Featured Application
A new and innovative steel-swarf-based electrode is proposed for the removal of heavy metal and total coliforms in sanitary landfill leachate.
Abstract
Sanitary landfill leachate (LL) composition varies according to climate variables variation, solid waste characteristics and composition, and landfill age. Leachate treatment is essentially carried out trough biological and physicochemical processes, which have showed variability in efficiency and appear a costly solution for the management authorities. Electrocoagulation (EC) seems a suitable solution for leachate treatment taking into account the characteristics of the liquor. One of the problems of EC is the electrode passivation, which affects the longevity of the process. One solution to this problem could be the replacement of the electrode by one made of recyclable material, which would make it possible to change it frequently and at a lower cost. The objective of the present work was to evaluate the removal of heavy metals (As, Ba, Cd, Cr, Cu, Fe, Pb, Mn, Ni, Se and Zn) and coliforms from a LL by EC using electrodes made from steel swarf (SfE) up to 8 h. Removal efficiencies of detected heavy metals were 51%(Cr), 59%(As), 71%(Cd), 72%(Zn), 92%(Ba), 95%(Ni) and >99%(Pb). The microbial load of coliforms in leachate was reduced from 10.76 × 104 CFU/mL (raw leachate) to less than 1 CFU/mL (after treatment with SfE) (i.e., approximately 100% reduction). The use of SfE in EC of LL is very effective in removing heavy metals and coliforms and can be used as alternative treatment solution for such effluents.
1. Introduction
Sanitary landfill leachate (LL) is a type of wastewater with high loads of organic and inorganic compounds, namely natural and synthetic organics, xenobiotics, ammonia, nitrate, pathogenic microorganisms, and heavy metals. The LL is composed of four different fractions: the natural humidity of the garbage, which increases in the rainy events; the aqueous constitution of organic matter, which is released during the decomposition processes; the bacteria in the trash, which secrete enzymes that dissolve organic matter with liquid formation; and from existing water sources in the area [1].
The leachate composition is very varied and heterogeneous, but generally contains [1,2,3]: High concentration of dissolved organic matter (as chemical oxygen demand (COD)), ammoniacal nitrogen (N-NH4+), nitrate nitrogen (N-N03−), chloride (Cl−), potassium (K+), sodium (Na+), calcium (Ca2+), iron (Fe2+), magnesium (Mg2+), manganese (Mn2+), sulfate (SO42−), hydrogen carbonate (HCO3−), heavy metals (lead (Pb2+), chromium (Cr3+) cadmium (Cd2+), nickel (Ni2+), copper (Cu2+) and zinc (Zn2+)) [3,4] and also xenobiotic organic compounds (e.g., phenols, aromatic hydrocarbons, pesticides [5], pharmaceutical compounds and personal use [6], antibiotics [7] and microplastics [8]).
The disposal of solid wastes in inappropriate places can cause significant negative impacts on soil and water bodies and public health problems, namely due to the groundwater and soil contamination with LL, and; therefore, the treatment of this effluent is necessary and important [4]. In general, the treatment of LL involves the use of biological processes (e.g., activated sludge, stabilization ponds and constructed wetlands) and physicochemical processes (e.g., coagulation, AOPs, ultrafiltration (UF) and reverse osmosis (RO)) [1,2,3,5,7]. However, the most used biological and physicochemical processes have showed variation in efficiency and high operation costs due to the use of UF and RO, and, additionally, the filtered material is returned to the landfill which will become a problem in the future [1,9].
Electrochemical processes such as electrocoagulation (EC) have proved to be a promising option in the treatment of various types of industrial effluents [10], for the removal of dyes [11], pesticides [12], heavy metals [13,14] and pharmaceutical compounds [15]. There still exist some studies on the use of conventional EC for leachate treatment [10,16]. Similar to other coagulation procedures, EC is based on sedimentation or flocculation of iron and aluminum multi-charged polynuclear complexes, with high adsorption properties. Nevertheless, in EC the coagulant ions, Fe3+ and Al3+ are electro generated by dissolution sacrifice anodes of stainless steel and aluminum, respectively [17,18]. The EC process is a relatively low-cost method, but the inherent metal lixiviation limits the durability of electrodes, thus increasing the operational cost [19]. An inexpensive solution for replacing the electrodes is to manufacture using industrial metallic residues (chips), since they have low cost and equal effectiveness to conventional electrodes [20]. Electrocoagulation with electrodes can be a lower cost option compared with UF and RO for removing heavy metals, micropollutants and pathogens. This technology can be used downstream of biological processes or physic-chemical processes for the removal of organic matter from LL.
Thus, the objective of this study was to investigate the use of electrodes obtained from metallurgical steel swarf (SfE) for the EC treatment of LL, in order to remove total coliforms and the following heavy metals: arsenic (As), barium (Ba), cadmium (Cd), chromium (Cr), cooper (Cu), iron (Fe), lead (Pb), manganese (Mn), nickel (Ni), selenium (Se) and zinc (Zn).
2. Materials and Methods
2.1. Electrode’s Production
The electrodes were manufactured using steel metallic residues from a local metallurgical industry that had more than 98% of iron in their composition. Steel was the chosen material, as it was the metal that showed the best treatment efficiency in a previous research work carried out by our group [20]. The preparation of filling electrodes of steel swarf (SfE) is presented in Figure 1.
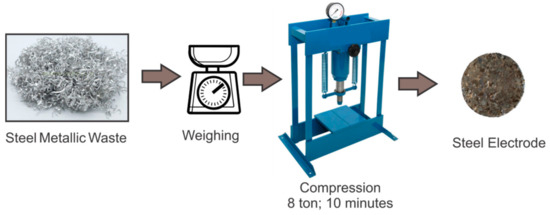
Figure 1.
Schematic preparation of steel-swarf-based (SfE) electrode.
The SfE was produced with suitable amounts of steel fillings, which was weighted, mixed and compressed in order to reach the same comparative dimensions, namely, 0.2 cm thickness and 2.5 cm diameter. Their final weigh was 2.5 g. The compression of 525.6 kgf cm−2 (8 ton) was carried out in a hydraulic press (Metal Técnica Bovenau, Brazil) for 10 min.
2.2. Electrocoagulation Experiments
The leachate was collected at a sanitary landfill located close the city of Goiânia (Brazil), using a previously cleaned container. The SfE were used as anode and cathode. The distance between the electrodes was 1 cm, as limited by the cell dimensions. The current was 0.1A and voltage of 5 V was applied by a DC power supply (HF-30035, Hikari, São Paulo, SP, Brazil).
An electrochemical cell with 250 mL capacity, containing 100 mL of LL for treatment was used. This small volume was used because it was a bench scale study that analyzed the behavior and efficiency of the designed electrodes. The experiments were carried out for 8 h, at room temperature (28 ± 2 °C). LL samples were collected at the beginning (raw LL) and after 8 h treatment for analysis of heavy metals (As, Ba, Cd, Cr, Cu, Fe, Pb, Mn, Ni, Se and Zn) and total coliforms. Total coliforms growth was also measured after 4 h of treatment. pH was measured for the raw and treated LL. The summary diagram of the electrocoagulation process is shown in Figure 2.

Figure 2.
Schematic of the experimental electrocoagulation process.
2.3. Analytical Methods
The analysis of heavy metals (As, Ba, Cd, Cr, Cu, Fe, Pb, Mn, Ni, Se and Zn) were performed using an ICP-OES spectrometer (Perkin-Elmer, Optima 8000, Waltham, MA, USA) following the Standard Methods [21].
Total coliforms were evaluated by pour plate method, using the MacConkey Agar (Scharlau®, Barcelona, Spain) as presented in the Standard Methods [9]. The volume of 1 mL of LL was placed separately in Petri dishes (in duplicate) and later the culture medium melted and cooled at 45 °C was placed. The entire procedure was carried out in a biological safety cabin. After the procedure, the plates were incubated in a bacteriological oven at 35–37 °C for 18–24 h for later counting of colony forming units (CFU/mL).
pH was measured through a pH meter PHS3-BW (BEL Engineering, Monza, Italy) before and after the EC treatment.
3. Results and Discussion
The results on heavy metals are shown in Table 1, as well as the standard limits for discharging treated LL in water bodies [22,23], reuse for irrigation [23] and discharging in municipal sewers [24]. pH for the raw and treated LL were 8.3 and 8.93, respectively.

Table 1.
Heavy metals concentration in the raw and treated landfill leachate (LL).
A very good removal efficiency (greater than 51%) can be observed for all the eight detected heavy metals (As, Ba, Cd, Cr, Fe, Pb, Ni and Zn). The metals Cu, Mn and Se were not detected either in the raw LL or in the treated LL. Therefore, there was not an increase in the concentration of these three metals in the treated LL, because it is known that EC can promote the electro-dissolution of the electrode used in the process.
The results show that the treated LL has a quality in agreement with several heavy metals standard limits for discharging in water bodies or irrigation. However, it is not suitable for discharging in public sewers due to higher values for Cr, Fe and Ni.
The research has evaluated the possibility of using treated LL in agriculture, since it is an effluent with a high concentration of ammonia [1,2,25]. The results of our research pointed out that the irrigation practice with treated LL, obtained through EC with SfE, would bring positive environmental advantages since it would reduce the use of water from surface waters and groundwater for the irrigation of green spaces, such as football fields, squares and gardens.
Some studies have analyzed the removal of heavy metals by EC, evaluating parameters such as pH, conductivity and electric current. Although the present study does not aim to analyze these parameters, but the behavior of electrodes produced from metallic residues, some correlations were observed between the cited parameters and the results obtained. A study that used an EC with Fe/Al electrodes for the removal of ions Cr3+, Cu2+, Ni2+ and Zn2+ showed a removal efficiency above 90% for all metals, in the pH range of 7.89 to 9.56 [26]. Therefore, for the pH range observed in this study (8.3 to 8.93), the removal efficiency of Cr and Zn was lower than the obtained by [26], but it was similar for Ni.
The influence of EC on the microbial load of total coliforms was analyzed and is present in Figure 3 and the growth of total coliforms in Petri dishes is shown in Figure 4.
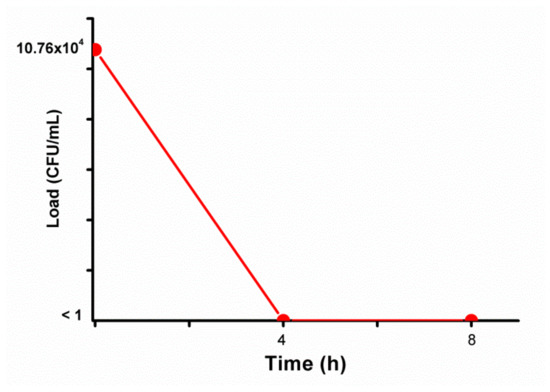
Figure 3.
Microbial load after electrocoagulation (EC) experiments using electrodes of steel swarf (SfE).
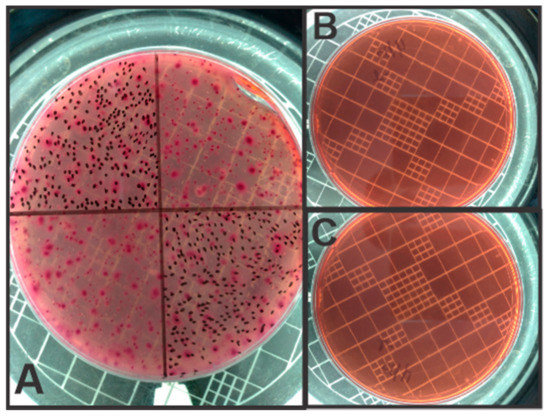
Figure 4.
Microbial load of LL: (A) Raw LL; (B) after 4 h of treatment and (C) after 8 h of treatment.
The initial coliform load of the LL was 10.76 × 104 CFU/mL, and, after EC treatment using SfE, that load was completely eliminated in the time of 4 h (<1 CFU/mL in the treated LL). Similar results were found in other studies [27,28]. Research [28] analyzed the influence of E. coli cell density in different treatment technologies applying electro-Fenton, electro-peroxi-coagulation and EC. Regarding the EC technology, these authors observed that the higher the microbial cell density (microbial load), the longer it takes to completely inactivate the microorganisms and, the higher is the current applied for a good inactivation.
In the present work, the applied current was 0.1A and it was enough for the almost total coliform inactivation in LL in 4 h treatment with SfE. Therefore, it seems this current and 4 h of contact time is enough to destroy the cytoplasm of the microorganisms present in LL, which makes this sensor very competitive in relation to other disinfection technologies, such as chlorination, with the additional advantage of not leaving by-products in the water. The exposure of a biological cell to an electric field can produce a wide variety of biochemical and physiological responses, and most of these responses are based on the modification of the membrane potential by the influence of the external electric field. If the strength of this electric field exceeds a certain limit (0.2–1 V), it results in the formation of pores in the plasma membrane, a phenomenon called electro-permeabilization or electroporation [29,30].
Another fact that contributes to the elimination of these microorganisms in an indirect way is the contact between bacteria and oxidants generated during the process and/or the adhesion of these microorganisms to the generated coagulants and consequent removal of the effluent by flotation or sedimentation. This phenomenon has been explained by other authors by observing live bacteria found in solids decanted throughout the EC process [29]. The results suggest that modifying parameters such as applied current, electrode size and treatment time the removal efficiency can be improved.
Therefore, taking into account the great efficiency of the EC process with SfE in the removal of heavy metals and coliforms from LL, as well as the need in valorizing metallic residues, the proposed SfE it arises as promising alternative for the treatment of such wastewaters. This solution could also result in lower operation costs in LL treatment plants in comparison with chemical coagulation along with UF and RO technologies that present highly operation costs.
4. Conclusions
The use of filling wastes from metallurgy in the manufacture of electrodes for electrocoagulation of LL was performed for the first time. The new electrodes produced from metallic residues were able to remove from 51% to 95% of heavy metals, a removal efficiency that can be improved with the modification of parameters such as applied current, electrode size and treatment time. The new steel-swarf-based electrode was able to remove 100% of the thermotolerant coliforms present in LL, for contact times between 4 and 8 h, showing that it can be very competitive in relation to other disinfection technologies. Therefore, metallic waste from metallurgic industries or machining facilities can be used for producing innovative and efficient electrodes for EC purposes, especially for removing heavy metals and thermotolerant coliforms from LL. Moreover, this sensor is a green technology and it seems an economical solution for industrial metal waste recycling.
Author Contributions
Conceptualization of new steel-swarf-based electrode was carried out by M.T.d.O., I.M.S.T. and E.d.S.G. The methodology and methods for electrode production were carried out by M.T.d.O., I.M.S.T. and E.d.S.G., whilst the characterization and execution of assay for heavy metal and total coliform characterization were defined by H.R., A.A. and P.S. Results presentation was in charge of M.T.d.O., I.M.S.T. and E.d.S.G. and data analysis, discussion and conclusions were carried out by all authors. All authors have read and agreed to the published version of the manuscript.
Funding
We acknowledge Capes and Fapeg for the funding students grants, CNPq for funding the research and Cassiano Pacheco from Aqualit S/A for the ICP-OES analysis.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The study has the support of the projects UIDB/00195/2020 (FibEnTech) and UIDB/04035/2020 (GeoBioTec), both funded by the Fundação para a Ciencia e Tecnologia (FCT).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fernandes, A.; Ciríaco, L.; Pacheco, M.J.; Lopes, A.; Albuquerque, A. Diagnosis and assessment of the management of sanitary landfill leachates in Portugal. In Proceedings of the WWASTES–Solutions, Treatments and Opportunities II: Selected Papers from the 4th Edition of the International Conference on Wastes: Solutions, Treatments and Opportunities, Porto, Portugal, 25–26 September 2017; CRC Press/Balkema: Boca Raton, FL, USA, 2018; pp. 87–92. [Google Scholar]
- Białowiec, A.; Davies, L.; Albuquerque, A.; Randerson, P.F. Nitrogen removal from landfill leachate in constructed wetlands with reed and willow: Redox potential in the root zone. J. Environ. Manag. 2012, 97, 22–27. [Google Scholar] [CrossRef]
- Costa, A.M.; Alfaia, R.G.; Campos, J.C. Landfill leachate treatment in Brazil—An overview. J. Environ. Manag. 2019, 232, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhu, X.; Chen, N.; Feng, C.; Wang, H.; Kuang, P.; Hu, W. Review on electrochemical system for landfill leachate treatment: Performance, mechanism, application, shortcoming, and improvement scheme. Sci. Total Environ. 2020, 745, 140768. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Duan, X.; Cao, G.; Zhu, S.; Ho, S.H. Graphitic nitride-catalyzed advanced oxidation processes (AOPs) for landfill leachate treatment: A mini review. Process Saf. Environ. Prot. 2020, 139, 230–240. [Google Scholar] [CrossRef]
- Yu, X.; Sui, Q.; Lyu, S.; Zhao, W.; Cao, X.; Wang, J.; Yu, G. Do high levels of PPCPs in landfill leachates influence the water environment in the vicinity of landfills? A case study of the largest landfill in China. Environ. Int. 2020, 135, 105404. [Google Scholar] [CrossRef]
- Yi, X.; Tran, N.H.; Yin, T.; He, Y.; Gin, K.Y.H. Removal of selected PPCPs, EDCs, and antibiotic resistance genes in landfill leachate by a full-scale constructed wetlands system. Water Res. 2017, 121, 46–60. [Google Scholar] [CrossRef]
- He, P.; Chen, L.; Shao, L.; Zhang, H.; Lü, F. Municipal solid waste (MSW) landfill: A source of microplastics? -Evidence of microplastics in landfill leachate. Water Res. 2019, 159, 38–45. [Google Scholar] [CrossRef]
- Košutić, K.; Dolar, D.; Strmecky, T. Treatment of landfill leachate by membrane processes of nanofiltration and reverse osmosis. Desalination Water Treat. 2015, 55, 2680–2689. [Google Scholar] [CrossRef]
- Shahedi, A.; Darban, A.K.; Taghipour, F.; Jamshidi-Zanjani, A. A review on industrial wastewater treatment via electrocoagulation processes. Curr. Opin. Electrochem. 2020, 22, 154–169. [Google Scholar] [CrossRef]
- Akhtar, A.; Aslam, Z.; Asghar, A.; Bello, M.M.; Raman, A.A.A. Electrocoagulation of Congo Red dye-containing wastewater: Optimization of operational parameters and process mechanism. J. Environ. Chem. Eng. 2020, 8, 104055. [Google Scholar] [CrossRef]
- Nasser Ghalwa, M.A.; Nader Farhat, B. Removal of Imidacloprid Pesticide by Electrocoagulation Process using Iron and aluminum Electrodes. J. Environ. Anal. Chem. 2015, 2, 1000154. [Google Scholar]
- Mansoorian, H.J.; Mahvi, A.H.; Jafari, A.J. Removal of lead and zinc from battery industry wastewater using electrocoagulation process: Influence of direct and alternating current by using iron and stainless steel rod electrodes. Sep. Purif. Technol. 2014, 135, 165–175. [Google Scholar] [CrossRef]
- El-Ashtoukhy, E.S.Z.; Amin, N.K.; Fouad, Y.O.; Hamad, H.A. Intensification of a new electrocoagulation system characterized by minimum energy consumption and maximum removal efficiency of heavy metals from simulated wastewater. Chem. Eng. Process. Process Intensif. 2020, 154, 108026. [Google Scholar] [CrossRef]
- Zaied, B.K.; Rashid, M.; Nasrullah, M.; Zularisam, A.W.; Pant, D.; Singh, L. A comprehensive review on contaminants removal from pharmaceutical wastewater by electrocoagulation process. Sci. Total Environ. 2020, 726, 138095. [Google Scholar] [CrossRef]
- Galvão, N.; de Souza, J.B.; de Sousa Vidal, C.M. Landfill leachate treatment by electrocoagulation: Effects of current density and electrolysis time. J. Environ. Chem. Eng. 2020, 8, 104368. [Google Scholar] [CrossRef]
- Alinsafi, A.; Khemis, M.; Pons, M.N.; Leclerc, J.P.; Yaacoubi, A.; Benhammou, A.; Nejmeddine, A. Electro-coagulation of reactive textile dyes and textile wastewater. Chem. Eng. Process. Process Intensif. 2005, 44, 461–470. [Google Scholar] [CrossRef]
- Moussa, D.T.; El-Naas, M.H.; Nasser, M.; Al-Marri, M.J. A comprehensive review of electrocoagulation for water treatment: Potentials and challenges. J. Environ. Manag. 2017, 186, 24–41. [Google Scholar] [CrossRef]
- Kobya, M.; Ozyonar, F.; Demirbas, E.; Sik, E.; Oncel, M.S. Arsenic removal from groundwater of Sivas-Şarkişla Plain, Turkey by electrocoagulation process: Comparing with iron plate and ball electrodes. J. Environ. Chem. Eng. 2015, 3, 1096–1106. [Google Scholar] [CrossRef]
- Oliveira, M.T.; Garcia, L.F.; Siqueira, A.C.R.; Somerset, V.; Gil, E.S. Electrocoagulation of the indigo carmine dye using electrodes produced from the compression of metallurgical filing wastes. Int. J. Environ. Sci. Technol. 2020, 17, 1657–1662. [Google Scholar] [CrossRef]
- American Public Health Association; American Waterworks Association; Water Environmental Federation. Standard Methods for the Examination of Water and Wastewater, 19th ed.; APHA: Washington, DC, USA, 1999. [Google Scholar]
- Conselho Nacional do Meio Ambiente Resolução No 430. Dispõe Sobre as Condições e Padrões de Lançamento de Efluentes; Diário Oficial da União: Brasilia, Brasil, 2011. [Google Scholar]
- Kinuthia, G.K.; Ngure, V.; Beti, D.; Lugalia, R.; Wangila, A.; Kamau, L. Levels of heavy metals in wastewater and soil samples from open drainage channels in Nairobi, Kenya: Community health implication. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Al-Musharafi, S.K.; Mahmoud, I.Y.; Al-Bahry, S.N. Heavy Metal Pollution from Treated Sewage Effluent. APCBEE Procedia 2013, 5, 344–348. [Google Scholar] [CrossRef]
- Rad, H.A.; Ziri, M.S.; Babaei, L. Investigation of landfill leachate treatability for reuse in agricultural purposes. Water Pract. Technol. 2017, 12, 224–233. [Google Scholar]
- Kim, T.; Kim, T.K.; Zoh, K.D. Removal mechanism of heavy metal (Cu, Ni, Zn, and Cr) in the presence of cyanide during electrocoagulation using Fe and Al electrodes. J. Water Process Eng. 2020, 33, 101109. [Google Scholar] [CrossRef]
- Hashim, K.S.; Ali, S.S.M.; AlRifaie, J.K.; Kot, P.; Shaw, A.; Al Khaddar, R.; Idowu, I.; Gkantou, M. Escherichia coli inactivation using a hybrid ultrasonic–electrocoagulation reactor. Chemosphere 2020, 247, 125868. [Google Scholar] [CrossRef] [PubMed]
- Kourdali, S.; Badis, A.; Boucherit, A.; Boudjema, K.; Saiba, A. Electrochemical disinfection of bacterial contamination: Effectiveness and modeling study of E. coli inactivation by electro-Fenton, electro-peroxi-coagulation and electrocoagulation. J. Environ. Manag. 2018, 226, 106–119. [Google Scholar] [CrossRef]
- Ricordel, C.; Miramon, C.; Hadjiev, D.; Darchen, A. Investigations of the mechanism and efficiency of bacteria abatement during electrocoagulation using aluminum electrode. Desalination Water Treat. 2014, 52, 5380–5389. [Google Scholar] [CrossRef]
- Wei, V.; Elektorowicz, M.; Oleszkiewicz, J.A. Influence of electric current on bacterial viability in wastewater treatment. Water Res. 2011, 45, 5058–5062. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).