The Rediscovery of Honey for Skin Repair: Recent Advances in Mechanisms for Honey-Mediated Wound Healing and Scaffolded Application Techniques
Abstract
:1. Introduction
2. Honey Physicochemical Composition
3. Honey Phenolic Fraction and Bioactive Compounds
4. Antimicrobial Properties of Honey
5. Anti-Inflammatory Properties of Honey
6. Latest Advances of Honey Applications in Wound Care
7. Commercially Available Honey-Based Products for Skin Repair
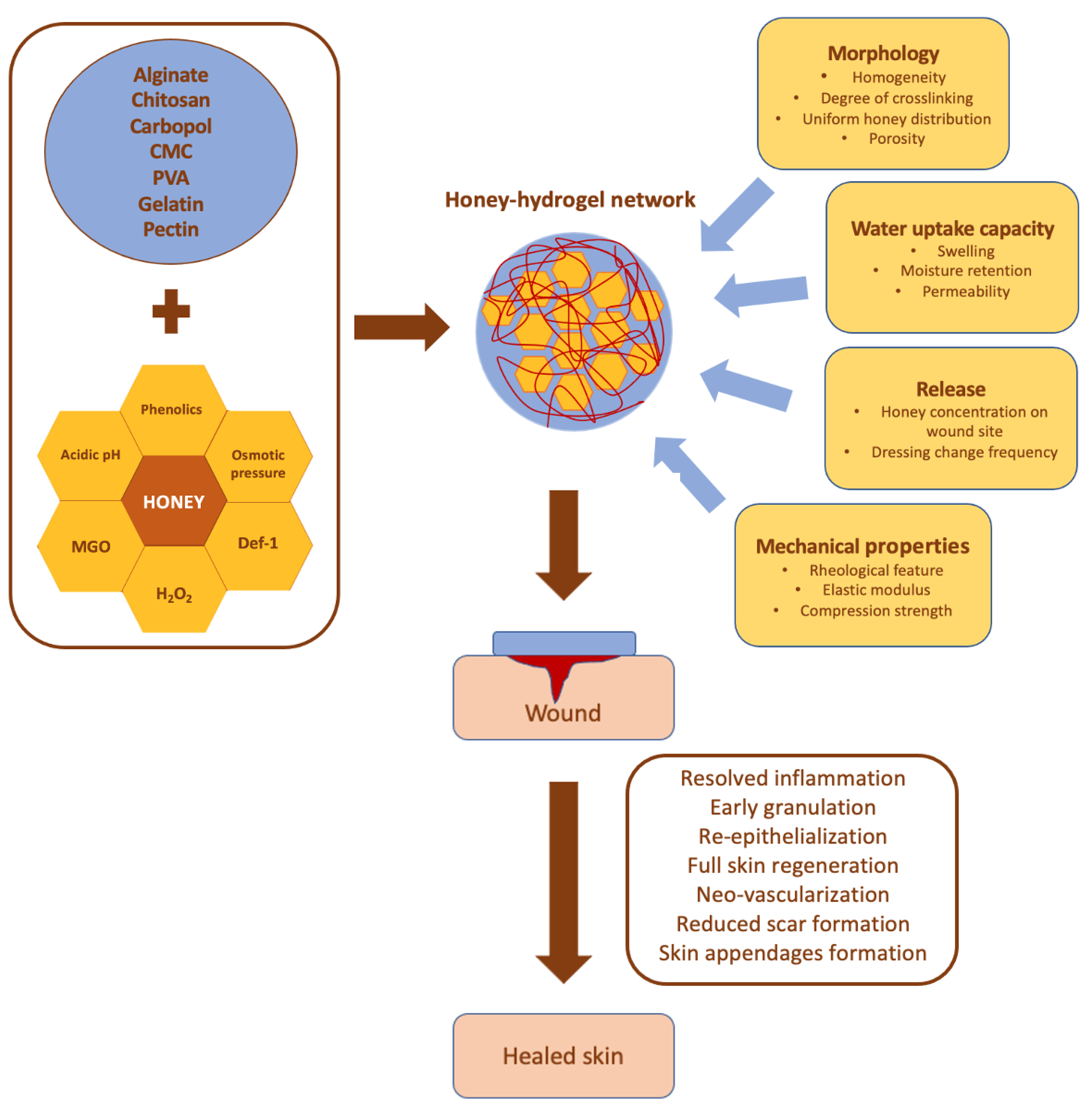
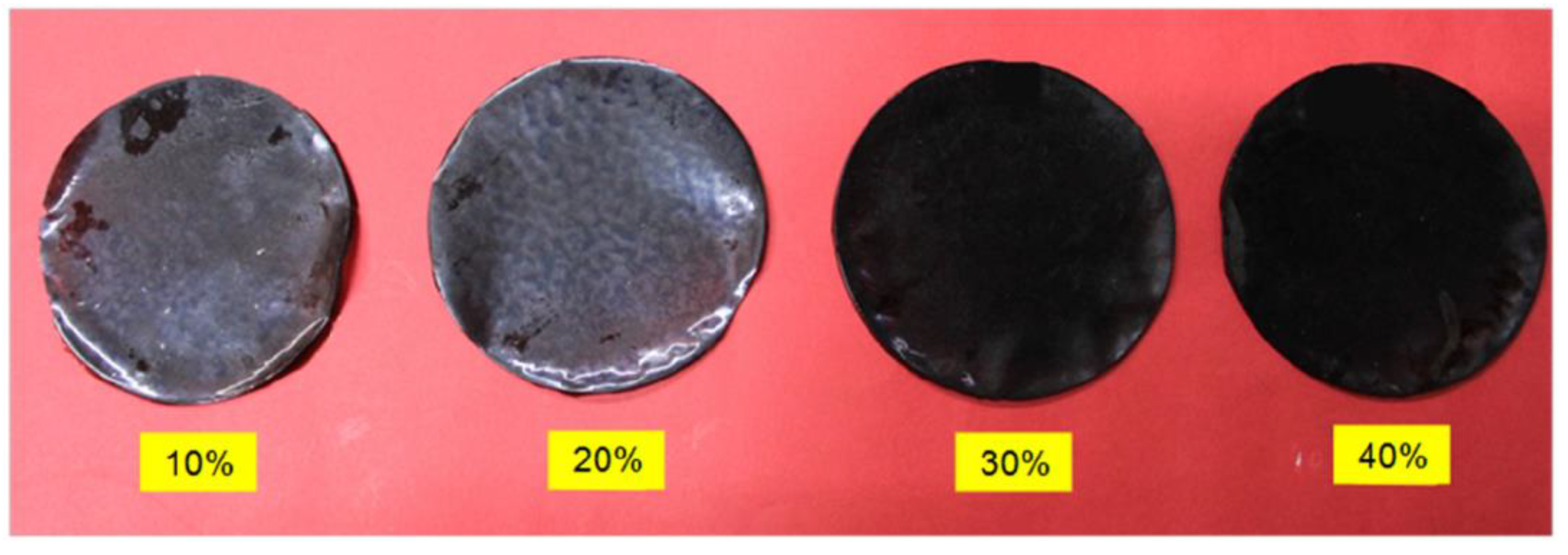
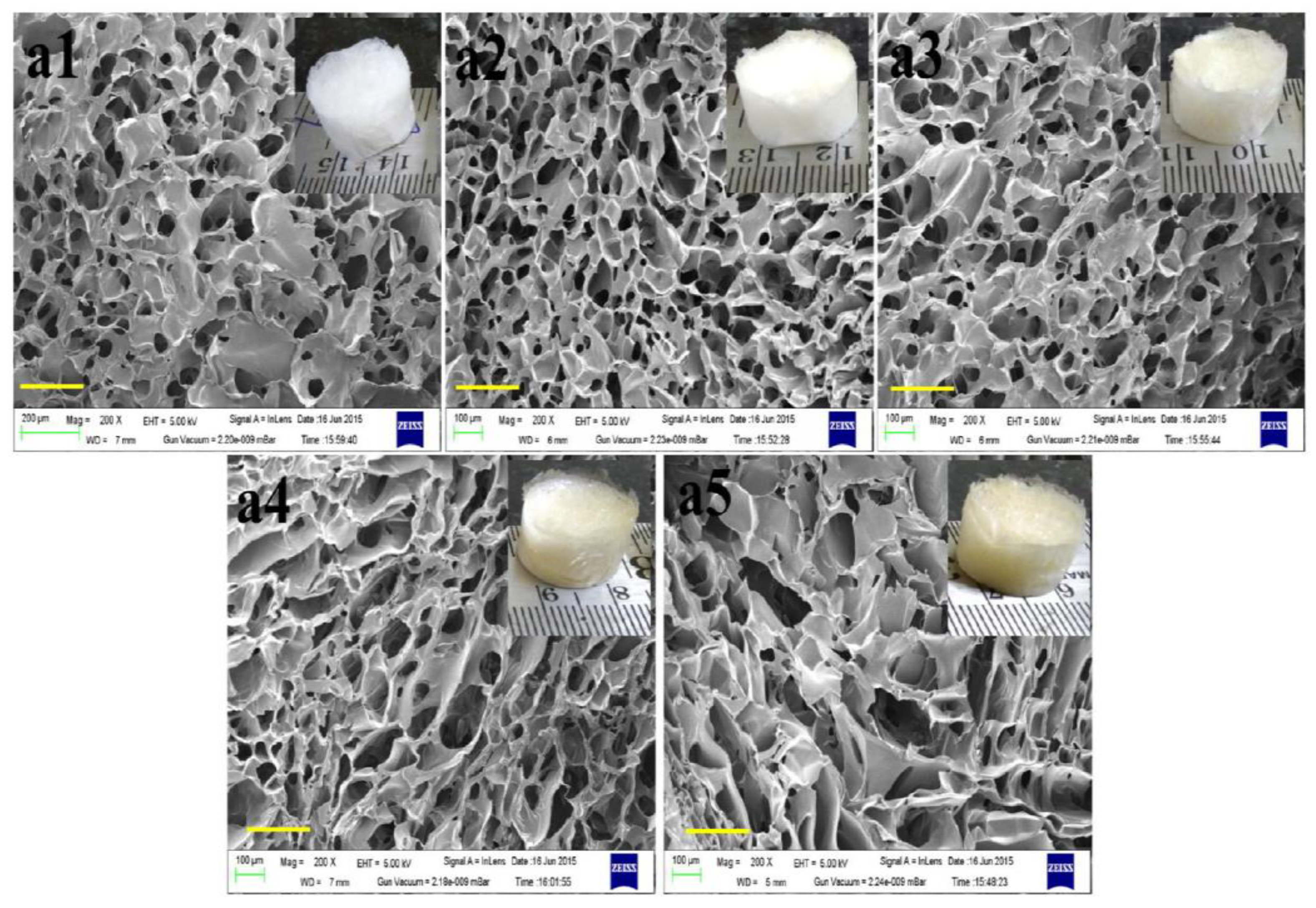
8. Functional Aspects of Honey-Loaded Scaffolds for Wound Healing
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegmund, B.; Urdl, K.; Jurek, A.; Leitner, E. “More than Honey”: Investigation on Volatiles from Monovarietal Honeys Using New Analytical and Sensory Approaches. J. Agric. Food Chem. 2017, 66, 2432–2442. [Google Scholar] [CrossRef]
- Marcazzan, G.L.; Mucignat-Caretta, C.; Marchese, C.M.; Piana, M.L. A review of methods for honey sensory analysis. J. Apic. Res. 2017, 57, 75–87. [Google Scholar] [CrossRef]
- Kortesniemi, M.; Rosenvald, S.; Laaksonen, O.; Vanag, A.; Ollikka, T.; Vene, K.; Yang, B. Sensory and chemical profiles of Finnish honeys of different botanical origins and consumer preferences. Food Chem. 2018, 246, 351–359. [Google Scholar] [CrossRef]
- Machado, A.M.; Miguel, M.G.; Vilas-Boas, M.; Figueiredo, A.C. Honey Volatiles as a Fingerprint for Botanical Origin—A Review on their Occurrence on Monofloral Honeys. Molecules 2020, 25, 374. [Google Scholar] [CrossRef] [Green Version]
- Kuropatnicki, A.K.; Kłósek, M.; Kucharzewski, M. Honey as medicine: Historical perspectives. J. Apic. Res. 2018, 57, 113–118. [Google Scholar] [CrossRef]
- Ou, H.-C.; Pandey, S.; Hung, M.-Y.; Huang, S.-H.; Hsu, P.-T.; Day, C.-H.; Pai, P.; Viswanadha, V.P.; Kuo, W.-W.; Huang, C.-Y. Luteolin: A Natural Flavonoid Enhances the Survival of HUVECs against Oxidative Stress by Modulating AMPK/PKC Pathway. Am. J. Chin. Med. 2019, 47, 541–557. [Google Scholar] [CrossRef] [PubMed]
- Dabeek, W.M.; Marra, M.V. Dietary Quercetin and Kaempferol: Bioavailability and Potential Cardiovascular-Related Bioactivity in Humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef] [Green Version]
- Nie, J.; Zhang, L.; Zhao, G.; Du, X. Quercetin reduces atherosclerotic lesions by altering the gut microbiota and reducing atherogenic lipid metabolites. J. Appl. Microbiol. 2019, 127, 1824–1834. [Google Scholar] [CrossRef] [PubMed]
- Ravishankar, D.; Salamah, M.; Attina, A.; Pothi, R.; Vallance, T.; Javed, M.; Williams, H.F.; Alzahrani, E.M.S.; Kabova, E.; Vaiyapuri, R.; et al. Ruthenium-conjugated chrysin analogues modulate platelet activity, thrombus formation and haemostasis with enhanced efficacy. Sci. Rep. 2017, 7, 5738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dludla, P.; Nkambule, B.B.; Jack, B.; Mkandla, Z.; Mutize, T.; Silvestri, S.; Orlando, P.; Tiano, L.; Louw, J.; Mazibuko-Mbeje, S.E. Inflammation and Oxidative Stress in an Obese State and the Protective Effects of Gallic Acid. Nutrients 2019, 11, 23. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Gu, P.; Shen, H. Gallic acid improved inflammation via NF-κB pathway in TNBS-induced ulcerative colitis. Int. Immunopharmacol. 2019, 67, 129–137. [Google Scholar] [CrossRef]
- Li, Z.-J.; Liu, M.; Dawuti, G.; Dou, Q.; Ma, Y.; Liu, H.-G.; Aibai, S. Antifungal Activity of Gallic Acid In Vitro and In Vivo. Phytother. Res. 2017, 31, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Ceci, C.; Lacal, P.M.; Tentori, L.; De Martino, M.G.; Miano, R.; Graziani, G. Experimental Evidence of the Antitumor, Antimetastatic and Antiangiogenic Activity of Ellagic Acid. Nutrients 2018, 10, 1756. [Google Scholar] [CrossRef] [Green Version]
- Song, X.; Tan, L.; Wang, M.; Ren, C.; Guo, C.; Yang, B.; Ren, Y.; Cao, Z.; Li, Y.; Pei, J. Myricetin: A review of the most recent research. Biomed. Pharmacother. 2021, 134, 111017. [Google Scholar] [CrossRef]
- Ci, Y.; Zhang, Y.; Liu, Y.; Lu, S.; Cao, J.; Li, H.; Zhang, J.; Huang, Z.; Zhu, X.; Gao, J.; et al. Myricetin suppresses breast cancer metastasis through down-regulating the activity of matrix metalloproteinase (MMP)-2/9. Phyther. Res. 2018, 32, 1373–1381. [Google Scholar] [CrossRef]
- Tharakan, T.; Bent, J.; Tavaluc, R. Honey as a Treatment in Otorhinolaryngology: A Review by Subspecialty. Ann. Otol. Rhinol. Laryngol. 2019, 128, 193–207. [Google Scholar] [CrossRef]
- Ramsay, E.I.; Rao, S.; Madathil, L.; Hegde, S.K.; Baliga-Rao, M.P.; George, T.; Baliga, M.S. Honey in oral health and care: A mini review. J. Oral Biosci. 2019, 61, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Abuelgasim, H.; Albury, C.; Lee, J. Effectiveness of honey for symptomatic relief in upper respiratory tract infections: A systematic review and meta-analysis. BMJ Evid. Based Med. 2021, 26, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Oduwole, O.; Udoh, E.E.; Oyo-Ita, A.; Meremikwu, M.M. Honey for Acute Cough in Children. Cochrane Database Syst. Rev. 2018. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.L.; Panyoyai, N.; Kasapis, S.; Pang, E.; Mantri, N. Honey and Its Role in Relieving Multiple Facets of Atherosclerosis. Nutrients 2019, 11, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olas, B. Honey and Its Phenolic Compounds as an Effective Natural Medicine for Cardiovascular Diseases in Humans? Nutrients 2020, 12, 283. [Google Scholar] [CrossRef] [Green Version]
- Battino, M.; Forbes-Hernández, T.Y.; Gasparrini, M.; Afrin, S.; Cianciosi, D.; Zhang, J.; Manna, P.P.; Reboredo-Rodríguez, P.; Varela-López, A.; Quiles, J.L.; et al. Relevance of functional foods in the Mediterranean diet: The role of olive oil, berries and honey in the prevention of cancer and cardiovascular diseases. Crit. Rev. Food Sci. Nutr. 2018, 59, 893–920. [Google Scholar] [CrossRef] [PubMed]
- Bobiş, O.; Dezmirean, D.S.; Moise, A.R. Honey and Diabetes: The Importance of Natural Simple Sugars in Diet for Preventing and Treating Different Type of Diabetes. Oxidative Med. Cell. Longev. 2018, 2018, 4757893. [Google Scholar] [CrossRef] [Green Version]
- Terzo, S.; Mulè, F.; Amato, A. Honey and obesity-related dysfunctions: A summary on health benefits. J. Nutr. Biochem. 2020, 82, 108401. [Google Scholar] [CrossRef]
- Ramli, E.S.M.; Sukalingam, K.; Kamaruzzaman, M.A.; Soelaiman, I.N.; Pang, K.-L.; Chin, K.-Y. Direct and Indirect Effect of Honey as a Functional Food Against Metabolic Syndrome and Its Skeletal Complications. Diabetes Metab. Syndr. Obesity Targets Ther. 2021, 14, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Ramli, N.Z.; Chin, K.-Y.; Zarkasi, K.A.; Ahmad, F. A Review on the Protective Effects of Honey against Metabolic Syndrome. Nutrients 2018, 10, 1009. [Google Scholar] [CrossRef] [Green Version]
- Masad, R.; Haneefa, S.; Mohamed, Y.; Al-Sbiei, A.; Bashir, G.; Fernandez-Cabezudo, M.; Al-Ramadi, B. The Immunomodulatory Effects of Honey and Associated Flavonoids in Cancer. Nutrients 2021, 13, 1269. [Google Scholar] [CrossRef]
- Badolato, M.; Carullo, G.; Cione, E.; Aiello, F.; Caroleo, M.C. From the hive: Honey, a novel weapon against cancer. Eur. J. Med. Chem. 2017, 142, 290–299. [Google Scholar] [CrossRef]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef]
- Oryan, A.; Alemzadeh, E.; Moshiri, A. Burn wound healing: Present concepts, treatment strategies and future directions. J. Wound Care 2017, 26, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Hesketh, M.; Sahin, K.B.; West, Z.E.; Murray, R.Z. Macrophage Phenotypes Regulate Scar Formation and Chronic Wound Healing. Int. J. Mol. Sci. 2017, 18, 1545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avishai, E.; Yeghiazaryan, K.; Golubnitschaja, O. Impaired wound healing: Facts and hypotheses for multi-professional considerations in predictive, preventive and personalised medicine. EPMA J. 2017, 8, 23–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahim, K.; Saleha, S.; Zhu, X.; Huo, L.; Basit, A.; Franco, O.L. Bacterial Contribution in Chronicity of Wounds. Microb. Ecol. 2016, 73, 710–721. [Google Scholar] [CrossRef]
- Bowler, P.G. Antibiotic resistance and biofilm tolerance: A combined threat in the treatment of chronic infections. J. Wound Care 2018, 27, 273–277. [Google Scholar] [CrossRef]
- Cambiaso-Daniel, J.; Boukovalas, S.; Bitz, G.; Branski, L.; Herndon, D.; Culnan, D. Topical Antimicrobials in Burn Care: Part 1—Topical Antiseptics. Ann. Plast. Surg. 2018. Online ahead of print.. [Google Scholar] [CrossRef]
- Baars, E.W.; Zoen, E.B.; Breitkreuz, T.; Martin, D.; Matthes, H.; von Schoen-Angerer, T.; Soldner, G.; Vagedes, J.; van Wietmarschen, H.; Patijn, O.; et al. The Contribution of Complementary and Alternative Medicine to Reduce Antibiotic Use: A Narrative Review of Health Concepts, Prevention, and Treatment Strategies. Evid. Based Complement. Altern. Med. 2019, 2019, 5365608. [Google Scholar] [CrossRef] [Green Version]
- Shedoeva, A.; Leavesley, D.; Upton, Z.; Fan, C. Wound Healing and the Use of Medicinal Plants. Evid. Based Complement. Altern. Med. 2019, 2019, 2684108. [Google Scholar] [CrossRef]
- Ibrahim, N.; Wong, S.K.; Mohamed, I.N.; Mohamed, N.; Chin, K.-Y.; Ima-Nirwana, S.; Shuid, A.N. Wound Healing Properties of Selected Natural Products. Int. J. Environ. Res. Public Health 2018, 15, 2360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chouhan, D.; Dey, N.; Bhardwaj, N.; Mandal, B.B. Emerging and innovative approaches for wound healing and skin regeneration: Current status and advances. Biomaterials 2019, 216, 119267. [Google Scholar] [CrossRef]
- Thomas, S.; Uzun, M. Testing dressings and wound management materials. In Advanced Textiles for Wound Care; Elsevier: Amsterdam, The Netherlands, 2019; pp. 23–54. [Google Scholar]
- Codex Alimentarius Commission. Standard For Honey Codex Stan 12-1981. Codex Stan 2001, 12, 1–8. [Google Scholar]
- Salonen, A.; Virjamo, V.; Tammela, P.; Fauch, L.; Julkunen-Tiitto, R. Screening bioactivity and bioactive constituents of Nordic unifloral honeys. Food Chem. 2017, 237, 214–224. [Google Scholar] [CrossRef] [Green Version]
- Pascual-Maté, A.; Osés, S.M.; Marcazzan, G.L.; Gardini, S.; Muiño, M.A.F.; Sancho, M.T. Sugar composition and sugar-related parameters of honeys from the northern Iberian Plateau. J. Food Compos. Anal. 2018, 74, 34–43. [Google Scholar] [CrossRef]
- Bucekova, M.; Jardekova, L.; Juricova, V.; Bugarova, V.; Di Marco, G.; Gismondi, A.; Leonardi, D.; Farkasovska, J.; Godocikova, J.; Laho, M.; et al. Antibacterial Activity of Different Blossom Honeys: New Findings. Molecules 2019, 24, 1573. [Google Scholar] [CrossRef] [Green Version]
- Bogdanov, S. Harmonised Methods of the International Honey Commission IHC. Available online: https://www.ihc-platform.net/ihcmethods2009.pdf (accessed on 8 April 2021).
- Kivima, E.; Tanilas, K.; Martverk, K.; Rosenvald, S.; Timberg, L.; Laos, K. The Composition, Physicochemical Properties, Antioxidant Activity, and Sensory Properties of Estonian Honeys. Foods 2021, 10, 511. [Google Scholar] [CrossRef]
- Grainger, M.N.; Owens, A.; Manley-Harris, M.; Lane, J.R.; Field, R.J. Kinetics of conversion of dihydroxyacetone to methylglyoxal in New Zealand mānuka honey: Part IV—Formation of HMF. Food Chem. 2017, 232, 648–655. [Google Scholar] [CrossRef]
- Nguyen, H.T.L.; Panyoyai, N.; Paramita, V.D.; Mantri, N.; Kasapis, S. Physicochemical and viscoelastic properties of honey from medicinal plants. Food Chem. 2018, 241, 143–149. [Google Scholar] [CrossRef]
- Kavanagh, S.; Gunnoo, J.; Passos, T.M.; Stout, J.C.; White, B. Physicochemical properties and phenolic content of honey from different floral origins and from rural versus urban landscapes. Food Chem. 2019, 272, 66–75. [Google Scholar] [CrossRef]
- Afrin, S.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Bompadre, S.; Quiles, J.L.; Sanna, G.; Spano, N.; Giampieri, F.; Battino, M. Strawberry-Tree Honey Induces Growth Inhibition of Human Colon Cancer Cells and Increases ROS Generation: A Comparison with Manuka Honey. Int. J. Mol. Sci. 2017, 18, 613. [Google Scholar] [CrossRef] [Green Version]
- Leyva-Jimenez, F.J.; Lozano-Sanchez, J.; Borras-Linares, I.; de la Luz Cadiz-Gurrea, M.; Mahmoodi-Khaledi, E. Potential antimicrobial activity of honey phenolic compounds against Gram positive and Gram negative bacteria. LWT 2019, 101, 236–245. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.M.; Giampieri, F.; Cordero, M.; Gasparrini, M.; Hernandez, T.Y.F.; Mazzoni, L.; Afrin, S.; Beltrán-Ayala, P.; González-Paramás, A.M.; Santos-Buelga, C.; et al. Activation of AMPK/Nrf2 signalling by Manuka honey protects human dermal fibroblasts against oxidative damage by improving antioxidant response and mitochondrial function promoting wound healing. J. Funct. Foods 2016, 25, 38–49. [Google Scholar] [CrossRef]
- Tuberoso, C.I.G.; Bifulco, E.; Jerkovi, Ć.I.; Caboni, P.; Cabras, P.; Floris, I. Methyl Syringate: A Chemical Marker of Asphodel (Asphodelus microcarpus Salzm. et Viv.) Monofloral Honey. J. Agric. Food Chem. 2009, 57, 3895–3900. [Google Scholar] [CrossRef]
- Di Petrillo, A.; Santos-Buelga, C.; Era, B.; González-Paramás, A.M.; Tuberoso, C.I.G.; Medda, R.; Pintus, F.; Fais, A. Sardinian honeys as sources of xanthine oxidase and tyrosinase inhibitors. Food Sci. Biotechnol. 2018, 27, 139–146. [Google Scholar] [CrossRef]
- Afrin, S.; Giampieri, F.; Cianciosi, D.; Pistollato, F.; Ansary, J.; Pacetti, M.; Amici, A.; Reboredo-Rodríguez, P.; Simal-Gandara, J.; Quiles, J.L.; et al. Strawberry tree honey as a new potential functional food. Part 1: Strawberry tree honey reduces colon cancer cell proliferation and colony formation ability, inhibits cell cycle and promotes apoptosis by regulating EGFR and MAPKs signaling pathways. J. Funct. Foods 2019, 57, 439–452. [Google Scholar] [CrossRef]
- Cabras, P.; Angioni, A.; Tuberoso, C.; Floris, I.; Reniero, F.; Guillou, C.; Ghelli, S. Homogentisic Acid: A Phenolic Acid as a Marker of Strawberry-Tree (Arbutus unedo) Honey. J. Agric. Food Chem. 1999, 47, 4064–4067. [Google Scholar] [CrossRef]
- Tuberoso, C.I.G.; Bifulco, E.; Caboni, P.; Cottiglia, F.; Cabras, P.; Floris, I. Floral Markers of Strawberry Tree (Arbutus unedo L.) Honey. J. Agric. Food Chem. 2010, 58, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Güneş, M.E.; Şahin, S.; Demir, C.; Borum, E.; Tosunoğlu, A. Determination of phenolic compounds profile in chestnut and floral honeys and their antioxidant and antimicrobial activities. J. Food Biochem. 2016, 41, e12345. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Afrin, S.; Gasparrini, M.; Reboredo-Rodriguez, P.; Manna, P.P.; Zhang, J.; Lamas, L.B.; Flórez, S.M.; Toyos, P.A.; et al. Phenolic Compounds in Honey and Their Associated Health Benefits: A Review. Molecules 2018, 23, 2322. [Google Scholar] [CrossRef] [Green Version]
- Działo, M.; Mierziak, J.; Korzun, U.; Preisner, M.; Szopa, J.; Kulma, A. The Potential of Plant Phenolics in Prevention and Therapy of Skin Disorders. Int. J. Mol. Sci. 2016, 17, 160. [Google Scholar] [CrossRef] [Green Version]
- Esposito, D.; Overall, J.; Grace, M.; Komarnytsky, S.; Lila, M.A. Alaskan Berry Extracts Promote Dermal Wound Repair Through Modulation of Bioenergetics and Integrin Signaling. Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zeng, R.; Hu, L.; Maffucci, K.G.; Ren, X.; Qu, Y. In vivo wound healing and in vitro antioxidant activities of Bletilla striata phenolic extracts. Biomed. Pharmacother. 2017, 93, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Sousa, J.M.; de Souza, E.L.; Marques, G.; Meireles, B.; de Magalhães Cordeiro, Â.T.; Gullón, B.; Pintado, M.M.; Magnani, M. Polyphenolic profile and antioxidant and antibacterial activities of monofloral honeys produced by Meliponini in the Brazilian semiarid region. Food Res. Int. 2016, 84, 61–68. [Google Scholar] [CrossRef]
- Fyfe, L.; Okoro, P.; Paterson, E.; Coyle, S.; McDougall, G.J. Compositional analysis of Scottish honeys with antimicrobial activity against antibiotic-resistant bacteria reveals novel antimicrobial components. LWT 2017, 79, 52–59. [Google Scholar] [CrossRef]
- Fratini, F.; Cilia, G.; Mancini, S.; Felicioli, A. Royal Jelly: An ancient remedy with remarkable antibacterial properties. Microbiol. Res. 2016, 192, 130–141. [Google Scholar] [CrossRef]
- Yang, Y.-C.; Chou, W.-M.; Widowati, D.A.; Lin, I.-P.; Peng, C.-C. 10-hydroxy-2-decenoic acid of royal jelly exhibits bactericide and anti-inflammatory activity in human colon cancer cells. BMC Complement. Altern. Med. 2018, 18, 202. [Google Scholar] [CrossRef]
- Peng, C.-C.; Sun, H.-T.; Lin, I.-P.; Kuo, P.-C.; Li, J.-C. The functional property of royal jelly 10-hydroxy-2-decenoic acid as a melanogenesis inhibitor. BMC Complement. Altern. Med. 2017, 17, 392. [Google Scholar] [CrossRef] [Green Version]
- Isidorow, W.; Witkowski, S.; Iwaniuk, P.; Zambrzycka, M.; Swiecicka, I. Royal Jelly Aliphatic Acids Contribute to Antimicrobial Activity of Honey. J. Apic. Sci. 2018, 62, 111–123. [Google Scholar] [CrossRef] [Green Version]
- Anand, S.; Deighton, M.; Livanos, G.; Morrison, P.D.; Pang, E.C.K.; Mantri, N. Antimicrobial Activity of Agastache Honey and Characterization of Its Bioactive Compounds in Comparison With Important Commercial Honeys. Front. Microbiol. 2019, 10, 263. [Google Scholar] [CrossRef]
- Pauliuc, D.; Dranca, F.; Oroian, M. Antioxidant Activity, Total Phenolic Content, Individual Phenolics and Physicochemical Parameters Suitability for Romanian Honey Authentication. Foods 2020, 9, 306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, L.; Xie, M.; Chen, G.; Qiao, J.; Zhang, H.; Zeng, X. Phenolics and Carbohydrates in Buckwheat Honey Regulate the Human Intestinal Microbiota. Evid. Based Complement. Altern. Med. 2020, 2020, 6432942. [Google Scholar] [CrossRef]
- Oroian, M.; Ropciuc, S. Honey authentication based on physicochemical parameters and phenolic compounds. Comput. Electron. Agric. 2017, 138, 148–156. [Google Scholar] [CrossRef]
- Poli, J.-P.; Guinoiseau, E.; Luciani, A.; Yang, Y.; Battesti, M.-J.; Paolini, J.; Costa, J.; Quilichini, Y.; Berti, L.; Lorenzi, V. Key role of hydrogen peroxide in antimicrobial activity of spring, Honeydew maquis and chestnut grove Corsican honeys on Pseudomonas aeruginosa DNA. Lett. Appl. Microbiol. 2018, 66, 427–433. [Google Scholar] [CrossRef]
- De Graft-Johnson, J.; Nowak, D. Effect of Selected Plant Phenolics on Fe2+-EDTA-H2O2 System Mediated Deoxyribose Oxidation: Molecular Structure-Derived Relationships of Anti- and Pro-Oxidant Actions. Molecules 2016, 22, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bucekova, M.; Buriova, M.; Pekarik, L.; Majtan, V.; Majtan, J. Phytochemicals-mediated production of hydrogen peroxide is crucial for high antibacterial activity of honeydew honey. Sci. Rep. 2018, 8, 9061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bueno-Costa, F.M.; Zambiazi, R.C.; Bohmer, B.W.; Chaves, F.C.; da Silva, W.P.; Zanusso, J.T.; Dutra, I. Antibacterial and antioxidant activity of honeys from the state of Rio Grande do Sul, Brazil. LWT 2016, 65, 333–340. [Google Scholar] [CrossRef] [Green Version]
- Ng, W.-J.; Sit, N.-W.; Ooi, P.A.-C.; Ee, K.-Y.; Lim, T.-M. The Antibacterial Potential of Honeydew Honey Produced by Stingless Bee (Heterotrigona itama) against Antibiotic Resistant Bacteria. Antibiotics 2020, 9, 871. [Google Scholar] [CrossRef] [PubMed]
- Minden-Birkenmaier, B.A.; Cherukuri, K.; Smith, R.A.; Radic, M.Z.; Bowlin, G.L. Manuka Honey Modulates the Inflammatory Behavior of a dHL-60 Neutrophil Model under the Cytotoxic Limit. Int. J. Biomater. 2019, 2019, 6132581. [Google Scholar] [CrossRef]
- Matzen, R.D.; Leth-Espensen, J.Z.; Jansson, T.; Nielsen, D.S.; Lund, M.; Matzen, S. The Antibacterial Effect In Vitro of Honey Derived from Various Danish Flora. Dermatol. Res. Pract. 2018, 2018, 7021713. [Google Scholar] [CrossRef] [Green Version]
- Ratiu, I.A.; Al-Suod, H.; Bukowska, M.; Ligor, M.; Buszewski, B. Correlation Study of Honey Regarding their Physicochemical Properties and Sugars and Cyclitols Content. Molecules 2019, 25, 34. [Google Scholar] [CrossRef] [Green Version]
- Albaridi, N.A. Antibacterial Potency of Honey. Int. J. Microbiol. 2019, 2019, 2464507. [Google Scholar] [CrossRef]
- Rosli, F.N.; Hazemi, M.H.F.; Akbar, M.A.; Basir, S.; Kassim, H.; Bunawan, H. Stingless Bee Honey: Evaluating Its Antibacterial Activity and Bacterial Diversity. Insects 2020, 11, 500. [Google Scholar] [CrossRef] [PubMed]
- Grecka, K.; Kuś, P.M.; Worobo, R.W.; Szweda, P. Study of the Anti-Staphylococcal Potential of Honeys Produced in Northern Poland. Molecules 2018, 23, 260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, S.-H.; Shen, C.-F.; Cheng, C.-M. Role of pH Value in Clinically Relevant Diagnosis. Diagnostics 2020, 10, 107. [Google Scholar] [CrossRef] [Green Version]
- Schade, H.; Marchionini, A. Der Säuremantel der Haut (Nach Gaskettenmessungen). J. Mol. Med. 1928, 7, 12–14. [Google Scholar] [CrossRef]
- Martinotti, S.; Laforenza, U.; Patrone, M.; Moccia, F.; Ranzato, E. Honey-mediated wound healing: H2O2 entry through AQP3 determines extracellular Ca2+ influx. Int. J. Mol. Sci. 2019, 20, 764. [Google Scholar] [CrossRef] [Green Version]
- Roshan, N.; Rippers, T.; Locher, C.; Hammer, K.A. Antibacterial activity and chemical characteristics of several Western Australian honeys compared to manuka honey and pasture honey. Arch. Microbiol. 2017, 199, 347–355. [Google Scholar] [CrossRef]
- Unique Mānuka Factor Honey Association. Grading System Explained. Available online: https://www.umf.org.nz/grading-system-explained/ (accessed on 7 April 2021).
- Grainger, M.N.; Manley-Harris, M.; Lane, J.R.; Field, R.J. Kinetics of conversion of dihydroxyacetone to methylglyoxal in New Zealand mānuka honey: Part I—Honey systems. Food Chem. 2016, 202, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Terio, V.; Bozzo, G.; Ceci, E.; Savarino, A.E.; Barrasso, R.; Di Pinto, A.; Mottola, A.; Marchetti, P.; Tantillo, G.; Bonerba, E. Methylglyoxal (MGO) in Italian Honey. Appl. Sci. 2021, 11, 831. [Google Scholar] [CrossRef]
- Hixon, K.R.; Lu, T.; McBride-Gagyi, S.H.; Janowiak, B.E.; Sell, S.A. A Comparison of Tissue Engineering Scaffolds Incorporated with Manuka Honey of Varying UMF. BioMed Res. Int. 2017, 2017, 4843065. [Google Scholar] [CrossRef] [Green Version]
- Girma, A.; Seo, W.; She, R.C. Antibacterial activity of varying UMF-graded Manuka honeys. PLoS ONE 2019, 14, e0224495. [Google Scholar] [CrossRef] [Green Version]
- Majtan, J.; Bohova, J.; Prochazka, E.; Klaudiny, J. Methylglyoxal May Affect Hydrogen Peroxide Accumulation in Manuka Honey Through the Inhibition of Glucose Oxidase. J. Med. Food 2014, 17, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Henatsch, D.; Hartog, G.J.D.; Duijvestijn, A.M.; Wolffs, P.F.; Phielix, E.; Stokroos, R.J.; Briedé, J. The contribution of α-dicarbonyl compound dependent radical formation to the antiseptic effect of honey. J. Funct. Foods 2018, 45, 239–246. [Google Scholar] [CrossRef]
- Majtan, J.; Klaudiny, J.; Bohova, J.; Kohutova, L.; Dzurova, M.; Sediva, M.; Bartosova, M.; Majtan, V. Methylglyoxal-induced modifications of significant honeybee proteinous components in manuka honey: Possible therapeutic implications. Fitoterapia 2012, 83, 671–677. [Google Scholar] [CrossRef]
- Stagos, D.; Soulitsiotis, N.; Tsadila, C.; Papaeconomou, S.; Arvanitis, C.; Ntontos, A.; Karkanta, F.; Adamou-Androulaki, S.; Petrotos, K.; Spandidos, D.; et al. Antibacterial and antioxidant activity of different types of honey derived from Mount Olympus in Greece. Int. J. Mol. Med. 2018, 42, 726–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bucekova, M.; Sojka, M.; Valachova, I.; Martinotti, S.; Ranzato, E.; Szep, Z.; Majtan, V.; Klaudiny, J.; Majtan, J. Bee-derived antibacterial peptide, defensin-1, promotes wound re-epithelialisation in vitro and in vivo. Sci. Rep. 2017, 7, 7340. [Google Scholar] [CrossRef] [Green Version]
- Omar, A.; Wright, J.B.; Schultz, G.; Burrell, R.; Nadworny, P. Microbial Biofilms and Chronic Wounds. Microorganisms 2017, 5, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shirlaw, O.; Billah, Z.; Attar, B.; Hughes, L.; Qasaymeh, R.M.; Seidel, V.; Efthimiou, G. Antibiofilm Activity of Heather and Manuka Honeys and Antivirulence Potential of Some of Their Constituents on the DsbA1 Enzyme of Pseudomonas aeruginosa. Antibiotics 2020, 9, 911. [Google Scholar] [CrossRef]
- Fernandes, L.; Oliveira, A.; Henriques, M.; Rodrigues, M.E. Honey as a Strategy to Fight Candida tropicalis in Mixed-Biofilms with Pseudomonas aeruginosa. Antibiotics 2020, 9, 43. [Google Scholar] [CrossRef] [Green Version]
- Tuksitha, L.; Chen, Y.-L.S.; Wong, K.-Y.; Peng, C.-C. Antioxidant and antibacterial capacity of stingless bee honey from Borneo (Sarawak). J. Asia-Pacific Èntomol. 2018, 21, 563–570. [Google Scholar] [CrossRef]
- Henatsch, D.; Nabuurs, C.H.; Van De Goor, R.M.; Wolffs, P.F.; Stokroos, R.J. Treatment of Recurrent Eczematous External Otitis with Honey Eardrops: A Proof-of-Concept Study. Otolaryngol. Neck Surg. 2017, 157, 696–699. [Google Scholar] [CrossRef]
- El–Gammal, E.; Di Nardo, V.; Daaboul, F.; Tchernev, G.; Wollina, U.; Lotti, J.; Lotti, T. Apitherapy as a New Approach in Treatment of Palmoplantar Psoriasis. Open Access Maced. J. Med Sci. 2018, 6, 1059–1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alangari, A.A.; Morris, K.; Lwaleed, B.A.; Lau, L.; Jones, K.; Cooper, R.; Jenkins, R. Honey is potentially effective in the treatment of atopic dermatitis: Clinical and mechanistic studies. Immun. Inflamm. Dis. 2017, 5, 190–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N.T. Reactive oxygen species (ROS) and wound healing: The functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int. Wound J. 2017, 14, 89–96. [Google Scholar] [CrossRef]
- Sanchez, M.C.; Lancel, S.; Boulanger, E.; Neviere, R. Targeting Oxidative Stress and Mitochondrial Dysfunction in the Treatment of Impaired Wound Healing: A Systematic Review. Antioxidants 2018, 7, 98. [Google Scholar] [CrossRef] [Green Version]
- Gasparrini, M.; Afrin, S.; Forbes-Hernández, T.Y.; Cianciosi, D.; Reboredo-Rodriguez, P.; Amici, A.; Battino, M.; Giampieri, F. Protective effects of Manuka honey on LPS-treated RAW 264.7 macrophages. Part 2: Control of oxidative stress induced damage, increase of antioxidant enzyme activities and attenuation of inflammation. Food Chem. Toxicol. 2018, 120, 578–587. [Google Scholar] [CrossRef]
- Afrin, S.; Gasparrini, M.; Forbes-Hernández, T.Y.; Cianciosi, D.; Reboredo-Rodriguez, P.; Manna, P.P.; Battino, M.; Giampieri, F. Protective effects of Manuka honey on LPS-treated RAW 264.7 macrophages. Part 1: Enhancement of cellular viability, regulation of cellular apoptosis and improvement of mitochondrial functionality. Food Chem. Toxicol. 2018, 121, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Floris, I.; Pusceddu, M.; Satta, A. The Sardinian Bitter Honey: From Ancient Healing Use to Recent Findings. Antioxidants 2021, 10, 506. [Google Scholar] [CrossRef]
- Afrin, S.; Forbes-Hernández, T.Y.; Cianciosi, D.; Pistollato, F.; Zhang, J.; Pacetti, M.; Amici, A.; Reboredo-Rodríguez, P.; Simal-Gandara, J.; Bompadre, S.; et al. Strawberry tree honey as a new potential functional food. Part 2: Strawberry tree honey increases ROS generation by suppressing Nrf2-ARE and NF-кB signaling pathways and decreases metabolic phenotypes and metastatic activity in colon cancer cells. J. Funct. Foods 2019, 57, 477–487. [Google Scholar] [CrossRef]
- Selders, G.S.; Fetz, A.E.; Radic, M.Z.; Bowlin, G.L. An overview of the role of neutrophils in innate immunity, inflammation and host-biomaterial integration. Regen. Biomater. 2017, 4, 55–68. [Google Scholar] [CrossRef]
- Jull, A.B.; Cullum, N.; Dumville, J.C.; Westby, M.J.; Deshpande, S.; Walker, N. Honey as a topical treatment for wounds. Cochrane Database Syst. Rev. 2015, CD005083. [Google Scholar] [CrossRef] [Green Version]
- Norman, G.; Christie, J.; Liu, Z.; Westby, M.J.; Jefferies, J.M.; Hudson, T.; Edwards, J.; Mohapatra, D.P.; A Hassan, I.; Dumville, J.C. Antiseptics for burns. Cochrane Database Syst. Rev. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Atalay, K. Treatment of corneal alkali burn with chestnut honey, royal jelly, and chestnut honey-royal jelly combination. Beyoglu Eye J. 2019. [Google Scholar] [CrossRef]
- Abderrahim, L.A.; Taïbi, K.; Abderrahim, N.A.; Boussaid, M.; Rios-Navarro, C.; Ruiz-Saurí, A. Euphorbia honey and garlic: Biological activity and burn wound recovery. Burns 2019, 45, 1695–1706. [Google Scholar] [CrossRef]
- Osés, S.; Pascual-Maté, A.; Muiño, M.A.F.; López-Díaz, T.; Sancho, M. Bioactive properties of honey with propolis. Food Chem. 2016, 196, 1215–1223. [Google Scholar] [CrossRef]
- Afonso, A.M.; Gonçalves, J.; Luís, Â.; Gallardo, E.; Duarte, A.P. Evaluation of the In Vitro Wound-Healing Activity and Phytochemical Characterization of Propolis and Honey. Appl. Sci. 2020, 10, 1845. [Google Scholar] [CrossRef] [Green Version]
- Jeffcoate, W.J.; Vileikyte, L.; Boyko, E.J.; Armstrong, D.G.; Boulton, A.J. Current Challenges and Opportunities in the Prevention and Management of Diabetic Foot Ulcers. Diabetes Care 2018, 41, 645–652. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.Z.M.; Ng, N.S.L.; Thomas, C. Prevention and treatment of diabetic foot ulcers. J. R. Soc. Med. 2017, 110, 104–109. [Google Scholar] [CrossRef] [Green Version]
- Lauri, C.; Leone, A.; Cavallini, M.; Signore, A.; Giurato, L.; Uccioli, L. Diabetic Foot Infections: The Diagnostic Challenges. J. Clin. Med. 2020, 9, 9. [Google Scholar] [CrossRef]
- Sudirjo, W.; Ali, S.; Nurpiyanti, A.; Kardiatun, T.; Jiu, C.K. Case report on the use of a honey on diabetic foot ulcer patients. Int. J. Indones. Natl. Nurses Assoc. 2018, 1, 98–104. [Google Scholar] [CrossRef] [Green Version]
- Koujalagi, R.S.; Uppin, V.M.; Shah, S.; Sharma, D. One year randomized controlled trial to compare the effectiveness of honey dressing versus povidone iodine dressing for diabetic foot ulcer at Dr. Prabhakar Kore Hospital and MRC, Belagavi. Int. Surg. J. 2020, 7, 506–513. [Google Scholar] [CrossRef] [Green Version]
- Nwabudike, L.C.; Maruhashi, E. Patient education, self-care and medical grade honey—managing a diabetic ulcer. Wounds Middle East 2017, 4, 32–35. [Google Scholar]
- Teobaldi, I.; Stoico, V.; Perrone, F.; Bruti, M.; Bonora, E.; Mantovani, A. Honey dressing on a leg ulcer with tendon exposure in a patient with type 2 diabetes. Endocrinol. Diabetes Metab. Case Rep. 2018, 2018, 18–117. [Google Scholar] [CrossRef]
- Karimi, Z.; Behnammoghadam, M.; Rafiei, H.; Abdi, N.; Zoladl, M.; Talebianpoor, M.S.; Arya, A.; Khastavaneh, M. Impact of olive oil and honey on healing of diabetic foot: A randomized controlled trial. Clin. Cosmet. Investig. Dermatol. 2019, 12, 347–354. [Google Scholar] [CrossRef] [Green Version]
- Tsang, K.-K.; Kwong, E.W.-Y.; To, T.S.-S.; Chung, J.W.-Y.; Wong, T.K.-S. A Pilot Randomized, Controlled Study of Nanocrystalline Silver, Manuka Honey, and Conventional Dressing in Healing Diabetic Foot Ulcer. Evid. Based Complement. Altern. Med. 2017, 2017, 5294890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Astrada, A.; Nakagami, G.; Jais, S.; Sanada, H. Successful treatment of a diabetic foot ulcer with exposed bone using Trigona honey: A case study. J. Wound Care 2019, 28, S4–S8. [Google Scholar] [CrossRef]
- Nair, H.K.R.; Tatavilis, N.; Pospíšilová, I.; Kučerová, J.; Cremers, N.A.J. Medical-Grade Honey Kills Antibiotic-Resistant Bacteria and Prevents Amputation in Diabetics with Infected Ulcers: A Prospective Case Series. Antibiotics 2020, 9, 529. [Google Scholar] [CrossRef]
- Takzaree, N.; Hassanzadeh, G.; Rouini, M.R.; Manayi, A.; Hadjiakhondi, A.; Zolbin, M.M. Evaluation of the Effects of Local Application of Thyme Honey in Open Cutaneous Wound Healing. Iran. J. Public Health 2017, 46, 545–551. [Google Scholar]
- Hermanns, R.; Mateescu, C.; Thrasyvoulou, A.; Tananaki, C.; Wagener, F.A.; Cremers, N.A. Defining the standards for medical grade honey. J. Apic. Res. 2019, 59, 125–135. [Google Scholar] [CrossRef]
- Giusto, G.; Vercelli, C.; Comino, F.; Caramello, V.; Tursi, M.; Gandini, M. A new, easy-to-make pectin-honey hydrogel enhances wound healing in rats. BMC Complement. Altern. Med. 2017, 17, 266. [Google Scholar] [CrossRef] [Green Version]
- Giusto, G.; Beretta, G.; Vercelli, C.; Valle, E.; Iussich, S.; Borghi, R.; Odetti, P.; Monacelli, F.; Tramuta, C.; Grego, E.; et al. Pectin-honey hydrogel: Characterization, antimicrobial activity and biocompatibility. Bio-Medical Mater. Eng. 2018, 29, 347–356. [Google Scholar] [CrossRef]
- Park, J.-S.; An, S.-J.; Jeong, S.-I.; Gwon, H.-J.; Lim, Y.-M.; Nho, Y.-C. Chestnut Honey Impregnated Carboxymethyl Cellulose Hydrogel for Diabetic Ulcer Healing. Polymers 2017, 9, 248. [Google Scholar] [CrossRef] [Green Version]
- Leyva-Gómez, G.; Santillan-Reyes, E.; Lima, E.; Madrid-Martínez, A.; Krötzsch, E.; Quintanar-Guerrero, D.; Garciadiego-Cázares, D.; Martínez-Jiménez, A.; Morales, M.H.; Ortega-Peña, S.; et al. A novel hydrogel of poloxamer 407 and chitosan obtained by gamma irradiation exhibits physicochemical properties for wound management. Mater. Sci. Eng. C 2017, 74, 36–46. [Google Scholar] [CrossRef]
- Nasef, S.M.; Khozemy, E.E.; Kamoun, E.A.; El-Gendi, H. Gamma radiation-induced crosslinked composite membranes based on polyvinyl alcohol/chitosan/AgNO3/vitamin E for biomedical applications. Int. J. Biol. Macromol. 2019, 137, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Horniackova, M.; Bucekova, M.; Valachova, I.; Majtan, J. Effect of gamma radiation on the antibacterial and antibiofilm activity of honeydew honey. Eur. Food Res. Technol. 2016, 243, 81–88. [Google Scholar] [CrossRef]
- Samraj, S.M.D.; Kirupha, S.D.; Elango, S.; Vadodaria, K. Fabrication of nanofibrous membrane using stingless bee honey and curcumin for wound healing applications. J. Drug Deliv. Sci. Technol. 2021, 63, 102271. [Google Scholar] [CrossRef]
- Mirzaei, B.; Etemadian, S.; Goli, H.R.; Bahonar, S.; Gholami, S.A.; Karami, P.; Farhadi, M.; Tavakoli, R. Construction and analysis of alginate-based honey hydrogel as an ointment to heal of rat burn wound related infections. Int. J. Burn. Trauma 2018, 8, 88–97. [Google Scholar]
- Advancis Medical Activon Manuka Honey—Wound Dressing Selection Guide. Available online: https://uk.advancismedical.com/uploads/files/files/ActivonWoundDressingGuide2015-A5V4.pdf (accessed on 28 May 2021).
- Zeleníková, R.; Vyhlídalová, D. Applying honey dressings to non-healing wounds in elderly persons receiving home care. J. Tissue Viability 2019, 28, 139–143. [Google Scholar] [CrossRef]
- L-Mesitran Products. Available online: https://mesitran.com/products/ (accessed on 28 May 2021).
- Smaropoulos, E.; Cremers, N.A. The pro-healing effects of medical grade honey supported by a pediatric case series. Complement. Ther. Med. 2019, 45, 14–18. [Google Scholar] [CrossRef]
- Smaropoulos, E.; Cremers, N.A.J. Treating severe wounds in pediatrics with medical grade honey: A case series. Clin. Case Rep. 2020, 8, 469–476. [Google Scholar] [CrossRef] [Green Version]
- Pullar, J.M.; Carr, A.C.; Vissers, M.C.M. The Roles of Vitamin C in Skin Health. Nutrients 2017, 9, 866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Sánchez, A.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Nutraceuticals for Skin Care: A Comprehensive Review of Human Clinical Studies. Nutrients 2018, 10, 403. [Google Scholar] [CrossRef] [Green Version]
- Hobson, R. Vitamin E and wound healing: An evidence-based review. Int. Wound J. 2016, 13, 331–335. [Google Scholar] [CrossRef]
- Sánchez, M.; González-Burgos, E.; Iglesias, I.; Gómez-Serranillos, M.P. Pharmacological Update Properties of Aloe Vera and its Major Active Constituents. Molecules 2020, 25, 1324. [Google Scholar] [CrossRef] [Green Version]
- Hermanns, R.; Cremers, N.A.; Leeming, J.P.; Van Der Werf, E.T. Sweet Relief: Determining the Antimicrobial Activity of Medical Grade Honey Against Vaginal Isolates of Candida albicans. J. Fungi 2019, 5, 85. [Google Scholar] [CrossRef] [Green Version]
- De Groot, T.; Janssen, T.; Faro, D.; Cremers, N.A.J.; Chowdhary, A.; Meis, J.F. Antifungal Activity of a Medical-Grade Honey Formulation against Candida auris. J. Fungi 2021, 7, 50. [Google Scholar] [CrossRef]
- Voss, G.T.; Gularte, M.S.; Vogt, A.G.; Giongo, J.L.; Vaucher, R.A.; Echenique, J.V.; Soares, M.P.; Luchese, C.; Wilhelm, E.A.; Fajardo, A.R. Polysaccharide-based film loaded with vitamin C and propolis: A promising device to accelerate diabetic wound healing. Int. J. Pharm. 2018, 552, 340–351. [Google Scholar] [CrossRef]
- Hemilä, H. Vitamin C and Infections. Nutrients 2017, 9, 339. [Google Scholar] [CrossRef] [Green Version]
- Fatima, Z.; Singh, S.; Hameed, S. Nanophytotherapeutic Potential of Essential Oils Against Candida Infections BT—NanoBioMedicine; Saxena, S.K., Khurana, S.M.P., Eds.; Springer: Singapore, 2020; pp. 315–331. ISBN 978-981-32-9898-9. [Google Scholar]
- Majtan, J.; Sojka, M.; Palenikova, H.; Bucekova, M.; Majtan, V. Vitamin C Enhances the Antibacterial Activity of Honey against Planktonic and Biofilm-Embedded Bacteria. Molecules 2020, 25, 992. [Google Scholar] [CrossRef] [Green Version]
- Molan, P. Medline TheraHoney Honey Wound Dressings. Available online: https://www.medline.com/media/catalog/Docs/MKT/LIT241_BRO_TheraHoney_1673178.pdf (accessed on 28 May 2021).
- Al Saeed, M. Prospective randomized comparison of controlled release ionic silver hydrophilic dressings and medicated honey-impregnated dressings in treating neuropathic diabetic foot ulcer. Saudi J. Health Sci. 2019, 8, 25. [Google Scholar] [CrossRef]
- Costeloe, A.; Vandjelovic, N.D.; Evans, M.A.; Saraiya, S.S. The use of honey in cochlear implant associated wounds in pediatric patients. Int. J. Pediatr. Otorhinolaryngol. 2018, 111, 80–83. [Google Scholar] [CrossRef]
- Integra LifeSciences—MediHoney Wound and Burn Dressing. Available online: https://www.integralife.com/ie/prepare/category/wound-reconstruction-care-outpatient-clinic-private-office-prepare (accessed on 28 May 2021).
- Elsass, F.T. A Sweet Solution: The Use of Medical-grade Honey on Oral Mucositis in the Pediatric Oncology Patient. Wounds 2017, 29, E115–E117. [Google Scholar]
- Faucett, E.A.; Reghunathan, S.; Jacob, A. Medicinal honey as treatment for skin reactions associated with bone-anchored hearing implant surgery. Laryngoscope 2014, 125, 1720–1723. [Google Scholar] [CrossRef]
- El-Kased, R.F.; Amer, R.I.; Attia, D.; Elmazar, M.M. Honey-based hydrogel: In vitro and comparative In vivo evaluation for burn wound healing. Sci. Rep. 2017, 7, 9692. [Google Scholar] [CrossRef] [Green Version]
- Ke, C.-L.; Deng, F.-S.; Chuang, C.-Y.; Lin, C.-H. Antimicrobial Actions and Applications of Chitosan. Polymers 2021, 13, 904. [Google Scholar] [CrossRef]
- Tian, B.; Hua, S.; Tian, Y.; Liu, J. Chemical and physical chitosan hydrogels as prospective carriers for drug delivery: A review. J. Mater. Chem. B 2020, 8, 10050–10064. [Google Scholar] [CrossRef]
- Bonifacio, M.A.; Cometa, S.; Cochis, A.; Gentile, P.; Ferreira, A.M.; Azzimonti, B.; Procino, G.; Ceci, E.; Rimondini, L.; De Giglio, E. Antibacterial effectiveness meets improved mechanical properties: Manuka honey/gellan gum composite hydrogels for cartilage repair. Carbohydr. Polym. 2018, 198, 462–472. [Google Scholar] [CrossRef] [Green Version]
- Datta, S.; Sarkar, R.; Vyas, V.; Bhutoria, S.; Barui, A.; Chowdhury, A.R.; Datta, P. Alginate-honey bioinks with improved cell responses for applications as bioprinted tissue engineered constructs. J. Mater. Res. 2018, 33, 2029–2039. [Google Scholar] [CrossRef]
- Singh, S.; Gupta, A.; Gupta, B. Scar free healing mediated by the release of aloe vera and manuka honey from dextran bionanocomposite wound dressings. Int. J. Biol. Macromol. 2018, 120, 1581–1590. [Google Scholar] [CrossRef]
- Sarhan, W.A.; Azzazy, H.M.; El-Sherbiny, I.M. The effect of increasing honey concentration on the properties of the honey/polyvinyl alcohol/chitosan nanofibers. Mater. Sci. Eng. C 2016, 67, 276–284. [Google Scholar] [CrossRef]
- Shi, C.; Wang, C.; Liu, H.; Li, Q.; Li, R.; Zhang, Y.; Liu, Y.; Shao, Y.; Wang, J. Selection of Appropriate Wound Dressing for Various Wounds. Front. Bioeng. Biotechnol. 2020, 8, 182. [Google Scholar] [CrossRef] [Green Version]
- Azam, N.A.N.M.; Amin, K.A.M. Influence of Manuka Honey on Mechanical Performance and Swelling Behaviour of Alginate Hydrogel Film. IOP Conf. Series: Mater. Sci. Eng. 2018, 440, 012024. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Rajput, M.; Barui, A.; Chatterjee, S.S.; Pal, N.K.; Chatterjee, J.; Mukherjee, R. Dual cross-linked honey coupled 3D antimicrobial alginate hydrogels for cutaneous wound healing. Mater. Sci. Eng. C 2020, 116, 111218. [Google Scholar] [CrossRef]
- Rajput, M.; Mandal, M.; Anura, A.; Mukhopadhyay, A.; Subramanian, B.; Paul, R.R.; Chatterjee, J. Honey loaded silk fibroin 3D porous scaffold facilitates homeostatic full-thickness wound healing. Materialia 2020, 12, 100703. [Google Scholar] [CrossRef]
- Kadakia, P.U.; Kalaf, E.A.G.; Dunn, A.J.; Shornick, L.P.; A Sell, S. Comparison of silk fibroin electrospun scaffolds with poloxamer and honey additives for burn wound applications. J. Bioact. Compat. Polym. 2018, 33, 79–94. [Google Scholar] [CrossRef]
- Esposito, L.; Barbosa, A.I.; Moniz, T.; Lima, S.C.; Costa, P.; Celia, C.; Reis, S. Design and Characterization of Sodium Alginate and Poly(vinyl) Alcohol Hydrogels for Enhanced Skin Delivery of Quercetin. Pharmaceutics 2020, 12, 1149. [Google Scholar] [CrossRef]

| Chemical Structure | Compound Name | Honey Varieties |
|---|---|---|
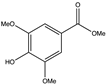 | Methylsyringate | Persian rose, Hawthorn, Thyme [51], Asphodel [53,54], Agastache [70], Manuka [52,65] |
 | Gallic acid | Strawberry tree [55], Chestnut [58], Mint, Raspberry [71] |
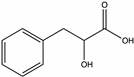 | Phenyllactic acid | Agastache, Jarrah [70], Manuka [65] |
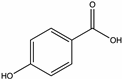 | para-Hydroxybenzoic acid | Strawberry tree [55], Chestnut [58], Raspberry, Sunflower, Mint [71], Buckwheat [72] |
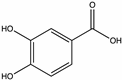 | Protocatechuic acid | Chestnut [58], Raspberry, Mint, Thyme, Honeydew [46,71], Buckwheat [72] |
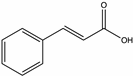 | Cinnamic acid | Chestnut [58], Heather [46] |
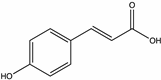 | Coumaric acid | Raspberry, Sunflower, Thyme [71], Buckwheat [42,72], Juazeiro [64], Tilia, Honeydew, Sunflower [73] |
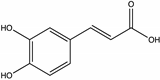 | Caffeic acid | Strawberry tree [55], Chestnut [58] |
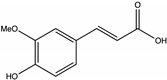 | Ferulic acid | Buckwheat [72], Juazeiro [64] |
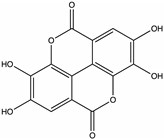 | Ellagic acid | Raspberry, Lingonberry [42], Juazeiro [64] |
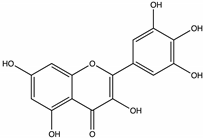 | Myricetin | Strawberry tree [55], Thyme, Rape, Mint, Raspberry, Sunflower [71], Malicia [64], Heather [46] |
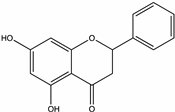 | Pinocembrin | White clover, Hawthorn, Black cumin [51], Manuka [52], Tilia, Acacia, Honeydew, Sunflower [73] |
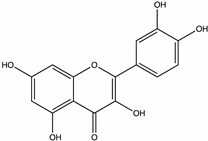 | Quercetin | Strawberry tree [55], Juazeiro, Malicia [64], Brassica [46], Honeydew, Acacia [73] |
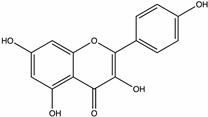 | Kaempferol | Strawberry tree [55], Juazeiro, Malicia [64], Brassica [46] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angioi, R.; Morrin, A.; White, B. The Rediscovery of Honey for Skin Repair: Recent Advances in Mechanisms for Honey-Mediated Wound Healing and Scaffolded Application Techniques. Appl. Sci. 2021, 11, 5192. https://doi.org/10.3390/app11115192
Angioi R, Morrin A, White B. The Rediscovery of Honey for Skin Repair: Recent Advances in Mechanisms for Honey-Mediated Wound Healing and Scaffolded Application Techniques. Applied Sciences. 2021; 11(11):5192. https://doi.org/10.3390/app11115192
Chicago/Turabian StyleAngioi, Roberta, Aoife Morrin, and Blánaid White. 2021. "The Rediscovery of Honey for Skin Repair: Recent Advances in Mechanisms for Honey-Mediated Wound Healing and Scaffolded Application Techniques" Applied Sciences 11, no. 11: 5192. https://doi.org/10.3390/app11115192
APA StyleAngioi, R., Morrin, A., & White, B. (2021). The Rediscovery of Honey for Skin Repair: Recent Advances in Mechanisms for Honey-Mediated Wound Healing and Scaffolded Application Techniques. Applied Sciences, 11(11), 5192. https://doi.org/10.3390/app11115192








