Systemic Antibiotic and Nonsteroidal Anti-Inflammatory Drug Treatment Decreases the Level of Endogenous Angiogenic Vascular Endothelial Growth Factor in Inflamed Human Periapical Tissues
Abstract
:1. Introduction
2. Materials and Methods
2.1. VEGF Quantification Using Enzyme-Linked Immunosorbent Assay
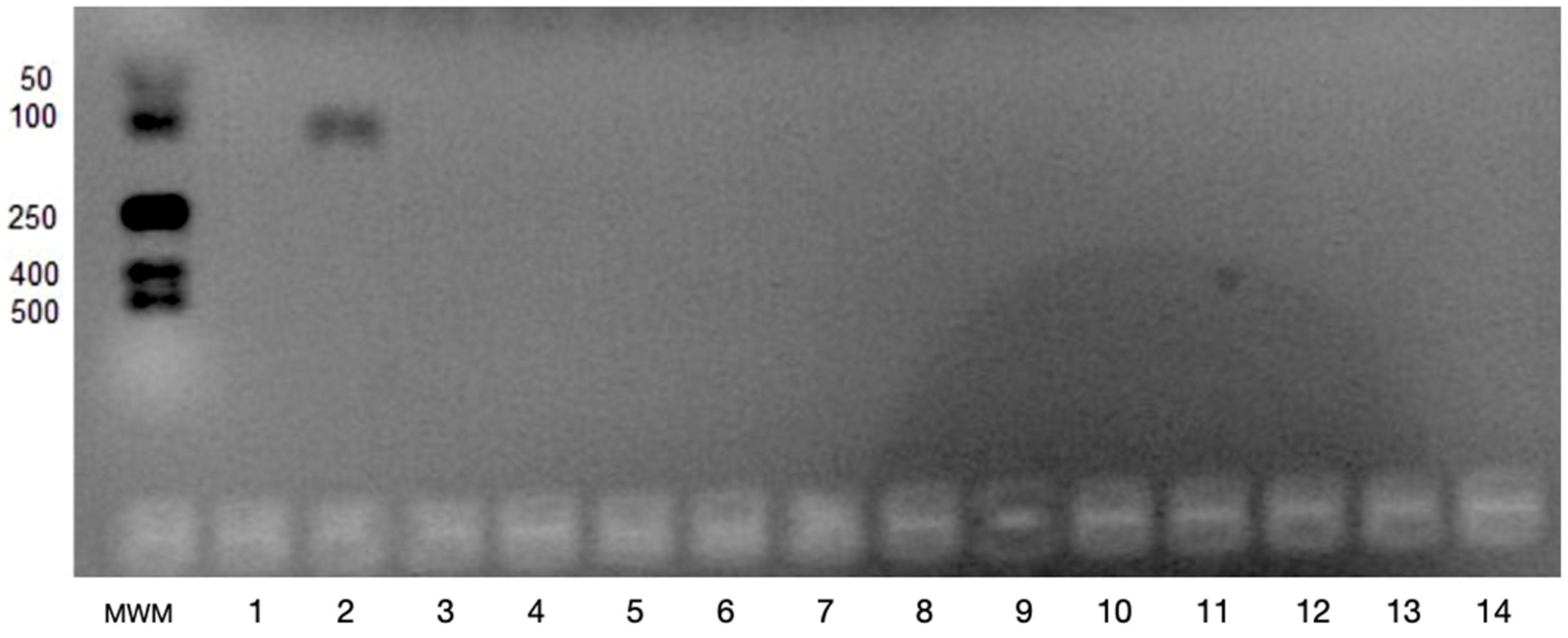
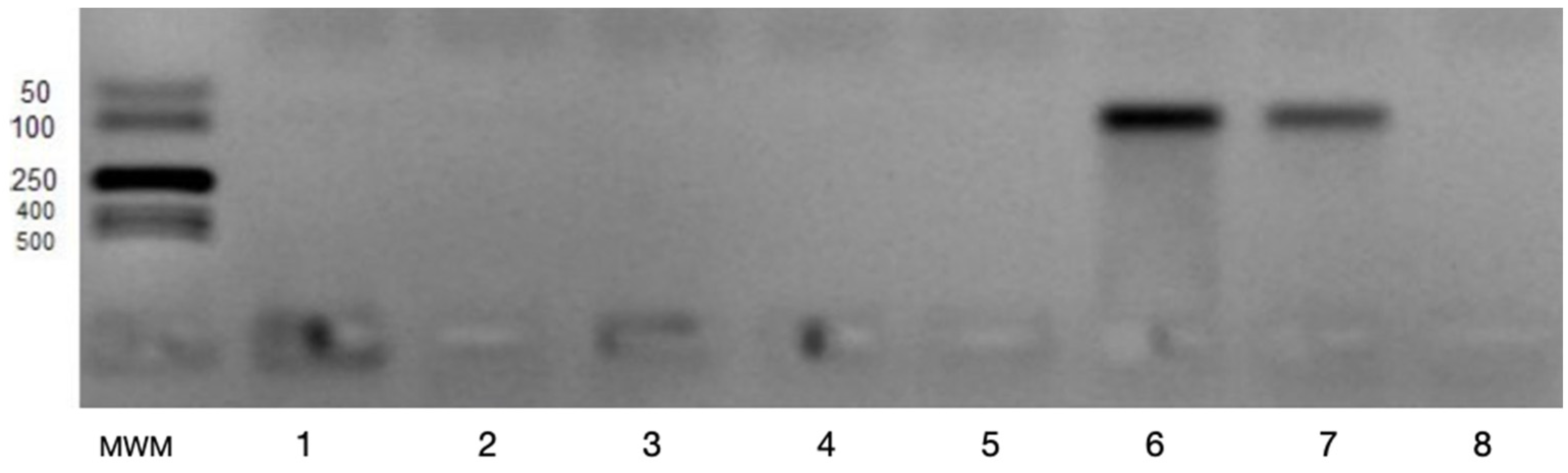
2.2. Determination of VEGFA and VEGFB Gene Expression
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Keles, G.C.; Cetinkaya, B.O.; Eroglu, C.; Simsek, S.B.; Kahraman, H. Vascular endothelial growth factor expression levels of gingiva in gingivitis and periodontitis patients with/without diabetes mellitus. Inflamm. Res. 2010, 59, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Silva, T.; Santos, C.C.O.; Alves, L.R.; Dias, L.C.; Brito-Junior, M.; De Paula, A.M.B.; Guimaraes, A.L.S. Detection and quantification of mast cell, vascular endothelial growth factor, and microvessel density in human inflammatory periapical cysts and granulomas. Int. Endod. J. 2012, 45, 859–864. [Google Scholar] [CrossRef]
- Rubini, C.; Arrese, L.; Zizzi, A.; Fioroni, M.; Ascani, G.; Poteri, G.; Stramazzotti, D.; Piccirilli, M.; Iezzi, G.; Piatelli, A. Immunohistochemical expression of vascular endothelial growth factor (VEGF) in different types of odontogenic cysts. Clin. Oral Investig. 2011, 15, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Bletsa, A.; Virtej, A.; Berggreen, E. Vascular endothelial growth factors and receptors are up-regulated during development of apical periodontitis. J. Endod. 2012, 38, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Veeriah, V.; Paone, R.; Chatterjee, S.; Teti, A.; Capulli, M. Osteoblasts Regulate Angiogenesis in Response to Mechanical Unloading. Calcif. Tissue Int. Epub. 2018. Ahead of Print. [Google Scholar] [CrossRef] [PubMed]
- Ergolu, Z.T.; Kurtis, B.; Altug, H.A.; Sahin, S.; Tuter, G.; Baris, E. Effect of topical ozonetherapy on gingival wound healing in pigs: Histological and immuno-histochemical analysis. J. Appl. Oral Sci. 2018, 10, 27. [Google Scholar] [CrossRef] [Green Version]
- Cetinkaya, B.O.; Keles, G.C.; Ayas, B.; Sakallioglu, E.E.; Acikgoz, G. The expression of vascular endothelial growth factor in a rat model at destruction and healing stages of periodontal disease. J. Periodontol. 2007, 78, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Angiogenesis: An organizing principle for drug discovery? Nat. Rev. Drug Discov. 2007, 6, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Kerbel, R.S. Angiogenesis as a therapeutic target. Nature 2005, 438, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Alitalo, K.; Tammela, T.; Petrova, T.V. Lymphangiogenesis in development and human disease. Nature 2005, 438, 946–953. [Google Scholar] [CrossRef]
- Lohela, M.; Bry, M.; Tammela, T.; Alitalo, K. VEGFs and receptors involved in angiogenesis versus lymphangiogenesis. Curr. Opin. Cell Biol. 2009, 21, 154–165. [Google Scholar] [CrossRef]
- Grimmond, S.; Lagercrantz, J.; Drinkwater, C.; Silins, G.; Townson, S.; Pollock, P.; Gotley, D.; Carson, E.; Rakar, S.; Nordenskj, O.M.; et al. Cloning and characterization of a novel human gene related to vascular endothelial growth factor. Genome Res. 1996, 6, 124–131. [Google Scholar] [CrossRef] [Green Version]
- Eriksson, L.X.; Novel, U. VEGF family members: VEGF-B, VEGF-C and VEGF-D. Int. J. Biochem. Cell Biol. 2001, 33, 421–426. [Google Scholar]
- Li, X.; Lee, C.; Tang, Z.; Zhang, F.; Arjunan, P.; Li, Y.; Hou, X.; Kumar, A.; Dong, L. VEGF-B: A survival, or an angiogenic factor? Cell Adh. Migr. 2009, 3, 322–327. [Google Scholar] [CrossRef] [Green Version]
- Zwolinska-Wcislo, M.; Krzysiek-Maczka, G.; Ptak-Belowska, A.; Karczewska, E.; Pajdo, R.; Sliwowski, Z.; Urbanczyk, K.; Drozdowicz, D.; Konturek, S.J.; Pawlik, W.W.; et al. Antibiotic treatment with ampicillin accelerates the healing of colonic damage impaired by aspirin and coxib in the experimental colitis. Importance of intestinal bacteria, colonic microcirculation and proinflammatory cytokines. J. Physiol. Pharmacol. 2011, 62, 357–368. [Google Scholar] [PubMed]
- Kwiatkowska, B.; Maślińska, M. Macrolide therapy in chronic inflammatory diseases. Mediat. Inflamm. 2012, 2012, 636157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathe, Z.; Dupraz, P.; Rinsch, C.; Thorens, B.; Bosco, D.; Zbinden, M.; Morel, P.; Berney, T.; Pepper, M.S. Tetracycline-regulated expression of VEGF-A in beta cells induces angiogenesis: Improvement of engraftment following transplantation. Cell Transplant. 2006, 15, 621–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palatynska-Ulatowska, A.; Michalska, M.; Łazarenkow, A. Fibrinogen, bFGF and VEGF levels during antibiotic therapy in gynecologic cancer: A preliminary report. Indian J. Biochem. Biophys. 2014, 51, 230–236. [Google Scholar] [PubMed]
- Pakneshan, P.; Birsner, A.E.; Adini, I.; Becker, C.M.; D’Amato, R.J. Differential suppression of vascular permeability and corneal angiogenesis by nonsteroidal anti-inflammatory drugs. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3909–3913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nadar, S.; Blann, A.D.; Lip, G.Y.H. Effects of aspirin on intra-platelet vascular endothelial growth factor angiopoietin-1, and P-selectin levels in hypertensive patients. Am. J. Hypertens. 2006, 19, 970–977. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Tao, K.; Huang, T. Effects of meloxicam on vascular endothelial growth factor and angiopoietin-2 expression in colon carcinoma cell line HT-29. J. Huazhong Univ. Sci. Med. 2000, 27, 399–402. [Google Scholar] [CrossRef]
- Jayadev, M.; Karunakar, P.; Vishwanath, B.; Soumya Chinmayi, S.; Siddhartha, P.; Chaitanya, B. Knowledge and pattern of antibiotic and non-narcotic analgesic prescription for pulpal and periapical pathologies—A survey among dentists. J. Clin. Diag. Res. 2014, 8, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Salako, N.O.; Rotimi, V.O.; Adib, S.M.; Al-Mutawa, S. Pattern of antibiotic prescription in the management of oral diseases among dentists in Kuwait. J. Dent. 2004, 32, 503–509. [Google Scholar] [CrossRef]
- Garcia, V.G.; Takano, R.Y.; Fernandes, L.A.; De Almeida, J.M.; Theodoro, L.H. Treatment of experimental periodontal disease by a selective inhibitor of cyclooxygenase-2 with scaling and root planing (SRP). Inflammopharmacology 2010, 18, 293–301. [Google Scholar]
- Addy, L.D.; Martin, M.V. Clindamycin and dentistry. Br. Dent. J. 2005, 199, 23–26. [Google Scholar] [PubMed] [Green Version]
- Virtej, A.; Løes, S.S.; Berggreen, E.; Bletsa, A. Localization and signaling patterns of vascular endothelial growth factors and receptors in human periapical lesions. J. Endod. 2013, 39, 605–611. [Google Scholar]
- Takeichi, O.; Hama, S.; Iwata, K.; Ito, K. Confocal immunolocalization of VE-Cadherin- and CXC chemokine-expressing endothelial cells in periapical granulomas. Int. Endod. J. 2008, 41, 401–407. [Google Scholar]
- Kudo, H.; Takeichi, O.; Hatori, K.; Makino, K.; Himi, K.; Ogiso, B.A. potential role for the silent information regulator 2 homologue 1 (SIRT1) in periapical periodontitis. Int. Endod. J. 2018, 51, 747–757. [Google Scholar]
- Prapulla, D.V.; Pai, B.S.; Pradeep, R. Gingival crevicular fluid VEGF levels in periodontal health and disease. J. Periodontol. 2007, 78, 1783–1787. [Google Scholar] [CrossRef] [PubMed]
- Samee, M.; Kasugai, S.; Kondo, H.; Ohya, K.; Shimokawa, H.; Kuroda, S. Bone morphogenetic protein-2 (BMP2) and vascular endothelial growth factor (VEGF) transfection to human periosteal cells enhances osteoblast differentiation and bone formation. J. Pharmacol. Sci. 2008, 108, 18–31. [Google Scholar]
- Yang, Y.Q.; Tan, Y.Y.; Wong, R.; Wenden, A.; Zhang, L.K.; Rabie, B.M. The role of vascular endothelial growth factor in ossification. Int. J. Oral Sci. 2012, 4, 64–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angelo, L.S.; Kurzrock, R. Vascular endothelial growth factor and its relationship to inflammatory mediators. Clin. Cancer Res. 2007, 13, 2825–2830. [Google Scholar]
- Heasman, P.; Hughes, J. Drugs, medications and periodontal disease. Br. Dent. J. 2014, 217, 411–419. [Google Scholar]
- Seymour, R.A. Effects of medications on the periodontal tissues in health and disease. Periodontology 2006, 40, 120–129. [Google Scholar] [CrossRef]
- Galiano, R.D.; Tepper, O.M.; Pelo, C.R.; Bhatt, K.A.; Callaghan, M.; Bastidas, N.; Bunting, S.; Steinmetz, H.G.; Gurtner, G.C. Topical vascular endothelial growth factor accelerates diabetic wound healing through increased angiogenesis and by mobilizing and recruiting bone marrow-derived cells. Am. J. Pathol. 2004, 164, 1935–1947. [Google Scholar]
- Takahashi, H.; Masabumi, S. The vascular endothelial growth factor (VEGF)/VEGF receptor system and its role under physiological and pathological conditions. Clin. Sci. 2005, 109, 227–241. [Google Scholar]
- Bao, P.; Kodra, A.; Tomic-Canic, M.; Golinko, M.S.; Ehrlich, H.P.; Brem, H. The role of vascular endothelial growth factor in wound healing. J. Surg. Res. 2009, 153, 347–358. [Google Scholar] [PubMed] [Green Version]
- Leszczyńska, A.; Buczko, P.; Buczko, W.; Pietruska, M. Periodontal pharmacotherapy—An updated review. Adv. Med. Sci. 2011, 56, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Harini, G.; Kaarthikeyan, G. Advanced drug delivery systems in treating periodontal diseases—A review. J. Dent. Med. Sci. 2014, 13, 2279–2861. [Google Scholar]
- Pejcic, A.; Kesic, L.; Brkic, Z.; Pesic, Z.; Mirkovic, D. Effect of periodontal treatment on lipoproteins levels in plasma in patients with periodontitis. South Med. J. 2011, 104, 547–552. [Google Scholar] [PubMed]
- Nair, P.N.R. Pathogenesis of apical periodontitis and the causes of endodontic failures. Crit. Rev. Oral Biol. Med. 2004, 15, 348–381. [Google Scholar] [CrossRef] [Green Version]
- Enholm, B.; Paavonen, K.; Ristimäki, A.; Kumar, V.; Gunji, Y.; Klefstrom, J.; Kivinen, L.; Laiho, M.; Olofsson, B.; Joukov, V.; et al. Comparison of VEGF, VEGF-B, VEGF-C and Ang-1 mRNA regulation by serum, growth factors, oncoproteins and hypoxia. Oncogene 1997, 14, 2475–2483. [Google Scholar] [CrossRef] [Green Version]
- Garlet, G.P.; Horwat, R.R., Jr.; Ray, H.L.J.; Garlet, T.P.; Silveira, E.M.; Campanelli, A.P.; Trombone, A.P.; Letra, A.; Silva, R.M. Expression Analysis of Wound Healing Genes in Human Periapical Granulomas of Progressive and Stable Nature. J. Endod. 2012, 38, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Nie, K.; Hu, P.; Wang, D.; Wei, Z.; Zeng, X.; Sun, G. Effects of rapamycin and deferoxamin on wound healing after ischemia and hypoxia. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2017, 31, 718–722. [Google Scholar] [PubMed]
- Kozakiewicz, M.; Wlazeł, R.N. Does Soluble Urokinase-Type Plasminogen Activator Receptor Level Predicts the Occurrence of Inflammatory Complications in Maxillofacial Surgery? Appl. Sci. 2021, 11, 2192. [Google Scholar] [CrossRef]
- Kozakiewicz, M.; Trzcińska-Kubik, M.; Wlazeł, R. Index of Body Inflammation for Maxillofacial Surgery Purpose-to Make the Soluble Urokinase-Type Plasminogen Activator Receptor Serum Level Independent on Patient Age. Appl. Sci. 2021, 11, 1345. [Google Scholar] [CrossRef]
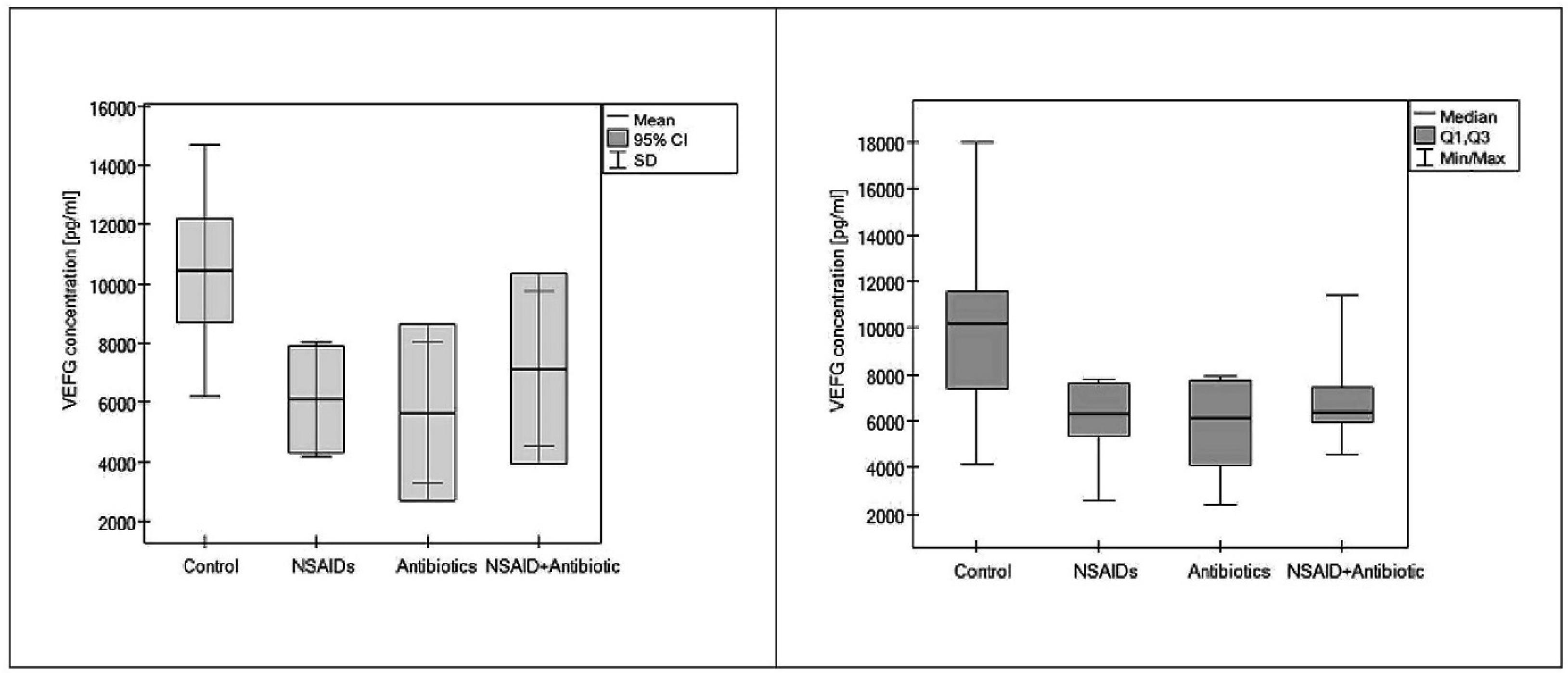
| Group | n | VEGF Concentration (ng/mL) Mean ± SD |
|---|---|---|
| 0 | 25 | 10.4324 ± 4257.2 |
| I | 7 | 6.0967 ± 1929.6 * |
| II | 5 | 5.6609 ± 2394.9 † |
| III | 5 | 7.1419 ± 2600.8 # |
| Gene VEGFA N (%) | Gene VEGFB N (%) | ||
|---|---|---|---|
| Presence of gene expression | 0 (0.0%) | Presence of gene expression | 26 (61.9%) |
| Absence of gene expression | 42 (100.0%) | Absence of gene expression | 16 (38.1%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palatyńska-Ulatowska, A.; Michalska, M.; Drelich, A.; Sałagacka-Kubiak, A.; Balcerczak, E.; Manowska, B.; Poli de Figueiredo, J.A. Systemic Antibiotic and Nonsteroidal Anti-Inflammatory Drug Treatment Decreases the Level of Endogenous Angiogenic Vascular Endothelial Growth Factor in Inflamed Human Periapical Tissues. Appl. Sci. 2021, 11, 4976. https://doi.org/10.3390/app11114976
Palatyńska-Ulatowska A, Michalska M, Drelich A, Sałagacka-Kubiak A, Balcerczak E, Manowska B, Poli de Figueiredo JA. Systemic Antibiotic and Nonsteroidal Anti-Inflammatory Drug Treatment Decreases the Level of Endogenous Angiogenic Vascular Endothelial Growth Factor in Inflamed Human Periapical Tissues. Applied Sciences. 2021; 11(11):4976. https://doi.org/10.3390/app11114976
Chicago/Turabian StylePalatyńska-Ulatowska, Aleksandra, Marta Michalska, Anna Drelich, Aleksandra Sałagacka-Kubiak, Ewa Balcerczak, Bogusława Manowska, and José Antonio Poli de Figueiredo. 2021. "Systemic Antibiotic and Nonsteroidal Anti-Inflammatory Drug Treatment Decreases the Level of Endogenous Angiogenic Vascular Endothelial Growth Factor in Inflamed Human Periapical Tissues" Applied Sciences 11, no. 11: 4976. https://doi.org/10.3390/app11114976






