
| AZC | Ammonium zirconium carbonate |
| CA | Cellulose acetate |
| CAB | Cellulose acetate butyrate |
| CAP | Cellulose acetate propionate |
| CMC | Carboxymethyl cellulose |
| CoO.ZnO | Cobalt(II) oxide/zinc oxide |
| EO | Essential oil |
| GHG | Greenhouse gas |
| HDPE | High density poly(ethylene) |
| HLCNC | High lignin-containing cellulose nanocrystals |
| HPMC | Hydroxypropylmethyl cellulose |
| LCA | Life cycle analysis |
| LDPE | Low density poly(ethelene) |
| PA | Poly(amide) |
| PBAT | Poly(butylene adipate terephthalate) |
| PBS | Poly(butylene succinate) |
| PCL | Poly(caprolactone) |
| PE | Poly(ethylene) |
| PHA | Poly(hydroxyalkanoate) |
| PHB | Poly(3-hydroxybutyrate) |
| PEF | Poly(ethylene furanoate) |
| PET | Poly(ethylene terephthalate) |
| PHBHHx | Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) |
| PHBV | Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) |
| PLA | Poly(lactic acid) |
| PP | Poly(propylene) |
| PS | Poly(styrene) |
| PTT | Poly(trimethylene terephthalate) |
| PVA | Poly(vinyl alcohol) |
| PVC | Poly(vinyl chloride) (PVC) |
| SA | Sodium alginate |
| SAM | Sustainable active microbiocidal (SAM) |
| SiO2 | Silicon dioxide |
| TAIC | Triallyl isocyanurate |
| WVP | Water vapor permeability |
Glossary
| Active packaging | Materials designed to deliberately incorporated components that would release or absorb substances into or from the packaged food or the environment surrounding the food. |
| Bioactive | Compound that has an effect on a living organism, tissue/cell. |
| Bio-based | Compound that is composed (in whole or in significant part) of biological products or renewable domestic agricultural or forestry materials (including plant, animal, and marine materials). |
| Biodegradable | Degradability achieved via microorganisms. |
| Bioplastics | Plastics that are either bio-base, biodegradable, or features both properties. |
| Biopolymers | Natural polymers produced by the cells of living organisms (e.g., forestry and agricultural crops, terrestrial and marine animals), examples are polysaccharides, proteins, and lignin. |
| Compostable | Compounds approved to be degradable by microorganisms at defined conditions (e.g., temperature, humidity, time). |
| Edible packing | Compounds approved to be metabolized by humans. |
| End-of-life options | Including re-use, recycling, recovery, disposal, and others (such as littering, ingestion). |
| European Green Deal | Action plan to boost the efficient use of resources by moving to a clean, circular economy, restore biodiversity, and cut pollution. |
| Fossil-based | Compounds obtained from crude oil, natural gas, brown or hard coal. |
| Renewable resource | Resource which will replenish to replace the portion depleted by usage and consumption, either through natural reproduction or other recurring processes in a finite amount of time in a human time scale. |
References
- Sharma, R.; Ghoshal, G. Emerging trends in food packaging. Nutr. Food Sci. 2018, 48, 764–779. [Google Scholar] [CrossRef]
- Youssef, A.M.; El-Sayed, S.M. Bionanocomposites materials for food packaging applications: Concepts and future outlook. Carbohydr. Polym. 2018, 193, 19–27. [Google Scholar] [CrossRef]
- Aggarwal, A.; Langowski, H.-C. Packaging functions and their role in technical development of food packaging systems: Functional equivalence in yoghurt packaging. Procedia CIRP 2020, 90, 405–410. [Google Scholar] [CrossRef]
- Kreyenschmidt, J.; Albrecht, A.; Braun, C.; Herbert, U.; Mack, M.; Rossaint, S.; Ritter, G.; Teitscheid, P.; Ilg, Y. Food waste in der Fleisch verarbeitenden Kette. Fleischwirtschaft 2013, 10, 57–63. [Google Scholar]
- Accorsi, R. A support-design procedure for sustainable food product-packaging systems. In Sustainable Food Supply Chains; Elsevier: Amsterdam, The Netherlands, 2019; pp. 61–81. ISBN 9780128134115. [Google Scholar]
- Coelho, P.M.; Corona, B.; Klooster, R.T.; Worrell, E. Sustainability of reusable packaging–Current situation and trends. Resour. Conserv. Recycl. X 2020, 6. [Google Scholar] [CrossRef]
- Barlow, C.Y.; Morgan, D.C. Polymer film packaging for food: An environmental assessment. Resour. Conserv. Recycl. 2013, 78, 74–80. [Google Scholar] [CrossRef]
- Matthews, C.; Moran, F.; Jaiswal, A.K. A review on European union’s strategy for plastics in a circular economy and its impact on food safety. J. Clean. Prod. 2021, 283, 125263. [Google Scholar] [CrossRef]
- Ellen MacArthur Foundation. The New Plastics Economy: Rethinking the Future of Plastics; Ellen MacArthur Foundation: Cowes, UK, 2016. [Google Scholar]
- Faraca, G.; Astrup, T. Plastic waste from recycling centres: Characterisation and evaluation of plastic recyclability. Waste Manag. 2019, 95, 388–398. [Google Scholar] [CrossRef]
- Ilg, Y.; Kreyenschmidt, J. Review: Nutzen und Risiken der Anwendung antimikrobieller Werkstoffe in der Lebensmittelkette. J. Food Saf. Food Qual. 2012, 63, 28–34. [Google Scholar]
- European Union. Commission Regulation (EC) No 450/2009 of 29 May 2009 on active and intelligent materials and articles intended to come into contact with food. Off. J. Eur. Union 2009, L135, 3. [Google Scholar]
- PlasticsEurope. Plastics—The Facts 2019: An Analysis of European Plastics Production, Demand and Waste Data; PlasticsEurope: Brussels, Belgium, 2019. [Google Scholar]
- European Commission. A European Strategy for Plastics in a Circular Economy; European Commission: Brussels, Belgium, 2018. [Google Scholar]
- Umweltbundesamt. Aufkommen und Verwertung von Verpackungsabfällen in Deutschland im Jahr 2018: Abschlussbericht; Umweltbundesamt: Vienna, Austria, 2020; Volume 166. [Google Scholar]
- Ragaert, K.; Delva, L.; van Geem, K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 2017, 69, 24–58. [Google Scholar] [CrossRef] [PubMed]
- Van Eygen, E.; Feketitsch, J.; Laner, D.; Rechberger, H.; Fellner, J. Comprehensive analysis and quantification of national plastic flows: The case of Austria. Resour. Conserv. Recycl. 2017, 117, 183–194. [Google Scholar] [CrossRef]
- Sohail, M.; Sun, D.-W.; Zhu, Z. Recent Developments in Intelligent Packaging for Enhancing Food Quality and Safety. Crit. Rev. Food Sci. Nutr. 2018, 58, 2650–2662. [Google Scholar] [CrossRef]
- Taleb, M.A.; Al Farooque, O. Towards a circular economy for sustainable development: An application of full cost accounting to municipal waste recyclables. J. Clean. Prod. 2021, 280. [Google Scholar] [CrossRef]
- European Commission. Closing the Loop—An EU Action Plan for the Circular Economy; European Commission: Brussels, Belgium, 2015. [Google Scholar]
- European Commission. On the Implementation of the Circular Economy Action Plan; European Commission: Brussels, Belgium, 2019. [Google Scholar]
- Pro Carton. Wie Wichtig Ist Nachhaltige Verpackung?: Die Einstellung von Konsumenten zu Verpackung und Nachhaltigkeit; Pro Carton: Vienna, Austria, 2010; Volume 3. [Google Scholar]
- Yildirim, S.; Röcker, B.; Pettersen, M.K.; Nilsen-Nygaard, J.; Ayhan, Z.; Rutkaite, R.; Radusin, T.; Suminska, P.; Marcos, B.; Coma, V. Active packaging applications for food. Compr. Rev. Food Sci. Food Saf. 2018, 17, 165–199. [Google Scholar] [CrossRef]
- Pauer, E.; Tacker, M.; Gabriel, V.; Krauter, V. Sustainability of flexible multilayer packaging: Environmental impacts and recyclability of packaging for bacon in block. Clean. Environ. Syst. 2020, 1. [Google Scholar] [CrossRef]
- Fabra, M.J.; López-Rubio, A.; Lagaron, J.M. Biopolymers for food packaging applications. In Smart Polymers and Their Applications; Elsevier: Amsterdam, The Netherlands, 2014; pp. 476–509. ISBN 9780857096951. [Google Scholar]
- Koller, M.; Sandholzer, D.; Salerno, A.; Braunegg, G.; Narodoslawsky, M. Biopolymer from industrial residues: Life cycle assessment of poly(hydroxyalkanoates) from whey. Resour. Conserv. Recycl. 2013, 73, 64–71. [Google Scholar] [CrossRef]
- Narodoslawsky, M. LCA of PHA Production—Identifying the Ecological Potential of Bio-plastic. Chem. Biochem. Eng. Q. 2015, 29, 299–305. [Google Scholar] [CrossRef]
- Ahorsu, R.; Medina, F.; Constantí, M. Significance and challenges of biomass as a suitable feedstock for bioenergy and biochemical production: A review. Energies 2018, 11. [Google Scholar] [CrossRef]
- Xu, C.; Arancon, R.A.D.; Labidi, J.; Luque, R. Lignin depolymerisation strategies: Towards valuable chemicals and fuels. Chem. Soc. Rev. 2014, 43, 7485–7500. [Google Scholar] [CrossRef] [PubMed]
- Daioglou, V.; Wicke, B.; Faaij, A.P.C.; van Vuuren, D.P. Competing uses of biomass for energy and chemicals: Implications for long-term global CO2 mitigation potential. GCB Bioenergy 2015, 7, 1321–1334. [Google Scholar] [CrossRef]
- Weiss, M.; Haufe, J.; Carus, M.; Brandão, M.; Bringezu, S.; Hermann, B.; Patel, M.K. A Review of the environmental impacts of biobased materials. J. Ind. Ecol. 2012, 16, 169–181. [Google Scholar] [CrossRef]
- Zhu, Y.; Romain, C.; Williams, C.K. Sustainable polymers from renewable resources. Nature 2016, 540, 354–362. [Google Scholar] [CrossRef]
- Broeren, M.L.; Kuling, L.; Worrell, E.; Shen, L. Environmental impact assessment of six starch plastics focusing on wastewater-derived starch and additives. Resour. Conserv. Recycl. 2017, 127, 246–255. [Google Scholar] [CrossRef]
- Rhim, J.-W.; Park, H.-M.; Ha, C.-S. Bio-nanocomposites for food packaging applications. Prog. Polym. Sci. 2013, 38, 1629–1652. [Google Scholar] [CrossRef]
- De Oliveira Filho, J.G.; Rodrigues, J.M.; Valadares, A.C.F.; de Almeida, A.B.; de Lima, T.M.; Takeuchi, K.P.; Alves, C.C.F.; de Figueiredo Sousa, H.A.; Da Silva, E.R.; Dyszy, F.H.; et al. Active food packaging: Alginate films with cottonseed protein hydrolysates. Food Hydrocoll. 2019, 92, 267–275. [Google Scholar] [CrossRef]
- Van Sluisveld, M.A.; Worrell, E. The paradox of packaging optimization—A characterization of packaging source reduction in the Netherlands. Resour. Conserv. Recycl. 2013, 73, 133–142. [Google Scholar] [CrossRef]
- Marra, A.; Silvestre, C.; Duraccio, D.; Cimmino, S. Polylactic acid/zinc oxide biocomposite films for food packaging application. Int. J. Biol. Macromol. 2016, 88, 254–262. [Google Scholar] [CrossRef]
- Hahladakis, J.N.; Iacovidou, E. Closing the loop on plastic packaging materials: What is quality and how does it affect their circularity? Sci. Total Environ. 2018, 630, 1394–1400. [Google Scholar] [CrossRef]
- Van den Oever, M.; Molenveld, K.; van der Zee, M.; Bos, H. Bio-Based and Biodegradable Plastics: Facts and Figures; Wageningen Food & Biobased Research: Wageningen, The Netherlands, 2017. [Google Scholar] [CrossRef]
- McMillin, K.W. Advancements in meat packaging. Meat Sci. 2017, 132, 153–162. [Google Scholar] [CrossRef]
- Molina-Besch, K.; Wikström, F.; Williams, H. The environmental impact of packaging in food supply chains—does life cycle assessment of food provide the full picture? Int. J. Life Cycle Assess 2019, 24, 37–50. [Google Scholar] [CrossRef]
- Wikström, F.; Verghese, K.; Auras, R.; Olsson, A.; Williams, H.; Wever, R.; Grönman, K.; Kvalvåg Pettersen, M.; Møller, H.; Soukka, R. Packaging Strategies That Save Food: A Research Agenda for 2030. J. Ind. Ecol. 2019, 23, 532–540. [Google Scholar] [CrossRef]
- Garcia-Arce, J.; Garrido, A.T.G.-P.; Prado-Prado, J.C. Implementing sustainable packaging logistics. An analysis in liquid detergents. Dir. Organ. 2016, 60, 47–56. [Google Scholar]
- Ma, X.; Park, C.; Moultrie, J. Factors for eliminating plastic in packaging: The European FMCG experts’ view. J. Clean. Prod. 2020, 256. [Google Scholar] [CrossRef]
- Dilkes-Hoffman, L.S.; Lane, J.L.; Grant, T.; Pratt, S.; Lant, P.A.; Laycock, B. Environmental impact of biodegradable food packaging when considering food waste. J. Clean. Prod. 2018, 180, 325–334. [Google Scholar] [CrossRef]
- Mulakkal, M.C.; Castillo Castillo, A.; Taylor, A.C.; Blackman, B.R.; Balint, D.S.; Pimenta, S.; Charalambides, M.N. Advancing mechanical recycling of multilayer plastics through finite element modelling and environmental policy. Resour. Conserv. Recycl. 2021, 166. [Google Scholar] [CrossRef]
- Statista. Recyclingquote Verschiedener Abfallarten in der EU im Jahr 2017; Statista: Hamburg, Germany, 2020. [Google Scholar]
- Statista. Recycling Rate of Plastic Packaging Waste in the European Union (EU-28) from 2006 to 2017; Statista: Hamburg, Germany, 2019. [Google Scholar]
- Statista. Recyclingquoten von Altpapier in Europa in den Jahren 1991 bis 2019; Statista: Hamburg, Germany, 2020. [Google Scholar]
- European Union. Directive (EU) 2018/852 of the European Parliament and of the Council of 30 May 2018 amending Directive 94/62/EC on packaging and packaging waste. Off. J. Eur. Union 2018, 150, 141. [Google Scholar]
- Sun, H.; Guo, G.; Memon, S.A.; Xu, W.; Zhang, Q.; Zhu, J.-H.; Xing, F. Recycling of carbon fibers from carbon fiber reinforced polymer using electrochemical method. Compos. Part A Appl. Sci. Manuf. 2015, 78, 10–17. [Google Scholar] [CrossRef]
- Deng, J.; Xu, L.; Liu, J.; Peng, J.; Han, Z.; Shen, Z.; Guo, S. Efficient method of recycling carbon fiber from the waste of carbon fiber reinforced polymer composites. Polym. Degrad. Stab. 2020, 182. [Google Scholar] [CrossRef]
- Mumbach, G.D.; Bolzan, A.; Machado, R.A.F. A closed-loop process design for recycling expanded polystyrene waste by dissolution and polymerization. Polymer 2020, 209. [Google Scholar] [CrossRef]
- Abdou, T.R.; Botelho, A.B., Jr.; Espinosa, D.C.R.; Tenório, J.A.S. Recycling of polymeric composites from industrial waste by pyrolysis: Deep evaluation for carbon fibers reuse. Waste Manag. 2021, 120, 1–9. [Google Scholar] [CrossRef]
- Khui, P.L.N.; Rahman, M.R.; Jayamani, E.; Bin Bakri, M.K. Recycling of sustainable polymers and composites. In Advances in Sustainable Polymer Composites; Elsevier: Amsterdam, The Netherlands, 2021; pp. 267–282. ISBN 9780128203385. [Google Scholar]
- Ingrao, C.; Gigli, M.; Siracusa, V. An attributional Life Cycle Assessment application experience to highlight environmental hotspots in the production of foamy polylactic acid trays for fresh-food packaging usage. J. Clean. Prod. 2017, 150, 93–103. [Google Scholar] [CrossRef]
- White, A.; Lockyer, S. Removing plastic packaging from fresh produce—What’s the impact? Nutr. Bull. 2020, 45, 35–50. [Google Scholar] [CrossRef]
- Bhat, R.; Jõudu, I. Emerging issues and challenges in agri-food supply chain. In Sustainable Food Supply Chains; Elsevier: Amsterdam, The Netherlands, 2019; pp. 23–37. ISBN 9780128134115. [Google Scholar]
- United Nations. Transforming our world: The 2030 Agenda for Sustainable Development. In General Assembly; A/Res/70/1; United Nations: New York, NY, USA, 2015. [Google Scholar]
- Mak, T.M.W.; Xiong, X.; Tsang, D.C.W.; Yu, I.K.M.; Poon, C.S. Sustainable food waste management towards circular bioeconomy: Policy review, limitations and opportunities. Bioresour. Technol. 2020, 297. [Google Scholar] [CrossRef]
- Bruckner, S.; Albrecht, A.; Petersen, B.; Kreyenschmidt, J. Characterization and comparison of spoilage processes in fresh pork and poultry. J. Food Qual. 2012, 35, 372–382. [Google Scholar] [CrossRef]
- Herbert, U.; Albrecht, A.; Kreyenschmidt, J. Definition of predictor variables for MAP poultry filets stored under different temperature conditions. Poult. Sci. 2015, 94, 424–432. [Google Scholar] [CrossRef]
- Caldeira, C.; de Laurentiis, V.; Corrado, S.; van Holsteijn, F.; Sala, S. Quantification of food waste per product group along the food supply chain in the European Union: A mass flow analysis. Resour. Conserv. Recycl. 2019, 149, 479–488. [Google Scholar] [CrossRef]
- Mena, C.; Adenso-Diaz, B.; Yurt, O. The causes of food waste in the supplier–retailer interface: Evidences from the UK and Spain. Resour. Conserv. Recycl. 2011, 55, 648–658. [Google Scholar] [CrossRef]
- Rossaint, S.; Kreyenschmidt, J. Intelligent label—A new way to support food waste reduction. Waste Resour. Manag. 2014, 168, 63–71. [Google Scholar] [CrossRef]
- Scherhaufer, S.; Moates, G.; Hartikainen, H.; Waldron, K.; Obersteiner, G. Environmental impacts of food waste in Europe. Waste Manag. 2018, 77, 98–113. [Google Scholar] [CrossRef] [PubMed]
- Williams, H.; Wikström, F. Environmental impact of packaging and food losses in a life cycle perspective: A comparative analysis of five food items. J. Clean. Prod. 2011, 19, 43–48. [Google Scholar] [CrossRef]
- Heller, M.C.; Selke, S.E.M.; Keoleian, G.A. Mapping the influence of food waste in food packaging environmental performance assessments. J. Ind. Ecol. 2019, 23, 480–495. [Google Scholar] [CrossRef]
- Verghese, K.; Lewis, H.; Lockrey, S.; Williams, H. Packaging’s role in minimizing food loss and waste across the supply chain. Packag. Technol. Sci. 2015, 28, 603–620. [Google Scholar] [CrossRef]
- Steinbuchel, A. Biopolymers: General Aspects and Special Applications; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Conte, A.; Cappelletti, G.M.; Nicoletti, G.M.; Russo, C.; Del Nobile, M.A. Environmental implications of food loss probability in packaging design. Food Res. Int. 2015, 78, 11–17. [Google Scholar] [CrossRef]
- Pettersen, M.K.; Grøvlen, M.S.; Evje, N.; Radusin, T. Recyclable mono materials for packaging of fresh chicken fillets: New design for recycling in circular economy. Packag. Technol. Sci. 2020, 33, 485–498. [Google Scholar] [CrossRef]
- Zhang, H.; Hortal, M.; Dobon, A.; Bermudez, J.M.; Lara-Lledo, M. The Effect of Active Packaging on Minimizing Food Losses: Life Cycle Assessment (LCA) of Essential Oil Component-enabled Packaging for Fresh Beef. Packag. Technol. Sci. 2015, 28, 761–774. [Google Scholar] [CrossRef]
- Van de Nadort, A. Opinion of the European Committee of the Regions—Communication on a European Strategy for Plastics in a circular economy. Off. J. Eur. Union 2018, 461, 30. [Google Scholar]
- Worrell, E.; Allwood, J.; Gutowski, T. The Role of Material Efficiency in Environmental Stewardship. Annu. Rev. Environ. Resour. 2016, 41, 575–598. [Google Scholar] [CrossRef]
- Verghese, K.; Lewis, H.; Fitzpatrick, L. Packaging for Sustainability; Springer: London, UK, 2012. [Google Scholar] [CrossRef]
- Popović, S.Z.; Lazić, V.L.; Hromiš, N.M.; Šuput, D.Z.; Bulut, S.N. Biopolymer Packaging materials for food shelf-life prolongation. In Biopolymers for Food Design; Elsevier: Amsterdam, The Netherlands, 2018; pp. 223–277. ISBN 9780128114490. [Google Scholar]
- Xia, C.; Wang, W.; Wang, L.; Liu, H.; Xiao, J. Multilayer zein/gelatin films with tunable water barrier property and prolonged antioxidant activity. Food Packag. Shelf Life 2019, 19, 76–85. [Google Scholar] [CrossRef]
- Wang, W.; Gu, F.; Deng, Z.; Zhu, Y.; Zhu, J.; Guo, T.; Song, J.; Xiao, H. Mulitlayer surface construction for enhancing barrier properties of cellulose-based packaging. Carbohydr. Polym. 2021, 255. [Google Scholar] [CrossRef]
- Biscarat, J.; Charmette, C.; Sanchez, J.; Pochat-Bohatier, C. Development of a new family of food packaging bioplastics from cross-linked gelatin based films. Can. J. Chem. Eng. 2015, 93, 176–182. [Google Scholar] [CrossRef]
- Asgher, M.; Urooj, Y.; Qamar, S.A.; Khalid, N. Improved exopolysaccharide production from Bacillus licheniformis MS3: Optimization and structural/functional characterization. Int. J. Biol. Macromol. 2020, 151, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Cazón, P.; Velazquez, G.; Ramírez, J.A.; Vázquez, M. Polysaccharide-based films and coatings for food packaging: A review. Food Hydrocoll. 2017, 68, 136–148. [Google Scholar] [CrossRef]
- Kumar, N.; Kaur, P.; Bhatia, S. Advances in bio-nanocomposite materials for food packaging: A review. Nutr. Food Sci. 2017, 47, 591–606. [Google Scholar] [CrossRef]
- Thakur, S.; Chaudhary, J.; Sharma, B.; Verma, A.; Tamulevicius, S.; Thakur, V.K. Sustainability of bioplastics: Opportunities and challenges. Curr. Opin. Green Sustain. Chem. 2018, 13, 68–75. [Google Scholar] [CrossRef]
- Vejdan, A.; Ojagh, S.M.; Adeli, A.; Abdollahi, M. Effect of TiO2 nanoparticles on the physico-mechanical and ultraviolet light barrier properties of fish gelatin/agar bilayer film. LWT Food Sci. Technol. 2016, 71, 88–95. [Google Scholar] [CrossRef]
- Bahrami, A.; Rezaei Mokarram, R.; Sowti Khiabani, M.; Ghanbarzadeh, B.; Salehi, R. Physico-mechanical and antimicrobial properties of tragacanth/hydroxypropyl methylcellulose/beeswax edible films reinforced with silver nanoparticles. Int. J. Biol. Macromol. 2019, 129, 1103–1112. [Google Scholar] [CrossRef]
- Davachi, S.M.; Shekarabi, A.S. Preparation and characterization of antibacterial, eco-friendly edible nanocomposite films containing Salvia macrosiphon and nanoclay. Int. J. Biol. Macromol. 2018, 113, 66–72. [Google Scholar] [CrossRef]
- Saral Sarojini, K.; Indumathi, M.P.; Rajarajeswari, G.R. Mahua oil-based polyurethane/chitosan/nano ZnO composite films for biodegradable food packaging applications. Int. J. Biol. Macromol. 2019, 124, 163–174. [Google Scholar] [CrossRef]
- Ma, P.; Hristova-Bogaerds, D.G.; Goossens, J.; Spoelstra, A.B.; Zhang, Y.; Lemstra, P.J. Toughening of poly(lactic acid) by ethylene-co-vinyl acetate copolymer with different vinyl acetate contents. Eur. Polym. J. 2012, 48, 146–154. [Google Scholar] [CrossRef]
- Kadam, D.M.; Thunga, M.; Wang, S.; Kessler, M.R.; Grewell, D.; Lamsal, B.; Yu, C. Preparation and characterization of whey protein isolate films reinforced with porous silica coated titania nanoparticles. J. Food Eng. 2013, 117, 133–140. [Google Scholar] [CrossRef]
- Youssef, A.M.; Malhat, F.M.; Abdel Hakim, A.A.; Dekany, I. Synthesis and utilization of poly (methylmethacrylate) nanocomposites based on modified montmorillonite. Arab. J. Chem. 2017, 10, 631–642. [Google Scholar] [CrossRef]
- Malathi, A.N.; Santhosh, K.S.; Nidoni, U. Recent trends of Biodegradable polymer: Biodegradable films for Food Packaging and application of Nanotechnology in Biodegradable Food Packaging. Curr. Trends Technol. Sci. 2014, 3, 73–79. [Google Scholar]
- Asgher, M.; Arshad, S.; Qamar, S.A.; Khalid, N. Improved biosurfactant production from Aspergillus niger through chemical mutagenesis: Characterization and RSM optimization. SN Appl. Sci. 2020, 2. [Google Scholar] [CrossRef]
- Ahmed, T.; Shahid, M.; Azeem, F.; Rasul, I.; Shah, A.A.; Noman, M.; Hameed, A.; Manzoor, N.; Manzoor, I.; Muhammad, S. Biodegradation of plastics: Current scenario and future prospects for environmental safety. Environ. Sci. Pollut. Res. 2018, 25, 7287–7298. [Google Scholar] [CrossRef] [PubMed]
- European Bioplastics. What are Bioplastics?: Material Types, Terminology, and Labels—An Introduction. Available online: https://docs.european-bioplastics.org/publications/fs/EuBP_FS_What_are_bioplastics.pdf (accessed on 26 February 2021).
- WRAP. Understanding Plastic Packaging and the Language We Use to Describe It; WRAP: Banbury, UK, 2018. [Google Scholar]
- Lambert, S.; Wagner, M. Environmental performance of bio-based and biodegradable plastics: The road ahead. Chem. Soc. Rev. 2017, 46, 6855–6871. [Google Scholar] [CrossRef] [PubMed]
- Asgher, M.; Qamar, S.A.; Bilal, M.; Iqbal, H.M.N. Bio-based active food packaging materials: Sustainable alternative to conventional petrochemical-based packaging materials. Food Res. Int. 2020, 137. [Google Scholar] [CrossRef]
- Peelman, N.; Ragaert, P.; Meulenaer, B.d.; Adons, D.; Peeters, R.; Cardon, L.; van Impe, F.; Devlieghere, F. Application of bioplastics for food packaging. Trends Food Sci. Technol. 2013, 32, 128–141. [Google Scholar] [CrossRef]
- Saha, T.; Hoque, M.E.; Mahbub, T. Biopolymers for Sustainable Packaging in Food, Cosmetics, and Pharmaceuticals. In Advanced Processing, Properties, and Applications of Starch and Other Bio-Based Polymers; Elsevier: Amsterdam, The Netherlands, 2020; pp. 197–214. ISBN 9780128196618. [Google Scholar]
- Kamdem, D.P.; Shen, Z.; Nabinejad, O.; Shu, Z. Development of biodegradable composite chitosan-based films incorporated with xylan and carvacrol for food packaging application. Food Packag. Shelf Life 2019, 21. [Google Scholar] [CrossRef]
- Tawakkal, I.S.M.A.; Cran, M.J.; Miltz, J.; Bigger, S.W. A review of poly(lactic acid)-based materials for antimicrobial packaging. J. Food Sci. 2014, 79, 1477–1490. [Google Scholar] [CrossRef]
- Rasal, R.M.; Janorkar, A.V.; Hirt, D.E. Poly(lactic acid) modifications. Prog. Polym. Sci. 2010, 35, 338–356. [Google Scholar] [CrossRef]
- Byun, Y.; Kim, Y.T. Utilization of Bioplastics for Food Packaging Industry. In Innovations in Food Packaging; Elsevier: Amsterdam, The Netherlands, 2014; pp. 369–390. ISBN 9780123946010. [Google Scholar]
- Jabeen, N.; Majid, I.; Nayik, G.A.; Yildiz, F. Bioplastics and food packaging: A review. Cogent Food Agric. 2015, 1. [Google Scholar] [CrossRef]
- Zinoviadou, K.G.; Gougouli, M.; Biliaderis, C.G. Innovative biobased materials for packaging sustainability. In Innovation Strategies in the Food Industry; Elsevier: Amsterdam, The Netherlands, 2016; pp. 167–189. ISBN 9780128037515. [Google Scholar]
- Auras, R.; Harte, B.; Selke, S. An overview of polylactides as packaging materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef]
- Benetto, E.; Jury, C.; Igos, E.; Carton, J.; Hild, P.; Vergne, C.; Di Martino, J. Using atmospheric plasma to design multilayer film from polylactic acid and thermoplastic starch: A screening Life Cycle Assessment. J. Clean. Prod. 2015, 87, 953–960. [Google Scholar] [CrossRef]
- Ahmadzadeh, S.; Khaneghah, A.M. Role of Green Polymers in Food Packaging. In Encyclopedia of Renewable and Sustainable Materials; Choudhury, I., Hashmi, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 305–319. ISBN 9780128131961. [Google Scholar]
- Ragaert, P.; Buntinx, M.; Maes, C.; Vanheusden, C.; Peeters, R.; Wang, S.; D’hooge, D.R.; Cardon, L. Polyhydroxyalkanoates for Food Packaging Applications. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780081005965. [Google Scholar]
- Crompton, T.R. Physical Testing of Plastics; Smithers Rapra: Shawbury, UK, 2012; ISBN 978-1-84735-487-7. [Google Scholar]
- Lange, J.; Wyser, Y. Recent innovations in barrier technologies for plastic packaging—A review. Packag. Technol. Sci. 2003, 16, 149–158. [Google Scholar] [CrossRef]
- Massey, L.K. Permeability Properties of Plastics and Elastomers: A Guide to Packaging and Barrier Materials, 2nd ed.; Elsevier Science: Norwich, UK, 2003; ISBN 9780815518518. [Google Scholar]
- Drieskens, M.; Peeters, R.; Mullens, J.; Franco, D.; Lemstra, P.J.; Hristova-Bogaerds, D.G. Structure versus properties relationship of poly(lactic acid). I. Effect of crystallinity on barrier properties. J. Polym. Sci. Part B Polym. Phys. 2009, 47, 2247–2258. [Google Scholar] [CrossRef]
- Rhim, J.-W.; Hong, S.-I.; Ha, C.-S. Tensile, water vapor barrier and antimicrobial properties of PLA/nanoclay composite films. LWT Food Sci. Technol. 2009, 42, 612–617. [Google Scholar] [CrossRef]
- Raquez, J.-M.; Habibi, Y.; Murariu, M.; Dubois, P. Polylactide (PLA)-based nanocomposites. Prog. Polym. Sci. 2013, 38, 1504–1542. [Google Scholar] [CrossRef]
- Panseri, S.; Martino, P.A.; Cagnardi, P.; Celano, G.; Tedesco, D.; Castrica, M.; Balzaretti, C.; Chiesa, L.M. Feasibility of biodegradable based packaging used for red meat storage during shelf-life: A pilot study. Food Chem. 2018, 249, 22–29. [Google Scholar] [CrossRef]
- Vanitha, R.; Kavitha, C. Development of natural cellulose fiber and its food packaging application. Mater. Today Proc. 2021, 36, 903–906. [Google Scholar] [CrossRef]
- Domenek, S.; Louaifi, A.; Guinault, A.; Baumberger, S. Potential of lignins as antioxidant additive in active biodegradable packaging materials. J. Polym. Environ. 2013, 21, 692–701. [Google Scholar] [CrossRef]
- Spiridon, I.; Tanase, C.E. Design, characterization and preliminary biological evaluation of new lignin-PLA biocomposites. Int. J. Biol. Macromol. 2018, 114, 855–863. [Google Scholar] [CrossRef]
- Gordobil, O.; Egüés, I.; Llano-Ponte, R.; Labidi, J. Physicochemical properties of PLA lignin blends. Polym. Degrad. Stab. 2014, 108, 330–338. [Google Scholar] [CrossRef]
- Kovalcik, A.; Pérez-Camargo, R.A.; Fürst, C.; Kucharczyk, P.; Müller, A.J. Nucleating efficiency and thermal stability of industrial non-purified lignins and ultrafine talc in poly(lactic acid) (PLA). Polym. Degrad. Stab. 2017, 142, 244–254. [Google Scholar] [CrossRef]
- Kumar, A.; Tumu, V.R.; Chowdhury, S.R.; Ramana Reddy, S.V.S. A green physical approach to compatibilize a bio-based poly (lactic acid)/lignin blend for better mechanical, thermal and degradation properties. Int. J. Biol. Macromol. 2019, 121, 588–600. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hegyesi, N.; Zhang, Y.; Mao, Z.; Feng, X.; Wang, B.; Pukánszky, B.; Sui, X. Poly(lactic acid)/lignin blends prepared with the Pickering emulsion template method. Eur. Polym. J. 2019, 110, 378–384. [Google Scholar] [CrossRef]
- Yang, W.; Fortunati, E.; Dominici, F.; Giovanale, G.; Mazzaglia, A.; Balestra, G.M.; Kenny, J.M.; Puglia, D. Effect of cellulose and lignin on disintegration, antimicrobial and antioxidant properties of PLA active films. Int. J. Biol. Macromol. 2016, 89, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Fortunati, E.; Dominici, F.; Giovanale, G.; Mazzaglia, A.; Balestra, G.M.; Kenny, J.M.; Puglia, D. Synergic effect of cellulose and lignin nanostructures in PLA based systems for food antibacterial packaging. Eur. Polym. J. 2016, 79, 1–12. [Google Scholar] [CrossRef]
- Wei, L.; Agarwal, U.P.; Matuana, L.; Sabo, R.C.; Stark, N.M. Performance of high lignin content cellulose nanocrystals in poly(lactic acid). Polymer 2018, 135, 305–313. [Google Scholar] [CrossRef]
- Armentano, I.; Fortunati, E.; Burgos, N.; Dominici, F.; Luzi, F.; Fiori, S.; Jiménez, A.; Yoon, K.; Ahn, J.; Kang, S.; et al. Bio-based PLA_PHB plasticized blend films: Processing and structural characterization. LWT Food Sci. Technol. 2015, 64, 980–988. [Google Scholar] [CrossRef]
- Andersson, C. New ways to enhance the functionality of paperboard by surface treatment—A review. Packag. Technol. Sci. 2008, 21, 339–373. [Google Scholar] [CrossRef]
- Alavi, S.; Thomas, S.; Sandeep, K.P.; Kalarikkal, N.; Varghese, J.; Yaragalla, S. Polymers for Packaging Applications; Apple Academic Press: Palm Bay, FL, USA, 2014. [Google Scholar]
- Siracusa, V.; Blanco, I.; Romani, S.; Tylewicz, U.; Rocculi, P.; Rosa, M.D. Poly(lactic acid)-modified films for food packaging application: Physical, mechanical, and barrier behavior. J. Appl. Polym. Sci. 2012, 125, E390–E401. [Google Scholar] [CrossRef]
- Vandewijngaarden, J.; Murariu, M.; Dubois, P.; Carleer, R.; Yperman, J.; Adriaensens, P.; Schreurs, S.; Lepot, N.; Peeters, R.; Buntinx, M. Gas Permeability Properties of Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate). J. Polym. Environ. 2014, 22, 501–507. [Google Scholar] [CrossRef]
- Kovalcik, A.; Machovsky, M.; Kozakova, Z.; Koller, M. Designing packaging materials with viscoelastic and gas barrier properties by optimized processing of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) with lignin. React. Funct. Polym. 2015, 94, 25–34. [Google Scholar] [CrossRef]
- Farmahini-Farahani, M.; Khan, A.; Lu, P.; Bedane, A.H.; Eic, M.; Xiao, H. Surface morphological analysis and water vapor barrier properties of modified Cloisite 30B/poly(3-hydroxybutyrate-co-3-hydroxyvalerate) composites. Appl. Clay Sci. 2017, 135, 27–34. [Google Scholar] [CrossRef]
- Siracusa, V.; Ingrao, C.; Karpova, S.G.; Olkhov, A.A.; Iordanskii, A.L. Gas transport and characterization of poly(3 hydroxybutyrate) films. Eur. Polym. J. 2017, 91, 149–161. [Google Scholar] [CrossRef]
- Maes, C.; Luyten, W.; Herremans, G.; Peeters, R.; Carleer, R.; Buntinx, M. Recent updates on the barrier properties of ethylene vinyl alcohol copolymer (EVOH): A review. Polym. Rev. 2018, 58, 209–246. [Google Scholar] [CrossRef]
- Anjum, A.; Zuber, M.; Zia, K.M.; Noreen, A.; Anjum, M.N.; Tabasum, S. Microbial production of polyhydroxyalkanoates (PHAs) and its copolymers: A review of recent advancements. Int. J. Biol. Macromol. 2016, 89, 161–174. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Samper, M.D.; López, J.; Jiménez, A. Combined effect of poly(hydroxybutyrate) and plasticizers on polylactic acid properties for film intended for food packaging. J. Polym. Environ. 2014, 22, 460–470. [Google Scholar] [CrossRef]
- Schutyser, W.; Renders, T.; van den Bosch, S.; Koelewijn, S.-F.; Beckham, G.T.; Sels, B.F. Chemicals from lignin: An interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem. Soc. Rev. 2018, 47, 852–908. [Google Scholar] [CrossRef]
- Rumpf, J.; Do, X.T.; Burger, R.; Monakhova, Y.B.; Schulze, M. Extraction of high-purity lignins via catalyst-free organosolv pulping from low-input crops. Biomacromolecules 2020, 21, 1929–1942. [Google Scholar] [CrossRef]
- Rinaldi, R.; Jastrzebski, R.; Clough, M.T.; Ralph, J.; Kennema, M.; Bruijnincx, P.C.A.; Weckhuysen, B.M. Paving the way for lignin valorisation: Recent advances in bioengineering, biorefining and catalysis. Angew. Chem. Int. Ed. 2016, 55, 8164–8215. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Ferdosian, F. Conversion of Lignin into Bio-Based Chemicals and Materials; Springer: Berlin/Heidelberg, Germany, 2017; ISBN 978-3-662-54957-5. [Google Scholar]
- Tribot, A.; Amer, G.; Abdou Alio, M.; Baynast, H.d.; Delattre, C.; Pons, A.; Mathias, J.-D.; Callois, J.-M.; Vial, C.; Michaud, P.; et al. Wood-lignin: Supply, extraction processes and use as bio-based material. Eur. Polym. J. 2019, 112, 228–240. [Google Scholar] [CrossRef]
- Kim, K.H.; Jeong, K.; Zhuang, J.; Jeong, H.J.; Kim, C.S.; Koo, B.; Yoo, C.G. Tandem conversion of lignin to catechols via demethylation and catalytic hydrogenolysis. Ind. Crop. Prod. 2021, 159. [Google Scholar] [CrossRef]
- Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. Moving towards the second generation of lignocellulosic biorefineries in the EU: Drivers, challenges, and opportunities. Renew. Sustain. Energy Rev. 2019, 101, 590–599. [Google Scholar] [CrossRef]
- Renders, T.; van den Bossche, G.; Vangeel, T.; van Aelst, K.; Sels, B. Reductive catalytic fractionation: State of the art of the lignin-first biorefinery. Curr. Opin. Biotechnol. 2019, 56, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Espinoza-Acosta, J.L.; Torres-Chávez, P.I.; Ramírez-Wong, B.; López-Saiz, C.M.; Montaño-Leyva, B. Antioxidant, antimicrobial, and antimutagenic properties of technical lignins and their applications. BioResources 2016, 11, 5452–5481. [Google Scholar] [CrossRef]
- Azadfar, M.; Gao, A.H.; Bule, M.V.; Chen, S. Structural characterization of lignin: A potential source of antioxidants guaiacol and 4-vinylguaiacol. Int. J. Biol. Macromol. 2015, 75, 58–66. [Google Scholar] [CrossRef]
- Michelin, M.; Marques, A.M.; Pastrana, L.M.; Teixeira, J.A.; Cerqueira, M.A. Carboxymethyl cellulose-based films: Effect of organosolv lignin incorporation on physicochemical and antioxidant properties. J. Food Eng. 2020, 285. [Google Scholar] [CrossRef]
- Hult, E.-L.; Ropponen, J.; Poppius-Levlin, K.; Ohra-Aho, T.; Tamminen, T. Enhancing the barrier properties of paper board by a novel lignin coating. Ind. Crop. Prod. 2013, 50, 694–700. [Google Scholar] [CrossRef]
- Klein, S.E.; Rumpf, J.; Alzagameem, A.; Rehahn, M.; Schulze, M. Antioxidant activity of unmodified kraft and organosolv lignins to be used as sustainable components for polyurethane coatings. J. Coat. Technol. Res. 2019, 55, 97. [Google Scholar] [CrossRef]
- Klein, S.E.; Alzagameem, A.; Rumpf, J.; Korte, I.; Kreyenschmidt, J.; Schulze, M. Antimicrobial activity of lignin-derived polyurethane coatings prepared from unmodified and demethylated lignins. Coatings 2019, 9, 494. [Google Scholar] [CrossRef]
- Hao, C.; Liu, T.; Zhang, S.; Brown, L.; Li, R.; Xin, J.; Zhong, T.; Jiang, L.; Zhang, J. A High-lignin-content, removable, and glycol-assisted repairable coating based on dynamic covalent bonds. ChemSusChem 2019, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Hambardzumyan, A.; Foulon, L.; Bercu, N.B.; Pernes, M.; Maigret, J.E.; Molinari, M.; Chabbert, B.; Aguié-Béghin, V. Organosolv lignin as natural grafting additive to improve the water resistance of films using cellulose nanocrystals. Chem. Eng. J. 2015, 264, 780–788. [Google Scholar] [CrossRef]
- Rastogi, V.; Samyn, P. Bio-based coatings for paper applications. Coatings 2015, 5, 887–930. [Google Scholar] [CrossRef]
- Flory, A.R.; Vicuna Requesens, D.; Devaiah, S.P.; Teoh, K.T.; Mansfield, S.D.; Hood, E.E. Development of a green binder system for paper products. BMC Biotechnol. 2013, 13, 28. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Chen, C.; Liang, Z.; He, S.; Kuang, Y.; Song, J.; Mi, R.; Chen, G.; Jiao, M.; Hu, L. Lignin as a wood-inspired binder enabled strong, water stable, and biodegradable paper for plastic replacement. Adv. Funct. Mater. 2020, 30, 1906307. [Google Scholar] [CrossRef]
- Alzagameem, A.; El Khaldi-Hansen, B.; Kamm, B.; Schulze, M. Lignocellulosic biomass for energy, biofuels, biomaterials, and chemicals. In Biomass and Green Chemistry: Building a Renewable Pathway; Vaz, S., Jr., Ed.; Springer International Publishing: Basel, Switzerland, 2018; pp. 95–132. ISBN 978-3-319-66736-2. [Google Scholar]
- Alzagameem, A.; Khaldi-Hansen, B.E.; Büchner, D.; Larkins, M.; Kamm, B.; Witzleben, S.; Schulze, M. Lignocellulosic biomass as source for lignin-based environmentally benign antioxidants. Molecules 2018, 23. [Google Scholar] [CrossRef]
- Alzagameem, A.; Klein, S.E.; Bergs, M.; Do, X.T.; Korte, I.; Dohlen, S.; Hüwe, C.; Kreyenschmidt, J.; Kamm, B.; Larkins, M.; et al. Antimicrobial activity of lignin and lignin-derived cellulose and chitosan composites against selected pathogenic and spoilage microorganisms. Polymers 2019, 11. [Google Scholar] [CrossRef]
- Galus, S.; Kadzińska, J. Food applications of emulsion-based edible films and coatings. Trends Food Sci. Technol. 2015, 45, 273–283. [Google Scholar] [CrossRef]
- Otoni, C.G.; Avena-Bustillos, R.J.; Olsen, C.W.; Bilbao-Sáinz, C.; McHugh, T.H. Mechanical and water barrier properties of isolated soy protein composite edible films as affected by carvacrol and cinnamaldehyde micro and nanoemulsions. Food Hydrocoll. 2016, 57, 72–79. [Google Scholar] [CrossRef]
- Cho, S.Y.; Lee, S.Y.; Rhee, C. Edible oxygen barrier bilayer film pouches from corn zein and soy protein isolate for olive oil packaging. LWT Food Sci. Technol. 2010, 43, 1234–1239. [Google Scholar] [CrossRef]
- Denavi, G.; Tapia-Blácido, D.R.; Añón, M.C.; Sobral, P.; Mauri, A.N.; Menegalli, F.C. Effects of drying conditions on some physical properties of soy protein films. J. Food Eng. 2009, 90, 341–349. [Google Scholar] [CrossRef]
- Popović, S.; Peričin, D.; Vaštag, Ž.; Lazić, V.; Popović, L. Pumpkin oil cake protein isolate films as potential gas barrier coating. J. Food Eng. 2012, 110, 374–379. [Google Scholar] [CrossRef]
- Umaraw, P.; Verma, A.K. Comprehensive review on application of edible film on meat and meat products: An eco-friendly approach. Crit. Rev. Food Sci. Nutr. 2017, 57, 1270–1279. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Q.; Rempel, C. Processing and characteristics of canola protein-based biodegradable packaging: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Acquah, C.; Zhang, Y.; Dubé, M.A.; Udenigwe, C.C. Formation and characterization of protein-based films from yellow pea (Pisum sativum) protein isolate and concentrate for edible applications. Curr. Res. Food Sci. 2020, 2, 61–69. [Google Scholar] [CrossRef]
- Wang, Y.; Xiong, Y.L.; Rentfrow, G.K.; Newman, M.C. Oxidation promotes cross-linking but impairs film-forming properties of whey proteins. J. Food Eng. 2013, 115, 11–19. [Google Scholar] [CrossRef]
- Ramos, Ó.L.; Silva, S.I.; Soares, J.C.; Fernandes, J.C.; Poças, M.F.; Pintado, M.E.; Malcata, F.X. Features and performance of edible films, obtained from whey protein isolate formulated with antimicrobial compounds. Food Res. Int. 2012, 45, 351–361. [Google Scholar] [CrossRef]
- Fratzl, P. Collagen: Structure and Mechanics, an Introduction. In Collagen; Fratzl, P., Ed.; Springer: New York, NY, USA, 2008; pp. 1–13. ISBN 978-0-387-73906-9. [Google Scholar]
- Fadini, A.L.; Rocha, F.S.; Alvim, I.D.; Sadahira, M.S.; Queiroz, M.B.; Alves, R.; Silva, L.B. Mechanical properties and water vapour permeability of hydrolysed collagen–cocoa butter edible films plasticised with sucrose. Food Hydrocoll. 2013, 30, 625–631. [Google Scholar] [CrossRef]
- Oechsle, A.M.; Bugbee, T.J.; Gibis, M.; Kohlus, R.; Weiss, J. Modification of extruded chicken collagen films by addition of co-gelling protein and sodium chloride. J. Food Eng. 2017, 207, 46–55. [Google Scholar] [CrossRef]
- Ciannamea, E.M.; Castillo, L.A.; Barbosa, S.E.; Angelis, M.G.d. Barrier properties and mechanical strength of bio-renewable, heat-sealable films based on gelatin, glycerol and soybean oil for sustainable food packaging. React. Funct. Polym. 2018, 125, 29–36. [Google Scholar] [CrossRef]
- Chentir, I.; Kchaou, H.; Hamdi, M.; Jridi, M.; Li, S.; Doumandji, A.; Nasri, M. Biofunctional gelatin-based films incorporated with food grade phycocyanin extracted from the Saharian cyanobacterium Arthrospira sp. Food Hydrocoll. 2019, 89, 715–725. [Google Scholar] [CrossRef]
- Amjadi, S.; Hamishehkar, H.; Ghorbani, M. A novel smart PEGylated gelatin nanoparticle for co-delivery of doxorubicin and betanin: A strategy for enhancing the therapeutic efficacy of chemotherapy. Mater. Sci. Eng. C 2019, 97, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Nur Hanani, Z.A.; Roos, Y.H.; Kerry, J.P. Use and application of gelatin as potential biodegradable packaging materials for food products. Int. J. Biol. Macromol. 2014, 71, 94–102. [Google Scholar] [CrossRef]
- Mojumdar, S.C.; Moresoli, C.; Simon, L.C.; Legge, R.L. Edible wheat gluten (WG) protein films. J. Therm. Anal. Calorim. 2011, 104, 929–936. [Google Scholar] [CrossRef]
- Kanatt, S.R. Development of active/intelligent food packaging film containing Amaranthus leaf extract for shelf life extension of chicken/fish during chilled storage. Food Packag. Shelf Life 2020, 24, 100506. [Google Scholar] [CrossRef]
- Li, Y.; Chen, H.; Dong, Y.; Li, K.; Li, L.; Li, J. Carbon nanoparticles/soy protein isolate bio-films with excellent mechanical and water barrier properties. Ind. Crop. Prod. 2016, 82, 133–140. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Li, Y.; Zhu, L.; Fang, Z.; Shi, Q. Physicochemical, mechanical and structural properties of composite edible films based on whey protein isolate/psyllium seed gum. Int. J. Biol. Macromol. 2020, 153, 892–901. [Google Scholar] [CrossRef]
- Syahida, N.; Fitry, I.; Zuriyati, A.; Hanani, N. Effects of palm wax on the physical, mechanical and water barrier properties of fish gelatin films for food packaging application. Food Packag. Shelf Life 2020, 23, 100437. [Google Scholar] [CrossRef]
- Nur Hanani, Z.A.; O’Mahony, J.A.; Roos, Y.H.; Oliveira, P.M.; Kerry, J.P. Extrusion of gelatin-based composite films: Effects of processing temperature and pH of film forming solution on mechanical and barrier properties of manufactured films. Food Packag. Shelf Life 2014, 2, 91–101. [Google Scholar] [CrossRef]
- Han, J.H. Edible Films and Coatings. In Innovations in Food Packaging; Elsevier: Amsterdam, The Netherlands, 2014; pp. 213–255. ISBN 9780123946010. [Google Scholar]
- Jiménez, A.; Fabra, M.J.; Talens, P.; Chiralt, A. Edible and biodegradable starch films: A review. Food Bioprocess Technol. 2012, 5, 2058–2076. [Google Scholar] [CrossRef]
- Elsabee, M.Z.; Abdou, E.S. Chitosan based edible films and coatings: A review. Mater. Sci. Eng. C 2013, 33, 1819–1841. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Chen, C.; Rosswurm, K.; Yao, T.; Janaswamy, S. A facile route to prepare cellulose-based films. Carbohydr. Polym. 2016, 149, 274–281. [Google Scholar] [CrossRef]
- Witzler, M.; Büchner, D.; Shoushrah, S.H.; Babczyk, P.; Baranova, J.; Witzleben, S.; Tobiasch, E.; Schulze, M. Polysaccharide-Based Systems for Targeted Stem Cell Differentiation and Bone Regeneration. Biomolecules 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Muscat, D.; Adhikari, B.; Adhikari, R.; Chaudhary, D.S. Comparative study of film forming behaviour of low and high amylose starches using glycerol and xylitol as plasticizers. J. Food Eng. 2012, 109, 189–201. [Google Scholar] [CrossRef]
- Dang, K.M.; Yoksan, R. Development of thermoplastic starch blown film by incorporating plasticized chitosan. Carbohydr. Polym. 2015, 115, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Dang, K.M.; Yoksan, R. Morphological characteristics and barrier properties of thermoplastic starch/chitosan blown film. Carbohydr. Polym. 2016, 150, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Nazan Turhan, K.; Şahbaz, F. Water vapor permeability, tensile properties and solubility of methylcellulose-based edible films. J. Food Eng. 2004, 61, 459–466. [Google Scholar] [CrossRef]
- Hay, W.T.; Fanta, G.F.; Peterson, S.C.; Thomas, A.J.; Utt, K.D.; Walsh, K.A.; Boddu, V.M.; Selling, G.W. Improved hydroxypropyl methylcellulose (HPMC) films through incorporation of amylose-sodium palmitate inclusion complexes. Carbohydr. Polym. 2018, 188, 76–84. [Google Scholar] [CrossRef]
- Jannatyha, N.; Shojaee-Aliabadi, S.; Moslehishad, M.; Moradi, E. Comparing mechanical, barrier and antimicrobial properties of nanocellulose/CMC and nanochitosan/CMC composite films. Int. J. Biol. Macromol. 2020, 164, 2323–2328. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Reddy, J.P.; Rhim, J.-W. Effect of lignin on water vapor barrier, mechanical, and structural properties of agar/lignin composite films. Int. J. Biol. Macromol. 2015, 81, 267–273. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Effect of CuS reinforcement on the mechanical, water vapor barrier, UV-light barrier, and antibacterial properties of alginate-based composite films. Int. J. Biol. Macromol. 2020, 164, 37–44. [Google Scholar] [CrossRef]
- Kurek, M.; Galus, S.; Debeaufort, F. Surface, mechanical and barrier properties of bio-based composite films based on chitosan and whey protein. Food Packag. Shelf Life 2014, 1, 56–67. [Google Scholar] [CrossRef]
- Hassan, B.; Chatha, S.A.S.; Hussain, A.I.; Zia, K.M.; Akhtar, N. Recent advances on polysaccharides, lipids and protein based edible films and coatings: A review. Int. J. Biol. Macromol. 2018, 109, 1095–1107. [Google Scholar] [CrossRef] [PubMed]
- Molavi, H.; Behfar, S.; Ali Shariati, M.; Kaviani, M.; Atarod, S. A review on biodegradable starch based film. J. Microbiol. Biotechnol. Food Sci. 2015, 4, 456–461. [Google Scholar] [CrossRef]
- Lumdubwong, N. Applications of Starch-Based Films in Food Packaging. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780081005965. [Google Scholar]
- Do Val Siqueira, L.; La Arias, C.I.F.; Maniglia, B.C.; Tadini, C.C. Starch-based biodegradable plastics: Methods of production, challenges and future perspectives. Curr. Opin. Food Sci. 2021, 38, 122–130. [Google Scholar] [CrossRef]
- Fakhouri, F.M.; Martelli, S.M.; Caon, T.; Velasco, J.I.; Mei, L.H.I. Edible films and coatings based on starch/gelatin: Film properties and effect of coatings on quality of refrigerated Red Crimson grapes. Postharvest Biol. Technol. 2015, 109, 57–64. [Google Scholar] [CrossRef]
- Thakur, R.; Pristijono, P.; Scarlett, C.J.; Bowyer, M.; Singh, S.P.; Vuong, Q.V. Starch-based films: Major factors affecting their properties. Int. J. Biol. Macromol. 2019, 132, 1079–1089. [Google Scholar] [CrossRef]
- Ojogbo, E.; Ogunsona, E.O.; Mekonnen, T.H. Chemical and physical modifications of starch for renewable polymeric materials. Mater. Today Sustain. 2020, 7, 100028. [Google Scholar] [CrossRef]
- Bhat, R.; Abdullah, N.; Din, R.H.; Tay, G.-S. Producing novel sago starch based food packaging films by incorporating lignin isolated from oil palm black liquor waste. J. Food Eng. 2013, 119, 707–713. [Google Scholar] [CrossRef]
- Miranda, C.S.; Ferreira, M.S.; Magalhães, M.T.; Bispo, A.P.G.; Oliveira, J.C.; Silva, J.B.; José, N.M. Starch-based films plasticized with glycerol and lignin from piassava fiber reinforced with nanocrystals from eucalyptus. Mater. Today Proc. 2015, 2, 134–140. [Google Scholar] [CrossRef]
- Miranda, C.S.; Ferreira, M.S.; Magalhães, M.T.; Santos, W.J.; Oliveira, J.C.; Silva, J.B.; José, N.M. Mechanical, thermal and barrier properties of starch-based films plasticized with glycerol and lignin and reinforced with cellulose nanocrystals. Mater. Today Proc. 2015, 2, 63–69. [Google Scholar] [CrossRef]
- Javed, A.; Ullsten, H.; Rättö, P.; Järnström, L. Lignin-containing coatings for packaging materials. Nord. Pulp Pap. Res. J. 2018, 33, 548–556. [Google Scholar] [CrossRef]
- Wang, S.; Lu, A.; Zhang, L. Recent advances in regenerated cellulose materials. Prog. Polym. Sci. 2016, 53, 169–206. [Google Scholar] [CrossRef]
- Guzman-Puyol, S.; Ceseracciu, L.; Tedeschi, G.; Marras, S.; Scarpellini, A.; Benítez, J.J.; Athanassiou, A.; Heredia-Guerrero, J.A. Transparent and robust all-cellulose nanocomposite packaging materials prepared in a mixture of trifluoroacetic acid and trifluoroacetic anhydride. Nanomaterials 2019, 9. [Google Scholar] [CrossRef]
- Mendes, F.R.; Bastos, M.S.; Mendes, L.G.; Silva, A.R.; Sousa, F.D.; Monteiro-Moreira, A.C.; Cheng, H.N.; Biswas, A.; Moreira, R.A. Preparation and evaluation of hemicellulose films and their blends. Food Hydrocoll. 2017, 70, 181–190. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, N.; Chen, M.; Wei, Y.; Liu, C. Functional packaging films originating from hemicelluloses laurate by direct transesterification in ionic liquid. Carbohydr. Polym. 2020, 229, 115336. [Google Scholar] [CrossRef]
- Dhall, R.K. Advances in edible coatings for fresh fruits and vegetables: A review. Crit. Rev. Food Sci. Nutr. 2013, 53, 435–450. [Google Scholar] [CrossRef]
- Bifani, V.; Ramírez, C.; Ihl, M.; Rubilar, M.; García, A.; Zaritzky, N. Effects of murta (Ugni molinae Turcz) extract on gas and water vapor permeability of carboxymethylcellulose-based edible films. LWT Food Sci. Technol. 2007, 40, 1473–1481. [Google Scholar] [CrossRef]
- Farshchi, E.; Pirsa, S.; Roufegarinejad, L.; Alizadeh, M.; Rezazad, M. Photocatalytic/biodegradable film based on carboxymethyl cellulose, modified by gelatin and TiO2-Ag nanoparticles. Carbohydr. Polym. 2019, 216, 189–196. [Google Scholar] [CrossRef]
- Hasheminya, S.-M.; Rezaei Mokarram, R.; Ghanbarzadeh, B.; Hamishekar, H.; Kafil, H.S. Physicochemical, mechanical, optical, microstructural and antimicrobial properties of novel kefiran-carboxymethyl cellulose biocomposite films as influenced by copper oxide nanoparticles (CuONPs). Food Packag. Shelf Life 2018, 17, 196–204. [Google Scholar] [CrossRef]
- Arnon, H.; Zaitsev, Y.; Porat, R.; Poverenov, E. Effects of carboxymethyl cellulose and chitosan bilayer edible coating on postharvest quality of citrus fruit. Postharvest Biol. Technol. 2014, 87, 21–26. [Google Scholar] [CrossRef]
- Lan, W.; He, L.; Liu, Y. Preparation and properties of sodium carboxymethyl cellulose/sodium alginate/chitosan composite film. Coatings 2018, 8, 291. [Google Scholar] [CrossRef]
- Šešlija, S.; Nešić, A.; Škorić, M.L.; Krušić, M.K.; Santagata, G.; Malinconico, M. Pectin/carboxymethylcellulose films as a potential food packaging material. Macromol. Symp. 2018, 378. [Google Scholar] [CrossRef]
- Moura, M.R.d.; Avena-Bustillos, R.J.; McHugh, T.H.; Krochta, J.M.; Mattoso, L.H.C. Properties of novel hydroxypropyl methylcellulose films containing chitosan nanoparticles. J. Food Sci. 2008, 73, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Sebti, I.; Chollet, E.; Degraeve, P.; Noel, C.; Peyrol, E. Water sensitivity, antimicrobial, and physicochemical analyses of edible films based on HPMC and/or chitosan. J. Agric. Food Chem. 2007, 55, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Küçük, G.S.; Çelik, Ö.F.; Mazi, B.G.; Türe, H. Evaluation of alginate and zein films as a carrier of natamycin to increase the shelf life of kashar cheese. Packag. Technol. Sci. 2020, 33, 39–48. [Google Scholar] [CrossRef]
- Ahmed, N.M.; Adel, A.M.; Diab, M.A. Packaging paper with improved mechanical and oil absorption properties based on novel ingredients. Packag. Technol. Sci. 2020, 33, 303–320. [Google Scholar] [CrossRef]
- Gammariello, D.; Incoronato, A.L.; Conte, A.; Del Nobile, M.A. Effect of sodium alginate coating with ascorbic acid on shelf life of raw pork meat. J. Food Technol. Res. 2016, 3, 1–11. [Google Scholar] [CrossRef]
- Sangsuwan, J.; Sutthasupa, S. Effect of chitosan and alginate beads incorporated with lavender, clove essential oils, and vanillin against Botrytis cinerea and their application in fresh table grapes packaging system. Packag. Technol. Sci. 2019, 32, 595–605. [Google Scholar] [CrossRef]
- Raafat, D.; Sahl, H.-G. Chitosan and its antimicrobial potential—A critical literature survey. Microb. Biotechnol. 2009, 2, 186–201. [Google Scholar] [CrossRef] [PubMed]
- Morganti, P.; Stoller, M. Chitin and lignin: Natural ingredients from waste materials to make innovative and healthy products for humans and plant. Chem. Eng. Trans. 2017, 60, 319–324. [Google Scholar] [CrossRef]
- Witzler, M.; Alzagameem, A.; Bergs, M.; Khaldi-Hansen, B.E.; Klein, S.E.; Hielscher, D.; Kamm, B.; Kreyenschmidt, J.; Tobiasch, E.; Schulze, M. Lignin-derived biomaterials for drug release and tissue engineering. Molecules 2018, 23. [Google Scholar] [CrossRef]
- Rai, S.; Dutta, P.K.; Mehrotra, G.K. Lignin incorporated antimicrobial chitosan film for food packaging application. J. Polym. Mater. 2017, 34, 171–183. [Google Scholar]
- Shahid-Ul-Islam; Butola, B.S. Recent advances in chitosan polysaccharide and its derivatives in antimicrobial modification of textile materials. Int. J. Biol. Macromol. 2019, 121, 905–912. [Google Scholar] [CrossRef]
- Haghighi, H.; Licciardello, F.; Fava, P.; Siesler, H.W.; Pulvirenti, A. Recent advances on chitosan-based films for sustainable food packaging applications. Food Packag. Shelf Life 2020, 26, 100551. [Google Scholar] [CrossRef]
- Lin, L.; Xue, L.; Duraiarasan, S.; Haiying, C. Preparation of ε-polylysine/chitosan nanofibers for food packaging against Salmonella on chicken. Food Packag. Shelf Life 2018, 17, 134–141. [Google Scholar] [CrossRef]
- Sharaf, O.M.; Al-Gamal, M.S.; Ibrahim, G.A.; Dabiza, N.M.; Salem, S.S.; El-Ssayad, M.F.; Youssef, A.M. Evaluation and characterization of some protective culture metabolites in free and nano-chitosan-loaded forms against common contaminants of Egyptian cheese. Carbohydr. Polym. 2019, 223. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Rhim, J.-W. Chitosan-based biodegradable functional films for food packaging applications. Innov. Food Sci. Emerg. Technol. 2020, 62. [Google Scholar] [CrossRef]
- Shen, Z.; Kamdem, D.P. Development and characterization of biodegradable chitosan films containing two essential oils. Int. J. Biol. Macromol. 2015, 74, 289–296. [Google Scholar] [CrossRef]
- Aider, M. Chitosan application for active bio-based films production and potential in the food industry: Review. LWT Food Sci. Technol. 2010, 43, 837–842. [Google Scholar] [CrossRef]
- Chen, L.; Tang, C.; Ning, N.; Wang, C.; Fu, Q.; Zhang, Q. Preparation and properties of chitosan/lignin composite films. Chin. J. Polym. Sci. 2009, 27, 739. [Google Scholar] [CrossRef]
- Crouvisier-Urion, K.; Bodart, P.R.; Winckler, P.; Raya, J.; Gougeon, R.D.; Cayot, P.; Domenek, S.; Debeaufort, F.; Karbowiak, T. Bio-based composite films from chitosan and lignin: Antioxidant activity related to structure and moisture. ACS Sustain. Chem. Eng. 2016, 4, 6371–6381. [Google Scholar] [CrossRef]
- Crouvisier-Urion, K.; Lagorce-Tachon, A.; Lauquin, C.; Winckler, P.; Tongdeesoontorn, W.; Domenek, S.; Debeaufort, F.; Karbowiak, T. Impact of the homogenization process on the structure and antioxidant properties of chitosan-lignin composite films. Food Chem. 2017, 236, 120–126. [Google Scholar] [CrossRef]
- Yang, W.; Owczarek, J.S.; Fortunati, E.; Kozanecki, M.; Mazzaglia, A.; Balestra, G.M.; Kenny, J.M.; Torre, L.; Puglia, D. Antioxidant and antibacterial lignin nanoparticles in polyvinyl alcohol/chitosan films for active packaging. Ind. Crop. Prod. 2016, 94, 800–811. [Google Scholar] [CrossRef]
- Soltani Firouz, M.; Mohi-Alden, K.; Omid, M. A critical review on intelligent and active packaging in the food industry: Research and development. Food Res. Int. 2021, 141. [Google Scholar] [CrossRef]
- Mousavi Khaneghah, A.; Hashemi, S.M.B.; Limbo, S. Antimicrobial agents and packaging systems in antimicrobial active food packaging: An overview of approaches and interactions. Food Bioprod. Process. 2018, 111, 1–19. [Google Scholar] [CrossRef]
- Appendini, P.; Hotchkiss, J.H. Review of antimicrobial food packaging. Innov. Food Sci. Emerg. Technol. 2002, 3, 113–126. [Google Scholar] [CrossRef]
- Han, J.H. Antimicrobial food packaging. In Novel Food Packaging Techniques; Elsevier: Amsterdam, The Netherlands, 2003; pp. 50–70. ISBN 9781855736757. [Google Scholar]
- Han, J.H. Antimicrobial packaging systems. In Innovations in Food Packaging; Han, J.H., Ed.; Elsevier: Amsterdam, The Netherlands, 2005; ISBN 0123116325. [Google Scholar]
- Coma, V. Bioactive packaging technologies for extended shelf life of meat-based products. Meat Sci. 2008, 78, 90–103. [Google Scholar] [CrossRef]
- Lavoine, N.; Givord, C.; Tabary, N.; Desloges, I.; Martel, B.; Bras, J. Elaboration of a new antibacterial bio-nano-material for food-packaging by synergistic action of cyclodextrin and microfibrillated cellulose. Innov. Food Sci. Emerg. Technol. 2014, 26, 330–340. [Google Scholar] [CrossRef]
- Cooksey, K. Effectiveness of antimicrobial food packaging materials. Food Addit. Contam. 2005, 22, 980–987. [Google Scholar] [CrossRef]
- Pereira, M.C.; Hill, L.E.; Zambiazi, R.C.; Mertens-Talcott, S.; Talcott, S.; Gomes, C.L. Nanoencapsulation of hydrophobic phytochemicals using poly (dl-lactide-co-glycolide) (PLGA) for antioxidant and antimicrobial delivery applications: Guabiroba fruit (Campomanesia xanthocarpa O. Berg) study. LWT Food Sci. Technol. 2015, 63, 100–107. [Google Scholar] [CrossRef]
- Qamar, S.A.; Asgher, M.; Bilal, M. Immobilization of alkaline protease from bacillus brevis using ca-alginate entrapment strategy for improved catalytic stability, silver recovery, and dehairing potentialities. Catal. Lett. 2020, 150, 3572–3583. [Google Scholar] [CrossRef]
- Ruiz-Navajas, Y.; Viuda-Martos, M.; Sendra, E.; Perez-Alvarez, J.A.; Fernández-López, J. In vitro antibacterial and antioxidant properties of chitosan edible films incorporated with Thymus moroderi or Thymus piperella essential oils. Food Control 2013, 30, 386–392. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Stretch, A.W. Antioxidant capacity in cranberry is influenced by cultivar and storage temperature. J. Agric. Food Chem. 2001, 49, 969–974. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Oxygen radical absorbing capacity of phenolics in blueberries, cranberries, chokeberries, and lingonberries. J. Agric. Food Chem. 2003, 51, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, J.; Vankar, P.S. Principal phenolic phytochemicals and antioxidant property in Eucalyptus bark. Nutr. Food Sci. 2012, 42, 412–421. [Google Scholar] [CrossRef]
- Carpena, M.; Nuñez-Estevez, B.; Soria-Lopez, A.; Garcia-Oliveira, P.; Prieto, M.A. Essential Oils and Their Application on Active Packaging Systems: A Review. Resources 2021, 10, 7. [Google Scholar] [CrossRef]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential oils’ chemical characterization and investigation of some biological activities: A critical review. Medicines 2016, 3. [Google Scholar] [CrossRef]
- Raut, J.S.; Karuppayil, S.M. A status review on the medicinal properties of essential oils. Ind. Crop. Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- Nieto, G. Biological activities of three essential oils of the lamiaceae family. Medicines 2017, 4. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Sauraj, K.B.; Deeba, F.; Kulshreshtha, A.; Negi, Y.S. Chitosan films incorporated with Apricot (Prunus armeniaca) kernel essential oil as active food packaging material. Food Hydrocoll. 2018, 85, 158–166. [Google Scholar] [CrossRef]
- Kamari, A.; Halim, A.L.A.; Yusoff, S.N.M.; Ishak, S. Gelatin film incorporated with banana leaf essential oil for food preservation. J. Phys. Conf. Ser. 2018, 1097. [Google Scholar] [CrossRef]
- Qin, Y.; Li, W.; Liu, D.; Yuan, M.; Li, L. Development of active packaging film made from poly (lactic acid) incorporated essential oil. Prog. Org. Coat. 2017, 103, 76–82. [Google Scholar] [CrossRef]
- Noshirvani, N.; Ghanbarzadeh, B.; Gardrat, C.; Rezaei, M.R.; Hashemi, M.; Le Coz, C.; Coma, V. Cinnamon and ginger essential oils to improve antifungal, physical and mechanical properties of chitosan-carboxymethyl cellulose films. Food Hydrocoll. 2017, 70, 36–45. [Google Scholar] [CrossRef]
- Kim, H.; Beak, S.-E.; Song, K.B. Development of a hagfish skin gelatin film containing cinnamon bark essential oil. LWT Food Sci. Technol. 2018, 96, 583–588. [Google Scholar] [CrossRef]
- Beak, S.; Kim, H.; Song, K.B. Sea Squirt Shell Protein and Polylactic Acid Laminated Films Containing Cinnamon Bark Essential Oil. J. Food Sci. 2018, 83, 1896–1903. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Gao, C.; Feng, X.; Yang, Y.; Shen, X.; Tang, X. Structure, physical and antioxidant properties of chitosan-gum arabic edible films incorporated with cinnamon essential oil. Int. J. Biol. Macromol. 2019, 134, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Fasihi, H.; Noshirvani, N.; Hashemi, M.; Fazilati, M.; Salavati, H.; Coma, V. Antioxidant and antimicrobial properties of carbohydrate-based films enriched with cinnamon essential oil by Pickering emulsion method. Food Packag. Shelf Life 2019, 19, 147–154. [Google Scholar] [CrossRef]
- Wu, J.; Sun, X.; Guo, X.; Ge, S.; Zhang, Q. Physicochemical properties, antimicrobial activity and oil release of fish gelatin films incorporated with cinnamon essential oil. Aquac. Fish. 2017, 2, 185–192. [Google Scholar] [CrossRef]
- Aisha, I.; Abdullahi, Y. Development of Whey Protein Concentrate Edible Membrane with Cinnamon Essential Oil. J. Adv. Biol. Biotechnol. 2017, 11, 1–14. [Google Scholar] [CrossRef]
- Yang, S.-Y.; Lee, K.-Y.; Beak, S.-E.; Kim, H.; Song, K.B. Antimicrobial activity of gelatin films based on duck feet containing cinnamon leaf oil and their applications in packaging of cherry tomatoes. Food Sci. Biotechnol. 2017, 26, 1429–1435. [Google Scholar] [CrossRef] [PubMed]
- Nisar, T.; Wang, Z.-C.; Yang, X.; Tian, Y.; Iqbal, M.; Guo, Y. Characterization of citrus pectin films integrated with clove bud essential oil: Physical, thermal, barrier, antioxidant and antibacterial properties. Int. J. Biol. Macromol. 2018, 106, 670–680. [Google Scholar] [CrossRef]
- De Figueiredo Sousa, H.A.; de Oliveira Filho, J.G.; Egea, M.B.; Da Rosa Silva, E.; Macagnan, D.; Pires, M.; Peixoto, J. Active film incorporated with clove essential oil on storage of banana varieties. Nutr. Food Sci. 2019, 49, 911–924. [Google Scholar] [CrossRef]
- Sharma, S.; Barkauskaite, S.; Jaiswal, S.; Duffy, B.; Jaiswal, A.K. Development of Essential Oil Incorporated Active Film Based on Biodegradable Blends of Poly (Lactide)/Poly (Butylene Adipate-co-Terephthalate) for Food Packaging Application. J Package Technol. Res. 2020, 4, 235–245. [Google Scholar] [CrossRef]
- Azadbakht, E.; Maghsoudlou, Y.; Khomiri, M.; Kashiri, M. Development and structural characterization of chitosan films containing Eucalyptus globulus essential oil: Potential as an antimicrobial carrier for packaging of sliced sausage. Food Packag. Shelf Life 2018, 17, 65–72. [Google Scholar] [CrossRef]
- Bonilla, J.; Poloni, T.; Lourenço, R.V.; Sobral, P.J. Antioxidant potential of eugenol and ginger essential oils with gelatin/chitosan films. Food Biosci. 2018, 23, 107–114. [Google Scholar] [CrossRef]
- Jamróz, E.; Juszczak, L.; Kucharek, M. Investigation of the physical properties, antioxidant and antimicrobial activity of ternary potato starch-furcellaran-gelatin films incorporated with lavender essential oil. Int. J. Biol. Macromol. 2018, 114, 1094–1101. [Google Scholar] [CrossRef]
- Song, X.; Zuo, G.; Chen, F. Effect of essential oil and surfactant on the physical and antimicrobial properties of corn and wheat starch films. Int. J. Biol. Macromol. 2018, 107, 1302–1309. [Google Scholar] [CrossRef]
- Akyuz, L.; Kaya, M.; Ilk, S.; Cakmak, Y.S.; Salaberria, A.M.; Labidi, J.; Yılmaz, B.A.; Sargin, I. Effect of different animal fat and plant oil additives on physicochemical, mechanical, antimicrobial and antioxidant properties of chitosan films. Int. J. Biol. Macromol. 2018, 111, 475–484. [Google Scholar] [CrossRef]
- Oliveira, S.P.L.F.; Bertan, L.C.; Rensis, C.M.V.B.d.; Bilck, A.P.; Vianna, P.C.B. Whey protein-based films incorporated with oregano essential oil. Polímeros 2017, 27, 158–164. [Google Scholar] [CrossRef]
- Sedlaříková, J.; Doležalová, M.; Egner, P.; Pavlačková, J.; Krejčí, J.; Rudolf, O.; Peer, P. Effect of oregano and marjoram essential oils on the physical and antimicrobial properties of chitosan based systems. Int. J. Polym. Sci. 2017, 2017, 2593863. [Google Scholar] [CrossRef]
- Ranjbar, M.; Azizi, M.H.; Hashtjin, A.M. Evaluation of physico-mechanical and antimicrobial properties of gelatincarboxymethyl cellulose film containing essential oil of bane (Pistacia atlantica). Nutr. Food Sci. Res. 2017, 4, 11–17. [Google Scholar]
- Akhter, R.; Masoodi, F.A.; Wani, T.A.; Rather, S.A. Functional characterization of biopolymer based composite film: Incorporation of natural essential oils and antimicrobial agents. Int. J. Biol. Macromol. 2019, 137, 1245–1255. [Google Scholar] [CrossRef]
- Mahcene, Z.; Khelil, A.; Hasni, S.; Akman, P.K.; Bozkurt, F.; Birech, K.; Goudjil, M.B.; Tornuk, F. Development and characterization of sodium alginate based active edible films incorporated with essential oils of some medicinal plants. Int. J. Biol. Macromol. 2020, 145, 124–132. [Google Scholar] [CrossRef]
- Hasheminya, S.-M.; Mokarram, R.R.; Ghanbarzadeh, B.; Hamishekar, H.; Kafil, H.S.; Dehghannya, J. Development and characterization of biocomposite films made from kefiran, carboxymethyl cellulose and Satureja Khuzestanica essential oil. Food Chem. 2019, 289, 443–452. [Google Scholar] [CrossRef]
- Kouravand, F.; Jooyandeh, H.; Barzegar, H.; Hojjati, M. Characterization of cross-linked whey protein isolate-based films containing Satureja Khuzistanica Jamzad essential oil. J. Food Process. Preserv. 2018, 42. [Google Scholar] [CrossRef]
- Abdollahi, M.; Damirchi, S.; Shafafi, M.; Rezaei, M.; Ariaii, P. Carboxymethyl cellulose-agar biocomposite film activated with summer savory essential oil as an antimicrobial agent. Int. J. Biol. Macromol. 2019, 126, 561–568. [Google Scholar] [CrossRef]
- Sharma, S.; Barkauskaite, S.; Duffy, B.; Jaiswal, A.K.; Jaiswal, S. Characterization and antimicrobial activity of biodegradable active packaging enriched with clove and thyme essential oil for food packaging application. Foods 2020, 9. [Google Scholar] [CrossRef]
- Zeid, A.; Karabagias, I.K.; Nassif, M.; Kontominas, M.G. Preparation and evaluation of antioxidant packaging films made of polylactic acid containing thyme, rosemary, and oregano essential oils. J. Food Process. Preserv. 2019, 83, 1299. [Google Scholar] [CrossRef]
- Karacabey, E.; Bayindirli, L.; Artik, N.; Mazza, G. Modeling solid-liquid extraction kinetics of trans -resveratrol and trans -ε-viniferin from grape cane. J. Food Process Eng. 2013, 36, 103–112. [Google Scholar] [CrossRef]
- Azwanida, N.N. A review on the extraction methods use in medicinal plants, principle, strength and limitation. Med. Aromat. Plants 2015, 4. [Google Scholar] [CrossRef]
- Aleksic Sabo, V.; Knezevic, P. Antimicrobial activity of Eucalyptus camaldulensis Dehn. plant extracts and essential oils: A review. Ind. Crop. Prod. 2019, 132, 413–429. [Google Scholar] [CrossRef] [PubMed]
- Havelt, T.; Brettschneider, S.; Do, X.T.; Korte, I.; Kreyenschmidt, J.; Schmitz, M. Sustainable extraction and characterisation of bioactive compounds from horse chestnut seed coats for the development of bio-based additives. Resources 2019, 8, 114. [Google Scholar] [CrossRef]
- Havelt, T.; Schmitz, M. Identifizierung und charakterisierung bioaktiver inhaltsstoffe in thymian: 8. tagung arznei- und gewürzpflanzenforschung, Bonn. Jul. Kühn Arch. 2018, 112–114. [Google Scholar] [CrossRef]
- Havelt, T.; Frase, J.N.; Pude, R.; Schmitz, M. Characterisation of bioactive ingredients in extracts of fresh and dried coniferous trees for the development of sustainable packaging materials. Processes 2020, 8, 1366. [Google Scholar] [CrossRef]
- Kimura, H.; Ogawa, S.; Ishihara, T.; Maruoka, M.; Tokuyama-Nakai, S.; Jisaka, M.; Yokota, K. Antioxidant activities and structural characterization of flavonol O-glycosides from seeds of Japanese horse chestnut (Aesculus turbinata BLUME). Food Chem. 2017, 228, 348–355. [Google Scholar] [CrossRef]
- Ay, E.B.; Gul, M.; Acikgoz, M.A.; Yarilgac, T.; Kara, S.M. Assessment of antioxidant activity of giant snowdrop (Galanthus elwesii Hook) extracts with their total phenol and flavonoid contents. Indian J. Pharm. Educ. Res. 2018, 52, 128–132. [Google Scholar] [CrossRef]
- Karimi, E.; Mehrabanjoubani, P.; Homayouni-Tabrizi, M.; Abdolzadeh, A.; Soltani, M. Phytochemical evaluation, antioxidant properties and antibacterial activity of Iranian medicinal herb Galanthus transcaucasicus Fomin. J. Food Meas. Charact. 2018, 12, 433–440. [Google Scholar] [CrossRef]
- Biswas, M.; Pal, A.; Dey, M.; Dey, A.; Bandyopadhyay, A. Influence of a biobased reagent on properties of industrial resin for printing ink application vis-à-vis comparison with standard commercial resin. Polym. Renew. Resour. 2018, 9, 59–74. [Google Scholar] [CrossRef]
- Veiga-Santos, P.; Silva, L.T.; Souza, C.O.d.; Da Silva, J.R.; Albuquerque, E.C.; Druzian, J.I. Coffee-cocoa additives for bio-based antioxidant packaging. Food Packag. Shelf Life 2018, 18, 37–41. [Google Scholar] [CrossRef]
- Wang, K.; Lim, P.N.; Tong, S.Y.; Thian, E.S. Development of grapefruit seed extract-loaded poly(ε-caprolactone)/chitosan films for antimicrobial food packaging. Food Packag. Shelf Life 2019, 22, 100396. [Google Scholar] [CrossRef]
- Wang, L.; Guo, H.; Wang, J.; Jiang, G.; Du, F.; Liu, X. Effects of Herba Lophatheri extract on the physicochemical properties and biological activities of the chitosan film. Int. J. Biol. Macromol. 2019, 133, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, A.; Jokar, M.; Mohammadi Nafchi, A. Preparation and characterization of biocomposite film based on chitosan and kombucha tea as active food packaging. Int. J. Biol. Macromol. 2018, 108, 444–454. [Google Scholar] [CrossRef]
- Sogut, E.; Seydim, A.C. The effects of Chitosan and grape seed extract-based edible films on the quality of vacuum packaged chicken breast fillets. Food Packag. Shelf Life 2018, 18, 13–20. [Google Scholar] [CrossRef]
- Piñeros-Hernandez, D.; Medina-Jaramillo, C.; López-Córdoba, A.; Goyanes, S. Edible cassava starch films carrying rosemary antioxidant extracts for potential use as active food packaging. Food Hydrocoll. 2017, 63, 488–495. [Google Scholar] [CrossRef]
- Mehdizadeh, T.; Langroodi, A.M. Chitosan coatings incorporated with propolis extract and Zataria multiflora Boiss oil for active packaging of chicken breast meat. Int. J. Biol. Macromol. 2019, 141, 401–409. [Google Scholar] [CrossRef]
- Langroodi, A.M.; Tajik, H.; Mehdizadeh, T.; Moradi, M.; Moghaddas Kia, E.; Mahmoudian, A. Effects of sumac extract dipping and chitosan coating enriched with Zataria multiflora Boiss oil on the shelf-life of meat in modified atmosphere packaging. LWT Food Sci. Technol. 2018, 98, 372–380. [Google Scholar] [CrossRef]
- Rezaeigolestani, M.; Misaghi, A.; Khanjari, A.; Basti, A.A.; Abdulkhani, A.; Fayazfar, S. Antimicrobial evaluation of novel poly-lactic acid based nanocomposites incorporated with bioactive compounds in-vitro and in refrigerated vacuum-packed cooked sausages. Int. J. Food Microbiol. 2017, 260, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Jafari, S.M.; Aadil, R.M.; Assadpour, E.; Randhawa, M.A.; Mahmood, S. Development of active food packaging via incorporation of biopolymeric nanocarriers containing essential oils. Trends Food Sci. Technol. 2020, 101, 106–121. [Google Scholar] [CrossRef]
- Rashed, M.M.; Zhang, C.; Ghaleb, A.D.; Li, J.; Nagi, A.; Majeed, H.; Bakry, A.M.; Haider, J.; Xu, Z.; Tong, Q. Techno-functional properties and sustainable application of nanoparticles-based Lavandula angustifolia essential oil fabricated using unsaturated lipid-carrier and biodegradable wall material. Ind. Crop. Prod. 2019, 136, 66–76. [Google Scholar] [CrossRef]
- Wen, P.; Zhu, D.-H.; Feng, K.; Liu, F.-J.; Lou, W.-Y.; Li, N.; Zong, M.-H.; Wu, H. Fabrication of electrospun polylactic acid nanofilm incorporating cinnamon essential oil/β-cyclodextrin inclusion complex for antimicrobial packaging. Food Chem. 2016, 196, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Chang, R.; Yang, J.; Ge, S.; Xiong, L.; Zhao, M.; Li, M.; Sun, Q. Preparation and characterization of essential oil-loaded starch nanoparticles formed by short glucan chains. Food Chem. 2016, 221, 1426–1433. [Google Scholar] [CrossRef]
- Dos Santos Paglione, I.; Galindo, M.V.; Medeiros, J.A.S.d.; Yamashita, F.; Alvim, I.D.; Ferreira Grosso, C.R.; Sakanaka, L.S.; Shirai, M.A. Comparative study of the properties of soy protein concentrate films containing free and encapsulated oregano essential oil. Food Packag. Shelf Life 2019, 22, 100419. [Google Scholar] [CrossRef]
- Mohsenabadi, N.; Rajaei, A.; Tabatabaei, M.; Mohsenifar, A. Physical and antimicrobial properties of starch-carboxy methyl cellulose film containing rosemary essential oils encapsulated in chitosan nanogel. Int. J. Biol. Macromol. 2018, 112, 148–155. [Google Scholar] [CrossRef]
- Khoobdel, M.; Ahsaei, S.M.; Farzaneh, M. Insecticidal activity of polycaprolactone nanocapsules loaded with Rosmarinus officinalis essential oil in Tribolium castaneum (Herbst). Entomol. Res. 2017, 47, 175–184. [Google Scholar] [CrossRef]
- Werdin González, J.O.; Jesser, E.N.; Yeguerman, C.A.; Ferrero, A.A.; Fernández Band, B. Polymer nanoparticles containing essential oils: New options for mosquito control. Environ. Sci. Pollut. Res. 2017, 24, 17006–17015. [Google Scholar] [CrossRef]
- Lin, L.; Mao, X.; Sun, Y.; Rajivgandhi, G.; Cui, H. Antibacterial properties of nanofibers containing chrysanthemum essential oil and their application as beef packaging. Int. J. Food Microbiol. 2019, 292, 21–30. [Google Scholar] [CrossRef]
- Maestrello, C.; Tonon, L.; Madrona, G.; Scapim, M.; Bergamasco, R. Production and Characterization of Biodegradable Films Incorporated with Clove Essential Oil/β-cyclodextrin Microcapsules. Chem. Eng. Trans. 2017, 57, 1274–1283. [Google Scholar] [CrossRef]
- Farshi, P.; Tabibiazar, M.; Ghorbani, M.; Mohammadifar, M.; Amirkhiz, M.B.; Hamishehkar, H. Whey protein isolate-guar gum stabilized cumin seed oil nanoemulsion. Food Biosci. 2019, 28, 49–56. [Google Scholar] [CrossRef]
- Karimirad, R.; Behnamian, M.; Dezhsetan, S. Development and characterization of nano biopolymer containing cumin oil as a new approach to enhance antioxidant properties of button mushroom. Int. J. Biol. Macromol. 2018, 113, 662–668. [Google Scholar] [CrossRef]
- Wu, Z.; Zhou, W.; Pang, C.; Deng, W.; Xu, C.; Wang, X. Multifunctional chitosan-based coating with liposomes containing laurel essential oils and nanosilver for pork preservation. Food Chem. 2019, 295, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Gu, Y.; Cui, H. Moringa oil/chitosan nanoparticles embedded gelatin nanofibers for food packaging against Listeria monocytogenes and Staphylococcus aureus on cheese. Food Packag. Shelf Life 2019, 19, 86–93. [Google Scholar] [CrossRef]
- Fraj, A.; Jaâfar, F.; Marti, M.; Coderch, L.; Ladhari, N. A comparative study of oregano (Origanum vulgare L.) essential oil-based polycaprolactone nanocapsules/ microspheres: Preparation, physicochemical characterization, and storage stability. Ind. Crop. Prod. 2019, 140, 111669. [Google Scholar] [CrossRef]
- Merino, N.; Berdejo, D.; Bento, R.; Salman, H.; Lanz, M.; Maggi, F.; Sánchez-Gómez, S.; García-Gonzalo, D.; Pagán, R. Antimicrobial efficacy of Thymbra capitata (L.) Cav. essential oil loaded in self-assembled zein nanoparticles in combination with heat. Ind. Crop. Prod. 2019, 133, 98–104. [Google Scholar] [CrossRef]
- Vafania, B.; Fathi, M.; Soleimanian-Zad, S. Nanoencapsulation of thyme essential oil in chitosan-gelatin nanofibers by nozzle-less electrospinning and their application to reduce nitrite in sausages. Food Bioprod. Process. 2019, 116, 240–248. [Google Scholar] [CrossRef]
- Lin, L.; Zhu, Y.; Cui, H. Electrospun thyme essential oil/gelatin nanofibers for active packaging against Campylobacter jejuni in chicken. LWT Food Sci. Technol. 2018, 97, 711–718. [Google Scholar] [CrossRef]
- Lee, M.H.; Seo, H.-S.; Park, H.J. Thyme oil encapsulated in halloysite nanotubes for antimicrobial packaging system. J. Food Sci. 2017, 82, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.-H.; Jang, S.-R.; Lee, G.-M.; Ryu, J.-H.; Park, S.-I.; Park, N.-H. Halloysite Nanocapsules Containing Thyme Essential Oil: Preparation, Characterization, and Application in Packaging Materials. J. Food Sci. 2017, 82, 2113–2120. [Google Scholar] [CrossRef] [PubMed]
- Ardekani, N.T.; Khorram, M.; Zomorodian, K.; Yazdanpanah, S.; Veisi, H.; Veisi, H. Evaluation of electrospun poly (vinyl alcohol)-based nanofiber mats incorporated with Zataria multiflora essential oil as potential wound dressing. Int. J. Biol. Macromol. 2019, 125, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Bos, H.; Meesters, K.; Conijn, S.; Corré, W.; Patel, M. Sustainability Aspects of Biobased Applications. Available online: http://edepot.wur.nl/170079 (accessed on 25 May 2019).
- Schumann, B.; Schmid, M. Packaging concepts for fresh and processed meat—Recent progresses. Innov. Food Sci. Emerg. Technol. 2018, 47, 88–100. [Google Scholar] [CrossRef]
- Golembiewski, B.; Sick, N.; Broering, S. The emerging research landscape on bioeconomy: What has been done so far and what is essential from a technology and innovation management perspective? Innov. Food Sci. Emerg. Technol. 2015, 29, 308–317. [Google Scholar] [CrossRef]
- Carraresi, L.; Berg, S.; Broering, S. Emerging value chains within the bioeconomy: Structural changes in the case of phosphate recovery. J. Clean. Prod. 2018, 183, 87–101. [Google Scholar] [CrossRef]
- Keegan, D.; Kretschmer, B.; Elbersen, B.; Panoutsou, C. Cascading use: A systematic approach to biomass beyond the energy sector. Biofuels Bioprod. Biorefining 2013, 7, 193–206. [Google Scholar] [CrossRef]
- Theinsathid, P.; Chandrachai, A.; Suwannathep, S.; Keeratipibul, S. Lead users and early adoptors of bioplastics: A market-led approach to innovative food packaging films. J. Biobased Mater. Bioenergy 2011, 5, 17–29. [Google Scholar] [CrossRef]
- Wensing, J.; Caputo, V.; Carraresi, L.; Bröring, S. The effects of green nudges on consumer valuation of bio-based plastic packaging. Ecol. Econ. 2020, 178, 106783. [Google Scholar] [CrossRef]
- Rossi, A.M.; Hinrichs, C.C. Hope and skepticism: Farmer and local community views on the socio-economic benefits of agricultural bioenergy. Biomass Bioenergy 2011, 35, 1418–1428. [Google Scholar] [CrossRef]
- Wensing, J.; Carraresi, L.; Broering, S. Do pro-environmental values, beliefs and norms drive farmers’ interest in novel practices fostering the Bioeconomy? J. Environ. Manag. 2019, 232, 858–867. [Google Scholar] [CrossRef]
- Van Eck, P.S.; Jager, W.; Leeflang, P.S.H. Opinion leaders’ role in innovation diffusion: A simulation study. J. Prod. Innov. Manag. 2011, 28, 187–203. [Google Scholar] [CrossRef]
- Xiong, H.; Payne, D.; Kinsella, S. Peer effects in the diffusion of innovations: Theory and simulation. J. Behav. Exp. Econ. 2016, 63, 1–13. [Google Scholar] [CrossRef]
- Berg, S.; Cloutier, L.M.; Broering, S. Collective stakeholder representations and perceptions of drivers of novel biomass-based value chains. J. Clean. Prod. 2018, 200, 231–241. [Google Scholar] [CrossRef]
- Sleenhoff, S.; Cuppen, E.; Osseweijer, P. Unravelling emotional viewpoints on a bio-based economy using Q methodology. Public Underst. Sci. 2015, 24, 858–877. [Google Scholar] [CrossRef] [PubMed]
- Sijtsema, S.J.; Onwezen, M.C.; Reinders, M.J.; Dagevos, H.; Partanen, A.; Meeusen, M. Consumer perception of bio-based products-An exploratory study in 5 European countries. Wagening J. Life Sci. 2016, 77, 61–69. [Google Scholar] [CrossRef]
- Stern, T.; Ploll, U.; Spies, R.; Schwarzbauer, P.; Hesser, F.; Ranacher, L. Understanding perceptions of the bioeconomy in Austria—An explorative case study. Sustainability 2018, 10. [Google Scholar] [CrossRef]
- Petljak, K.; Naletina, D.; Bilogrević, K. Considering ecologically sustainable packaging during decision-making while buying food products. Ekon. Poljopr. 2019, 66, 107–126. [Google Scholar] [CrossRef]
- Koutsimanis, G.; Harte, J.; Almenar, E. Freshness maintenance of cherries ready for consumption using convenient, microperforated, bio-based packaging. J. Sci. Food Agric. 2015, 95, 972–982. [Google Scholar] [CrossRef]
- Herbes, C.; Beuthner, C.; Ramme, I. Consumer attitudes towards biobased packaging—A cross-cultural comparative study. J. Clean. Prod. 2018, 194, 203–218. [Google Scholar] [CrossRef]
- Scherer, C.; Emberger-Klein, A.; Menrad, K. Biogenic product alternatives for children: Consumer preferences for a set of sand toys made of bio-based plastic. Sustain. Prod. Consum. 2017, 10, 1–14. [Google Scholar] [CrossRef]
- Scherer, C.; Emberger-Klein, A.; Menrad, K. Consumer preferences for outdoor sporting equipment made of biobased plastics: Results of a choice-based-conjoint experiment in Germany. J. Clean. Prod. 2018, 203, 1085–1094. [Google Scholar] [CrossRef]
- Klein, F.; Emberger-Klein, A.; Menrad, K.; Moehring, W.; Blesin, J.-M. Influencing factors for the purchase intention of consumers choosing bioplastic products in Germany. Sustain. Prod. Consum. 2019, 19, 33–43. [Google Scholar] [CrossRef]
- Rumm, S. Verbrauchereinschätzungen zu Biokunststoffen: Eine Analyse vor dem Hintergrund des Heuristic-Systematic Model. Available online: https://mediatum.ub.tum.de/1306582 (accessed on 18 October 2018).
- Müller, G.; Hanecker, E.; Blasius, K.; Seidemann, C.; Tempel, L.; Sadocco, P.; Pozo, B.F.; Boulougouris, G.; Lozo, B.; Jamnicki, S.; et al. End-of-life Solutions for Fibre and Bio-based Packaging Materials in Europe. Packag. Technol. Sci. 2014, 27, 1–15. [Google Scholar] [CrossRef]
- Yue, C.; Hall, C.R.; Behe, B.K.; Campbell, B.L.; Dennis, J.H.; Lopez, R.G. Are consumers willing to pay more for biodegradable containers than for plastic ones? Evidence from hypothetical conjoint analysis and nonhypothetical experimental auctions. In Proceedings of the 2010 Annual Meeting, Denver, CO, USA, 25–27 July 2010; pp. 757–772. [Google Scholar]
- Klaiman, K.; Ortega, D.L.; Garnache, C. Consumer preferences and demand for packaging material and recyclability. Resour. Conserv. Recycl. 2016, 115, 1–8. [Google Scholar] [CrossRef]
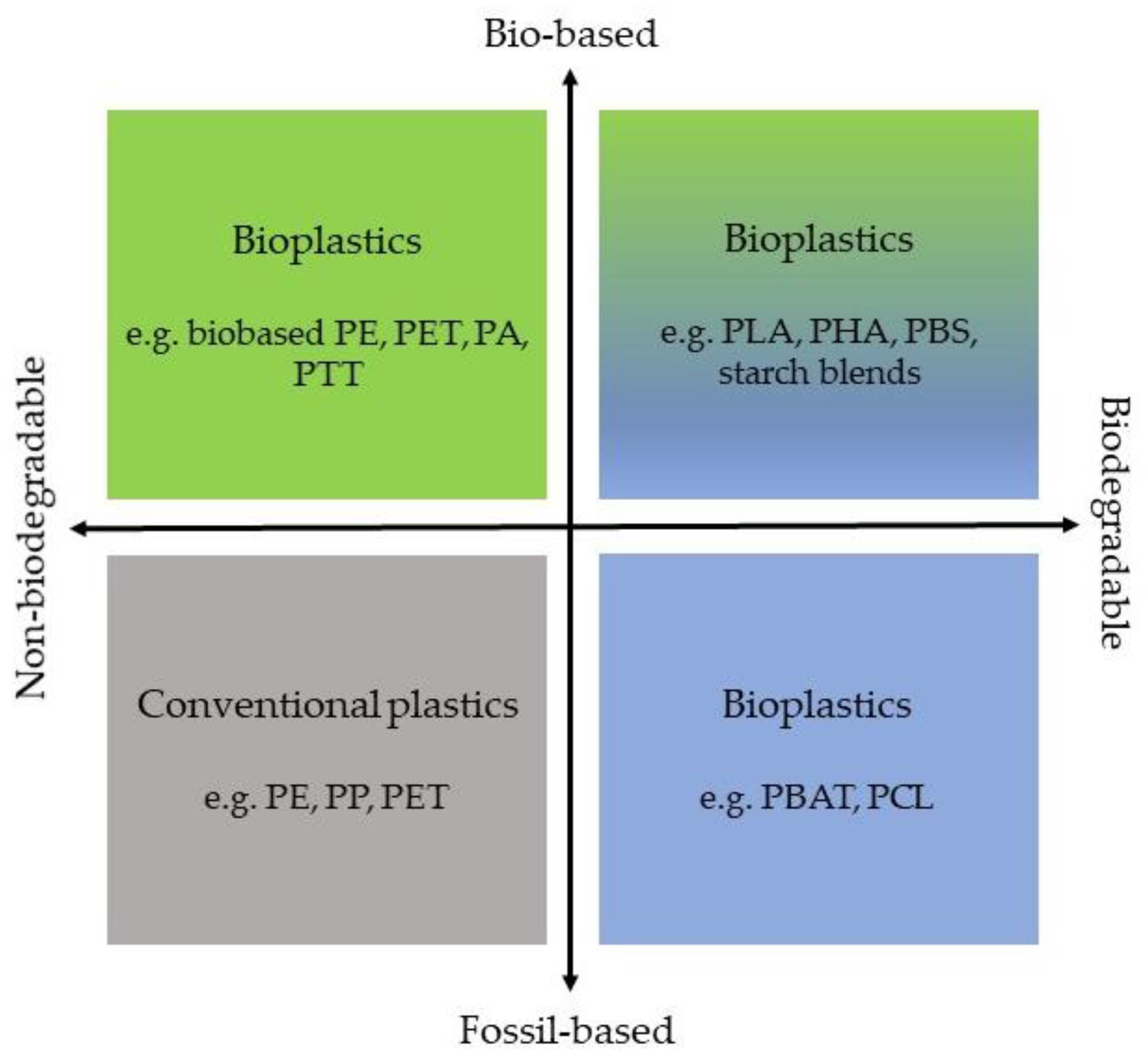
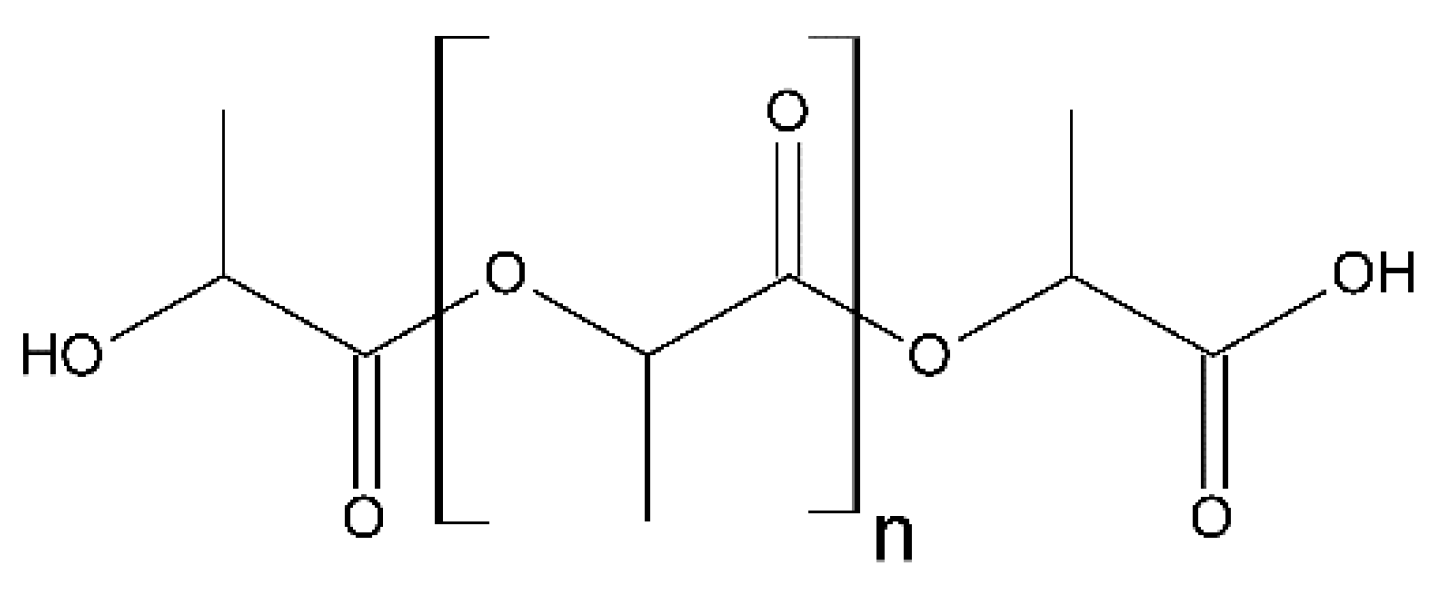

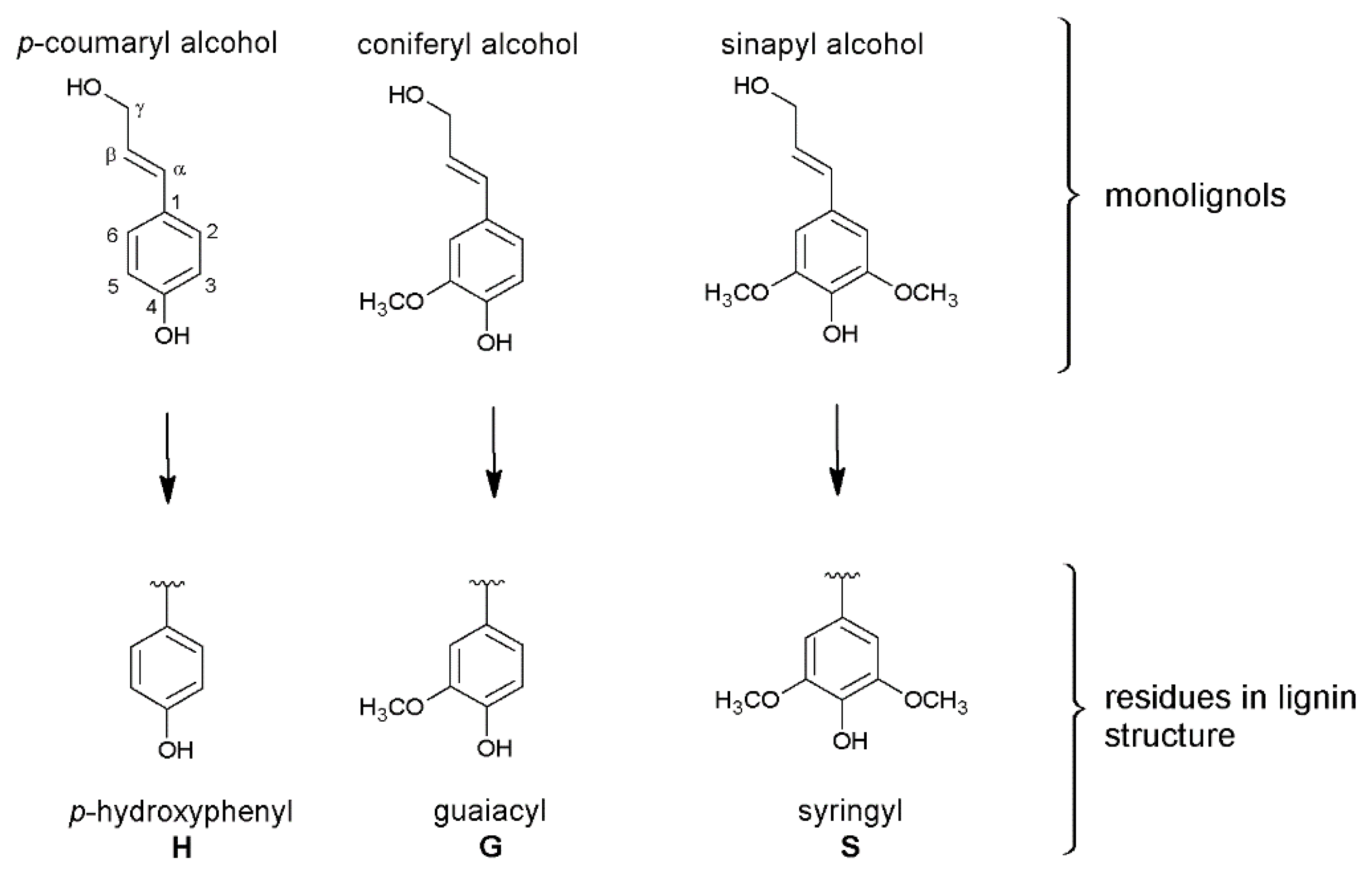

| Film Composition | Permeability | Mechanical Properties | References | |||
|---|---|---|---|---|---|---|
| O2 | CO2 | H2O Vapor | Tensile Strength | Elongation at Break | ||
| [cm3·mm/(m2·d·atm)] | [g·mm/(m2·d·atm)] | [MPa] | [%] | |||
| Synthetic Polymers | ||||||
| HDPE | 44–91 (20 °C, 65% RH) | 100 (23 °C, 0% RH) | 0.15 (38 °C, 90% RH) | 32 | 150 | [110,111] |
| LPDE | 98–183 (23 °C, 50% RH) | NR | 0.44 (38 °C, 90% RH) | 10 | 400 | [110,111] |
| PE | 50–200 (23 °C, 50% or 0% RH) | 100–1000 (23-25 °C, unknown RH) | 0.5–2 (23 °C, 85% RH) | 18 | 350 | [111,112,113] |
| PET | 1–5 (23 °C, 50% or 0% RH) | 3–7 (23 °C, 75% RH) | 0.5–2 (23 °C, 85% RH) | 55 | 300 | [111,112,113] |
| PP | 50–100 (23 °C, 50% or 0% RH) | 200–900 (unknown conditions) | 0.2–0.4 (23 °C, 85% RH) | 26 | 80 | [111,112,113] |
| Biomass-derived chemically synthesized materials | ||||||
| PLA | 3.5–15 (23 °C, 50% or 0% RH) | 32.9–72 (23 °C, 0% RH) | 1.6–3.6 (38 °C, 85% RH) | 50.45 | 3 | [110,114,115] |
| Film Composition | Permeability | Mechanical Properties | References | |||
|---|---|---|---|---|---|---|
| O2 | CO2 | H2O Vapor | Tensile Strength | Elongation at Break | ||
| [cm3·mm/(m2·d·atm)] | [g·mm/(m2·d atm)] | [MPa] | [%] | |||
| PHB (P3HB) | 2–11.4 (23 °C, 0% RH) | 3–28.9 (23 °C, 0% RH) | 1–5 (unknown conditions) | 35–40 | 3–8 | [110,137] |
| PHBV (P(3HB-co-3HV)) | 4.9–16.7 (25 °C, 0% RH) | 146 (25 °C, 0% RH) | 1.5 (38 °C, 90% RH) | 38 | NR | [110,137] |
| PHBHHx | 8.3 (23 °C, 0% RH) | 54 (23 °C, 0% RH) | 1.42 (23 °C, 0% RH) | 20 | 850 | [132,137] |
| Film Composition | Permeability | Mechanical Properties | References | |||
|---|---|---|---|---|---|---|
| O2 | CO2 | H2O Vapor | Tensile Strength | Elongation at Break | ||
| [g·mm/(m2·d·atm)] * | [MPa] | [%] | ||||
| Soy protein isolate film with glycerol (casting method) | NR | NR | 1710 (23 °C, 50% RH) | 6.97 | 113.94 | [180] |
| Whey protein isolate film with glycerol | 92.448 g/(m2·d) (25 °C, 90% RH) | NR | 1680 (25 °C, 75% RH) | 3 | 13 | [181] |
| Pumpkin oil cake protein isolate film with glycerol | 16.06 cm3/(m2·d·atm) (23 °C; unknown RH) | 21.15 cm3/(m2·d·atm) * (23 °C; unknown RH) | NR | 0.86–6.56 | 22.2–196.61 | [165] |
| Fish gelatin film with glycerol | NR | NR | 1040 (24 °C, 50% RH) | 9.08 | 44.93 | [182] |
| Beef skin gelatin films with corn oil (extrusion) | 0.8–4.7 × 10−4 cm3·mm/(m2·d·atm) (23 °C, 50% RH) | NR | 4.05–8.61 × 106 (23 °C, 50% RH) | 1.43–5.37 | 1.68–2.60 | [183] |
| Film Composition | Permeability | Mechanical Properties | References | ||
|---|---|---|---|---|---|
| O2 | H2O Vapor | Tensile Strength | Elongation at Break | ||
| [g·mm/(m2·d·atm)] * | [MPa] | [%] | |||
| High amylose cornstarch films without plasticizer (amylose:amylopectin ratio 80:20) | NR | 1260 (20 °C; 52.9% RH) | 34.32 | 1.41 | [189] |
| Low amylose cornstarch films without plasticizer (amylose:amylopectin ratio 25:75) | NR | 1430 (20 °C; 52.9% RH) | 44.38 | 2.40 | [189] |
| Thermoplastic (cassava) starch (extrusion) | 0.182 (Ambient temp., 0% RH) | 36.8 (25 °C, 50% RH) | 5.8 | 78 | [190,191] |
| Methylcellulose mixtures in ethanol | NR | 446–945 (25 °C; 52% RH) | 25–33 | 29–14 | [192] |
| Hydroxypropyl methylcellulose without plasticizer | NR | 974,000 (23 °C, 50% RH) | 61.04 | 29.51 | [193] |
| Carboxymethyl cellulose film with glycerol (casting method) | NR | 683 (25 °C, 52.8% RH) | 6.10 | 201.73 | [194] |
| CMC-film with 50 wt% ethanol organosolv lignin with glycerol (casting method) | NR | 2570 (20 °C, 0% RH) | 20 | 5.92 | [149] |
| Agar/10% lignin composite film with glycerol | NR | 13,400 (25 °C, 50% RH) | 51.8 | 22.1 | [195] |
| (Sodium) alginate film with glycerol (casting method) | NR | 13,600 (25 °C, 50% RH) | 41.1 | 8.5 | [196] |
| Chitosan film with glycerol | 0.188 × 10−2 (25 °C, <50% RH) | 210–3020 (25 °C, 0–100% RH) | 8.9 | 38.5 | [197] |
| Material | Advantage | Disadvantage | Reference |
|---|---|---|---|
| PLA-based | Renewable, biodegradable, biocompatible Usable for mono- and multilayer applications Desirable mechanical properties (stiffness, tensile strength) Good gas permeability Transparent | Expensive (synthesis) Limited to rigid packaging | [39,83,104,107,108,109] |
| PHA-based | Water-resistant surfaces by coating Functionalize grease resistance and sealability Good thermomechanical properties Desirable gas permeability and WVP | Not transparent | [106,110,129,130] |
| Lignocellulose biomass and/or lignin-based | Abundance in nature Antioxidant activity with long-term stability Improves mechanical, thermal, and barrier properties Reduction in WVP | Deficient quality (technical lignins) Copolymerization requires functionalization | [147,149,158,159,160] |
| Protein-based | Biodegradable Abundance in nature Good film-forming properties Desirable barrier properties Transparent Low cost/cost-effective | Low mechanical strength Lack of heat stability | [83,90,165,166,167,168] |
| Polysaccharide-based | Environmentally compatible, biodegradable Abundance in nature Effective oxygen barriers (intermediate to low humidity) Good mechanical properties Transparent Potential for edible packaging | Poor water vapor barrier Sensitive to moisture | [2,82,106,184,187,198] |
| Biomass | Packaging Matrix | Results | Reference |
|---|---|---|---|
| Apricot kernel EO | Chitosan | Prepared films showed better water resistance and improved antioxidant, antimicrobial, and mechanical properties; fungal growth on packaged bread is inhibited | [260] |
| Banana leaf EO | Gelatin | Improved antimicrobial properties (against E. coli and S. aureus) of gelatin films enriched with banana leaf EO; improvements on mechanical properties observed | [261] |
| Bergamot, lemongrass, rosemary, and clove EOs | PLA | Enhanced mechanical and antimicrobial properties against E. coli | [262] |
| Cinnamon and ginger EOs | CMC and Chitosan | Decreased water vapor permeability (particularly for cinnamon EO), antifungal activity against A. niger with a higher efficacy of cinnamon EO | [263] |
| Cinnamon bark EO | Gelatin | Antioxidant and antimicrobial effects observed (against S. typhimurium and L. monocytogenes), water resistance is increased | [264] |
| Cinnamon bark EO | PLA and Sea squirt (Halocynthia roretzi) shell protein | Enhanced antioxidant activity and antimicrobial effects against L. monocytogenes, S. aureus, E. coli and S. typhimurium; change of mechanical properties, decrease of water solubility, and water permeability | [265] |
| Cinnamon EO | Chitosan and Gum arabic | Better water barrier properties with a decrease in mechanical properties; high antioxidant effect when applying appropriate ratios of chitosan, gum Arabic, and cinnamon EO | [266] |
| Cinnamon EO | CMC and PVA | Enhancement of antioxidant and photostabilizing properties; highly effective against Penicillium digitatum; shelf life of packaged bread was increased | [267] |
| Cinnamon EO | Gelatin | Water vapor permeability and light-absorbing properties of enriched films increase while water content and elongation at break decrease; antifungal and antimicrobial activity against E. coli, S. aureus, A. niger, Rhizopus oryzae, and Paecilomyces varioti observed | [268] |
| Cinnamon EO | Whey protein | Effect of enriched films against S. aureus, no effect against E. coli observed | [269] |
| Cinnamon leaf oil | Gelatin | Antimicrobial effect against foodborne pathogens reported (E. coli, S. typhimurium, S. aureus, L. monocytogenes) | [270] |
| Clove bud EO | Pectin | Antioxidant and antimicrobial effects observed (against S. aureus, E. coli, and L. monocytogenes); improved mechanical properties (flexibility, resistance to breakage, water barrier properties, and heat stability) | [271] |
| Clove EO | Starch | Antifungal activity against Colletotrichum gloeosporioides and Colletotrichum musae, but not against Saccharomyces bourladii; enhanced shelf life of packaged bananas | [272] |
| Eucalyptus and Cinnamon EOs | PLA and PBAT | Antimicrobial activities against E. coli and S. aureus observed for both EOs with cinnamon EO showing a higher antimicrobial effect, increased biofilm inhibition and decreased UV-light transmission | [273] |
| Eucalyptus globulus EO | Chitosan | Antibacterial effects against S. entertidis, E. coli, B. cereus, and S. aureus observed (especially in liquid phase); lower antibacterial effect in vapor phase | [274] |
| Ginger EO and Eugenol | Gelatin and Chitosan | Antioxidant effect observed for both Eugenol and ginger EO (depending on film formulation); comparable water vapor permeability with increased elasticity | [275] |
| Lavender EO | Starch, Furcellaran, and Gelatin | Enhanced antioxidant and antimicrobial effects against E. coli and S. aureus; change of mechanical properties with addition of Lavender EO (decrease of tensile strength, water absorption, etc.) | [276] |
| Lemon EO | Starch | Optical and mechanical properties examined; antimicrobial effects against S. aureus and E. coli | [277] |
| Olive oil, corn oil, sunflower oil | Chitosan | Particularly olive oil enriched films showed better mechanical properties and a high antibacterial activity | [278] |
| Oregano EO | Whey protein | Higher amounts of EO resulted in higher water vapor permeability and film flexibility; antimicrobial activity against Penicillium commune | [279] |
| Origanum vulgare, O. majorana EOs | Chitosan | Antimicrobial effect against S. aureus and B. cereus observed with both EOs, significantly enhanced effects for O. vulgare EO | [280] |
| Pistacia atlantica EO | CMC and Gelatin | Antimicrobial effect against E. coli, S. aureus, Clostridium sporogenes, and particularly Salmonella enterica; reduction of e.g., water vapor permeability, film thickness, and tensile strength | [281] |
| Rosemary and mint EOs | Chitosan, Pectin, and Starch | Rosemary and mint EOs improved water barrier properties and inhibited Bacillus subtilis, E. coli, and L. monocytogenes; both EOs resulted in enhanced antioxidant effects | [282] |
| Rosmarinus officinalis, Artemisia herba-alba, Ocimum basilicum and Mentha pulegium EOs | Alginate | Strong antibacterial activity against S. aureus, E. coli, Salmonella enterica, Enterococcus faecium, Klebsiella pneumoniae, and Enterococcus faecalis; physical properties analyzed, antioxidant effect observed | [283] |
| Satureja Khuzestanica EO | Kefiran and CMC | Antimicrobial effects against S. aureus and E. coli, significant antioxidant properties, change in physical properties (e.g., decrease in water vapor permeability) | [284] |
| Satureja Khuzistanica Jamzad EO | Whey protein | Antimicrobial effect particularly against S. aureus with Pseudomonas aeruginosa showing the highest resistance of analyzed bacteria; increased elongation at break and water vapor permeability | [285] |
| Summer savory EO | CMC and Agar | Antimicrobial effects particularly against S. aureus, B. cereus, and L. monocytogenes with lower effects against E. coli; alteration of physical properties (increased water vapor permeability, improved mechanical flexibility) | [286] |
| Thyme and Clove EOs | PLA and PBAT | Positive properties observed for both EOs, but particularly for clove EO films, including UV-blocking and highly antimicrobial effects (inhibition of E. coli, complete killing of S. aureus) | [287] |
| Thyme, rosemary, and oregano EOs | PLA | Significant antioxidant effect on packaged minced fish with moderate alteration of mechanical properties | [288] |
| Extracted biomass | Packaging matrix | Results | Reference |
|---|---|---|---|
| Coffee beans and de-fatted cocoa beans | Starch | Synergistic antioxidant effect, decreased water vapor permeability, increased shelf life of palm oil | [299] |
| Grapefruit seed extract | PCL and Chitosan | Films showed better mechanical properties and inhibited bacterial growth of E. coli and P. aeruginosa for up to 6 days; successful tests with packaged salmon and bread | [300] |
| Herba Lophatheri extract | Chitosan | Moisture and oil resistance are enhanced, both antioxidant and antimicrobial activities observed (E. coli, S. aureus) | [301] |
| Kombucha tea extract | Chitosan | Decreased water vapor permeability and improved antioxidant, photoabsorbing, and antimicrobial effects (against E. coli and S. aureus); 3 days extended shelf life for packaged minced beef | [302] |
| Grape seed extract | Chitosan | Enhanced antioxidant and antimicrobial activity (total mesophilic aerobic bacteria, coliforms, E. coli, L. monocytogenes, S. aureus, and P. aeruginosa; shelf-life extension of refrigerated, vacuum-packed chicken breast fillets) | [303] |
| Rosemary extract | Starch | Significant antioxidant effect, increased UV-stability | [304] |
| Propolis extract and Zataria multiflora EO | Chitosan | Antimicrobial effects measured for mesophilic total viable plate counts, lactic acid bacteria, psychotropic bacteria, and Pseudomonas; synergistic effects observed; lower microbial load on packaged chicken | [305] |
| Sumac extract and Zataria multiflora EO | Chitosan | Antioxidant effects and prolonged shelf life on packaged meat observed; antimicrobial activity against different bacteria (e.g., Pseudomonas spp.) | [306] |
| Propolis extract and Zataria multiflora EO | PLA | Increased shelf life of packaged sausages, antimicrobial effects against common food pathogens (S. aureus, E. coli, Vibrio parahaemolyticus, L. monocytogenes) | [307] |
| Biomass | Packaging Matrix | Encapsulation Details | Results | Reference |
|---|---|---|---|---|
| Chrysanthemum EO | - | Chitosan nanofibers | Antioxidant and antimicrobial effect against L. monocytogenes observed e.g., on packaged beef, prolongation of shelf life possible | [316] |
| Cinnamon EO | PLA | Nanofibers | Better antimicrobial effect against S. aureus and E. coli observed for encapsulated EO; encapsulation process is more suitable formulation method to maintain EO properties; shelf life of packaged pork was prolonged | [310] |
| Clove EO | Alginate | Inclusion complex | Successful incorporation of clove EO complexes; resulting in less transparent and flexible films, decreased elasticity, increased water vapor permeability | [317] |
| Cumin seed oil | - | Nanoemulsion (Whey protein, Guar gum) | Antimicrobial effect of encapsulated oil against S. aureus, E. coli, and A. flavus | [318] |
| Cuminum cyminum EO | - | Chitosan nanoparticles | Significant antioxidant effect in packaged white button mushrooms observed, resulting in presumed shelf-life prolongation | [319] |
| Laurel EO and silver nanoparticles | PE | Liposomes in Chitosan | Antioxidant properties observed during 7 days of storage with only about 30% of EO released from liposomes; antimicrobial effect against S. aureus and E. coli results in 6 days prolonged shelf life of packaged pork | [320] |
| Lavandula angustofolia EO | - | Nanoemulsion (Whey protein) | Encapsulation enhanced thermal stability of EO; antibacterial effect is observed | [309] |
| Menthone, Oregano, Cinnamon, Lavender and Citral EOs | - | Starch nanoparticles | Enhanced stability of antioxidants against thermal influence after encapsulation; antimicrobial effects against E. coli and S. aureus are prolonged | [311] |
| Moringa oil | Gelatin nanofibers | Chitosan nanoparticles | High antimicrobial activity of encapsulated Moringa oil against L. monocytogenes and S. aureus for 10 days without affecting the sensory properties of packaged cheese | [321] |
| Oregano EO | - | PCL nanocapsules | High retention of encapsulated Rosemary EO (determined via carvacrol content) observed, suitability for long-term delivery of carvacrol can be assumed | [322] |
| Oregano EO | Soy protein | Microencapsulation by ionic gelation | Strong antioxidant and antimicrobial properties against E. coli and S. aureus; enhanced effects and mechanical properties with microencapsulated EO in contrast to free EOs | [312] |
| Rosemary EO | Starch and CMC | Chitosan nanogel | Films with encapsulated EO show higher water vapor permeability, higher transparency, and tensile strength; immediate (free EO) and gradual (encapsulated EO) antimicrobial effects against S. aureus were observed | [313] |
| Thymbra capitata EO | - | Zein nanoparticles | Both free and encapsulated EO are effective against E. coli and L. monocytogenes; presumably due to controlled release, encapsulated EO showed lower antimicrobial efficacy compared to free EO | [323] |
| Thyme EO | - | Nanofibers (Chitosan, Gelatin) | Both free and encapsulated thyme EO has antioxidant and antimicrobial effects against Clostridium perfringens; tests show that such nanofibers could be used to substitute nitrite in meat products | [324] |
| Thyme EO | Gelatin | Nanofibers | Antimicrobial effect against Campylobacter jejuni in packaged chicken observed | [325] |
| Thyme EO | Ink (for paper packaging) | Halloysite nanotubes | Strong antibacterial activity against E. coli, mesophilic aerobic bacteria, molds, and yeasts for up to 10 days after encapsulation in Halloysite nanotubes | [326,327] |
| Zataria multiflora EO | PVA | Nanofibers (Chitosan, PVA, Gelatin) | Encapsulated Zataria multiflora EO completely inhibited growth of S. aureus, P. aeruginosa, and Candida albicans for 24 h; tested material is developed for use as wound dressing | [328] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korte, I.; Kreyenschmidt, J.; Wensing, J.; Bröring, S.; Frase, J.N.; Pude, R.; Konow, C.; Havelt, T.; Rumpf, J.; Schmitz, M.; et al. Can Sustainable Packaging Help to Reduce Food Waste? A Status Quo Focusing Plant-Derived Polymers and Additives. Appl. Sci. 2021, 11, 5307. https://doi.org/10.3390/app11115307
Korte I, Kreyenschmidt J, Wensing J, Bröring S, Frase JN, Pude R, Konow C, Havelt T, Rumpf J, Schmitz M, et al. Can Sustainable Packaging Help to Reduce Food Waste? A Status Quo Focusing Plant-Derived Polymers and Additives. Applied Sciences. 2021; 11(11):5307. https://doi.org/10.3390/app11115307
Chicago/Turabian StyleKorte, Imke, Judith Kreyenschmidt, Joana Wensing, Stefanie Bröring, Jan Niklas Frase, Ralf Pude, Christopher Konow, Thomas Havelt, Jessica Rumpf, Michaela Schmitz, and et al. 2021. "Can Sustainable Packaging Help to Reduce Food Waste? A Status Quo Focusing Plant-Derived Polymers and Additives" Applied Sciences 11, no. 11: 5307. https://doi.org/10.3390/app11115307
APA StyleKorte, I., Kreyenschmidt, J., Wensing, J., Bröring, S., Frase, J. N., Pude, R., Konow, C., Havelt, T., Rumpf, J., Schmitz, M., & Schulze, M. (2021). Can Sustainable Packaging Help to Reduce Food Waste? A Status Quo Focusing Plant-Derived Polymers and Additives. Applied Sciences, 11(11), 5307. https://doi.org/10.3390/app11115307









