Mini-Review: Recent Technologies of Electrode and System in the Enzymatic Biofuel Cell (EBFC)
Abstract
1. Introduction
2. The Enzyme, Support, and Substrate in EBFC
3. Current Development on the Bioanode and Biocathode in EBFC
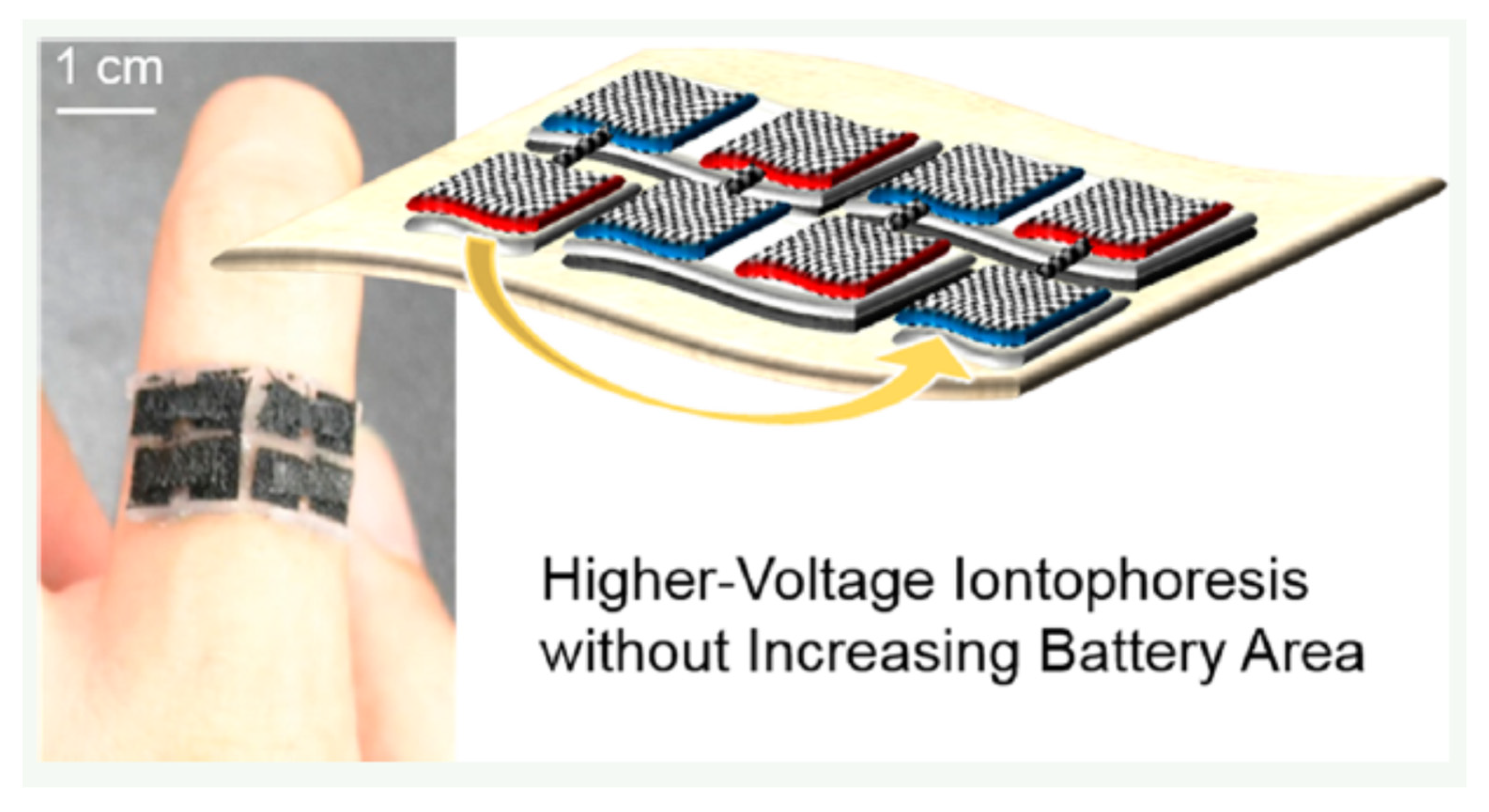
4. System in EBFC
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shakeel, N.; Ahmad, A.; Ahamed, M.I.; Asiri, A.M. Kraton based polymeric nanocomposite bioanode for the application in a biofuel cell. Enzym. Microb. Technol. 2019, 127, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Arjun, A.M.; Vimal, M.; Sandhyarani, N. A hybrid hydrogel separated biofuel cell with a novel enzymatic anode and glucose tolerant cathode. Int. J. Hydrog. Energy 2019, 44, 27056–27066. [Google Scholar] [CrossRef]
- Li, X.; Li, D.; Zhang, Y.; Lv, P.; Feng, Q.; Wei, Q. Encapsulation of enzyme by metal-organic framework for single-enzymatic biofuel cell-based self-powered biosensor. Nano Energy 2020, 68, 104308. [Google Scholar] [CrossRef]
- Southcott, M.; MacVittie, K.; Halámek, J.; Halámková, L.; Jemison, W.D.; Lobel, R.; Katz, E. A pacemaker powered by an implantable biofuel cell operating under conditions mimicking the human blood circulatory system—Battery not included. Phys. Chem. Chem. Phys. 2013, 15, 6278–6283. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; McGourty, K.D.; Magner, E. Enzymatic biofuel cells for self-powered, controlled drug release. J. Am. Chem. Soc. 2020, 142, 11602–11609. [Google Scholar] [CrossRef]
- Jeon, W.-Y.; Lee, J.-H.; Dashnyam, K.; Choi, Y.-B.; Kim, T.-H.; Lee, H.-H.; Kim, H.-W.; Kim, H.-H. Performance of a glu-cose-reactive enzyme-based biofuel cell system for biomedical applications. Sci. Rep. 2019, 9, 10872. [Google Scholar] [CrossRef]
- Li, X.; Lv, P.; Yao, Y.; Feng, Q.; Mensah, A.; Li, D.; Wei, Q. A novel single-enzymatic biofuel cell based on highly flexible conductive bacterial cellulose electrode utilizing pollutants as fuel. Chem. Eng. J. 2020, 379, 122316. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, S.; Pandey, L.M.; Chandra, P. Nanoengineered material based biosensing electrodes for enzymatic biofuel cells applications. Mater. Sci. Energy Technol. 2018, 1, 38–48. [Google Scholar] [CrossRef]
- Kwon, C.H.; Ko, Y.; Shin, D.; Kwon, M.; Park, J.; Bae, W.K.; Lee, S.W.; Cho, J. High-power hybrid biofuel cells using layer-by-layer assembled glucose oxidase-coated metallic cotton fibers. Nat. Commun. 2018, 9, 4479. [Google Scholar] [CrossRef]
- Franco, J.H.; Klunder, K.J.; Lee, J.; Russell, V.; de Andrade, A.R.; Minteer, S.D. Enhanced electrochemical oxidation of ethanol using a hybrid catalyst cascade architecture containing pyrene-tempo, oxalate decarboxylase and carboxylated multi-walled carbon nanotube. Biosens. Bioelectron. 2020, 154, 112077. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhou, M.; Wen, D.; Bai, L.; Lou, B.; Dong, S. Small-size biofuel cell on paper. Biosens. Bioelectron. 2012, 35, 155–159. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, F.; Han, Z.; Huang, K.; Wang, X.; Liu, Z.; Wang, S.; Lu, Y. Construction of sandwiched self-powered biosensor based on smart nanostructure and capacitor: Toward multiple signal amplification for thrombin detection. Sens. Actuators B Chem. 2020, 304, 127418. [Google Scholar] [CrossRef]
- Wang, F.-T.; Wang, Y.-H.; Xu, J.; Huang, K.-J. A high-energy sandwich-type self-powered biosensor based on DNA bioconjugates and a nitrogen doped ultra-thin carbon shell. J. Mater. Chem. B 2020, 8, 1389–1395. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, M.; Kuwahara, K.; Tsushida, M.; Shida, K. Cellulose nanofiber-based electrode as a component of an enzyme-catalyzed biofuel cell. RSC Adv. 2020, 10, 22120–22125. [Google Scholar] [CrossRef]
- Shakeel, N.; Ahamed, M.I.; Ahmed, A.; Rahman, M.M.; Asiri, A.M. Functionalized magnetic nanoparticle-reduced graphene oxide nanocomposite for enzymatic biofuel cell applications. Int. J. Hydrog. Energy 2019, 44, 28294–28304. [Google Scholar] [CrossRef]
- Alamry, K.A. Application of electrically conducting nanocomposite material polythiophene@nio/frt/gox as anode for enzymatic biofuel cells. Materials 2020, 13, 1823. [Google Scholar]
- Duong, N.B.; Wang, C.-L.; Huang, L.Z.; Fang, W.T.; Yang, H. Development of a facile and low-cost chitosan-modified carbon cloth for efficient self-pumping enzymatic biofuel cells. J. Power Sources 2019, 429, 111–119. [Google Scholar] [CrossRef]
- Jayapiriya, U.S.; Rewatkar, P.; Goel, S. Miniaturized polymeric enzymatic biofuel cell with integrated microfluidic device and enhanced laser ablated bioelectrodes. Int. J. Hydrog. Energy 2021, 46, 3183–3192. [Google Scholar]
- Bahar, T.; Yazici, M.S. Assessment of glucose oxidase based enzymatic fuel cells integrated with newly developed chitosan membranes by electrochemical impedance spectroscopy. Electroanalysis 2020, 32, 1304–1314. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, H.; Liu, M.; Lu, H.; Zhao, G. A novel self-powered aptasensor for environmental pollutants detection based on simple and efficient enzymatic biofuel cell. Sens. Actuators B Chem. 2020, 305, 127468. [Google Scholar] [CrossRef]
- Shakeel, N.; Ahamed, M.I.; Ahmed, A.; Kanchi, S.; Kashmery, H.A. Hydrothermally synthesized defective nimose2 nanoplates decorated on the surface of functionalized swcnts doped polypyrrole scaffold for enzymatic biofuel cell applications. Int. J. Hydrog. Energy 2021, 46, 3240–3250. [Google Scholar] [CrossRef]
- Wang, X.; Kim, J.H.; Choi, Y.B.; Kim, H.-H.; Kim, C.-J. Fabrication of optimally configured layers of swcnts, gold nanoparticles, and glucose oxidase on ito electrodes for high-power enzymatic biofuel cells. Korean J. Chem. Eng. 2019, 36, 1172–1183. [Google Scholar] [CrossRef]
- Choi, H.S.; Yang, X.; Kim, D.S.; Yang, J.H.; Han, S.O.; Park, C.; Kim, S.W. Power generation from cheese whey using enzymatic fuel cell. J. Clean. Prod. 2020, 254, 120181. [Google Scholar] [CrossRef]
- Chu, T.-F.; Rajendran, R.; Kuznetsova, I.; Wang, G.-J. High-power, non-enzymatic glucose biofuel cell based on a nano/micro hybrid-structured au anode. J. Power Sources 2020, 453, 227844. [Google Scholar] [CrossRef]
- Ohayon, D.; Nikiforidis, G.; Savva, A.; Giugni, A.; Wustoni, S.; Palanisamy, T.; Chen, X.; Maria, I.P.; Di Fabrizio, E.; Costa, P.M.F.J.; et al. Biofuel powered glucose detection in bodily fluids with an n-type conjugated polymer. Nat. Mater. 2020, 19, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Babadi, A.A.; Wan-Mohtar, W.A.A.Q.I.; Chang, J.-S.; Ilham, Z.; Jamaludin, A.A.; Zamiri, G.; Akbarzadeh, O.; Basirun, W.J. High-performance enzymatic biofuel cell based on three-dimensional graphene. Int. J. Hydrog. Energy 2019, 44, 30367–30374. [Google Scholar] [CrossRef]
- Franco, J.H.; de Almeida, P.Z.; Abdellaoui, S.; Hickey, D.P.; Ciancaglini, P.; de Lourdes, T.M.; Polizeli, M.; Minteer, S.D.; de Andrade, A.R. Bioinspired architecture of a hybrid bifunctional enzymatic/organic electrocatalyst for complete ethanol oxidation. Bioelectrochemistry 2019, 130, 107331. [Google Scholar] [CrossRef] [PubMed]
- Hyun, K.; Kang, S.; Kim, J.; Kwon, Y. New biocatalyst including a 4-nitrobenzoic acid mediator embedded by the crosslinking of chitosan and genipin and its use in an energy device. ACS Appl. Mater. Interfaces 2020, 12, 23635–23643. [Google Scholar] [CrossRef]
- Munauwarah, R.; Bojang, A.A.; Wu, H.S. Characterization of enzyme immobilized carbon electrode using covalent-entrapment with polypyrrole. J. Chin. Inst. Eng. 2018, 41, 710–719. [Google Scholar] [CrossRef]
- Conghaile, P.Ó.; Kumar, R.; Ferrer, M.L.; Leech, D. Glucose oxidation by enzyme electrodes using genipin to crosslink chitosan, glucose oxidase and amine-containing osmium redox complexes. Electrochem. Commun. 2020, 113, 106703. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, X.; Zhang, L.; Si, Y.; Guo, S.; Su, H.; Liu, J. Layer-by-layer assembly for immobilizing enzymes in enzymatic biofuel cells. Sustain. Energy Fuels 2020, 4, 68–79. [Google Scholar] [CrossRef]
- Miki, K.; Watanabe, T.; Koh, S. Electrochemical characterization of cvd-grown graphene for designing electrode/biomolecule interfaces. Crystals 2020, 10, 241. [Google Scholar] [CrossRef]
- Galindo-de-la-Rosa, J.; Álvarez, A.; Gurrola, M.P.; Rodríguez-Morales, J.A.; Oza, G.; Arriaga, L.G.; Ledesma-García, J. Alcohol dehydrogenase immobilized on tio2 nanotubes for ethanol microfluidic fuel cells. ACS Sustain. Chem. Eng. 2020, 8, 10900–10910. [Google Scholar] [CrossRef]
- Mohamad, N.R.; Marzuki, N.H.C.; Buang, N.A.; Huyop, F.; Wahab, R.A. An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnol. Biotechnol. Equip. 2015, 29, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Minteer, S.D.; Atanassov, P.; Luckarift, H.R.; Johnson, G.R. New materials for biological fuel cells. Mater. Today 2012, 15, 166–173. [Google Scholar] [CrossRef]
- Lee, J.Y.Y.; Elouarzaki, K.; Sabharwal, H.S.; Fisher, A.C.; Lee, J.-M. A hydrogen/oxygen hybrid biofuel cell comprising an electrocatalytically active nanoflower/laccase-based biocathode. Catal. Sci. Technol. 2020, 10, 6235–6243. [Google Scholar] [CrossRef]
- Sakthivel, M.; Ramaraj, S.; Chen, S.-M.; Chen, T.-W.; Ho, K.-C. Transition-metal-doped molybdenum diselenides with defects and abundant active sites for efficient performances of enzymatic biofuel cell and supercapacitor applications. ACS Appl. Mater. Interfaces 2019, 11, 18483–18493. [Google Scholar] [CrossRef]
- Ma, C.; Wu, R.; Huang, R.; Jiang, W.; You, C.; Zhu, L.; Zhu, Z. Directed evolution of a 6-phosphogluconate dehydrogenase for operating an enzymatic fuel cell at lowered anodic phs. J. Electroanal. Chem. 2019, 851, 113444. [Google Scholar] [CrossRef]
- Kang, Z.; Job Zhang, Y.-H.P.; Zhu, Z. A shriveled rectangular carbon tube with the concave surface for high-performance enzymatic glucose/o2 biofuel cells. Biosens. Bioelectron. 2019, 132, 76–83. [Google Scholar] [CrossRef]
- Jayapiriya, U.S.; Goel, S. Surface modified 3d printed carbon bioelectrodes for glucose/o2 enzymatic biofuel cell: Comparison and optimization. Sustain. Energy Technol. Assess. 2020, 42, 100811. [Google Scholar]
- Ji, J.; Ro, S.; Kwon, Y. Membraneless biofuel cells using new cathodic catalyst including hemin bonded with amine functionalized carbon nanotube and glucose oxidase sandwiched by poly(dimethyl-diallylammonium chloride). J. Ind. Eng. Chem. 2020, 87, 242–249. [Google Scholar] [CrossRef]
- Díaz-González, J.-c.M.; Escalona-Villalpando, R.A.; Arriaga, L.G.; Minteer, S.D.; Casanova-Moreno, J.R. Effects of the cross-linker on the performance and stability of enzymatic electrocatalytic films of glucose oxidase and dimethylferrocene-modified linear poly(ethyleneimine). Electrochim. Acta 2020, 337, 135782. [Google Scholar] [CrossRef]
- Ji, J.; Woo, J.; Chung, Y.; Joo, S.H.; Kwon, Y. Dual catalytic functions of biomimetic, atomically dispersed iron-nitrogen doped carbon catalysts for efficient enzymatic biofuel cells. Chem. Eng. J. 2020, 381, 122679. [Google Scholar] [CrossRef]
- Niiyama, A.; Murata, K.; Shigemori, Y.; Zebda, A.; Tsujimura, S. High-performance enzymatic biofuel cell based on flexible carbon cloth modified with mgo-templated porous carbon. J. Power Sources 2019, 427, 49–55. [Google Scholar] [CrossRef]
- Tang, J.; Werchmeister, R.M.L.; Preda, L.; Huang, W.; Zheng, Z.; Leimkühler, S.; Wollenberger, U.; Xiao, X.; Engelbrekt, C.; Ulstrup, J.; et al. Three-dimensional sulfite oxidase bioanodes based on graphene functionalized carbon paper for sulfite/o2 biofuel cells. ACS Catal. 2019, 9, 6543–6554. [Google Scholar] [CrossRef]
- Trifonov, A.; Stemmer, A.; Tel-Vered, R. Power generation by selective self-assembly of biocatalysts. ACS Nano 2019, 13, 8630–8638. [Google Scholar] [CrossRef] [PubMed]
- Fritea, L.; Gross, A.J.; Reuillard, B.; Gorgy, K.; Cosnier, S.; Le Goff, A. A diethyleneglycol-pyrene-modified ru(ii) catalyst for the design of buckypaper bioelectrodes and the wiring of glucose dehydrogenases. ChemElectroChem 2019, 6, 3621–3626. [Google Scholar] [CrossRef]
- Shen, F.; Pankratov, D.; Halder, A.; Xiao, X.; Toscano, M.D.; Zhang, J.; Ulstrup, J.; Gorton, L.; Chi, Q. Two-dimensional graphene paper supported flexible enzymatic fuel cells. Nanoscale Adv. 2019, 1, 2562–2570. [Google Scholar] [CrossRef]
- Rocha, I.M.; Soares, O.S.G.P.; Fernandes, D.M.; Freire, C.; Figueiredo, J.L.; Pereira, M.F.R. N-doped carbon nanotubes for the oxygen reduction reaction in alkaline medium: Synergistic relationship between pyridinic and quaternary nitrogen. ChemistrySelect 2016, 1, 2522–2530. [Google Scholar] [CrossRef]
- Wan, J.; Mi, L.; Tian, Z.; Li, Q.; Liu, S. A single-liquid miniature biofuel cell with boosting power density via gas diffusion bioelectrodes. J. Mater. Chem. B 2020, 8, 3550–3556. [Google Scholar] [CrossRef]
- Rewatkar, P.; Goel, S. Microfluidic paper based membraneless biofuel cell to harvest energy from various beverages. J. Electrochem. Sci. Eng. 2020, 10, 49–54. [Google Scholar] [CrossRef]
- Gross, A.J.; Holzinger, M.; Cosnier, S. Buckypaper bioelectrodes: Emerging materials for implantable and wearable biofuel cells. Energy Environ. Sci. 2018, 11, 1670–1687. [Google Scholar] [CrossRef]
- Tsuruoka, N.; Soto, S.S.; Tahar, A.B.; Zebda, A.; Tsujimura, S. Mediated electrochemical oxidation of glucose via poly(methylene green) grafted on the carbon surface catalyzed by flavin adenine dinucleotide-dependent glucose dehydrogenase. Colloids Surf. B Biointerfaces 2020, 192, 111065. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, Y.; Ding, F.; Chen, D.; Wang, Y.; Jin, X.; Zhu, X. Rational design of electroactive redox enzyme nanocapsules for high-performance biosensors and enzymatic biofuel cell. Biosens. Bioelectron. 2021, 174, 112805. [Google Scholar] [CrossRef] [PubMed]
- Korkut, S.; Kiliç, M.S.; Hazer, B. Newly designed bioanode for glucose/o2 biofuel cells to generate renewable energy. Asia-Pac. J. Chem. Eng. 2019, 14, e2374. [Google Scholar] [CrossRef]
- Shakeel, N. Optimization of rgo-pei/naph-sh/agnws/frt/gox nanocomposite anode for biofuel cell applications. Sci. Rep. 2020, 10, 8919. [Google Scholar] [CrossRef]
- Herkendell, K.; Stemmer, A.; Tel-Vered, R. Magnetically induced enzymatic cascades—Advancing towards multi-fuel direct/mediated bioelectrocatalysis. Nanoscale Adv. 2019, 1, 1686–1692. [Google Scholar] [CrossRef]
- Herkendell, K.; Stemmer, A.; Tel-Vered, R. Extending the operational lifetimes of all-direct electron transfer enzymatic biofuel cells by magnetically assembling and exchanging the active biocatalyst layers on stationary electrodes. Nano Res. 2019, 12, 767–775. [Google Scholar] [CrossRef]
- Werchmeister, R.M.L.; Tang, J.; Xiao, X.; Wollenberger, U.; Hjuler, H.A.; Ulstrup, J.; Zhang, J. Three-dimensional bioelectrodes utilizing graphene based bioink. J. Electrochem. Soc. 2019, 166, G170–G177. [Google Scholar] [CrossRef]
- Adachi, T.; Fujii, T.; Honda, M.; Kitazumi, Y.; Shirai, O.; Kano, K. Direct electron transfer-type bioelectrocatalysis of fad-dependent glucose dehydrogenase using porous gold electrodes and enzymatically implanted platinum nanoclusters. Bioelectrochemistry 2020, 133, 107457. [Google Scholar] [CrossRef]
- Shakeel, N.; Ahamed, M.I.; Kanchi, S.; Kashmery, H.A. Green synthesis of zno nanoparticles decorated on polyindole functionalized-mcnts and used as anode material for enzymatic biofuel cell applications. Sci. Rep. 2020, 10, 5052. [Google Scholar]
- Sakamoto, H.; Koto, A.; Takamura, E.-I.; Asakawa, H.; Fukuma, T.; Satomura, T.; Suye, S.-I. Development of biofuel cell using a complex of highly oriented immobilized his-tagged enzyme and carbon nanotube surface through a pyrene derivative. J. Nanosci. Nanotechnol. 2019, 19, 3551–3557. [Google Scholar] [CrossRef]
- Duong, N.B.; Truong, V.M.; Li, Y.-S.; Wang, C.-L.; Yang, H. Improving the immobilization of glucose oxidase on carbon cloth via a hybrid approach of crosslinked chitosan/tpp matrices with na polymers for high-performance self-pumping enzyme-based biofuel cells. Energy Fuels 2020, 34, 10050–10058. [Google Scholar] [CrossRef]
- ul Haque, S.; Nasar, A.; Rahman, M.M. Applications of chitosan (chi)-reduced graphene oxide (rgo)-polyaniline (pani) conducting composite electrode for energy generation in glucose biofuel cell. Sci. Rep. 2020, 10, 10428. [Google Scholar] [CrossRef]
- Lv, C.; Li, S.; Liu, L.; Zhu, X.; Yang, X. Enhanced electrochemical characteristics of the glucose oxidase bioelectrode constructed by carboxyl-functionalized mesoporous carbon. Sensors 2020, 20, 3365. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Choi, H.S.; Yang, X.; Yang, J.H.; Lee, J.H.; Yoo, H.Y.; Lee, J.; Park, C.; Kim, S.W. Improvement of power generation of enzyme fuel cell by novel go/co/chitosan electrodeposition. J. Ind. Eng. Chem. 2020, 81, 108–114. [Google Scholar] [CrossRef]
- Butsyk, O.; Olejnik, P.; Romero, E.; Plonska-Brzezinska, M.E. Postsynthetic treatment of carbon nano-onions: Surface modification by heteroatoms to enhance their capacitive and electrocatalytic properties. Carbon 2019, 147, 90–104. [Google Scholar] [CrossRef]
- Tang, J.; Yan, X.; Huang, W.; Engelbrekt, C.; Duus, J.Ø.; Ulstrup, J.; Xiao, X.; Zhang, J. Bilirubin oxidase oriented on novel type three-dimensional biocathodes with reduced graphene aggregation for biocathode. Biosens. Bioelectron. 2020, 167, 112500. [Google Scholar] [CrossRef] [PubMed]
- Kuroishi, K.; Doi, T.; Yonaha, Y.; Kusajima, I.; Nishioka, Y.; Imai, S. Enzymatic biofuel cell using grooved gel of fructose between graphene-coated carbon fiber cloth electrodes. IEICE Trans. Electron. 2019, 102, 151–154. [Google Scholar] [CrossRef]
- Shen, F.; Pankratov, D.; Pankratova, G.; Toscano, M.D.; Zhang, J.; Ulstrup, J.; Chi, Q.; Gorton, L. Supercapacitor/biofuel cell hybrid device employing biomolecules for energy conversion and charge storage. Bioelectrochemistry 2019, 128, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Kizling, M.; Dzwonek, M.; Więckowska, A.; Stolarczyk, K.; Bilewicz, R. Biosupercapacitor with an enzymatic cascade at the anode working in a sucrose solution. Biosens. Bioelectron. 2021, 186, 113248. [Google Scholar] [CrossRef]
- Ji, J.; Woo, J.; Chung, Y.; Joo, S.H.; Kwon, Y. Membraneless enzymatic biofuel cells using iron and cobalt co-doped ordered mesoporous porphyrinic carbon based catalyst. Appl. Surf. Sci. 2020, 511, 145449. [Google Scholar] [CrossRef]
- Mashayekhi Mazar, F.; Alijanianzadeh, M.; Molaei Rad, A.; Heydari, P. Power harvesting from physiological serum in microfluidic enzymatic biofuel cell. Microelectron. Eng. 2020, 219, 111159. [Google Scholar] [CrossRef]
- Kwon, C.H.; Ko, Y.; Shin, D.; Lee, S.W.; Cho, J. Highly conductive electrocatalytic gold nanoparticle-assembled carbon fiber electrode for high-performance glucose-based biofuel cells. J. Mater. Chem. A 2019, 7, 13495–13505. [Google Scholar] [CrossRef]
- Kizling, M.; Dzwonek, M.; Nowak, A.; Tymecki, Ł.; Stolarczyk, K.; Więckowska, A.; Bilewicz, R. Multi-substrate biofuel cell utilizing glucose, fructose and sucrose as the anode fuels. Nanomaterials 2020, 10, 1534. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Kong, X.; Gu, C.; Gai, P.; Li, F. Ultrasensitive self-powered biosensors with visual self-checking function for pathogenic bacteria detection. Sens. Actuators B Chem. 2020, 307, 127618. [Google Scholar] [CrossRef]
- Seok, S.; Wang, C.; Lefeuvre, E.; Park, J. Autonomous energy harvester based on textile-based enzymatic biofuel cell for on-demand usage. Sensors 2020, 20, 5009. [Google Scholar] [CrossRef] [PubMed]
- Rewatkar, P.; U.S., J.; Goel, S. Optimized shelf-stacked paper origami-based glucose biofuel cell with immobilized enzymes and a mediator. ACS Sustain. Chem. Eng. 2020, 8, 12313–12320. [Google Scholar] [CrossRef]
- Khan, H.; Kim, C.M.; Kim, S.Y.; Goel, S.; Dwivedi, P.K.; Sharma, A.; Kim, Y.H.; Kim, G.M. Fabrication of enzymatic biofuel cell with electrodes on both sides of microfluidic channel. Int. J. Precis. Eng. Manuf. Green Technol. 2019, 6, 511–520. [Google Scholar] [CrossRef]
- Rewatkar, P.; Bandapati, M.; Goel, S. Miniaturized additively manufactured co-laminar microfluidic glucose biofuel cell with optimized grade pencil bioelectrodes. Int. J. Hydrog. Energy 2019, 44, 31434–31444. [Google Scholar] [CrossRef]
- Rewatkar, P.; Goel, S. Next-generation 3d printed microfluidic membraneless enzymatic biofuel cell: Cost-effective and rapid approach. IEEE Trans. Electron Devices 2019, 66, 3628–3635. [Google Scholar] [CrossRef]
- Rewatkar, P.; Kothuru, A.; Goel, S. Pdms-based microfluidic glucose biofuel cell integrated with optimized laser-induced flexible graphene bioelectrodes. IEEE Trans. Electron Devices 2020, 67, 1832–1838. [Google Scholar] [CrossRef]
- Gai, P.; Gu, C.; Kong, X.; Li, F. Anode-driven controlled release of cathodic fuel via ph response for smart enzymatic biofuel cell. iScience 2020, 23, 101133. [Google Scholar] [CrossRef] [PubMed]
- Varničić, M.; Zasheva, I.N.; Haak, E.; Sundmacher, K.; Vidaković-Koch, T. Selectivity and sustainability of electroenzymatic process for glucose conversion to gluconic acid. Catalysts 2020, 10, 269. [Google Scholar] [CrossRef]
- Yin, S.; Liu, X.; Kobayashi, Y.; Nishina, Y.; Nakagawa, R.; Yanai, R.; Kimura, K.; Miyake, T. A needle-type biofuel cell using enzyme/mediator/carbon nanotube composite fibers for wearable electronics. Biosens. Bioelectron. 2020, 165, 112287. [Google Scholar] [CrossRef]
- Yoshida, S.; Mizuno, T.; Kusama, S.; Sato, K.; Raut, B.; Nishizawa, M. Series-connected flexible biobatteries for higher voltage electrical skin patches. ACS Appl. Electron. Mater. 2020, 2, 170–176. [Google Scholar] [CrossRef]
- Shitanda, I.; Fujimura, Y.; Nohara, S.; Hoshi, Y.; Itagaki, M.; Tsujimura, S. Paper-based disk-type self-powered glucose biosensor based on screen-printed biofuel cell array. J. Electrochem. Soc. 2019, 166, B1063–B1068. [Google Scholar] [CrossRef]
- Marković, N.; Conzuelo, F.; Szczesny, J.; González García, M.B.; Hernández Santos, D.; Ruff, A.; Schuhmann, W. An air-breathing carbon cloth-based screen-printed electrode for applications in enzymatic biofuel cells. Electroanalysis 2019, 31, 217–221. [Google Scholar] [CrossRef]
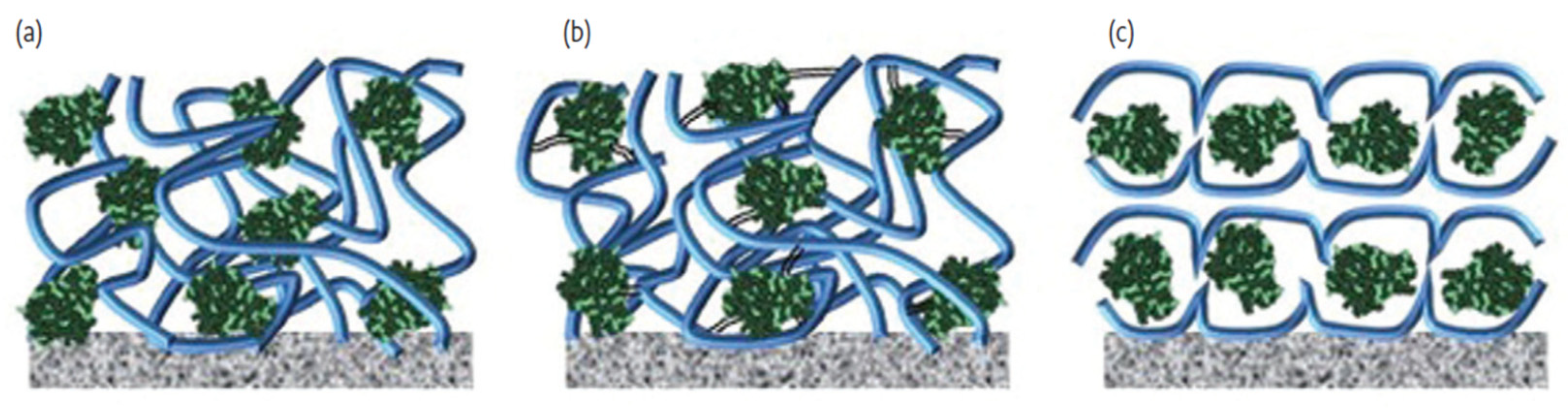


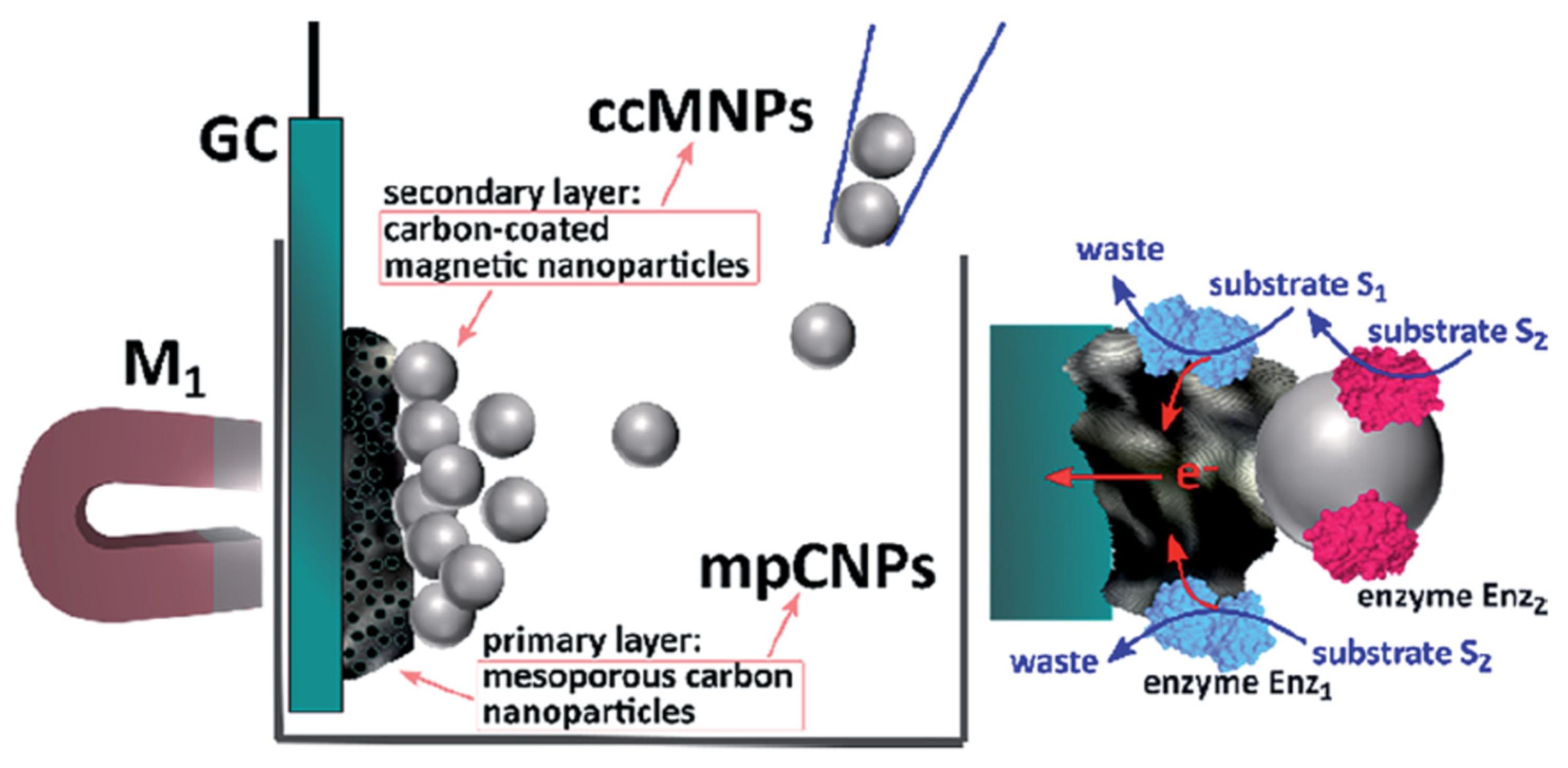
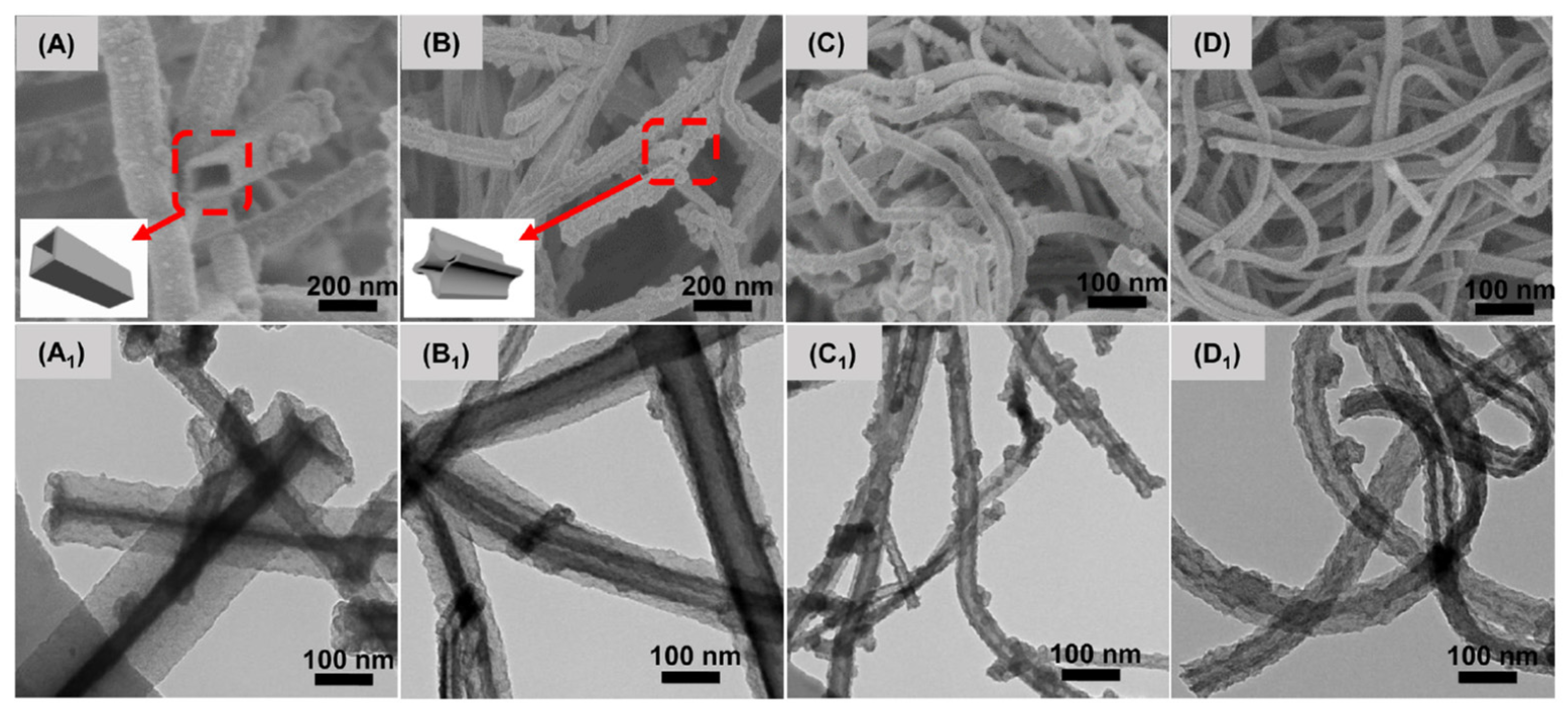

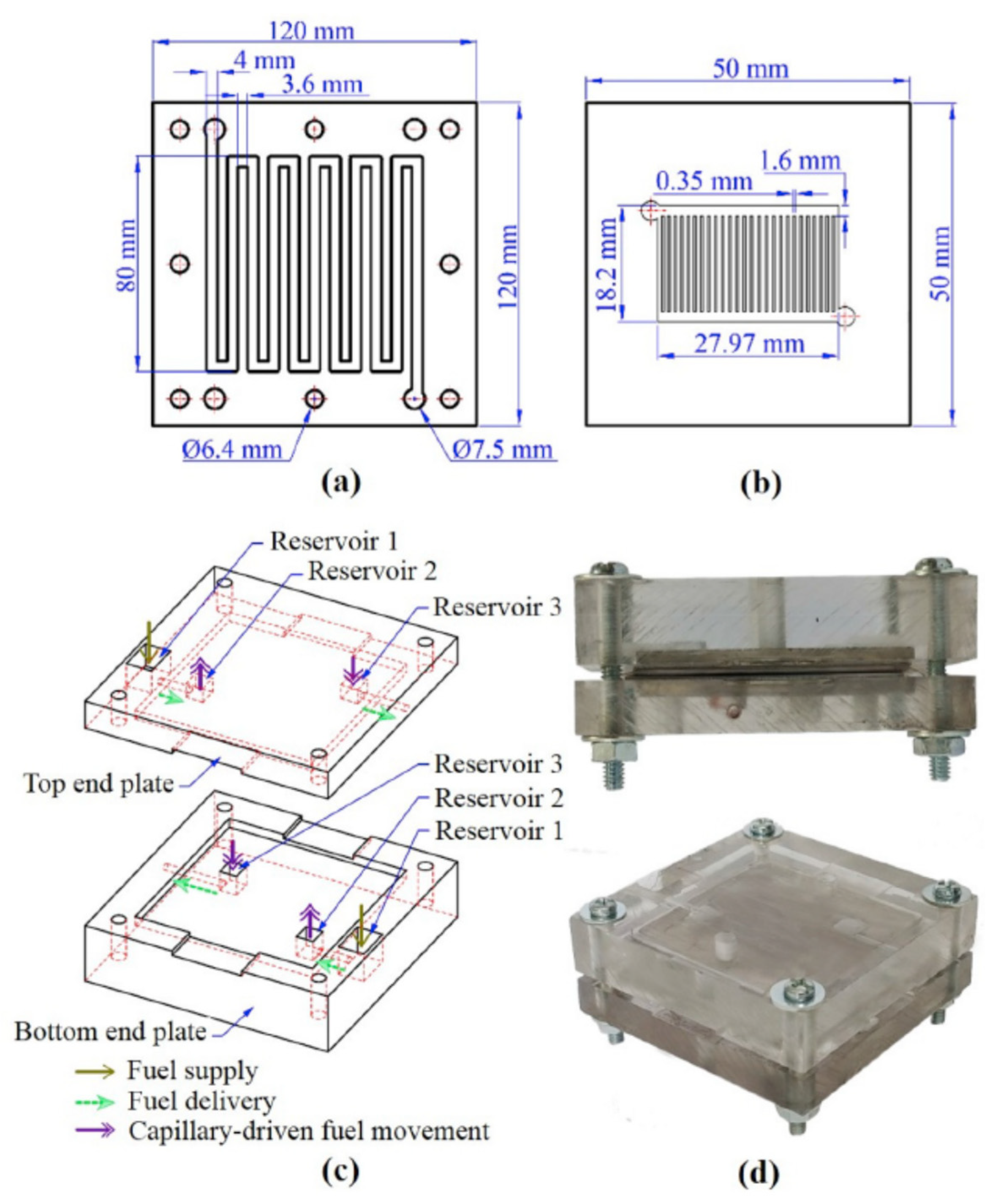
| Enzyme | Electrode Materials | Electrode Size | Storage Conditions/ Lifetime/Stability | Working Conditions/Response Time/Measurement Range | Performance | Ref |
|---|---|---|---|---|---|---|
| Glucose oxidase (GOx) | Kraton/MWCNTs; mediator = ferritin (Frt); glassy carbon electrode (GCE) | 3 mm diameter | - | 1M PBS (pH 7.0) solution at an ambient temperature at a scan rate of 100 mV/s | Current density = 1.14 mA/cm2 at 60 mM | [1] |
| Glucose oxidase (GOx) | Multi-walled carbon nanotube-pyrene carboxylic acid (MWCNTePCA) nanocomposite; carbon cloth (CC) | 4 cm2 | - | PBS solution (pH 7.4) (N2 saturated) containing 500 mM glucose | OCP = 140 mV, peak power density = 6.25 µW/cm2 at 60 µA/cm2 | [2] |
| Glucose oxidase (GOx) and Laccase (LAC) | Zeolitic imidazolate framework-8 (ZIF-8), bacterial cellulose (BC)/carboxylated multi-walled carbon nanotubes (c-MWCNTs) | - | After being stored at 4 °C for 20 days, the residual activity of free LAC retained was only 38%, while the ZIF-8-LAC retained fairly residual activity at 53% | Linear dynamic range from 0.01 to 0.4 mM with a lower detection limit of 1.95 × 10−3 mM for BPA concentrations | Maximal power density of 3.68 W/m3 | [3] |
| Glucose oxidase (GOx); glucose dehydrogenase (GDH; favin adenine dinucleotide (FAD)-dependent), and laccase (LAC) | Mediator (PAA-PVI-[Os(dmobpy)2Cl]+/2+), and PEGDGE (4:4:1 v/v%); BOD (10 U/mL in PBS), PAA-PVI-[Os(dCl-bpy)2Cl]+/2+ (0.5 mg/mL in DW), and PEGDGE (10.0 mg/mL in DW) in a 4:4:1 volume ratio (v/v%) | - | 20% of the power density remained after 24 h incubation in 25 mM glucose (in 1X PBS) compared to the initial power density | −0.4 to 0.8 V scan range and 0.01 V/s scan rate at 25 °C; increases in cell viability (~150%) and cell migration (~90%) with a relatively low inflammatory response | Power densities of 15.26 to 38.33 nW/cm2 depending on the enzyme concentration in media supplemented with 25 mM glucose; extreme cytotoxicity (~10%) due to the lethal concentration of H2O2 byproducts (~1500 µM) | [6] |
| Laccase (LAC) | Carbon nanotubes (CNTs), bacterial cellulose (BC), amidoxime-modified BC, carboxylated multi-walled CNTs | - | The residual activities of AOBC/c-MWCNTs-LAC and AOBC-LAC/c-MWCNTs remained at 44% and 61% of the initial catalytic activity after 10 reuse times, respectively | Anode and cathode were separated by a proton exchange membrane of Nafion (5 wt%), buffer solution (pH 4.5) acted as the electrolyte in cathode chamber | OCV = 0.14 V, power density at 1.897 W/cm3 | [7] |
| Glucose oxidase (GOx) | Metallic cotton fibers, gold nanoparticles | Diameter 200 μm, length 5.0-mm, active external surface area 3.14 mm2 | Scan rate of 5 mV/s in a phosphate-buffered saline (PBS) solution | Power density = 3.7 mW/cm2 | [9] | |
| Oxalate decarboxylase (ethanol as fuel) | Pyrene-TEMPO (2,2,6,6-tetramethylpiperidinyl-N-oxyl), carboxylated multi-walled carbon nanotube, carbon paper | 1 cm2 | A stable amperometric curve and an excellent current density value over a duration of 10 h; after 30 days of storage the electrode showed 14% loss in power density | Able to oxidize ethanol to CO2 after 10 h of electrolysis | OCP = 598 mV, power density = 388 W/cm2 | [10] |
| Glucose oxidase (GOx) | SnS2 nanoflowers/Au nanoparticles, DNA–carbon nanotubes bioconjugate, aptamer | CP (0.5 cm × 0.5 cm) | Continuous operation for 2000 s, EOCV remained about 98%, demonstrating the self-powered biosensor has a good stability | Sensitivity of 42.4 μA/(ng/mL) can be discharged with an increase of 18.4 times that of pure EBFCs; exhibited a wide linear range (0.02–5 ng/mL) and a low detection limit (7.90 pg/mL) | - | [11] |
| Glucose oxidase (GOx) and bilirubin oxidase (BOD) | Nitrogen-doped ultra-thin carbon shell/gold nanoparticles | Carbon paper (CP) electrode (1 cm × 1 cm) | The EOCV kept almost unchanged after 5-days save, and it still remained at 98.63% after two weeks, suggesting good stability | A wide linear range of 0.1–2000 ng/mL with a low detection limit of 21.5 pg/mL (S/N = 3) | - | [12] |
| Flavin adenine dinucleotide-dependent glucose dehydrogenase | Cellulose nanofiber, multi-walled carbon nanotubes, | 10 × 5 mm2 | - | 30 mmol dm 3 glucose at a potential sweep rate of 10 mV/s. Temperature range: 15–18 °C | The maximum voltage and maximum current density of the biofuel cell were 434 mV and 176 mA/cm2, respectively, at room temperature (15–18 °C). The maximum power output was 27 mW/cm2 | [14] |
| Glucose oxidase (GOx) | Reduced graphene oxide (rGO) and functionalized magnetic nanoparticles (f-Fe3O4 NPs) in polyaniline matrix | 3 mm diameter glassy carbon electrode | The lifetime of the rGO/PANI/f-Fe3O4/Frt/GOx bioelectrode when stored at 4 °C was estimated to be 45 days | 0.3 M [K4Fe(CN)6] as supporting electrolyte at ambient conditions | Maximum current density of 32.9 mA/cm2 at the optimum glucose concentration of 50 mM | [15] |
| Glucose oxidase (GOx) | Polythiophene@NiO/Frt/Gox, nano-inspired nickel oxide nanoparticles (NiO) and polythiophene (Pth), mediator ferritin | Glassy carbon electrode (GCE) | Electrode was kept in the refrigerator at 4 °C prior to use | 40 mM glucose dissolved in PBS of pH 7.0 | The current density of Pth@NiO/Frt/GOx bioanode was found to be 5.4 mA/cm2 | [16] |
| Glucose oxidase (GOx) | Chitosan-modified carbon cloth via tripolyphosphate | CCs (8.2 × 8.2 cm2) | The cell right after each testing was stored in a refrigerator at −4 °C, as suggested by the enzyme manufacturer, to prevent the degradation of GOx from ambient temperature | Phosphate-buffered saline (PBS, pH ¼ 7) with and without 0.1 M C6H12O6 at 100 mV/s | 53% improvement in area power density; efficient area and volume power density of 0.549 mW/cm2 and 114.52 mW/cm3 | [17] |
| Glucose dehydrogenase (GDH) | Cathode = aptamers (Apt) and Au, anode = carboxylated multi-walled carbon nanotubes | Anode = glassy carbon electrode (GCE); cathode = Au electrode (d = 3 mm) | Stored at 4 °C; the relative power output ratio and the relative ratio (R)—R% values in the presence of these pollutants were all less than 10%; the EOCV was maintained at over 95% after 9 h of continuous operation | ATZ detection limit 7.5 nM | The self-powered Pmax reached 15.3 μW/cm2 | [20] |
| Glucose oxidase (GOx) | Defective NiMoSe2 nanoplates, functionalized SWCNTs doped polypyrrole, | GCE 0.07 cm2 | - | PBS (pH 7.0) as supporting electrolyte at ambient conditions; 50 mM glucose concentration | Open circuit potential (OCV) of 0.35 V and delivered the maximum current density of 9.01 mA/cm2 in 50 mM glucose concentration | [21] |
| Glucose oxidase (GOx) | SWCNTs, gold nanoparticles | Indium tin oxide (ITO) electrodes 1.25 cm × 2.75 cm | Cell voltage was maintained at 0.54 V under 66.7 uA/cm2 of discharge current density for 48 h | PBS (pH 7.0) supplemented with 30 mM of glucose at scan rate of 10 mV/s | Maximum power density of 38.2 ± 2.0 µW/cm2 at 0.57 ± 0.03 V of a cell voltage | [22] |
| Glucose oxidase (GOx) | Three-dimensional graphene | GCE, diameter 3 mm | - | Scan rate 50 mV/s and the solution PBS | Power density of 164 mW/cm2 at 0.4 V | [26] |
| Oxalate oxidase (ethanol as fuel) | TEMPO-modified linear poly(ethylenimine) (LPEI), carboxylated multi-walled carbon nanotubes (MWCNT-COOH) | GC electrode | - | 50 mM citric acid-phosphate buffers, pH = 5.5, ν = 10 mV/s and 25 °C | - | [27] |
| Glucose oxidase (GOx) | Cross-linking of chitosan and genipin, 4-nitrobenzoic acid mediator, carbon nanotube | - | - | 0.01 M PBS (pH7.4) electrolyte, while 20 mM glucose was injected | Anodic current (331 μA/cm2 at 0.3 V vs. Ag/AgCl) with a low onset potential (0.05 V vs. Ag/AgCl); open-circuit voltage of 0.54 V and a maximum power density of 38 μW/cm2 | [28] |
| Glucose oxidase (GOx) | Fe3(CN)6,polypyrrole, CNB | Carbon paper 1 cm2 | Stored at 4 °C | The measurements were performed at a working temperature of 37 °C with phosphate-buffered solution of pH 7 as an electrolyte, and 10 mM glucose was added to the anode as a fuel | Continuous 16 h, the maximum power density achieved for a hydrophobic electrode was approximately 80 μW/cm2 at 0.13 V | [29] |
| Glucose oxidase (GOx) | Amine-containing osmium redox complexes, genipin to crosslink chitosan, Functionalised MWCNTs | - | Genipin cross-linked hydrogels delivered a 3-fold increase in stability for continuous amperometric current production over a 20 h period; 13% activity retained after 20 h; very low ГOs retention (16% retained after 20 h) | 50 mM phosphate-buffered saline (150 mM NaCl, pH 7.4, 37 °C) containing 100 mM glucose | Glucose oxidation current densities of 730 μA/cm2 at an applied potential of 0.45 V (vs. Ag/AgCl) | [30] |
| Alcohol deshydrogenase (ADH) | Tetrabutylammonium bromide and Nafion and subsequently immobilized on TiO2 nanotubes (TNT), NAD+ | 2 × 0.3 cm titanium dioxide nanotube plate | Optimal conditions for preserving 70% of the enzymatic activity | The assays were carried out at 25 °C, using 15 min of reaction time; pH 8.86 and 35 °C | Open circuit potential greater than 0.9 V; 5.93 mW/cm2 of power density operating at 1.0 V | [33] |
| Glucose oxidase (GOx); laccase | Transition-metal-doped molybdenum diselenides (NiMoSe2); | Nickel foam 1 × 1 cm2 | The oxidation current response was recorded before and after 24 h electrode storage in electrolyte solution; cell kept 89.5% of its initial performance after 3 days | 1 mg of enzyme/1 mL of phosphate buffer, pH 5 | open-circuit voltage (VOC = 0.6 V) and a short-circuit current density (JSC = 8.629 mA/cm2) with a maximum power density (Pmax) of 1.2 mW/cm2; electrochemical pseudocapacitor application, the proposed NiMoSe2/NF exhibited excellent specific capacitance (535.74 F/g), with 86.7% rate performance | [37] |
| Two dehydrogenases and a diaphorase | - | 1 cm2 carbon felt | The Tm6PGDHmutant 3-3 exhibited a 42-fold increase in catalytic efficiency at pH 5.4 compared to the original enzyme. | Anodic pH of 5.4 | Maximum power density of 0.13 mW/cm2 at pH 5.4 | [38] |
| Glucose oxidase (GOx)- or laccase (Lac)-modified | Rectangular carbon tube polypyrrole (RPPy) | Glassy carbon electrode (φ 3 mm); 0.5 × 0.5 cm2 nickel foam; 2 × 2 cm2 nickel foam | Stored at 4 °C; after 14 days, the power density was still 82.02% | Anodic compartment was 50 mL Ar-saturated 0.1 M SDA (pH 5.0) with 0.05 M glucose; the cathodic compartment was filled with 50 mL 0.1M B-R buffer (pH 5.0) with 0.5 mM ABTS and continuously bubbled with oxygen; discharge time reached 49.9 h at a discharge current of 0.2 mA before the voltage was lower than 0.8 V | Open-circuit voltage reached 1.16 V; power density was measured to 0.350 mW/cm2, which correlated to the gravimetric power density of 0.265 mW/mg (per mg of GOx) at 0.85 V | [39] |
| GOx for bioanode and laccase for biocathode | 3D-printed carbon bioelectrodes | 20 mm × 2 mm × 1 mm (length × width × height) | Stored at 4 °C; CB bioanode exhibited almost 65.5% of current response of its first day whereas AM bioanode showed about 50% of performance of its first day | Enzyme solutions of glucose oxidase (5 mg/mL, pH 7) and laccase (5 mg/mL, pH 5); bioelectrodes were dried in atmospheric conditions for 2 h and preserved in PBS at 4 °C until use; the electrolyte solutions consisted of mediators such as PBQ (1 mM, pH 7) and ABTS (1 mM, pH 5) in anolyte and catholyte, respectively. | CB bioelectrodes gave a power density of 0.1 μW/cm2 with a current density of 3 μA/cm2 at an open circuit potential of 105 mV | [40] |
| Glucose oxidase (GOx) | Hemin bonded with amine-functionalized carbon nanotube; poly(dimethyl-diallylammonium chloride) | GCE, diameter of 5 mm | Activity preserved 82.1% after four weeks | 0.01 M PBS (pH 7.4) was used as electrolyte and potential scan rate was 20 mV/s at N2 state condition | Membraneless EBFC adopting this catalyst is measured, maximum power density is 24.1 mW/cm2 | [41] |
| Glucose oxidase (GOx) | Dimethylferrocene-modified linear poly(ethyleneimine); either glutaraldehyde (GA) or ethylene glycol diglycidyl ether (EGDGE) | - | Stored at 2–8 °C for 24 h; 48% of the initial OCP value is retained after 21 days of storage; 60% of the initial current density was lost in that period of time | Solutions of b-D-glucose in 0.1 M pH ¼ 7.4 PB were prepared at concentrations ranging from 0 to 10 mM in 2 mM increments; applying potentials from 0.3 to þ0.3 V vs. a Ag/AgCl pseudoreference at scan rates (n) of 5e500 mV/s | OCP of around 0.82 V, and a maximum current and power of about 440 mA/cm2 and 86 mW/cm2 | [42] |
| Glucose oxidase (GOx) | Iron–nitrogen doped carbon nanotube (Fe–N/CNT); polyethylenimine (PEI) | - | Preserving 81.2% of its initial value even after four weeks | 0.01 M phosphate-buffered solution (PBS, pH 7.4); 0.03 M glucose solution (air purge) was circulated from an external bottle to the EBFC kit at a flow rate of 0.1 mL/min, while within the cathode of the membrane EBFC, 0.01 M of pH-adjusted PBS | Onset potential and current density (0.17 V and 74.3 μA/cm2) with the injection of 8 mM glucose solution; constant and maximum current density were 139.4 mM and 347.1 μA/cm2 | [43] |
| Flavin adenine dinucleotide-dependent glucose dehydrogenase | Carbon cloth modified with MgO-templated porous carbon; 1,4-naphthoquinone | 1.0 or 4.0 cm2 | FAD-GDH exhibited 30% of the initial activity | mV s−1 in 1.0 M phosphate-buffered pH 7.0; 1.0 M glucose | Open circuit potential was 0.75 V and maximum output power density was 2 mW/cm2 at 0.4 V | [44] |
| Sulfite oxidase (sulfite) | Three-dimensional sulfite oxidase; graphene-functionalized carbon paper | 0.50 × 0.50 cm2 | Loss rate remained at 4–5% of the initial signal | Oxygen-free Tris-acetate buffer solutions (750 mM, pH 8.4) and 1.0 mM Na2SO3; scan rate, 5 mV/s | Open-circuit voltage (OCV) of 0.64 1 V and a maximum power density of 61 μW/cm2 (122 mW/m3) at 30 °C | [45] |
| Pyrroloquinoline quinone-dependent glucose dehydrogenase (PQQ-GDH) and bilirubin oxidase (BOx) | Two-dimensional graphene paper, Meldola blue (MB) | 0.25 cm2 | Stored at 4 °C | 2D-GP electrode was immersed in a 10 mM MB aqueous solution and left overnight; 10 mM phosphate-buffered (PB) solution at pH 7.0 was used as the electrolyte for electrochemical experiments | Open circuit voltage = 0.665 V; maximum power density = 4 mW/cm2 | [48] |
| Glucose oxidase (GOx) or laccase (Lac) | Porous structured carbon paper (CP) | Diameter: 2 cm; thickness: 4 mm for one piece and 1 mm for the other 4 pieces | Stored at 4 °C; the cell was operated continuously for 2000 s in 5 mM glucose containing PB under ambient air; it maintained 75% of its power | Cyclic voltammetry (CV) in 0.1 M, pH 7.4 PB containing 0.4 mM HAuCl4 solution with a potential scan rate of 50 mV/s and a scanning range of 1.0 to 0.5 V for 20 cycles | 9.64 mW/cm2 at 0.43 V and 53.0 mW/cm2 at 0.45 V for the cell in 5 mM glucose | [50] |
| Flavin adenine dinucleotide-dependent glucose dehydrogenase (FAD-GDH) | Poly(methylene green) grafted on the carbon surface; glassy carbon electrode | 0.196 cm2 | - | Cyclic voltammetry measurements using poly(phenothiazine)-modified GC electrodes as working electrodes were carried out in a 0.1 M phosphate buffer between −300 and 600 mV at a scan rate of 10 mV/s in the presence and absence of 0.1 M glucose and 1.0 μMFAD-GDH; response time in 10% using five different electrodes | 3 mA/cm2 of glucose oxidation current | [53] |
| Glucose oxidase (GOx) | GOx nanocapsule with SFAD-containing polymeric network (n(GOx-SFAD-PAM)) | - | Stored at room temperature; n(GOx-SFAD-PAM) retained most activity at 60 and 70 °C, retaining more than one third of activity at 80 °C; at pH 3.0 n(GOx-SFAD-PAM) retained activity 70.3%; 30-day storage at room temperature n(GOx-SFAD-PAM) still maintained 90% | Response time in 3~5 s | Low detection potential (−0.4 vs. Ag/AgCl), high sensitivity (64.97 μA mM−1cm−2); high maximum power density (1011.21 μW/cm2) | [54] |
| Glucose oxidase; bilirubin oxidase | Copolymer poly(methyl methacrylate-co-vinylferrocene) | Gold (diameter = 2 mm) | The peak currents were the same up to 38 cycles, declined only about 7 μA at the last 12 cycles | The aerated 100 mM, pH 7.4 phosphate buffer; −1 and +1 V | Power density of 323 μW/cm2 at 10 mM glucose at 0.4 V | [55] |
| Glucose oxidase (GOx) | rGO-PEI/Naph-SH/AgNWs/Frt/Gox | 3 mm diameter glassy carbon electrode (GCE) | Stored at 4 °C | Limiting glucose concentration of 50 mM in PBS (pH 7.0) as supporting electrolyte at a scan rate of 100 mV/s | Maximum current density 19.9 mA/cm2 | [56] |
| Glucose oxidase (GOD) | Matrix of reduced graphene oxides (RGOs), polyethylenimine (PEI), and ferrocene carboxylic acid (FcCOOH) on carbon paper (CP) | 1.0 × 5.0 cm2 | Stored at 4 °C; the bioelectrodes maintained comparable activity with the freshly prepared electrode after two days’ storage; the activity decreased by 28%, while the noncatalytic current density dropped slightly after one-week’s storage | GOD-graphene electrode in 20 mM PBS with pH ranging from 5.3 to 8.1 | Maximum power density of 5.1 μW/cm2 and an open circuit voltage of 0.40 V at 25 °C | [59] |
| FAD-dependent glucose dehydrogenase | Porous gold electrodes; platinum nanoclusters | Au electrodes (3 mm in diameter) | Stored 2 h at 4 °C | 0.1 M phosphate buffer (pH 7.0) at 25 C under quiescent conditions in an Ar atmosphere at v = 10 mV s 1 | Current density with PtNCs (~1 mA cm2 at 0 V vs. Ag|AgCl|sat. KCl) was considerably higher than that without PtNCs | [60] |
| Glucose oxidase (GOD) | ZnO nanoparticles decorated on polyindole-functionalized MCNTs; ferritin | Glassy carbon electrode (GCE) of diameter 3 mm | Stored at 4 °C | 50 mM glucose concentration in phosphate-buffered saline (PBS) (pH 7.4) as the testing solution by applying 100 mV/s scan rates | Maximum current density of 4.9 mA/cm2 | [61] |
| PQQ-glucose dehydrogenase (GDH) | Carbon nanotube; multi-walled carbon nanotubes (MWCNT); pyrene butyric acid N-hydroxysuccinimide ester, and then N-(5-amino-1-carboxypentyl) iminodiacetic acid (AB-NTA) and NiCl2 were added to modify the NTA-Ni2+ complex on the CNT surface | Gold (geometrical area: 0.02 cm2) | - | Anode cell, 0.1 M HEPES buffer, pH 7.5, was used as an electrolyte along with 20 mM D-glucose | Power density 32 µW/cm2 | [62] |
| Glucose oxidase (GOD) | Cross-linked chitosan/TPP matrices with Na® polymers; carbon cloth (CC) | CC (3 × 2 cm2, 131.5 mg) | Retained 89.2% of its beginning performance after 240 h testing | 0.1 M PBS (pH 7) at 100 mV/s | Higher peak power density (1.077 mW/cm2) than that utilizing GOx[CS/TPP]CC (0.776 mW/cm2) and GOx[CS/Na]CC (0.682 mW/cm2) | [63] |
| Glucose oxidase (GOD) | Chitosan (CHI)-reduced graphene (rGO) polyaniline (PAni)/ferritin (Frt)/glucose oxidase (GOx) | Glassy carbon (GC) electrode with 0.07 cm2 surface area | Stored at 6 °C; better storage stability after one week and it retained 95% of its initial current response | 0.1 M PBS of pH 7.0 at a sweep rate of 100 mV/s | A stable current response of 3.5 ± 0.02 mA/cm2 in 20 mM glucose. The coverage of enzyme on 0.07 cm2 area of electrode modified with CHI@rGO-PAni/Frt was calculated to be 3.80 × 10−8 mol/cm2 | [64] |
| Glucose oxidase (GOD) | Carboxyl-functionalized mesoporous carbon | 2.25 cm2 | Stored at 4 °C | CV scans were performed at 20–200 mV/s scan in the potential range from −1.0 to 1.0 V (v.s. Ag/AgCl) in air-saturated 0.1 M pH 7.0 PBS supplemented with 10 mM glucose at room temperature | The Gox immobilization and enzyme activity in MC-COOH increased 140.72 and 252.74% | [65] |
| Glucose oxidase (GOD) | Graphite oxide/cobalt/chitosan | - | Stored at 4 °C | - | Potential voltage of 0.548 V vs. Ag/AgCl with power density of 1198.09 mW/cm2 | [66] |
| Bilirubin oxidase | RGO on three-dimensional (3D) carbon paper electrodes | 0.50 × 0.50 cm2 | Stored at 4 °C; after two-week storage, the RGO-A bioelectrodes retained 50% of initial catalytic response while the RGO and RGO-A(N) bioelectrodes only retained 25% of the initial value; RGO-A bioelectrode showed superior operational stability with a half-lifetime of 55 h compared to RGO (13 h) and RGO-A_ads/BOD (40 h) | CVs at 50 mV/s of electrodes in 100 mM O2-free PBS (pH 7.0) | Maximum power density of 22 μW/cm2 and an open circuit voltage of 0.51 V | [68] |
| Bilirubin oxidase and pyrroloquinoline quinone-dependent glucose dehydrogenase (PQQ-GDH) | Supercapacitor/biofuel cell | Stored 4 °C for 2 h; 80% residual activity after 50 charge/discharge pulses | CV scanning −0.2–0.5 Ag/AgCl, 3mM glucose | Power density = 4.5 µW/cm2 | ||
| Glucose oxidase (GOD) | Iron and cobalt co-doped ordered mesoporous porphyrinic carbon (FeCo-OMPC) | GCE, diameter of 5 mm | - | 0.01 M PBS (pH 7.4) was used as the electrolyte and the potential scan rate was 10 mV/s | Maximum power density of 21.3 ± 2.97 µW/cm2 with open circuit voltage (OCV) of 0.17 ± 0.016 V | [72] |
| Glucose oxidase (GOD) | RGO/AuNPs/PNR | 1 × 15 mm2 | Retained activity in 2 h | 25 °C and 50 uL/min of serum stream flows | Open circuit voltage (OCV) and maximum power density = 0.2 V and 3.6 µW/cm2 at a flow rate of 50 uL/min | [73] |
| Glucose oxidase (GOD) | Gold nanoparticle-modified carbon nanotube hybrid fibers | - | Operating stability (~85% of the initial power performance after 15 days) | 50 mL PBS solution (20 mmol L−1 phosphate, 0.14 mol/L NaCl, pH: ~7.4) at 37 °C | Power output of 1.2 µW/cm2 under a fixed external resistance (cyclic voltammetry measurement ~2.1 mW/cm2) at 300 mmol/L glucose | [72] |
| Invertase, mutarotase, flavine adenine dinucleotide (FAD)-dependent glucose dehydrogenase and fructose dehydrogenase | Gold nanoparticles—covalently bound naphthoquinone moieties—cellulose/polypyrrole (CPPy) paper | - | - | McIlvaine buffer, pH 5.5 at a scan rate of 1 mV/s | Power density = 0.81 mW/cm2 | [75] |
| Bilirubin oxidase | Prussian blue (PB)/russian white (PW); carbon nanotubes | - | - | Electrolyte solution was 0.1 M PBS (0.1 M, pH = 6.5) with 2 mM NAD+/NADH and 5 mM glucose | The EOCV decreased while the color of the mediated electrode would return back to blue | [76] |
| Glucose oxidase | Carbon cloth with Prussian blue (PB) nanoparticle | Single electrode sizing of 1 cm2 | 4 °C and 24 h | 0.1 M acetate buffer solution (mixed solution of acetic acid and sodium acetate) at pH 5 and GOD concentrations (6 mg/mL) | 5 stacks produced a maximal power of 13 W with an output voltage of 0.88 V when load resistance was 40 kW | [77] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
A. Karim, N.; Yang, H. Mini-Review: Recent Technologies of Electrode and System in the Enzymatic Biofuel Cell (EBFC). Appl. Sci. 2021, 11, 5197. https://doi.org/10.3390/app11115197
A. Karim N, Yang H. Mini-Review: Recent Technologies of Electrode and System in the Enzymatic Biofuel Cell (EBFC). Applied Sciences. 2021; 11(11):5197. https://doi.org/10.3390/app11115197
Chicago/Turabian StyleA. Karim, Nabila, and Hsiharng Yang. 2021. "Mini-Review: Recent Technologies of Electrode and System in the Enzymatic Biofuel Cell (EBFC)" Applied Sciences 11, no. 11: 5197. https://doi.org/10.3390/app11115197
APA StyleA. Karim, N., & Yang, H. (2021). Mini-Review: Recent Technologies of Electrode and System in the Enzymatic Biofuel Cell (EBFC). Applied Sciences, 11(11), 5197. https://doi.org/10.3390/app11115197







