Protective Effect of Wheat and Barley Grass Against the Acute Toxicological Effects of the Concurrent Administration of Excessive Heavy Metals in Drinking Water on the Rats Liver and Brain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pre-Survey Study
- (1)
- A sample from an outlet drain of a clothes dye factory;
- (2)
- A sample from a pump 60 m from the drain, with a pump depth of 42 m;
- (3)
- A sample from a pump 200 m away from the factory;
- (4)
- A sample from a house 500 m away from the factory;
- (5)
- A sample from a house 1 km away from the factory.
- (1)
- A sample from the water station treatment before treatment and after about 3 km from the water intake from the Nile;
- (2)
- A sample from the water station after treatment;
- (3)
- A sample from a house 200 m away from the station;
- (4)
- A sample from a house 400 m away from the station;
- (5)
- A sample from a house 600 m away from the station;
- (6)
- A sample from a house 800 m away from the station;
- (7)
- A sample from a house 1000 m away from the station;
- (8)
- A sample of a house 1200 m away from the station;
- (9)
- A sample from a house 1400 m away from the station;
- (10)
- A sample from a house 1600 m away from the station.
Heavy Metals Analysis
2.2. Plants Germination and Composition
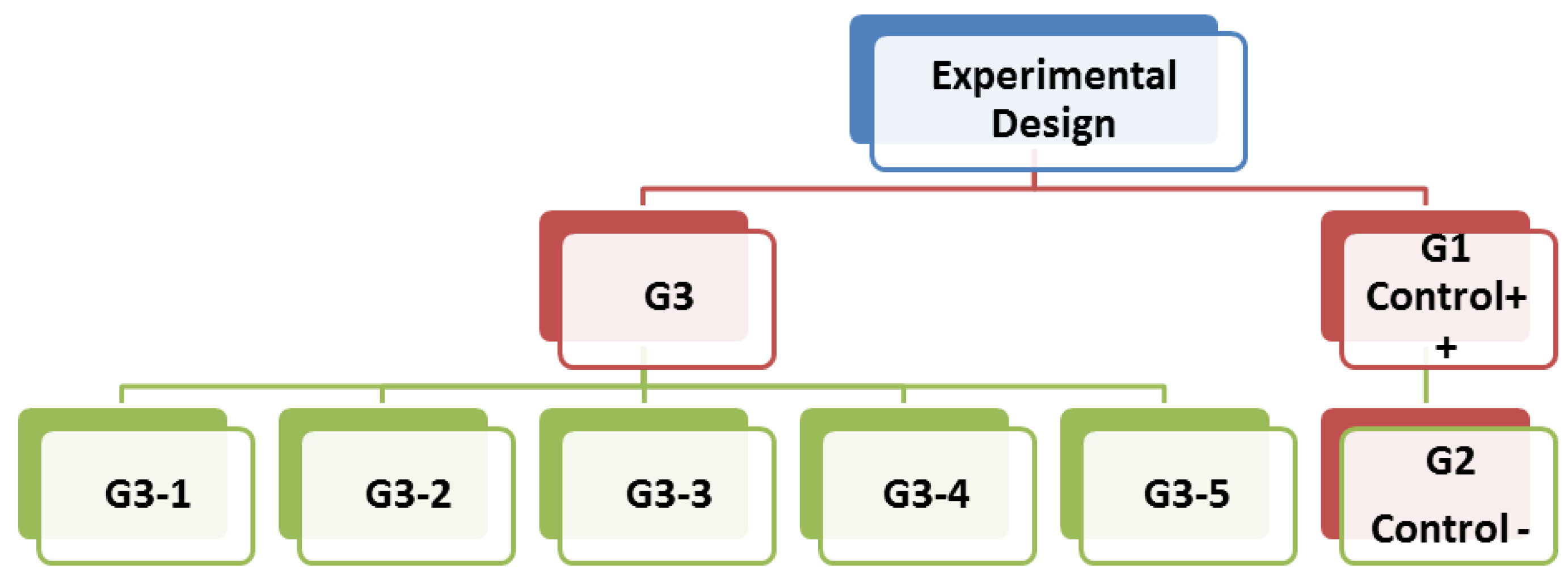
2.3. Animals and Experimental Design
- (1)
- G3-1: Subjected to prepared drinking water;
- (2)
- G3-2: Subjected to prepared drinking water + wheatgrass (250 mg kg−1 day−1);
- (3)
- G3-3: Subjected to prepared drinking water + wheatgrass (500 mg kg−1 day−1);
- (4)
- G3-4: Subjected to prepared drinking water + barley grass (250 mg kg−1 day−1);
- (5)
- G3-5: Subjected to prepared drinking water + barley grass (500 mg kg−1 day−1) as in Figure 1.
2.3.1. Biochemical Analyses of Examined Rats
2.3.2. Histopathological Examination of Liver
2.3.3. Antioxidants Levels and Oxidative Markers in Liver Tissues
2.3.4. Determination of Acetylcholinesterase (ACHE) Activity in the Brain
2.3.5. Comet Assay (Antimutagenic Test)
2.3.6. Molecular Analysis
2.3.7. Heavy Metals in Rats’ Serum and Liver
2.3.8. Statistical Analysis
3. Results and Discussions
3.1. Chemical Composition and GC-MS Analysis of Wheat and Barley
3.2. Liver Biomarkers and Hepatocellular Injury Indicators
3.3. Protein Fractions
3.4. The Concentrations of Cu, Mn, and Zn in Rats Serum and Liver Tissues
3.5. Antioxidant SOD, CAT, and Lipid Peroxide (MDA)
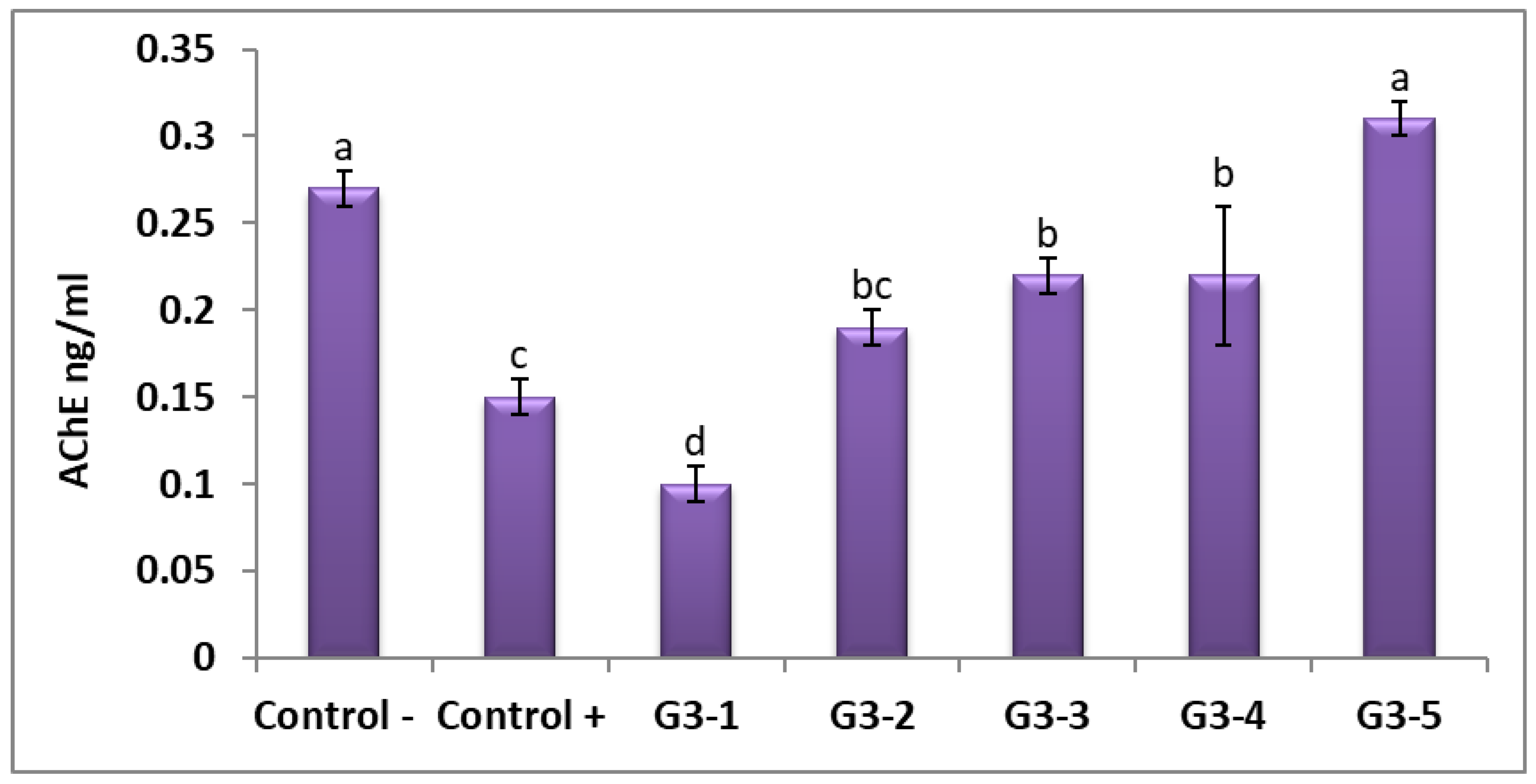
3.6. Acetylcholineesterase (AChE) Level in Rat Brains
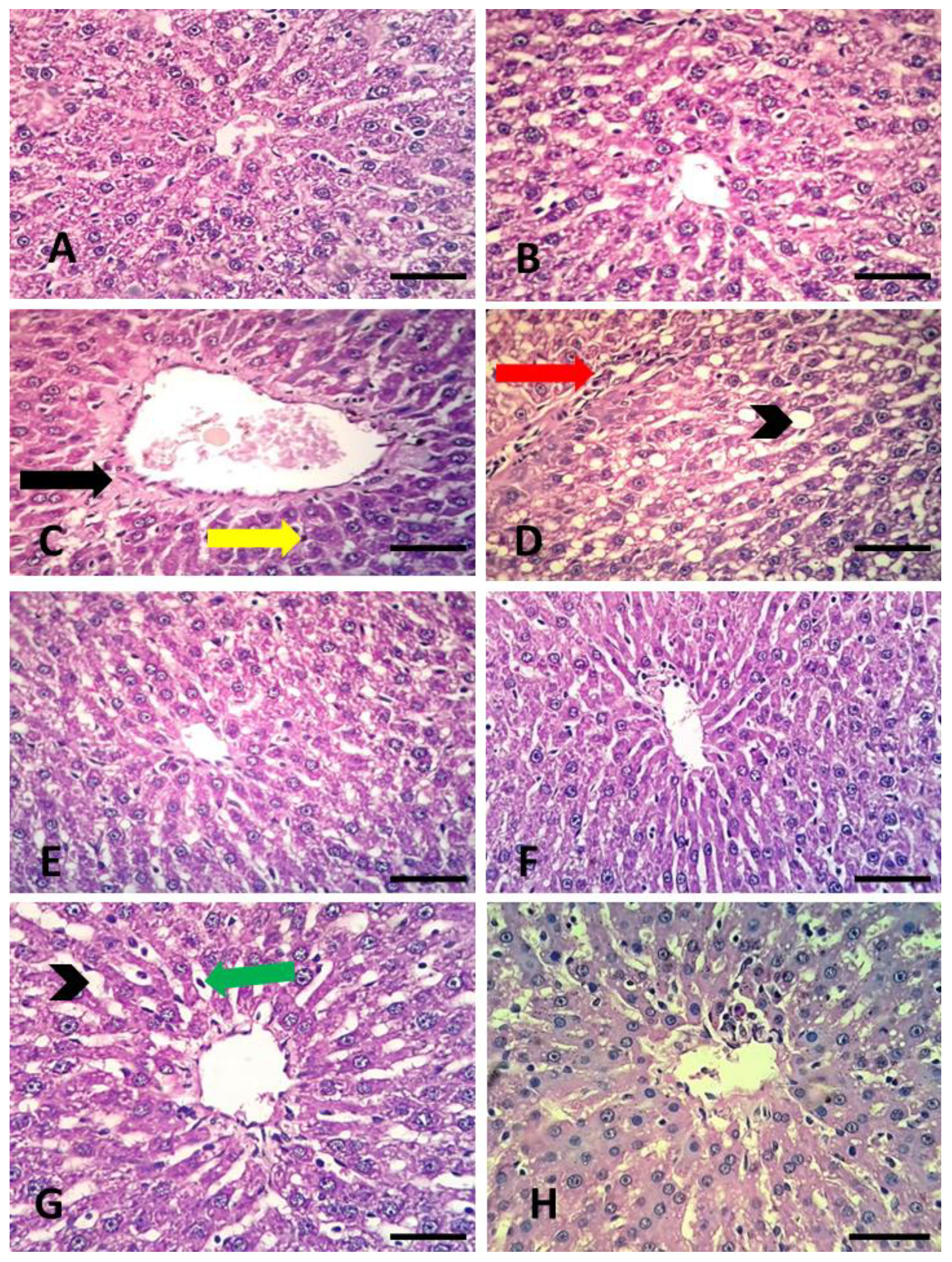
3.7. Histopathological Observations of Rat Livers Exposed to Contaminated Water and Grasses of Wheat and Barley
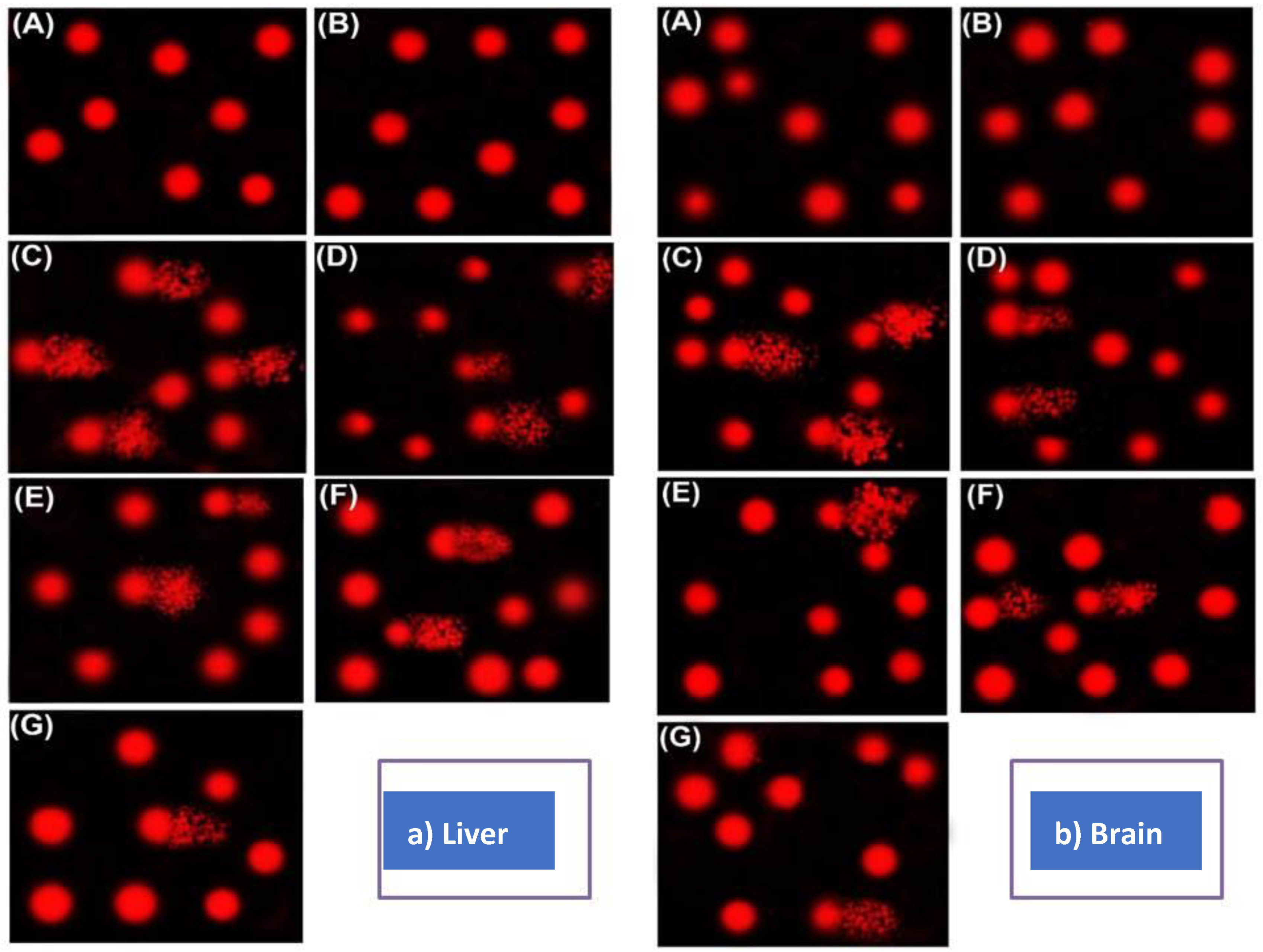
3.8. Comet Assay in Rats Liver Subjected To an Excess of Cu, Mn, and Zn and Germinated Wheat and Barley Grasses
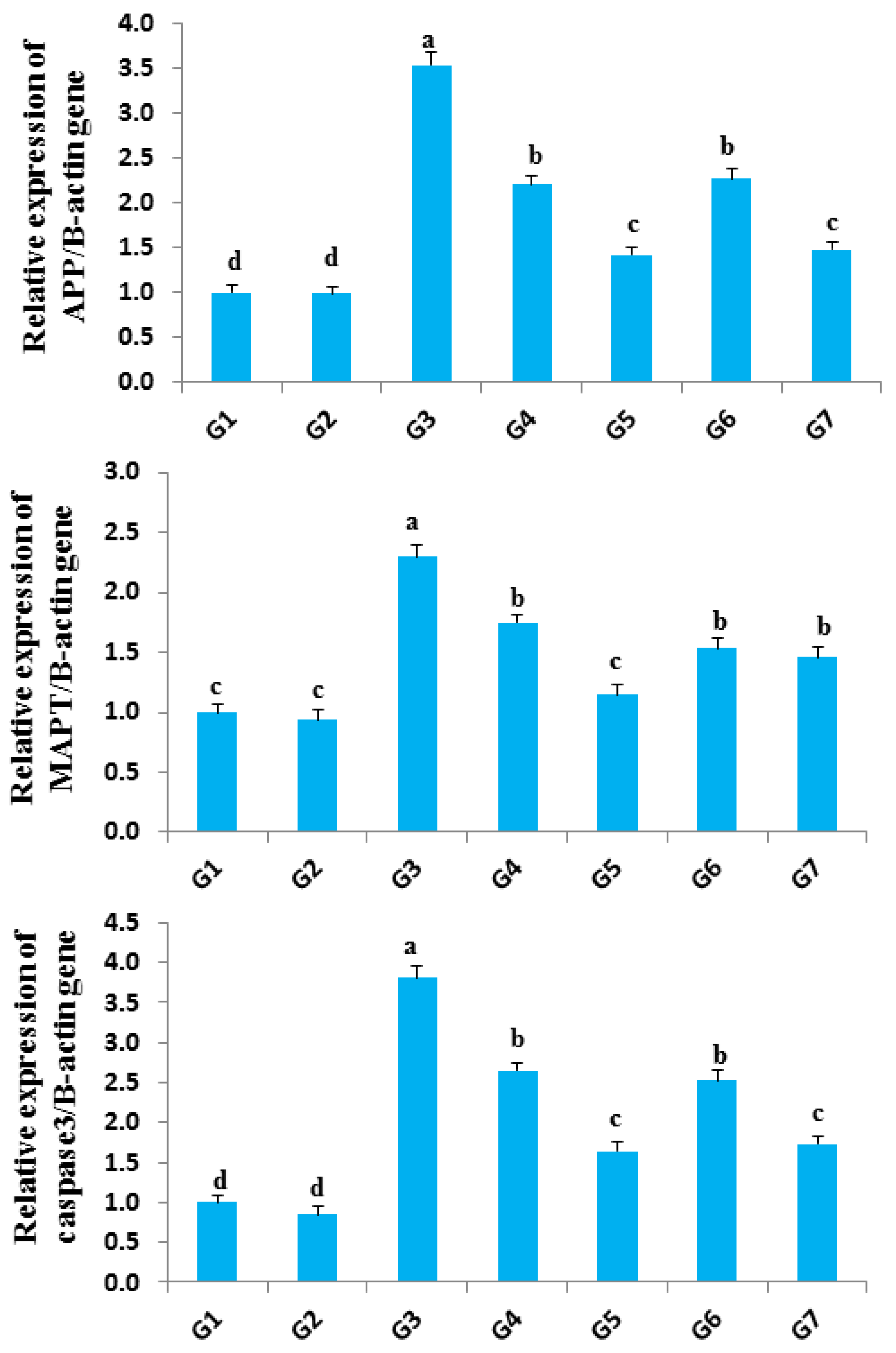
3.9. Gene Expression of APP, MAPT, and Caspase-3 under Mixed Chemical Minerals Stress in Rat Brain
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mandour, R.A. Human health impacts of drinking water (surface and ground) pollution Dakahlyia Governorate, Egypt. Appl. Water Sci. 2012, 2, 157–163. [Google Scholar] [CrossRef] [Green Version]
- EL-Bady, M.S. Toxic levels of some heavy metals in drinking network surface water of Damietta governorate, Egypt. Drink. Water Eng. Sci. Discuss. 2016, 1–8. [Google Scholar]
- Pecina, V.; Brtnický, M.; Baltazár, T.; Juřička, D.; Kynický, J.; Galiová, M.V. Human health and ecological risk assessment of trace elements in urban soils of 101 cities in China: A meta-analysis. Chemosphere 2020, 267, 129215. [Google Scholar] [CrossRef]
- Ullah, I.; Ditta, A.; Imtiaz, M.; Mehmood, S.; Rizwan, M.; Rizwan, M.S.; Ahmad, I. Assessment of health and ecological risks of heavy metal contamination: A case study of agricultural soils in Thall, Dir-Kohistan. Environ. Monit. Assess. 2020, 192, 1–19. [Google Scholar] [CrossRef]
- Masoumi, H.; Ghaemi, A.; Gilani, H.G. Evaluation of hyper-cross-linked polymers performances in the removal of hazardous heavy metal ions: A Review. Sep. Purif. Technol. 2020, 260, 118221. [Google Scholar] [CrossRef]
- Keshavarzi, A.; Kumar, V.; Ertunç, G.; Brevik, E.C. Ecological risk assessment and source apportionment of heavy metals contamination: An appraisal based on the Tellus soil survey. Environ. Geochem. Health 2021, 43, 1–22. [Google Scholar] [CrossRef]
- Schlutow, A.; Schröder, W.; Scheuschner, T. Assessing the relevance of atmospheric heavy metal deposition with regard to ecosystem integrity and human health in Germany. Environ. Sci. Eur. 2021, 33, 1–34. [Google Scholar] [CrossRef]
- Singh, B.P.; Tandon, P.K. Effect of river water pollution on hematological parameters of fish, Wallagoattu. Res. Environ. Life Sci. 2009, 2, 211–214. [Google Scholar]
- Reddy, U.A.; Prabhakar, P.V.; Rao, G.S.; Rao, P.R.; Sandeep, K.; Rahman, M.F.; Mahboob, M. Biomarkers of oxidative stress in rat for assessing toxicological effects of heavy metal pollution in river water. Environ. Sci. Pollut. Res. 2015, 22, 13453–13463. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guo, S.; Liu, J.; Zhang, Z.; Song, K.; Tan, C.; Li, H. A study on the removal of copper (II) from aqueous solution using lime sand bricks. Appl. Sci. 2019, 9, 670. [Google Scholar] [CrossRef] [Green Version]
- Sadeghi, P.; Savari, A.; Movahedinia, A.; Safahieh, A.; Azhdari, D. An assessment of hematological and biochemical responses in the tropical fish Epinephelus stoliczkae of Chabahar Bay and Gulf of Oman under chromium exposure: Ecological and experimental tests. Environ. Sci. Pollut. Res. 2014, 21, 6076–6088. [Google Scholar] [CrossRef]
- Elbasiouny, H.; Elbehiry, F. Mobility and Potential Ecological Risk Assessment of Copper and Zinc in Alluvial and Marine Soils in The North Nile Delta, Egypt. Environ. Biodivers. Soil Secur. 2019, 3, 255–268. [Google Scholar] [CrossRef]
- Tang, H.; Xu, M.; Luo, J.; Zhao, L.; Ye, G.; Shi, F.; Lv, C.; Chen, H.; Wang, Y.; Li, Y. Liver toxicity assessments in rats following sub-chronic oral exposure to copper nanoparticles. Environ. Sci. Eur. 2019, 31, 30. [Google Scholar] [CrossRef] [Green Version]
- WHO. Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Huang, Y.; Wang, B.; Liu, G.; Ge, W.; Zhang, M.; Yue, B.; Kong, M. Effects of Bacillus Subtilis-Zinc on Rats with Congenital Zinc Deficiency. Biol. Trace Element Res. 2020, 194, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Wilk, A.; Szypulska-Koziarska, D.; Marchelek-Myśliwiec, M.; Głazek, W.; Wiszniewska, B. Serum Selenium, Iron, Zinc, and Copper Concentrations in Renal Transplant Recipients Treated with Mycophenolate Mofetil. Biol. Trace Element Res. 2020, 198, 371–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdeldayem, R. A preliminary study of yy metals pollution risk in water. Appl. Water Sci. 2020, 10, 1. [Google Scholar] [CrossRef] [Green Version]
- O’Neal, S.L.; Zheng, W. Manganese toxicity upon overexposure: A decade in review. Curr. Environ. Health Rep. 2015, 2, 315–328. [Google Scholar] [CrossRef] [Green Version]
- Pakfetrat, S.; Amiri, S.; Radi, M.; Abedi, E.; Torri, L. Reduction of phytic acid, aflatoxins and other mycotoxins in wheat during germination. J. Sci. Food Agric. 2019, 99, 4695–4701. [Google Scholar] [CrossRef]
- Benedetti, S.; Primiterra, M.; Tagliamonte, M.C.; Carnevali, A.; Gianotti, A.; Bordoni, A.; Canestrari, F. Counteraction of oxidative damage in the rat liver by an ancient grain (Kamut brand khorasan wheat). Nutrition 2012, 28, 436–441. [Google Scholar] [CrossRef]
- Nepali, S.; Ki, H.H.; Lee, J.H.; Cha, J.Y.; Lee, Y.M.; Kim, D.K. Triticum aestivum sprout-derived polysaccharide exerts hepatoprotective effects against ethanol-induced liver damage by enhancing the antioxidant system in mice. Int. J. Mol. Med. 2017, 40, 1243–1252. [Google Scholar] [CrossRef] [Green Version]
- Stevenson, L.; Phillips, F.; O’Sullivan, K.; Walton, J. Wheat bran: Its composition and benefits to health, a European perspective. Int. J. Food Sci. Nutr. 2012, 63, 1001–1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leoncini, E.; Prata, C.; Malaguti, M.; Marotti, I.; Segura-Carretero, A.; Catizone, P.; Dinelli, G.; Hrelia, S. Phytochemical profile and nutraceutical value of old and modern common wheat cultivars. PLoS ONE 2012, 7, e45997. [Google Scholar] [CrossRef] [Green Version]
- Quan, M.; Li, Q.; Zhao, P.; Tian, C. Chemical composition and hepatoprotective effect of free phenolic extract from barley during malting process. Sci. Rep. 2018, 8, 4460. [Google Scholar] [CrossRef] [Green Version]
- Obadi, M.; Sun, J.; Xu, B. Highland barley: Chemical composition, bioactive compounds, health effects, and applications. Food Res. Int. 2021, 140, 110065. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Kim, S.H.; Lee, S.; Kim, K.M.; Jung, J.C.; Son, T.G.; Ki, S.H.; Seo, W.D.; Kwak, J.H.; Hong, J.T.; et al. Antioxidant effect of barley sprout extract via enhancement of nuclear factor-erythroid 2 related factor 2 activity and glutathione synthesis. Nutrients 2017, 9, 1252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Zhao, L.; Cheng, Q.; Ji, B.; Yang, M.; Sanidad, K.Z.; Wang, C.; Zhou, F. Structurally different flavonoid subclasses attenuate high-fat and high-fructose diet induced metabolic syndrome in rats. J. Agric. Food Chem. 2018, 66, 12412–12420. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.S.; Cho, W.; Tak, S.B.; Kim, S.; Hong, S.P.; Kim, S.O. Triticum aestivum ethanolic extract improves non-alcoholic fatty liver disease in mice fed a choline-deficient or high-fat diet. J. Sci. Food Agric. 2019, 99, 2602–2609. [Google Scholar] [CrossRef] [PubMed]
- Egyptian Ministry of Health, E.M.H. Standards and Specifications of Water Quality for Drinking and Domestic Uses; Internal Report; Egyptian Ministry of Health: Cairo, Egypt, 2007; pp. 1–8.
- American Public Health Association. Standard Methods for the Examination of Water and Waste Water, 22nd ed.; American Water Works Association, Water Environment Federation: Washington, DC, USA, 2012. [Google Scholar]
- Jain, G.; Argal, A. Hepatoprotective potential of young leaves of Triticum aestivum Linn. against CCl4 induced hepatotoxicity. Int. J. Pharm. Sci. Res. 2014, 5, 4751–4755. [Google Scholar]
- Malla, S.; Mourya, M.K.; Halder, D.; Gomroki, F.; Mohammed, H. Healing effects of Wheat Grass (Triticum aestivum L) extracts on RBC Membrane Damage. Am. J. Life Sci. 2014, 2, 22. [Google Scholar] [CrossRef]
- American Association of Cereal Chemists. Approved Methods of American Association of Cereal Chemists, 10th ed.; AACC: St. Paul, MN, USA, 2000. [Google Scholar]
- Abed, K.A.K.; Yaqoob, K.; Abdoh, A.O.O.; Mohammed, S.M.; Pankaj, T.; Hakeem, S.M.A.; Mamoon, H.S. Investigation of Antigenotoxic Potential of Wheatgrass (Triticumaestivum) Powder on Cyclophosphamide Induced Genotoxicity and Oxidative Stress in Mice. Austin J. Pharmacol. Ther. 2017, 5, 1098. [Google Scholar]
- Reitman, S.; Frankel, S.A. Colorimetric method for the determination of sGOT and sGPT. Am. J. Clin. Pathol. 1957, 28, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Klein, B.; Read, P.A.; Babson, L.A. Effects of Ocimum basilicum on tissue anti-oxidant pathways in normal and streptozotocin-diabetic rats. Clin. Chem. 1960, 6, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Walters, M.I.; Gerard, H.W. An ultramicromethod for the determination of conjugated and total bilirubin in serum or plasma. Microchem. J. 1970, 15, 231–243. [Google Scholar] [CrossRef]
- Bowers, L.; Wong, E. Kinetic serum creatinine assays II. A critical evaluation and review. Clin. Chem. 1980, 26, 555–561. [Google Scholar] [CrossRef]
- Doumas, B.T.; Watson, W.A.; Biggs, H.G. Albumin standards and the measurement of serum albumin with bromocresol green. Clin. Chim. Acta 1977, 31, 87–96. [Google Scholar] [CrossRef]
- Allain, C.C.; Poon, L.S.; Chan, C.S.; Richmond, W.; Fu, P.C. Enzymaticdetermination of total cholesterol. Clin. Chem. 1974, 20, 470–475. [Google Scholar] [CrossRef]
- Albers, J.J.; Warnick, G.R.; Cheung, M.C. Quantification of high density lipoproteins. Lipids 1978, 13, 926–932. [Google Scholar] [CrossRef]
- Bucolo, G.; David, H. Quantitativedetermination of serum triglycer-331ides by use of enzymes. Clin. Chem. 1973, 19, 476–482. [Google Scholar] [CrossRef]
- Warnick, G.R.; Knopp, R.H.; Fitzpatrick, V.; Branson, L. Estimatinglow-density lipoprotein cholesterol by the Friedewald equation is408adequate for classifying patients on the basis of nationally recommended cut points. Clin. Chem. 1990, 36, 15–19. [Google Scholar] [CrossRef]
- Suvarna, K.S.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques, 7th ed.; Churchill Livingstone; Elsevier: Oxford, UK, 2013; p. 654. [Google Scholar]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay of lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Archer, S. Measurement of nitric oxide in biological models. FASEB J. 1993, 7, 340–360. [Google Scholar] [CrossRef] [PubMed]
- Carageorgiou, H.; Tzotzes, V.; Sideris, A.; Zarros, A.; Tsakiris, S. Cadmium effects on brain acetylcholinesterase activity and antioxidant status of adult rats: Modulation by zinc, calcium and L-cysteine co-administration. Basic Clin. Pharmacol. Toxicol. 2005, 97, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Olive, P.L.; Bánath, J.P. The comet assay: A method to measure DNA damage in individual cells. Nat. Protoc. 2006, 1, 23–29. [Google Scholar] [CrossRef]
- Pozos, P.G.I.; Ruiz-López, M.A.; Zamora Natera, J.F.; Alvarez Moya, C.; Barrientos Ramirez, L.; Reynoso Silva, M.; Vargas Radillo, J.J. Antioxidant Capacity and Antigenotoxic Effect of Hibiscus sabdariffa L. Extracts Obtained with Ultrasound-Assisted Extraction Process. Appl. Sci. 2020, 10, 560. [Google Scholar] [CrossRef] [Green Version]
- Abd-Allah, S.H.; Shalaby, S.M.; Abd-Elbary, E.; Saleh, A.A.; Abu El-Magd, M. Human peripheral blood CD34+ cells attenuate oleic acid–induced acute lung injury in rats. Cytotherapy 2015, 17, 443–453. [Google Scholar] [CrossRef]
- Khamis, A.A.; Ali, E.M.; El-Moneim, M.A.A.; Abd-Alhaseeb, M.; Abu El-Magd, M.; Salim, E.I. Hesperidin, piperine and bee venom synergistically potentiate the anticancer effect of tamoxifen against breast cancer cells. Biomed. Pharmacother. 2018, 105, 1335–1343. [Google Scholar] [CrossRef]
- El-Magd, M.A.; Khamis, A.; Eldeen, S.K.N.; Ibrahim, W.M.; Salama, A.F. Trehalose enhances the antitumor potential of methotrexate against mice bearing Ehrlich ascites carcinoma. Biomed. Pharmacother. 2017, 92, 870–878. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, Y.; Li, Z.; Hua, Q.; Wang, L.; Song, X.; Tang, C. Joint toxicity of a multi-heavy metal mixture and chemoprevention in sprague dawley rats. Int. J. Environ. Res. Public Health 2020, 17, 1451. [Google Scholar] [CrossRef] [Green Version]
- Shakya, G.; Pajaniradje, S.; Hoda, M.; Durairaj, V.; Rajagopalan, R. GC-MS analysis, in vitro antioxidant and cytotoxic studies of wheatgrass extract. Am. J. Phytomed. Clin. Ther. 2014, 2, 877–893. [Google Scholar]
- Richard, D.; Kefi, K.; Bausero, P.; Visioli, F. Polyunsaturated fatty acids as antioxidants. Pharmacol. Res. 2008, 57, 451–455. [Google Scholar] [CrossRef]
- Banerjee, S.; Katiyar, P.; Kumar, V.; Waghmode, B.; Nathani, S.; Krishnan, V.; Sircar, D.; Roy, P. Wheatgrass inhibits the lipopolysaccharide-stimulated inflammatory effect in RAW 264.7 macrophages. Curr. Res. Toxicol. 2021, 2, 116–127. [Google Scholar] [CrossRef]
- Panthi, M.; Kumar, R.; Raut, S.; Khanal, D.P.; Koirala, N. Bioactivity evaluations of leaf extract fractions from young barley grass and correlation with their phytochemical profiles. BMC Complement. Med. Ther. 2020, 20, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erdinest, N.; Shmueli, O.; Grossman, Y.; Ovadia, H.; Solomon, A. Anti-inflammatory effects of alpha linolenic acid on human corneal epithelial cells. Investig. Opthalmol. Vis. Sci. 2012, 53, 4396–4406. [Google Scholar] [CrossRef]
- Bamidele, A.; Ayannuga, S.; Olugbenga, O. Hepatoprotective potentials of methanolic extract of the leaf of momordicacharantialinn on cadmium -induced hepatotoxicity in rats. J. Nat. Sci. Res. 2012, 2, 41–47. [Google Scholar]
- Lala, V.; Goyal, A.; Bansal, P.; Minter, D.A. Liver Function Tests; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar] [PubMed]
- Jang, D.H.; Hoffman, R.S. Heavy metal chelation in neurotoxic exposures. Neurol. Clin. 2011, 29, 607–622. [Google Scholar] [CrossRef] [PubMed]
- Adal, A.; Wiener, S.W. Heavy Metal Toxicity. Medscape. 2013. Available online: http://emedicine.medscape.com/article/814960-overview (accessed on 10 August 2020).
- Calzuola, I.; Valeria, M.; Gianfranceschi, G.L. Synthesis of antioxidants in wheat sprouts. J. Agric. Food Chem. 2014, 52, 5201–5206. [Google Scholar] [CrossRef] [PubMed]
- Kamboj, J.K.; Rana, S.V.; Ola, R.P.; Dhawan, D.K.; Vahiphei, K. Wheatgrass and Antioxidant Levels in Carbon Tetrachloride-induced Hepatotoxicity in Rats. J. Clin. Exp. Hepatol. 2011, 1, 43. [Google Scholar] [CrossRef]
- Lahouar, L.; El-Bok, S.; Achour, L. Therapeutic potential of young green barley leaves in prevention and treatment of chronic diseases: An overview. Am. J. Chin. Med. 2015, 43, 1311–1329. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Kim, J.H.; Kim, S.H.; Oh, J.Y.; Seo, W.D.; Kim, K.M.; Jung, J.C.; Jung, Y.S. Barley sprouts extract attenuates alcoholic fatty liver injury in mice by reducing inflammatory response. Nutrients 2016, 8, 440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simeonova, R.; Kondeva-Burdina, M.; Vitcheva, V.; Krasteva, I.; Manov, V.; Mitcheva, M. Protective effects of the apigenin-O/C-diglucoside saponarin from Gypsophila trichotoma on carbone tetrachloride-induced hepatotoxicity in vitro/in vivo in rats. Phytomedicine 2014, 21, 148–154. [Google Scholar] [CrossRef]
- Ajiboye, B.O.; Oloyede, H.O.B.; Salawu, M.O. Antidiabetic activity of Triticum aestivum seed-based diet on alloxan-induced diabetic rats. J. Diet. Suppl. 2020, 17, 133–149. [Google Scholar] [CrossRef]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free radicals: Properties, sources, targets, and their implication in various diseases. Ind. J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef] [Green Version]
- Foda, M. Biochemical Studies on Antioxidants Extracted from young Green Barley Leaves. Master’s Thesis, Department of Biochemistry, Faculty of Agriculture, Benha University, Benha, Egypt, 2010. [Google Scholar]
- Stamoulis, I.; Kouraklis, G.; Theocharis, S. Zinc and the Liver: An Active Interaction. Dig. Dis. Sci. 2007, 52, 1595–1612. [Google Scholar] [CrossRef] [PubMed]
- Dung, D.D.; Godwin, R.; Nolan, I.V. Barley Grain and Sprouted Barley. J. Anim. Vet. Adv. 2010, 9, 2485–2492. [Google Scholar]
- Wilhelm Filho, D.; Júnior, S.Á.; Possamai, F.P.; Parisotto, E.B.; Moratelli, A.M.; Garlet, T.R.; Inácio, D.B.; Torres, M.A.; Colepicolo, P.; Dal-Pizzol, F. Antioxidant therapy attenuates oxidative stress in the blood of subjects exposed to occupational airborne contamination from coal mining extraction and incineration of hospital residues. Ecotoxicology 2010, 19, 1193–1200. [Google Scholar] [CrossRef]
- Deng, L.; Feng, G.; Gao, Y.; Shen, Y.; Li, H.; Gu, Y.; Luan, H. Phytochemical Constituents and Antioxidant Enzyme Activity Profiles of Different Barley (Hordeum Vulgare L.) Cultivars at Different Developmental Stages. Agronomy 2020, 10, 37. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Y.; Pu, X.; Yang, X.; Yang, J.; Du, J.; Yang, T.; Li, X. Strategies of functional foods for heart disease prevention in human beings. In Proceedings from the ICERP 2016; Sciendo Migration: Warsaw, Poland; pp. 108–123.
- Thatiparthi, J.; Dodoala, S.; Koganti, B.; Kvsrg, P. Barley grass juice (Hordeum vulgare L.) inhibits obesity and improves lipid profile in high fat diet-induced rat model. J. Ethnopharmacol. 2019, 238, 111843. [Google Scholar] [CrossRef]
- Mohamed, R.S.; Diaa, M.A.; Salah, H.S.; Ahmed, H.Z.; Ihab, A.S.; Abdel Razik, F.H.; Ahmed, A.M. Hypoglycemic, hypolipidemic and antioxidant effects of green sprouts juice and functional dairy micronutrients against streptozotocin-induced oxidative stress and diabetes in rats. Heliyon 2019, 5, 01197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luyen, B.T.; Tai, B.H.; Thao, N.P.; Cha, J.Y.; Lee, Y.M.; Kim, Y.H. A new phenolic component from Triticum aestivum sprouts and its effects on LPS-stimulated production of nitric oxide and TNF-alpha in RAW 264.7 cells. Phytother. Res. 2014, 28, 1064–1070. [Google Scholar] [CrossRef]
- Chauhan, M. A pilot study on wheat grass juice for its phytochemical, nutritional and therapeutic potential on chronic diseases. Int. J. Chem. Stud. 2014, 2, 27–34. [Google Scholar]
- Richetti, S.K.; Rosemberg, D.B.; Ventura-Lima, J.; Monserrat, J.M.; Bogo, M.R.; Bonan, C.D. Acetylcholinesterase activity and antioxidant capacity of zebrafish brain is altered by heavy metal exposure. Neurotoxicology 2011, 32, 116–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.; Backus, C.; Oh, S.; Hayes, J.M.; Feldman, E.L. Increased tau phosphorylation and cleavage in mouse models of type 1 and type 2 diabetes. Endocrinology 2009, 150, 5294–5301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira-Vieira, T.H.; Guimaraes, I.M.; Silva, F.R.; Ribeiro, F.M. Alzheimer’s disease: Targeting the Cholinergic System. Curr. Neuropharmacol. 2016, 14, 101–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rinne, J.O.; Kaasinen, V.; Järvenpää, T.; Någren, K.; Roivainen, A.; Yu, M.; Oikonen, V.; Kurki, T. Brain acetylcholinesterase activity in mild cognitive impairment and early Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 2003, 74, 113–115. [Google Scholar] [CrossRef]
- Van Dyk, J.C.; Pieterse, G.M.; Van Vuren, J.H. Histological changes in the liver of Oreochromis mossambicus (Cichlidae) after exposure to cadmium and zinc. Ecotoxicol. Environ. Saf. 2007, 66, 432–440. [Google Scholar] [CrossRef]
- Xu, D.; Hu, L.; Su, C.; Xia, X.; Zhang, P.; Fu, J.; Wang, W.; Xu, D.; Du, H.; Hu, Q.; et al. Tetrachloro-p-benzoquinone induces hepatic oxidative damage and inflammatory response, but not apoptosis in mouse: The prevention of curcumin. Toxicol. Appl. Pharmacol. 2014, 280, 305–313. [Google Scholar] [CrossRef]
- Kotoh, K.; Enjoji, M.; Kohjima, M.; Nakamuta, M.; Takayanagi, R. A new parameter using serum lactate dehydrogenase and alanine aminotransferase level is useful for predicting the prognosis of patients at an early stage of acute liver injury: A retrospective study. Comp. Hepatol. 2008, 7, 6. [Google Scholar] [CrossRef] [Green Version]
- Poudel, B.; Nepali, S.; Xin, M.; Ki, H.; Kim, D.; Lee, Y. Flavonoids from Triticum aestivum inhibit adipogenesis in 3T3-L1 cells by upregulating the insig pathway. Mol. Med. Rep. 2015, 12, 3139–3145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.H.; Lee, Y.M.; Le, H.S.; Kim, D.K. Anti-oxidative and anti-hyperglycemia effects of Triticum aestivum wheat sprout water extracts on the streptozotocin-induced diabetic mice. Korean J. Pharmacogn. 2009, 40, 408–414. [Google Scholar]
- Guecheva, T.; Henriques, J.A.; Erdtmann, B. Genotoxic effects of copper sulphate in freshwater planarian in vivo, studied with the single-cell gel test (comet assay). Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2001, 497, 19–27. [Google Scholar] [CrossRef]
- Bolognesi, C.; Landini, E.; Roggieri, P.; Fabbri, R.; Viarengo, A. Genotoxicity biomarkers in the assessment of heavy metal effects in mussels: Experimental studies. Environ. Mol. Mutagen. 1999, 33, 287–292. [Google Scholar] [CrossRef]
- Aschner, M.; Guilarte, T.R.; Schneider, J.S.; Zheng, W. Manganese: Recent advances in understanding its transport and neurotoxicity. Toxicol. Appl. Pharmacol. 2007, 221, 131–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, J.; Qi, Y.M.; Fu, J.L.; Zhou, Z.C. Manganese-induced single strand breaks of mitochondrial DNA in vitro and in vivo. Environ. Toxicol. Pharmacol. 2008, 26, 123–127. [Google Scholar] [CrossRef]
- Huang, C.C.; Chu, N.S.; Lu, C.S.; Wang, J.D.; Tsai, J.L.; Tzeng, J.L.; Wolters, E.C.; Calne, D.B. Chronic manganese intoxication. Arch. Neurol. 1989, 46, 1104–1106. [Google Scholar] [CrossRef]
- Lee, S.H.; Jew, S.S.; Chang, P.S.; Hong, I.J.; Hwang, E.S.; Kim, K.S.; Kim, K.T. Free radical scavenging effect and antioxidant activities of barley leaves. Food Sci. Biotechnol. 2003, 12, 268–273. [Google Scholar]
- Kulkami, S.D.; Tilak, J.C.; Acharya, R.; Rajurkar, N.S.; Devasagayam, T.P.; Reddy, A.V. Evaluation of the antioxidant activity of wheatgrass (Triticum aestivum, L.) as a function of growth under different conditions. Phytother. Res. 2006, 20, 218–227. [Google Scholar]
- Paulíčková, I.; Ehrenbergerová, J.; Fiedlerová, V.; Gabrovska, D.; Havlova, P.; Holasova, M.; Kopáček, J.; Ouhrabková, J.; Pinkrová, J.; Rysová, J.; et al. Evaluation of barley grass as a potential source of some nutritional substances. Czech J. Food Sci. 2007, 25, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Durairaj, V.; Hoda, M.; Shakya, G.; Babu, S.P.; Rajagopalan, R. Phytochemical screening and analysis of antioxidant properties of aqueous extract of wheatgrass. Asian Pac. J. Trop. Med. 2014, 7, S398–S404. [Google Scholar] [CrossRef]
- Armendariz, A.D.; Gonzalez, M.; Loguinov, A.V.; Vulpe, C.D. Gene expression profiling in chronic copper overload reveals upregulation of Prnp and App. Physiol. Genom. 2004, 20, 45–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Basha, M.R.; Brock, B.; Cox, D.P.; Cardozo-Pelaez, F.; McPherson, C.A.; Harry, J.; Rice, D.C.; Maloney, B.; Chen, D.; et al. Alzheimer’s disease (AD)-like pathology in aged monkeys after infantile exposure to environmental metal lead (Pb): Evidence for a developmental origin and environmental link for AD. J. Neurosci. 2008, 28, 3–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.J.; Park, M.K.; Seo, Y.R. Pathogenic Mechanisms of Heavy Metal Induced-Alzheimer’s Disease. Toxicol. Environ. Health Sci. 2018, 10, 1–10. [Google Scholar] [CrossRef]
- Ballatore, C.; Lee, V.M.; Trojanowski, J.Q. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat. Rev. Neurosci. 2007, 8, 663–672. [Google Scholar] [CrossRef]
- Chakravarthy, M.; Chen, S.; Wang, T.; Veedu, R.N. Development of novel chemically-modified nucleic acid molecules for efficient inhibition of human MAPT gene expression. Genes 2020, 11, 667. [Google Scholar] [CrossRef] [PubMed]
- Caillet-Boudin, M.L.; Buée, L.; Sergeant, N.; Lefebvre, B. Regulation of human MAPT gene expression. Mol. Neurodegener. 2015, 10, 28. [Google Scholar] [CrossRef] [Green Version]
- Alquezar, C.; Felix, J.B.; McCandlish, E.; Buckley, B.T.; Caparros-Lefebvre, D.; Karch, C.M.; Golbe, L.I.; Kao, A.W. Heavy metals contaminating the environment of a progressive supranuclear palsy cluster induce tau accumulation and cell death in cultured neurons. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
| Sample Number | Cu | Mn | Zn |
|---|---|---|---|
| 1 | 1.63 | 7.15 | 5.07 |
| 2 | nd * | 0.56 | 0.88 |
| 3 | 0.12 | 0.32 | 0.64 |
| 4 | 0.06 | 0.90 | 0.95 |
| 5 | 0.05 | 0.32 | 0.72 |
| 6 | 1.50 | 2.08 | 4.38 |
| 7 | 1.15 | 0.58 | 2.45 |
| 8 | 1.03 | 0.85 | 4.48 |
| 9 | 0.73 | 0.30 | 1.68 |
| 10 | 0.73 | 0.70 | 3.35 |
| 11 | 0.55 | 0.68 | 2.33 |
| 12 | 0.78 | 0.95 | 3.25 |
| 13 | 0.78 | 0.28 | 3.00 |
| 14 | 0.20 | 0.53 | 3.30 |
| 15 | 0.15 | 4.55 | 1.83 |
| Permissible Limits of WHO (2011) | 2.0 | 0.4 | 3.0 |
| Permissible Limits of EMH (2011) | 2.0 | 0.4 | 3.0 |
| Gene | Forward Primer (′5–′3) | Reverse Primer (′5–′3) |
|---|---|---|
| APP | AACGAAGACCACTGTGGAGC | CGTCGACAGGCTCAACTTCA |
| MAPT | TGGCTTAAAAGCTGAAGAAGCA | GCCCTTGGCTTTCTTCTCGT |
| Caspase 3 | GGTATTGAGACAGACAGTGG | CATGGGATCTGTTTCTTTGC |
| β-actin | AAGTCCCTCACCCTCCCAAAAG | AAGCAATGCTGTCACCTTCCC |
| Grass | Proteins | Fats | Fibers | Carbohydrates | Ash |
|---|---|---|---|---|---|
| Wheat | 21.71 | 1.62 | 18.73 | 31.69 | 12.60 |
| Barely | 22.55 | 1.58 | 22.65 | 24.01 | 15.32 |
| RT | Compound Name | Area % | % Peak Area | Formula | MW |
|---|---|---|---|---|---|
| Wheat | |||||
| 5.40 | 3-Octadecena | 4.32 | 72,153,576 | C18H34O | 266 |
| 8.93 | Dodecane | 4.20 | 70,048,549 | C12H26 | 170 |
| 24.32 | 2,2,3,3,4,4 Hexadeutero octadecanal | 2.91 | 48,550,405 | C18H30D6O | 274 |
| 27.30 | 17-Octadecynoic acid | 4.80 | 80,057,445 | C18H32O2 | 280 |
| 27.54 | 2-Pentadecanone, 6,10,14-trimethyl | 5.31 | 88,654,291 | C18H36O | 268 |
| 27.82 | Ethanol, 2-(9-octadecenyloxy) | 2.68 | 44,757,657 | C20H40O2 | 312 |
| 28.20 | 13-Heptadecyn-1-ol | 1.68 | 27,956,548 | C17H32O | 252 |
| 29.20 | Cyclopropanebutanoic acid, 2-[[2-[[2-[(2-pentylcyclopropyl)methyl]cyclopropyl]methyl]cyclopropyl] methyl]-, methyl ester | 2.52 | 42,105,270 | C25H42O2 | 374 |
| 30.92 | 9-Octadecenoic acid | 2.00 | 33,361,130 | C18H34O2 | 282 |
| 32.40 | 7-Methyl-Z-tetradecen-1-ol acetate | 6.34 | 105,891,316 | C17H32O2 | 268 |
| 34.20 | 9,12-Octadecadienoic acid | 2.24 | 37,389,728 | C18H32O2 | 280 |
| 44.13 | Ethyl iso-allocholate | 3.31 | 55,267,353 | C26H44O5 | 436 |
| Barley | |||||
| 8.92 | Dodecane | 17.03 | 126,128,138 | C12H26 | 170 |
| 32.54 | 9Octadecenoic acid | 42.19 | 312,497,160 | C18H34O2 | 282 |
| 44.49 | Ethyliso-allocholate | 40.78 | 302,104,307 | C26H44O5 | 346 |
| Parameters Groups | Liver Weight (g) | ALT(U L−1) | AST(U L−1) | ALP (U L−1) |
|---|---|---|---|---|
| Control - | 9.70 ± 0.88 a | 63.86 ± 4.07 e | 111.40 ± 2.7 e | 70.45 ± 3.76 c |
| Control + | 4.21 ± 0.06 d | 92.73 ± 2.20 b | 180.2 ± 3.6 b | 94.02 ± 5.03 b |
| G3-1 | 4.28 ± 0.22 d | 115.43 ± 4.8 a | 201.4 ± 7.5 a | 108.0 ± 13.2 a |
| G3-2 | 5.51 ± 0.33 c | 83.14 ± 2.4 c | 144.9 ± 5.0 c | 64.34 ± 4.7 d |
| G3-3 | 4.57 ± 0.29 d | 73.47 ± 2.6 d | 126.21 ± 3.35 d | 62.72 ± 5.7 d |
| G3-4 | 5.82 ± 0.34 c | 86.64 ± 1.4 c | 146.33 ± 3.21 c | 78.64 ± 5.2 cd |
| G3-5 | 7.38 ± 0.28 b | 63.44 ± 3.0 e | 119.0 ± 1.0 d | 64.92 ± 4.8 d |
| Parameters Groups | T.B (mg dL−1) | D.B (mg dL−1) | ID.B (mg dL−1) |
|---|---|---|---|
| Control− | 0.55 ± 0.09 cd | 0.09 ± 0.02 c | 0.46 ± 0.08 bc |
| Control+ | 0.85 ± 0.10 ab | 0.18 ± 0.02 b | 0.67 ± 0.12 a |
| G3-1 | 0.87 ± 0.09 a | 0.22 ± 0.02 a | 0.65 ± 0.12 a |
| G3-2 | 0.70 ± 0.05 bc | 0.16 ± 0.01 b | 0.53 ± 0.04 ab |
| G3-3 | 0.55 ± 0.07 cd | 0.12 ± 0.01 c | 0.43 ± 0.06 bc |
| G3-4 | 0.72 ± 0.10 ab | 0.16 ± 0.01 b | 0.56 ± 0.10 ab |
| G3-5 | 0.44 ± 0.07 d | 0.11 ± 0.02 c | 0.33 ± 0.05 c |
| Parameters Groups | T.P (g dl−1) | ALB (g dl−1) | G (g dl−1) | A/G |
|---|---|---|---|---|
| Control− | 7.08 ± 0.15 abc | 3.52 ± 0.10 ab | 3.56 ± 0.25 ab | 0.99 ± 0.10 b |
| Control+ | 7.20 ± 0.07 a | 3.46 ± 0.12 ab | 3.73 ± 0.20 a | 0.93 ± 0.08 bc |
| G3-1 | 6.99 ± 0.11 bc | 3.65 ± 0.11 a | 3.34 ± 0.19 b | 1.09 ± 0.08 a |
| G3-2 | 7.15 ± 0.06 ab | 3.61 ± 0.12 ab | 3.53 ± 0.16 ab | 1.02 ± 0.07 ab |
| G3-3 | 6.95 ± 0.05 cd | 3.49 ± 0.06 ab | 3.46 ± 0.06 ab | 1.01 ± 0.02 ab |
| G3-4 | 7.22 ± 0.04 a | 3.62 ± 0.04 ab | 3.59 ± 0.07 ab | 1.01 ± 0.03 ab |
| G3-5 | 6.79 ± 0.14 d | 3.43 ± 0.15 b | 3.36 ± 0.28 b | 1.03 ± 0.13 ab |
| Parameters Groups | Zn μg dL−1 | Cu μg dL−1 | Mn μg dL−1 |
|---|---|---|---|
| Liver | |||
| Control− | 13.68 ± 2.31 f | 111.5 ± 10.03 e | 0.29 ± 0.03 d |
| Control+ | 31.75 ± 2.04 b | 169 ± 6.55 b | 1.08 ± 0.07 b |
| G3-1 | 40.94 ± 3.94 a | 250.6 ± 11.01 a | 1.78 ± 0.51 a |
| G3-2 | 29.07 ± 2.03 bc | 153.66 ± 3.21 c | 0.95 ± 0.04 cb |
| G3-3 | 23.72 ± 1.48 ed | 135 ± 5.00 d | 0.69 ± 0.19 cb |
| G3-4 | 26.03 ± 1.57 cd | 157.33 ± 2.51 cb | 1.004 ± 0.09 cb |
| G3-5 | 20.08 ± 1.19 e | 132.9 ± 8.5 d | 0.63 ± 0.1 cd |
| Brain | |||
| Control− | 10.29 ± 0.93 e | 77.5 ± 3.9 f | 0.13 ± 0.004 e |
| Control+ | 33.62 ± 0.94 b | 117.65 ± 6.05 b | 0.26 ± 0.006 b |
| G3-1 | 42.58 ± 5.54 a | 135 ± 9 a | 0.35 ± 0.044 a |
| G3-2 | 20.03 ± 1.6 c | 99.09 ± 0.98 c | 0.23 ± 0.007 cb |
| G3-3 | 14.45 ± 1.05 ed | 79.65 ± 4.5 ef | 0.19 ± 0.021 d |
| G3-4 | 22.84 ± 2.1 c | 91.65 ± 3.47 cd | 0.22 ± 0.009 dc |
| G3-5 | 15.52 ± 0.88 d | 88.66 ± 5.1 de | 0.19 ± 0.10 d |
| Parameters | CAT (ng mg−1) | GSH (ng mg−1) | NO (nmol mg−1) | MDA (nmol mg−1) |
|---|---|---|---|---|
| Control− | 0.29 ± 0.01 a | 0.31 ± 0.02 a | 0.10 ± 0.005 e | 0.10 ± 0.01 f |
| Control+ | 0.15 ± 0.01 e | 0.15 ± 0.01 e | 0.19 ± 0.01 b | 0.26 ± 0.01 b |
| G3-1 | 0.10 ± 0.003 f | 0.11 ± 0.003 f | 0.24 ± 0.01 a | 0.32 ± 0.004 a |
| G3-2 | 0.20 ± 0.01 d | 0.19 ± 0.01 d | 0.16 ± 0.01 c | 0.20 ± 0.01 c |
| G3-3 | 0.23 ± 0.001 c | 0.23 ± 0.01 c | 0.13 ± 0.01 d | 0.17 ± 0.002 d |
| G3-4 | 0.21 ± 0.01 d | 0.20 ± 0.01 d | 0.18 ± 0.006 b | 0.18 ± 0.01 d |
| G3-5 | 0.25 ± 0.003 b | 0.26 ± 0.01 b | 0.12 ± 0.01 d | 0.13 ± 0.01 e |
| Group | Tailed % | Untailed % | Tails Length µm | Tail DNA% | Tail Moment | |
|---|---|---|---|---|---|---|
| Liver | ||||||
| A | Control − | 1.75 | 98.25 | 1.25 ± 0.26 e | 1.41 | 1.76 |
| B | Control + | 2.25 | 97.75 | 1.36 ± 0.24 e | 1.45 | 1.97 |
| C | G3-1 | 14.00 | 86.00 | 4.05 ± 0.56 a | 4.94 | 20.00 |
| D | G3-2 | 9.00 | 91.00 | 2.94 ± 0.42 b | 3.43 | 10.08 |
| E | G3-3 | 6.00 | 94.00 | 2.18 ± 0.29 cd | 2.62 | 5.71 |
| F | G3-4 | 7.00 | 93.00 | 2.33 ± 0.34 c | 2.90 | 6.76 |
| G | G3-5 | 5.00 | 95.00 | 2.04 ± 0.24 d | 2.46 | 5.02 |
| Brain | ||||||
| A | Control − | 2 | 98 | 1.31 ± 0.24 d | 1.39 | 1.82 |
| B | Control + | 2.5 | 97.5 | 1.35 ± 0.26 d | 1.44 | 1.94 |
| C | G3-1 | 16 | 84 | 4.93 ± 0.58 a | 5.37 | 26.47 |
| D | G3-2 | 10 | 90 | 3.85 ± 0.48 b | 3.94 | 15.17 |
| E | G3-3 | 7 | 93 | 2.35 ± 0.32 c | 2.89 | 6.79 |
| F | G3-4 | 9 | 91 | 3.59 ± 0.40 b | 3.58 | 12.85 |
| G | G3-5 | 6 | 94 | 2.11 ± 0.29 c | 2.60 | 5.49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eldamaty, H.S.E.; Elbasiouny, H.; Elmoslemany, A.M.; Abd El-Maoula, L.M.; El-Desoky, O.I.; Rehan, M.; Abd El Moneim, D.; Zedan, A. Protective Effect of Wheat and Barley Grass Against the Acute Toxicological Effects of the Concurrent Administration of Excessive Heavy Metals in Drinking Water on the Rats Liver and Brain. Appl. Sci. 2021, 11, 5059. https://doi.org/10.3390/app11115059
Eldamaty HSE, Elbasiouny H, Elmoslemany AM, Abd El-Maoula LM, El-Desoky OI, Rehan M, Abd El Moneim D, Zedan A. Protective Effect of Wheat and Barley Grass Against the Acute Toxicological Effects of the Concurrent Administration of Excessive Heavy Metals in Drinking Water on the Rats Liver and Brain. Applied Sciences. 2021; 11(11):5059. https://doi.org/10.3390/app11115059
Chicago/Turabian StyleEldamaty, Hanan S.E., Heba Elbasiouny, Amira M. Elmoslemany, Lamiaa M. Abd El-Maoula, Ola Ibrahim El-Desoky, Medhat Rehan, Diaa Abd El Moneim, and Amina Zedan. 2021. "Protective Effect of Wheat and Barley Grass Against the Acute Toxicological Effects of the Concurrent Administration of Excessive Heavy Metals in Drinking Water on the Rats Liver and Brain" Applied Sciences 11, no. 11: 5059. https://doi.org/10.3390/app11115059
APA StyleEldamaty, H. S. E., Elbasiouny, H., Elmoslemany, A. M., Abd El-Maoula, L. M., El-Desoky, O. I., Rehan, M., Abd El Moneim, D., & Zedan, A. (2021). Protective Effect of Wheat and Barley Grass Against the Acute Toxicological Effects of the Concurrent Administration of Excessive Heavy Metals in Drinking Water on the Rats Liver and Brain. Applied Sciences, 11(11), 5059. https://doi.org/10.3390/app11115059







