3.3. Elements in Ash and Deposit
The behaviour of trace elements during combustion depends on their concentration, the form of occurrence, and how they are bonded to the organic or inorganic components of the coal and the combustion conditions [
61]. Elements bonded in organic matter are vaporised and subsequently escape into the atmosphere or are adsorbed on small particles of ash during combustion in the boiler. Most elements that have a higher affinity for the inorganic matrix are usually non-volatile and tend to stay in the ash or fly ash [
62].
Vassilev et al. [
43] sorted the elements by the concentration of ash-forming elements in plant ash (141 biomass varieties) in the following order: O > Ca > K > Si > Mg > Al > Cl > P > Fe > Na > S > Mn > Ti. Elements Ca, Cl, K, Mg, Mn, Na, P, and S in ash from natural biomass normally show higher contents than the respective values for coal ashes, while Al, Fe, Si, and Ti show significantly lower values [
43].
Based on the concentrations of major elements in ash from WM briquette combustion, the elements show a similar arrangement of Ca >> Si = K > Fe > Al > Mn > S > P > Ti as elements in ash from plant ash [
43]. The series of trace elements in ash from WM burning is as follows, according to trace element concentration: Zn > Cl > Cu > Pb > Ni = Cr = As. Calcium levels in ash are up to five times higher than those of Si, a ratio that is maintained in deposits. The ash from WM has a significantly higher Fe content than plant ash [
43]. Differences in Fe content are already evident in WM briquettes, which contain up to 15 times more Fe than wood biomass, according to Jensen et al. [
63]. In addition, Si has about nine times higher content in WM, and Al up to twenty times higher content than Jensen et al. [
63] reported for wood. Increased Fe, Al, and Si concentrations may be related to the brushing of furniture wood. Garnet, aluminium oxide, silicon carbide and alumina-zirconia are used as abrasives.
When comparing the concentrations of elements in fuel (
Table 7) and ash, the position of P has changed because it is highly volatile. The order of trace elements in ash has remained unchanged compared to the input fuel.
3.4. Release of Elements into the Atmosphere during Combustion
The amounts of element release were quantified by a mass balance based on weight measurements and chemical analysis of the fuel and ash obtained, similarly to Sommersacher et al. [
64]. The calculation was made on the basis of data on the total amount of fuel burned and the concentrations of elements in the fuel, the amounts of by-products of combustion, and the concentrations of elements captured in deposits and ash. The results, expressed as the percentage of the element released into the atmosphere from the burnt fuel, are highly relevant to the assessment of the environmental performance of domestic boilers (
Figure 3). The amounts of elements captured in ash are shown in
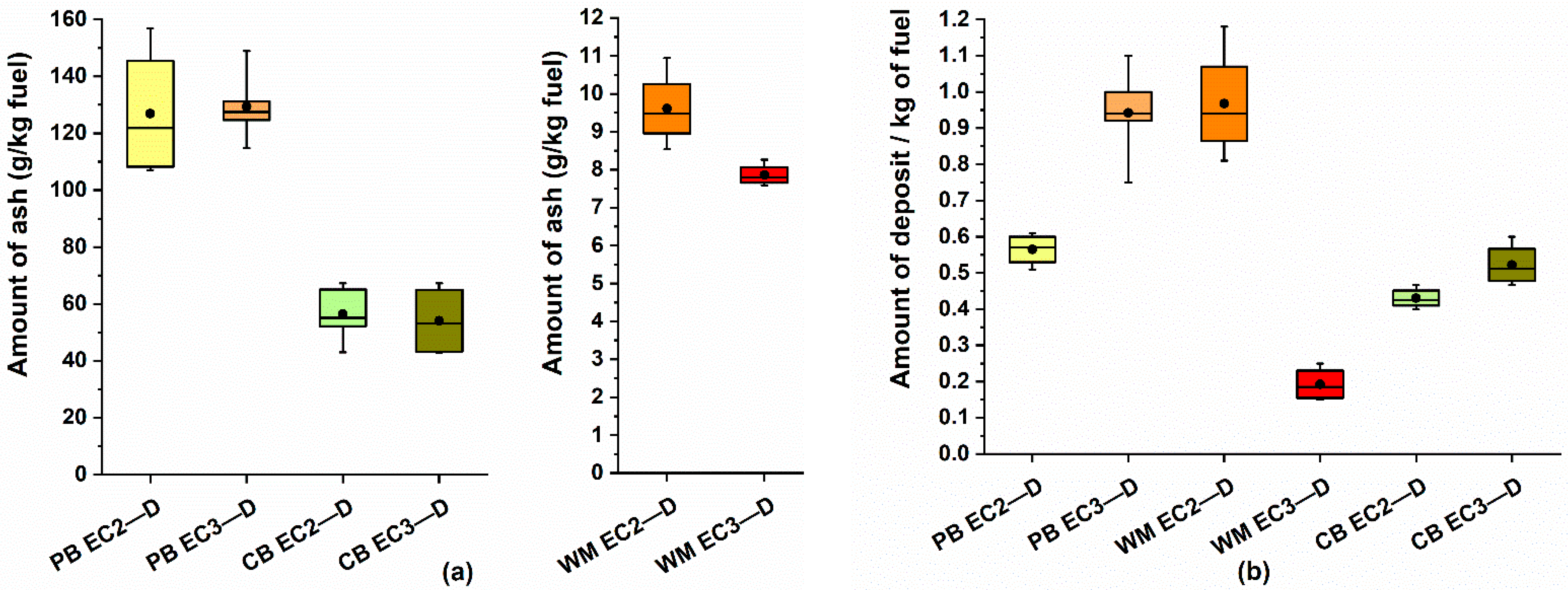
Table 8. Due to the small production and collection of particles in deposits (
Table 2), the amounts of the captured elements are low. For PB and CB, the amounts of elements captured are <1% except for Cl and Zn (2.3%) for CB. In the case of WM, the production of deposits per kg/fuel was higher (8–10 g/kg), and the capture of elements is more significant, ranging between 10–15%.
The binding of elements in ash and their release into the atmosphere is primarily influenced by the form of the element in the fuel, which is probably more significant in terms of their release into the environment than the technological solution of domestic boilers.
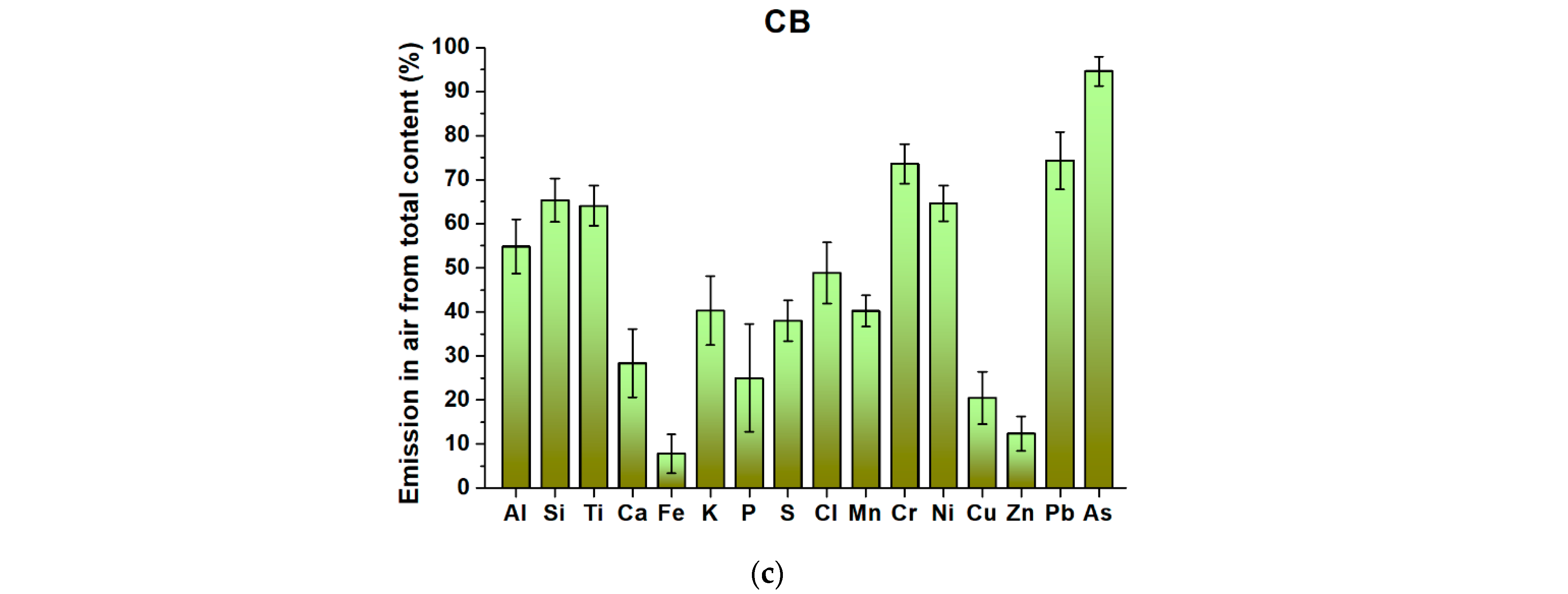
The most significant amounts of the major elements Al, Si, Ti, and P are released into the atmosphere from WM. The largest amounts of Ca, Fe, S, K, and Cl are released into the atmosphere from PB. Major elements are released in the lowest amounts from CB (
Figure 3). Of the trace elements, the most significant amounts of Ni. The largest amounts of Cu and Zn are released from WM. The largest amounts of Mn and Pb are released from paper briquettes (PB). The largest amounts of As and Cr are released from CB. These results correspond to the mass balances from the joint evaluation of the two boilers, which were similar for both BEC2 and BEC3. Cases with a difference in the amounts of elements released into the atmosphere for the compared boilers > 20% are shown in
Table 9.
The higher amount of Al released from the BEC3 boiler is probably related to the higher temperature that allows the decomposition of aluminium sulfate used in the production of surface treatment paper, which decomposes at 770–860 °C [
65]. Manganese (Mn) in biomass occurs as positive metal ions, which can be in the form of free ions or complexes. Metal ions may also be organically associated with biomass fibres [
52] and can only be released into the air when they burn out.
The higher amount of Ca released from the BEC2 boiler during WM combustion is related to the degradation of calcium oxalate crystals [
66] and the subsequent reaction of Ca ions with CO
2 to form calcite at >479 °C. Calcite decomposes at a temperature higher than 750 °C [
67] for the creation of CaO. CaO has not been identified in ash and deposit products from BEC2 and BEC3 by the X-ray-diffraction method. The mineralogical analysis of ash shows that in the case of ash and deposits from BEC3, a greater amount of Ca is bound in an amorphous phase, which was formed at a higher temperature than was present in the BEC2 boiler. A surplus of Ca ions was released into the atmosphere. Wood preservatives containing As (arsenate) break down in the temperature range from 750–800 °C during de-arsenation [
68]. The amount of sulfur in the BEC2 and BEC3 boiler emissions is affected by the thermal degradation of thioxanthene, which has been identified in the deposit products and in the input material by the Py-GC/MS method. Thioxanthene is a pyrolysis product formed by the thermal degradation of the thiophenol/formaldehyde resins [
69] contained in WM. In addition to thioxanthene, other sulfur-containing organic compounds have been identified in the deposits from BEC2 from WM combustion—thiourea, tetramethyl thiourea, 3 methyl thiophene and dihydro 2,3H thiophenone, which are also produced during the thermal degradation of thiophenol/formaldehyde resins. For example, thiourea starts to isomerize to ammonium thiocyanate (NH
4SCN) at temperatures ranging from 140 to 180 °C [
70]. The absence of these compounds in deposits from BEC3 explains the degradation of sulfur-containing organic compounds in the BEC3 boiler, and hence the higher sulfur emissions.
Results of the mineralogical analysis were also used to identify the differences between element release during the combustion of CB in BEC2 and BEC3. The results indicate that the amounts of maghemite in deposits from BEC2 (13.45%) and BEC3 (20.45%) vary significantly. In addition, hematite is also present in BEC3 (8.68%). Maghemite levels in ash from the combustion in both BEC2 and BEC3 are comparable (14.55 and 16.31%). Generally, higher amounts of Fe are bound in deposits from BEC3 in mineral Fe-phases than in BEC2. During combustion, iron distribution into individual phases—hematite, magnetite and Fe-glass phases—is affected by the O
2/CO
2 ratio [
71]. With a higher O
2/CO
2 concentration, the proportion of magnetite increases and hematite decreases, while the amount of Fe-glass phase does not change. The amount of unburned carbon affects oxidation processes involving the formation of magnetite, maghemite, and hematite. After the complete burning of carbon, the magnetite present in the ash becomes completely oxidised to hematite [
72]. Part of hematite and magnetite (approximately 8% of Fe) crystallises from the molten glass phases during combustion and is not significantly affected by changing combustion conditions [
71]. Some trace elements are bonded to newly formed Fe-phases when coal is burned. For the deposits from BEC2 and BEC3, a polynomial dependence between Fe and Cu concentration was confirmed (r = 0.84,
n = 9, α = 0.005). Adsorption of heavy metals on the surface of iron oxides (mainly magnetite) in preferential order of Pb, Zn, Cu, Cr, and Cd or their substitution in minerals with spinel structure is known [
73]. In the case of Zn, correlation between Zn and Ca has been demonstrated (r = 0.81,
n = 9, α = 0.01).
Differences in the behaviour of both major and trace elements during the combustion in BEC2 and BEC3 are primarily due to the presence of elements in the input fuel and the decomposition ability of phases in which both major and trace elements are bound and influenced by temperature and oxidation-reduction conditions.
In recalculation on the element content of the input fuel, up to twice as many major elements (55 g/kg) were released from PB during combustion compared to WM (28 g/kg fuel for BEC2 and 19 g/kg for BEC3) and CB (BEC2 21 g/kg and BEC3 23 g/kg). Calcium (up to 37 g/kg of fuel) accounts for the major proportion of elements released when PB is burned (
Figure 4). The concentration of emitted elements when burning PB decreases in the following order: Si > Al > Ca > Ti > S > K = Fe > P, and for trace elements: Mn > Cl > Zn > Cu > Pb > Ni = Cr = As. The high sulphur emissions from burning PB are due to the presence of organically bound sulfur, which is part of lignin sulfonate, a by-product of the sulfite pulping process [
74] used to glue cartons [
75]. The thermal stability of sulfonic groups is rather weak, as indicated by the low-temperature evolution of SO
2. The decomposition is done in two stages at a temperature of between 120 and 360 °C, and the lignin component [
76] decomposes from 360 to 700 °C. Cations (Na
+ and NH
4+) bonded to the sulphonic groups strongly affect the decomposition of lignosulphonates. In the case of sodium sulfonate, sulphur is bound to Na
2SO
3, or Na
2SO
4 and sulfides are formed under reduction conditions. The sulfonic group with NH
4+ releases NH
3 at 270 °C, breaking down the sulfonate group into SO
2 [
76].
The comparison of individual fuels shows that when burning PB, 1 kg of fuel releases the highest amount of trace elements 630–830 mg/kg (
Figure 5). The value is affected by a high concentration of Mn. Other monitored elements (As, Cu, Cr, Ni, Pb, and Zn are emitted at a concentration of 121–128 mg/kg, which is lower than WM combustion emissions. In the case of printed cartons, the content of other elements of Sn and Sb is also increased in the flue gas [
77]. The amounts of trace elements released when burning waste office paper and corrugated cardboard increases with temperature [
78]. When burning waste paper at 750–950 °C, manganese (68–205 mg/kg of fuel) and lead (38–54 mg/kg of fuel) pass into flue gas [
78]. In our case, the amount of Mn released into the air was about twice as high, with input paper briquettes containing Mn in concentrations up to 520 mg/kg. The combustion in the EC2 and EC3 boiler released Mn in amounts of 300 to 470 mg/kg of fuel and Pb in amounts of 18 to 20 mg/kg of fuel. Lead and zinc have a lower volatility of Zn during the combustion of waste paper at a lower rate of combustion. The behaviour of Cu is not affected by the rate of combustion [
79]. When burning WM in BEC2 at the fuel ratios 2.5 kg/h and 4 kg fuel/h (BEC3), the differences in Cu, Pb, and Zn emitted were not significant.
When burning WM, the series of released elements is P > Si > Ca > Al > Fe > K > S > Ti, and for trace elements: Mn > Cl > Zn > Cu > Ni > Pb > Ni = Cr = As. Phosphorus was released in the highest amounts; it can be completely released as gaseous phosphorus oxides during the combustion process when it is organically bound due to the high volatility of organic phosphorus compounds [
80,
81]. Potassium release into the air from burning waste wood was studied by Sommersacher et al. [
64], who found lower amounts of K release for waste wood (18.5 ± 3.2%), hardwood chips (32.8 ± 12.0%), and softwood chips (24.9 ± 8.1%) than was demonstrated for the PB burning (54–56%). The contamination caused by the presence of soil particles in biomass in the feedstock also affects potassium behaviour during the combustion process of compressed wood mass, where K is bound in silicate minerals, and thus the amount of produced K
2SO
4 is decreased, resulting in an increase in SO
x emissions [
54].
According to decreasing concentrations, the different concentration series of major elements released into the atmosphere was found during burning CB. Due to silicates contained in the coal briquettes, the order of elements is as follows: Si > Al > Ca > Ti > S > K = Fe > P, for trace elements Mn > Cl > Cr > Ni > Cu = Zn = Pb = As. The combustion of coal briquettes emits the lowest amounts of major elements (21–23 g/kg), and for trace elements 204–215 mg/kg (with Mn), and about 58 mg/kg without Mn. In terms of both major and trace element emissions, coal briquettes are a “cleaner” fuel than other fuels using waste products.
3.5. Relative Enrichment Factor Elements in Ash and Deposits
It is known from the literature that in the ash from coalburning, the concentration of trace elements increases with decreasing particle size, which is influenced by the specific surface and sorption processes [
59,
82]. Similar relationships are known for major elements (Ca, P, Mg, and Si) in ash from biomass combustion but for K, the opposite has been shown [
43].
The highest amounts of fine-grained particles are present in samples of WM-D deposits, followed by PB-D (
Figure 6). The comparison of the mean grain size (d
050) for WM-D (0.114 mm) and WM-A (0.265 mm) shows that the WM ash particles have a coarser character. PB combustion products show a similar trend. The mean particle size (d
050) for PB-D is 0.122 mm, and for PB-A, it is 0.249 mm. A completely different shape of particle size distribution curves was found for deposits from CB combustion (
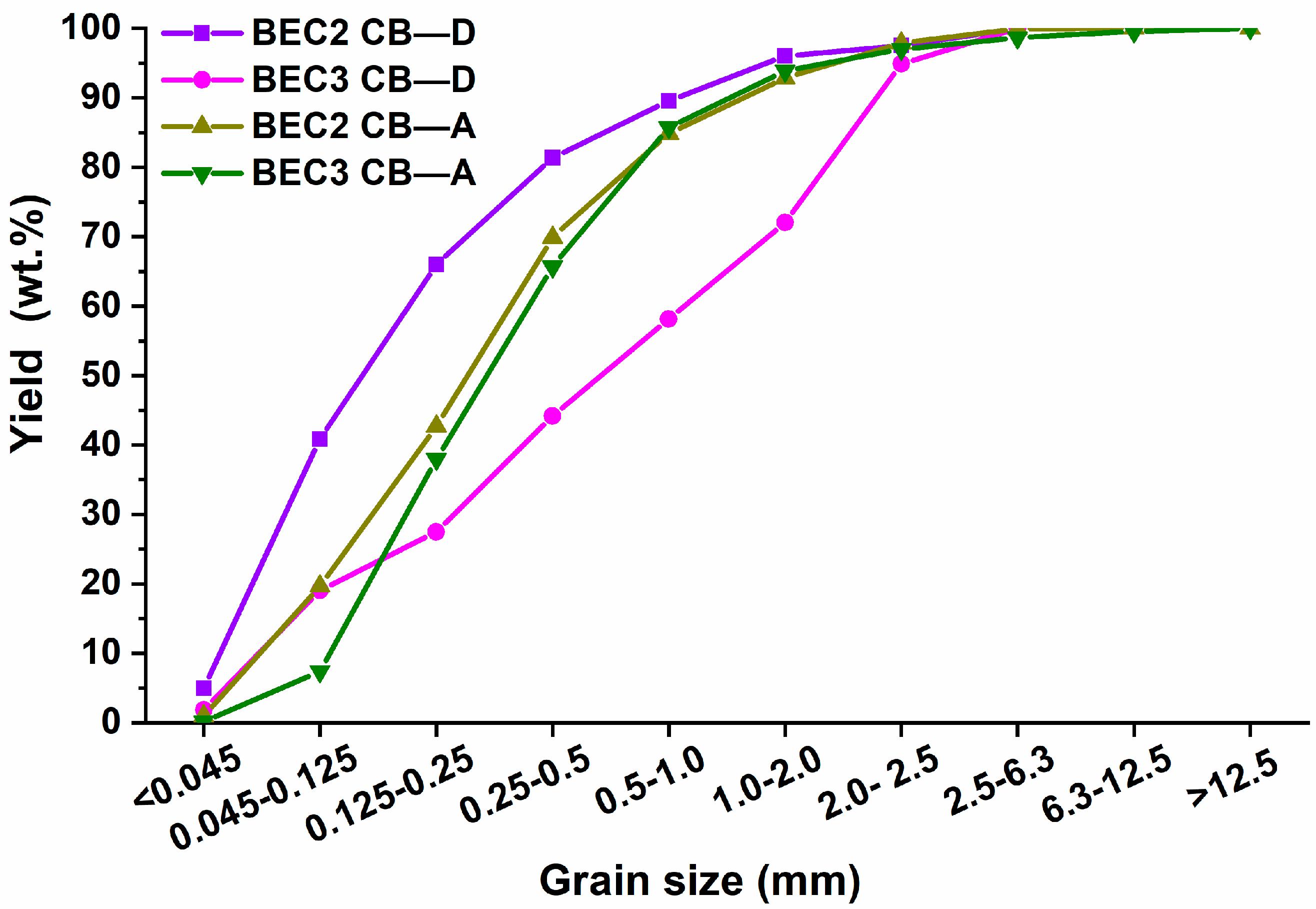
Figure 7), where BEC3 CB-D has a mean grain value of 0.71 mm (specked particles are present), while BEC2 CB-D has a mean grain size value of 0.42 mm. The shapes of the ash grain curves are almost identical; the mean particle size for BEC2 CB-A is 0.254 mm, and for BEC3 CB-A, it is 0.287 mm.
The average size of the particles that make up the deposits is significantly smaller than the ash particles except for BEC3 CB-D. Therefore, it can be assumed that there could be enrichment by both major and trace elements, even in this case. The relative enrichment factor (RE) defined by Meij [
83] was used to monitor the acquisition of by-products of combustion. Meij [
83] classified trace elements contained in coal on the basis of the relationship between the temperature and degree of volatilisation during the combustion in large energy sources (
Table 10).
During the combustion of PB, higher values of RE were found for ash (except S, Mn, and Cl). When burning CB, major elements Al, Si, Ti, and Fe, and selected trace elements Cr, Ni and Cu have higher values of RE in ash, while cations Ca and K and trace elements Pb, Zn, and As have higher values of RE in deposits (
Figure 8). Completely different results were obtained when burning WM. All monitored elements (both major and trace) have a higher relative enrichment factor RE in deposits (
Figure 9). The highest values were found for Pb and Zn.
Some regularities resulting from the behaviour of elements during the burning of coal [
83] cannot be used to identify unauthorised fuels burned in domestic boilers for solid fuels, as documented by the results obtained from the burning of paper briquettes. In the case of PB, all major elements and most trace elements (except Cu) have RE < 0.7, placing them among the volatile elements. In fuels where elements occur bound in the form of salts, they are released into the atmosphere during combustion, which reduces their concentration in the by-products of combustion, and they cannot be used to identify the fuel originally burned. However, identification of unauthorised fuels on the basis of the presence of organic compounds in deposits is possible [
26].
The ratios of the mean concentration values of elements in ash and deposits are shown in
Figure 10. Ratio values > 1 were found for Al, Si, Ca, Ti, Cr, Ni, Cu, and Fe, Mn (CB and WM only), and P (PB and CB only). The values of A/D concentration ratio > 1 confirm that the element is preferentially bound to ash. The other elements (S, K, Zn, Pb, Cl, and As) have a ratio value of < 1 for all fuels due to their preferred concentration in deposits. The results confirmed the higher volatility of trace elements Zn, Pb and As.
3.6. The Influence of Principles of the Circular Economy for Residential Heating Fuels
Primarily, the aim of the circular economy (CE) is to protect primary raw material resources, including wood. CE is a regenerative method in which raw material input, waste generation, emissions, and energy are minimized by promoting the circularity of material and energy achieved by repair, reuse, remanufacture, refurbishing, and recycling [
84]. The use of wood residues for energy is a natural part of the circular economy of the forest and wood industry. The partial replacement of wood or wood by-products (sawdust, shavings) with wood-based waste paper and cardboard could make a significant contribution to saving primary raw materials, especially in areas where waste paper and cardboard cannot be reused for the paper industry. At the same time, this option reduces the proportion of waste dumped ineffectively in landfills.
With the addition of 50% cardboard to wood chips, the price of the fuel input drops by 50% [
85]. A similar price drop in the use of cardboard for briquette production has not been confirmed in the Czech Republic, and in the production of mono-fuel from cardboard, the price is even higher than for wood briquettes or sawmill briquettes. The price of briquettes made from sawdust and kindling from the production of wood (WOOD briquettes) in the Czech Republic is EUR 2.20/10 kg, and the price of “MIXI” briquettes made with the addition of 20% pure cardboard paper is EUR 1.92/10 kg. The price difference is only 13% [
86]. NIGHT briquettes are only made from waste cardboard, are the most expensive (EUR 2.71/10 kg), and have the worst thermal parameters. WOOD briquettes have the lowest ash content of <1.47% and the best higher heating value of 18.8 MJ/kg, MIXI briquettes contain <6.5% ash, and their higher heating value reaches 16.5 MJ/kg, and NIGHT briquettes have the highest ash content of 13.04% and a higher heating value of 15 MJ/kg. The use of “NIGHT” briquettes is specifically targeted at citizens who “want to be environmentally friendly”. The prices of briquettes on the market in the Czech Republic are highly variable. Novak & Novak Eko Co. (Česká Skalice, the Czech Republic) supplies briquette “beech cubes” at a price of EUR 1.62/10 kg, and briquettes from deciduous trees at EUR 1.42/10 kg. Moreover, the price of briquettes changes significantly during the year. It is about 20% lower in the summer months. The price for 10 kg of EN-certified pellets plus A1 ranges from EUR 2.1–2.3/10 kg for one of the largest producers in the Czech Republic (BIOMAC Co.) and between EUR 1.8–2.0/10 kg for non-certified pellets [
87]. The prices of pellets and briquettes are comparable. Their overproduction influences the price of both pellets and briquettes in the Czech Republic. In 2016, a total of 300,000 tonnes of pellets were produced, and 25,000 pellet boilers were put into operation. In 2020, forty factories in the Czech Republic produced 486,000 tonnes of pellets, and 40,000 pellet boilers were in operation. The greater part (67%) of their annual production goes to exports. Almost all (96%) of the pellets produced are EN plus A1-certified internationally [
88]. Another important factor is the low price—the average price of wood pellets on the Ukrainian market is EUR 80–100, while the prices of this product in the European Union countries vary from EUR 180 to 220 for a tonne.
While there is an increasing pressure to use RES (Renewable Energy Sources) for residential combustion across Europe, the market price trends did not foresee any significant increase [
89]. Prices fell between 2012 and 2016 from USD 208 to 190, and then rose slightly to USD 200 in 2018. A gradual increase in the cost of industrial wood pellets is expected to be likely, reaching above USD 250 per million tonnes by 2030.
The market situation was influenced by the global COVID-19 pandemic for almost all commodities, such as lumber, where prices have increased by up to 200% over the past three months. Compared to the situation before the pandemic, COVID-19 lumber prices are up a staggering 188% [
90]. The price increase for lumber and wood products was also demonstrated by the Producer Price Index used in the USA, which rose to 400 (in 1982, the index value was set at 100), compared to 200 in 2015 [
91].
However, many factors play a role in the choice of heating, and the price is one of several significant factors. Recently, the importance of circular economics principles has been increasing. The increasing demand for the use of chipboard panels for construction purposes will increase the pressure to recycle them, although this depends on the possibility of finding ways to detoxify them before recycling or utilization of natural resins as a replacement for synthetic resins [
92]. These missing proportions of waste wood could be replaced by waste paper and cardboard. Currently, the technology and waste-reclaiming process that turns cellulose residue from cardboard into a fully recyclable construction material (Honext) are already available.
In terms of local approach, to ensure self-sufficiency in the biomass supply for residential combustion, and, based on experimental results on the amounts of elements emitted when burning paper briquettes, we recommend the production of briquettes with a maximum of 10% added cardboard. This will ensure compliance with the requirements for briquette quality according to “EN ISO 17225-3 Solid biofuels—Fuel specifications and classes—Part 3: Graded wood briquettes (3% ash for briquettes group B)”. It is also possible to combine it with the otherwise unused material with a minimal negative impact on the environment.