Current View on Green Tea Catechins Formulations, Their Interactions with Selected Drugs, and Prospective Applications for Various Health Conditions
Abstract
:1. Introduction
2. Background of Tea Preparation’s Technological Aspects
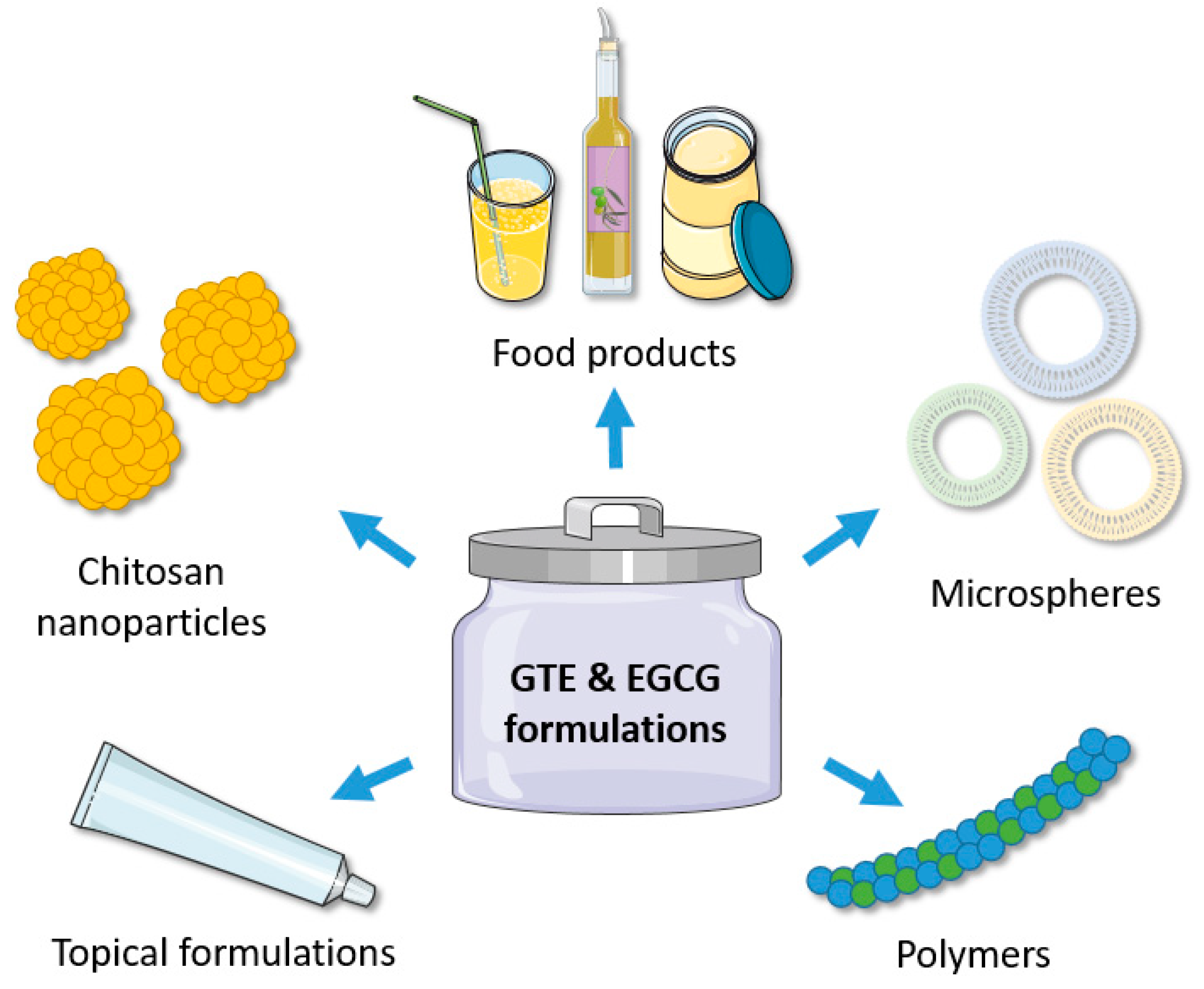
3. Formulations of Green Tea Extracts and EGCG in Food Industry
4. Formulations of Green Tea Extracts and EGCG in Pharmaceutical and Cosmetic Applications
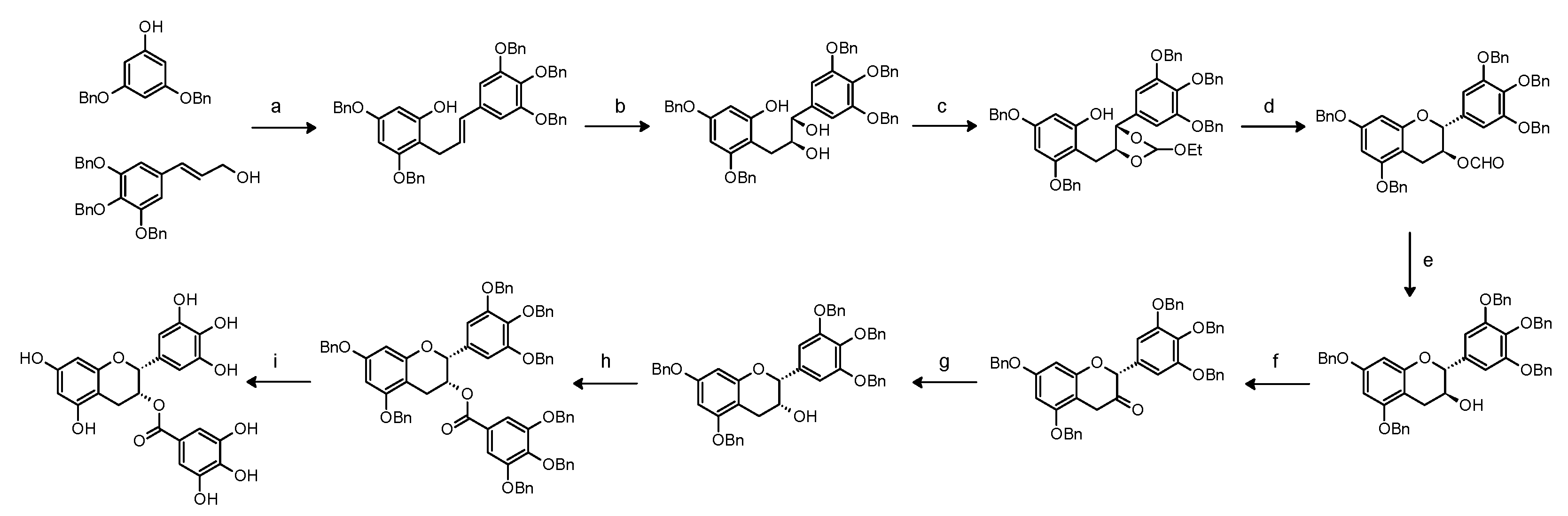
5. De Novo Synthesis of EGCG and Methods of Isolation
6. Interactions of Green Tea Extracts and EGCG with Selected Active Pharmaceutical Ingredients
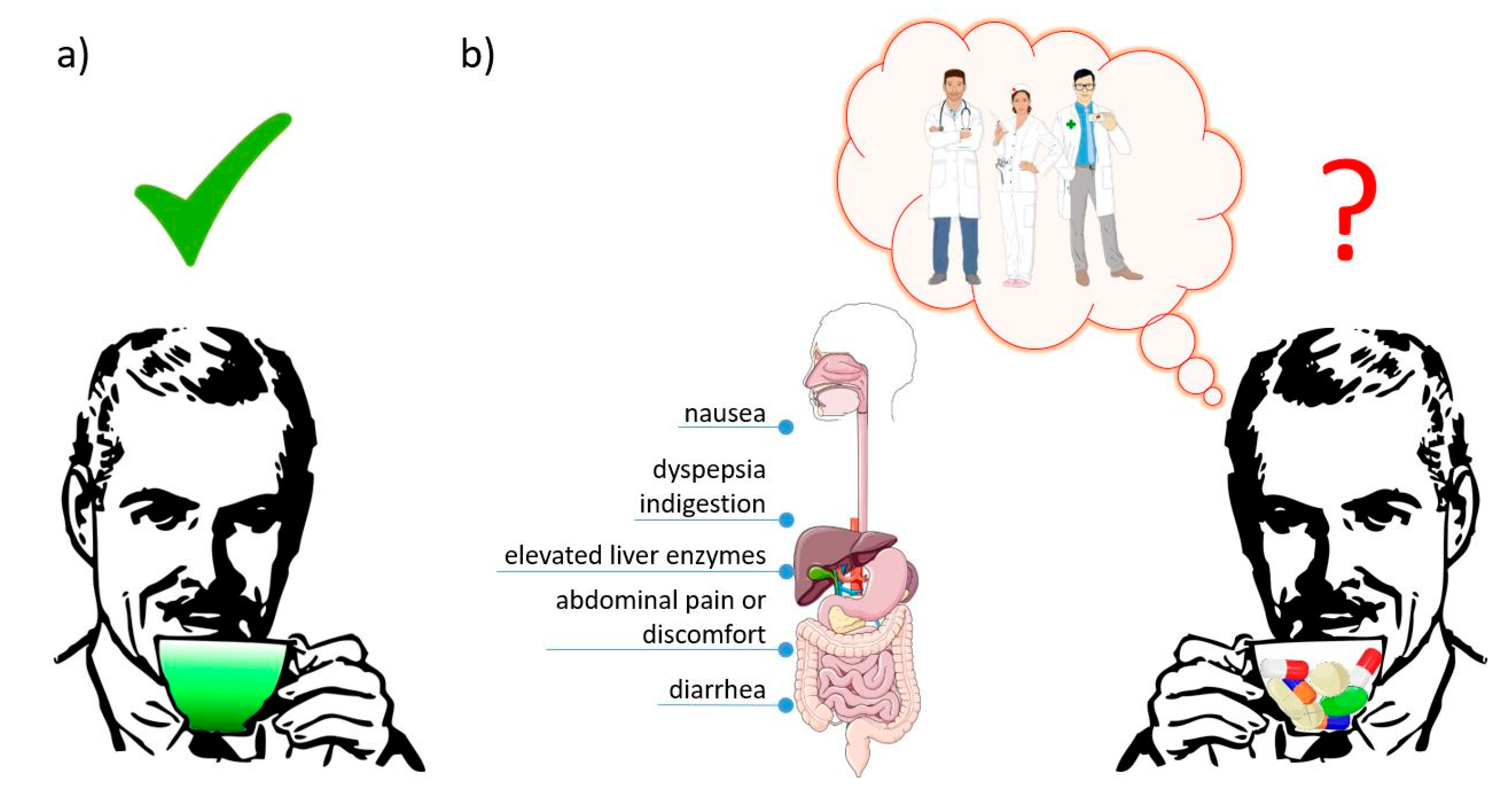
7. Human Safety Data with an Indication of the Role of Health Care Providers
8. Techno Economic Challenges and Future Research Directions
9. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Namal Senanayake, S.P.J. Green tea extract: Chemistry, antioxidant properties and food applications—A review. J. Funct. Foods 2013, 5, 1529–1541. [Google Scholar] [CrossRef]
- Shi, M.; Shi, Y.-L.; Li, X.-M.; Ma, S.-C.; Ye, J.-H.; Lu, J.-L.; Liang, Y.-R.; Zheng, X.-Q. Food-Grade Encapsulation Systems for (−)-Epigallocatechin Gallate. Molecules 2018, 23, 445. [Google Scholar] [CrossRef] [Green Version]
- Krupkova, O.; Ferguson, S.J.; Wuertz-Kozak, K. Stability of (−)-epigallocatechin gallate and its activity in liquid formula-tions and delivery systems. J. Nutr. Biochem. 2016, 37, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Deng, J.; Man, Y.; Qu, Y. Green Tea Extracts Epigallocatechin-3-gallate for Different Treatments. Biomed Res. Int. 2017, 2017, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Krstin, S.; Wink, M. Modulation of multidrug resistant in cancer cells by EGCG, tannic acid and curcumin. Phytomedicine 2018, 50, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Jiang, Y.; Yang, X.; Wang, S.; Xie, C.; Li, X.; Li, Y.; Chen, Y.; Wang, X.; Meng, Y.; et al. Wnt/β-catenin pathway mediates (−)-Epigallocatechin-3-gallate (EGCG) inhibition of lung cancer stem cells. Biochem. Biophys. Res. Commun. 2017, 482, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, Y.; Terashita, N.; Muraguchi, T.; Fukusato, T.; Kubota, S. Effects of Epigallocatechin-3-gallate (EGCG) on A549 Lung Cancer Tumor Growth and Angiogenesis. Biosci. Biotechnol. Biochem. 2013, 77, 1799–1803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rady, I.; Mohamed, H.; Rady, M.; Siddiqui, I.A.; Mukhtar, H. Cancer preventive and therapeutic effects of EGCG, the major polyphenol in green tea. Egypt. J. Basic Appl. Sci. 2018, 5, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Moreira, L.; Araújo, I.; Costa, T.; Correia-Branco, A.; Faria, A.; Martel, F.; Keating, E. Quercetin and epigallocatechin gallate inhibit glucose uptake and metabolism by breast cancer cells by an estrogen receptor-independent mechanism. Exp. Cell Res. 2013, 319, 1784–1795. [Google Scholar] [CrossRef]
- Jankun, J.; Keck, R.W.; Selman, S.H. Epigallocatechin-3-gallate prevents tumor cell implantation/growth in an experimental rat bladder tumor model. Int. J. Oncol. 2014, 44, 147–152. [Google Scholar] [CrossRef]
- Green Tea Market Size, Share & COVID-19 Impact Analysis, Form (Tea Bags, Loose Leaves, Loose Powder, Ready-to-drink, and Capsules & Tablets), Flavor (Flavored and Unflavored), Distribution Channel (Supermarkets/Hypermarkets, Convenience Stores, Specialty Stores, and Online Retail) and Regional Forecast, 2020–2027. Market Research Report. Fortune Business Insights 2020. Available online: https://www.fortunebusinessinsights.com/industry-reports/organic-tea-market-100804 (accessed on 20 May 2021).
- CBI Product Factsheet: Tea in Poland. Available online: https://www.cbi.eu/sites/default/files/market-information/cbi_2016_-_tea_-_pfs_pl_-_final_draft.pdf (accessed on 20 May 2021).
- Karwowska, K.; Śmiechowska, M. Dietary Supplements Containing Tea in Commodity Aspect. Towarozn. Probl. Jakości 2017, 3, 38–48. [Google Scholar]
- Cerbin-Koczorowska, M.; Przymuszala, P.; Zielinska-Tomczak, L.; Wawrzyniak, E.; Marciniak, R. Is there a time and place for health education in chain pharmacies? Perspectives of Polish community pharmacists. Health Soc. Care Community 2020, 00, 1–11. [Google Scholar]
- Lu, H.; Zhang, J.; Yang, Y.; Yang, X.; Xu, B.; Yang, W.; Tong, T.; Jin, S.; Shen, C.; Rao, H.; et al. Earliest tea as evidence for one branch of the Silk Road across the Tibetan Plateau. Sci. Rep. 2016, 6, 18955. [Google Scholar] [CrossRef] [Green Version]
- Heiss, M.L.; Heiss, R.J. The Tea Enthusiast’s Handbook: A Guide to Enjoying the World’s Best Teas; Ten Speed Press: Berkeley, CA, USA, 2010. [Google Scholar]
- Meegahakumbura, M.K.; Wambulwa, M.C.; Li, M.-M.; Thapa, K.K.; Sun, Y.-S.; Möller, M.; Xu, J.-C.; Yang, J.-B.; Liu, J.; Liu, B.-Y.; et al. Domestication Origin and Breeding History of the Tea Plant (Camellia sinensis) in China and India Based on Nuclear Microsatellites and cpDNA Sequence Data. Front. Plant Sci. 2018, 8, 2270. [Google Scholar] [CrossRef] [Green Version]
- Monsanto, M.F.M. Separation of polyphenols from aqueous green and black tea. Tech. Univ. Eindh. 2015. [Google Scholar] [CrossRef]
- Henning, S.M.; Fajardo-Lira, C.; Lee, H.W.; Youssefian, A.A.; Go, V.L.W.; Herber, D. Catechin Content of 18 Teas and a Green Tea Extract Supplement Correlates with the Antioxidant Capacity. Nutr. Cancer 2003, 45, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Komes, D.; Horžić, D.; Belščak, A.; Ganić, K.K.; Vulić, I. Green tea preparation and its influence on the content of bioactive compounds. Food Res. Int. 2010, 43, 167–176. [Google Scholar] [CrossRef]
- Huo, C.; Wan, S.B.; Lam, W.H.; Li, L.; Wang, Z.; Landis-Piwowar, K.R.; Chen, D.; Dou, Q.P.; Chan, T.H. The challenge of developing green tea polyphenols as therapeutic agents. Inflammopharmacol 2008, 16, 248–252. [Google Scholar] [CrossRef] [Green Version]
- Dettlaff, K.; Stawny, M.; Ogrodowczyk, M.; Jelińska, A.; Bednarski, W.; Wątróbska-Świetlikowska, D.; Keck, R.W.; Khan, O.A.; Mostafa, I.H.; Jankun, J. Formulation and characterization of EGCG for the treatment of superficial bladder cancer. Int. J. Mol. Med. 2017, 40, 329–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, J.H.; Choi, D.H. Effects of Oral Epigallocatechin Gallate on the Oral Pharmacokinetics of Verapamil in Rats. Biopharm. Drug Dispos. 2009, 30, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Peters, C.M.; Green, R.J.; Janle, E.M.; Ferruzzi, M.G. Formulation with ascorbic acid and sucrose modulates catechin bioavailability from green tea. Food Res. Int. 2010, 43, 95–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, R.J.; Murphy, A.S.; Schulz, B.; Watkins, B.A.; Ferruzzi, M.G. Common tea formulations modulate in vitro digestive recovery of green tea catechins. Mol. Nutr. Food Res. 2007, 59, 1152–1162. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, J.; Ferruzzi, M.G.; Taylor, L.S.; Mauer, L.J. Interaction of Environmental Moisture with Powdered Green Tea Formulations: Effect on Catechin Chemical Stability. J. Agric. Food Chem. 2008, 56, 4068–4077. [Google Scholar] [CrossRef]
- Lavelli, V.; Vantaggi, C.; Corey, M.; Kerr, W. Formulation of a Dry Green Tea-Apple Product: Study on Antioxidant and Color Stability. J. Food Sci. 2010, 75, C184–C190. [Google Scholar] [CrossRef] [PubMed]
- Menaa, F.; Menaa, A.; Menaa, B. Polyphenols Nano-Formulations for Topical Delivery and Skin Tissue Engineering. In Polyphenols in Human Health and Disease; Elsevier: Amsterdam, The Netherlands; pp. 839–848.
- Garcia, J.; Hsieh, M.-F.; Doma, B.; Peruelo, D.; Chen, I.-H.; Lee, H.-M. Synthesis of Gelatin-γ-Polyglutamic Acid-Based Hydrogel for the In Vitro Controlled Release of Epigallocatechin Gallate (EGCG) from Camellia sinensis. Polymers 2013, 6, 39–58. [Google Scholar] [CrossRef]
- dal Belo, S.E.; Gaspar, L.R.; Maia Campos, P.M.B.G.; Marty, J.-P. Skin Penetration of Epigallocatechin-3-Gallate and Quercetin from Green Tea and Ginkgo biloba Extracts Vehiculated in Cosmetic Formulations. Ski. Pharmacol. Physiol. 2009, 22, 299–304. [Google Scholar] [CrossRef]
- Dvorakova, K.; Dorr, R.T.; Valcic, S.; Timmermann, B.; Alberts, D.S. Pharmacokinetics of the green tea derivative, EGCG, by the topical route of administration in mouse and human skin. Cancer Chemother. Pharmacol. 1999, 43, 331–335. [Google Scholar] [CrossRef]
- Scalia, S.; Trotta, V.; Bianchi, A. In vivo human skin penetration of (–)-epigallocatechin-3-gallate from topical formulations. Acta Pharm. 2014, 64, 257–265. [Google Scholar] [CrossRef] [Green Version]
- Gianeti, M.D.; Mercurio, D.G.; Maia Campos, P.M.B.G. The use of green tea extract in cosmetic formulations: Not only an antioxidant active ingredient: The use of green tea extract in cosmetics. Dermatol. Ther. 2013, 26, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.D.; Kim, D.H.; Zheng, R.; Yang, C.S. Transdermal delivery of (−)-epigallocatechin-3-gallate, a green tea polyphenol, in mice. J. Pharm. Pharmacol. 2006, 58, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Isnan, A.P.; Jufri, M. Formulation of niosomal gel containing green tea extract (Camelia sinensis, L. Kuntze) using thin-layer hydration. Int. J. Appl. Pharm. 2017, 9, 38. [Google Scholar] [CrossRef] [Green Version]
- Ramadon, D.; Pramesti, S.S.; Anwar, E. Formulation, stability tformulation, stability test and in vitro penetration study of transethosomal gel containing green tea (Camellia sinensis L. Kuntze) leaves extractest and in vitro penetration study of transethosomal gel containing green tea (Camellia sinensis L. Kuntze) leaves extract. Int. J. Appl. Pharm. 2017, 9, 91. [Google Scholar]
- Ramadon, D.; Wirarti, G.A.; Anwar, E. Novel Transdermal Ethosomal Gel Containing Green Tea (Camellia sinensis, L. Kuntze) Leaves Extract: Formulation and In vitro Penetration Study. JYP 2017, 9, 336–340. [Google Scholar] [CrossRef] [Green Version]
- Mittal, A.; Piyathilake, C.; Hara, Y.; Katiyar, S.K. Exceptionally High Protection of Photocarcinogenesis by Topical Application of (−)-Epigallocatechin-3-Gallate in Hydrophilic Cream in SKH-1 Hairless Mouse Model: Relationship to Inhibition of UVB-Induced Global DNA Hypomethylation. Neoplasia 2003, 5, 555–565. [Google Scholar] [CrossRef] [Green Version]
- Bianchi, A.; Marchetti, N.; Scalia, S. Photodegradation of (−)-epigallocatechin-3-gallate in topical cream formulations and its photostabilization. J. Pharm. Biomed. Anal. 2011, 56, 692–697. [Google Scholar] [CrossRef]
- Scalia, S.; Marchetti, N.; Bianchi, A. Comparative Evaluation of Different Co-Antioxidants on the Photochemical- and Functional-Stability of Epigallocatechin-3-gallate in Topical Creams Exposed to Simulated Sunlight. Molecules 2013, 18, 574–587. [Google Scholar] [CrossRef]
- Puri, A.; Nguyen, H.X.; Banga, A.K. Microneedle-mediated intradermal delivery of epigallocatechin-3-gallate. Int. J. Cosmet. Sci. 2016, 38, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.-H.; Jung, E.Y.; Noh, D.O.; Suh, H.J. Physiological effects of formulation containing tannase-converted green tea extract on skin care: Physical stability, collagenase, elastase, and tyrosinase activities. Integr. Med. Res. 2014, 3, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Dahiya, S.; Rani, R.; Kumar, S.; Dhingra, D.; Dilbaghi, N. Chitosan-Gellan Gum Bipolymeric Nanohydrogels—a Potential Nanocarrier for the Delivery of Epigallocatechin Gallate. BioNanoSci 2017, 7, 508–520. [Google Scholar] [CrossRef]
- Dube, A.; Nicolazzo, J.A.; Larson, I. Chitosan nanoparticles enhance the plasma exposure of (−)-epigallocatechin gallate in mice through an enhancement in intestinal stability. Eur. J. Pharm. Sci. 2011, 44, 422–426. [Google Scholar] [CrossRef]
- Liang, J.; Yan, H.; Wang, X.; Zhou, Y.; Gao, X.; Puligundla, P.; Wan, X. Encapsulation of epigallocatechin gallate in zein/chitosan nanoparticles for controlled applications in food systems. Food Chem. 2017, 231, 19–24. [Google Scholar] [CrossRef]
- Laudadio, E.; Minnelli, C.; Amici, A.; Massaccesi, L.; Mobbili, G.; Galeazzi, R. Liposomal Formulations for an Efficient Encapsulation of Epigallocatechin-3-Gallate: An In-Silico/Experimental Approach. Molecules 2018, 23, 441. [Google Scholar] [CrossRef] [Green Version]
- Ramadass, S.K.; Anantharaman, N.V.; Subramanian, S.; Sivasubramanian, S.; Madhan, B. Paclitaxel/Epigallocatechin gallate coloaded liposome: A synergistic delivery to control the invasiveness of MDA-MB-231 breast cancer cells. Colloids Surf. B Biointerfaces 2015, 125, 65–72. [Google Scholar] [CrossRef]
- Rashidinejad, A.; Birch, E.J.; Sun-Waterhouse, D.; Everett, D. WDelivery of green tea catechin and epigallocatechin gallate in liposomes incorporated into low-fat hard cheese. Food Chem. 2014, 156, 176–183. [Google Scholar] [CrossRef]
- Liang, R.; Chen, L.; Yokoyama, W.; Williams, P.A.; Zhong, F. Niosomes Consisting of Tween-60 and Cholesterol Improve the Chemical Stability and Antioxidant Activity of (−)-Epigallocatechin Gallate under Intestinal Tract Conditions. J. Agric. Food Chem. 2016, 64, 9180–9188. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Li, D.; Zhou, Y.; Yang, J.; Yang, W.; Zhou, G.; Wen, J. Enhanced uptake and transport of (+)-catechin and (−)-epigallocatechin gallate in niosomal formulation by human intestinal Caco-2 cells. Int. J. Nanomed. 2014, 9, 2157–2165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anwar, E.; Farhana, N. Formulation and Evaluation of Phytosome-Loaded Maltodextrin-Gum Arabic Microsphere System for Delivery of Camellia sinensis Extract. JYP 2018, 10, S56–S62. [Google Scholar] [CrossRef] [Green Version]
- Donsì, F.; Voudouris, P.; Veen, S.J.; Velikov, K.P. Zein-based colloidal particles for encapsulation and delivery of epigallocatechin gallate. Food Hydrocoll. 2017, 63, 508–517. [Google Scholar] [CrossRef]
- Gómez-Mascaraque, L.G.; Hernández-Rojas, M.; Tarancón, P.; Tenon, M.; Feuillère, N.; Vélez Ruiz, J.F.; Fiszman, S.; López-Rubio, A. Impact of microencapsulation within electrosprayed proteins on the formulation of green tea extract-enriched biscuits. LWT Food Sci. Technol. 2017, 81, 77–86. [Google Scholar] [CrossRef] [Green Version]
- Shutava, T.G.; Balkundi, S.S.; Lvov, Y.M. (−)-Epigallocatechin gallate/gelatin layer-by-layer assembled films and microcapsules. J. Colloid Interface Sci. 2009, 330, 276–283. [Google Scholar] [CrossRef]
- Gonçalves, V.S.S.; Poejo, J.; Matias, A.A.; Rodríguez-Rojo, S.; Cocero, M.J.; Duarte, C.M. MUsing different natural origin carriers for development of epigallocatechin gallate (EGCG) solid formulations with improved antioxidant activity by PGSS-drying. RSC Adv. 2016, 6, 67599–67609. [Google Scholar] [CrossRef]
- Lazzeroni, M.; Guerrieri-Gonzaga, A.; Gandini, S.; Johansson, H.; Serrano, D.; Cazzaniga, M.; Aristarco, V.; Macis, D.; Mora, S.; Caldarella, P.; et al. A Presurgical Study of Lecithin Formulation of Green Tea Extract in Women with Early Breast Cancer. Cancer Prev. Res. 2017, 9, 363–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, C.; Gu, C.; Fang, Q.; Wang, Q.; Xia, Q. Transdermal solid delivery of epigallocatechin-3-gallate using self-double-emulsifying drug delivery system as vehicle: Formulation, evaluation and vesicle-skin interaction. J. Biomater. Appl. 2016, 30, 1080–1091. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Wang, Q.; Zhao, G.; Yao, W.; Xia, Q. Improved oral absorption of (−)-epigallocatechin-3-gallate via self-double-emulsifying solid formulation: Improved oral absorption of EGCG-SDEDDS solid formulation. Eur. J. Lipid Sci. Technol. 2016, 118, 1115–1124. [Google Scholar] [CrossRef]
- Jackson, J.K. The Effective Solubilization of Hydrophobic Drugs Using Epigallocatechin Gallate or Tannic Acid-Based Formulations. J. Pharm. Sci. 2016, 105, 3143–3152. [Google Scholar] [CrossRef] [PubMed]
- Lemarié, F.; Chang, C.W.; Blatchford, D.R.; Amor, R.; Norris, G.; Tetley, L.; McConnell, G.; Dufès, C. Antitumor activity of the tea polyphenol epigallocatechin-3-gallate encapsulated in targeted vesicles after intravenous administration. Nanomedicine 2013, 8, 181–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onoue, S.; Ochi, M.; Yamada, S. Development of (−)-epigallocatechin-3-gallate (EGCG)-loaded enteric microparticles with intestinal mucoadhesive property. Int. J. Pharm. 2011, 410, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Klinski, E. Block Copolymer Based Composition of Epigallocatechin-3-gallate with Improved Oral Bioavailability as a Way to Increase its Therapeutic Activity. J. Nanomed. Biotherapeut. Discov. 2013, 3. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Nie, S.; Martinez-Zaguilan, R.; Sennoune, S.R.; Wang, S. Formulation, characteristics and antiatherogenic bioactivities of CD36-targeted epigallocatechin gallate (EGCG)-loaded nanoparticles. J. Nutr. Biochem. 2016, 30, 14–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Chan, T.H. Enantioselective Synthesis of Epigallocatechin-3-gallate (EGCG), the Active Polyphenol Component from Green Tea. Org. Lett. 2001, 3, 739–741. [Google Scholar] [CrossRef]
- Graham, H.N. Green tea composition, consumption, and polyphenol chemistry. Prev. Med. 1992, 21, 334–350. [Google Scholar] [CrossRef]
- Banerjee, S.; Chatterjee, J. Efficient extraction strategies of tea (Camellia sinensis) biomolecules. J. Food Sci. Technol. 2015, 52, 3158–3168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oda, K.; Murakami, T. Pharmacokinetic interaction of green tea beverage containing cyclodextrins and high concentration catechins with P-glycoprotein substrates in LLC-GA5-COL150 cells in vitro and in the small intestine of rats in vivo. J. Pharm. Pharmacol. 2017, 69, 1736–1744. [Google Scholar] [CrossRef] [PubMed]
- Lecumberri, E.; Dupertuis, Y.M.; Miralbell, R.; Pichard, C. Green tea polyphenol epigallocatechin-3-gallate (EGCG) as adjuvant in cancer therapy. Clin. Nutr. 2013, 32, 894–903. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Chen, D.; Zhu, K. SOX2OT variant 7 contributes to the synergistic interaction between EGCG and Doxorubicin to kill osteosarcoma via autophagy and stemness inhibition. J. Exp. Clin. Cancer Res. 2018, 37, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Tang, J.; Du, Y.; Ding, J.; Liu, J.-Y. The green tea polyphenol EGCG potentiates the antiproliferative activity of sunitinib in human cancer cells. Tumor Biol. 2016, 37, 8555–8566. [Google Scholar] [CrossRef]
- Yuan, L.; Liu, M.; Liu, G.; Li, D.; Wang, Z.; Wang, B.; Han, J.; Zhang, M. Competitive binding of (−)-epigallocatechin-3-gallate and 5-fluorouracil to human serum albumin: A fluorescence and circular dichroism study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 173, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Lubecka, K.; Kaufman-Szymczyk, A.; Cebula-Obrzut, B.; Smolewski, P.; Szemraj, J.; Fabianowska-Majewska, K. Novel Clofarabine-Based Combinations with Polyphenols Epigenetically Reactivate Retinoic Acid Receptor Beta, Inhibit Cell Growth, and Induce Apoptosis of Breast Cancer Cells. Int. J. Mol. Sci. 2018, 19, 3970. [Google Scholar] [CrossRef] [Green Version]
- Shervington, A.; Pawar, V.; Menon, S.; Thakkar, D.; Patel, R. The sensitization of glioma cells to cisplatin and tamoxifen by the use of catechin. Mol. Biol. Rep. 2009, 36, 1181–1186. [Google Scholar] [CrossRef]
- Kim, T.E.; Ha, N.; Kim, Y.; Kim, H.; Lee, J.W.; Jeon, J.-Y.; Kim, M.-G. Effect of epigallocatechin-3-gallate, major ingredient of green tea, on the pharmacokinetics of rosuvastatin in healthy volunteers. Drug Des. Dev. Ther. 2017, 11, 1409–1416. [Google Scholar] [CrossRef] [Green Version]
- Misaka, S.; Yatabe, J.; Müller, F.; Takano, K.; Kawabe, K.; Glaeser, H.; Yatabe, M.S.; Onoue, S.; Werba, J.P.; Watanabe, H.; et al. Green tea ingestion greatly reduces plasma concentrations of nadolol in healthy subjects. Clin. Pharmacol. Ther. 2014, 95, 432–438. [Google Scholar] [CrossRef]
- Han, X.; Zhang, H.; Hao, H.; Li, H.; Guo, X.; Zhang, D. Effect Of epigallocatechin-3-gallate on the pharmacokinetics of amlodipine in rats. Xenobiotica 2019, 49, 970–974. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Burm, J.P. Effects of oral epigallocatechin gallate on the pharmacokinetics of nicardipine in rats. Arch. Pharmacal Res. 2009, 32, 1721–1725. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Choi, J.S. Effects of epigallocatechin gallate on the bioavailability and pharmacokinetics of diltiazem in rats. Pharmazie 2008, 63, 815–818. [Google Scholar] [PubMed]
- Hegazy, S. The Effect of Green Tea on Sildenafil Pharmacokinetics in Egyptian Healthy Volunteers. Br. J. Pharm. Res. 2014, 4, 289–300. [Google Scholar] [CrossRef]
- Werba, J.P.; Misaka, S.; Giroli, M.G.; Yamada, S.; Cavalca, V.; Kawabe, K.; Squellerio, I.; Laguzzi, F.; Onoue, S.; Veglia, F.; et al. Overview of Green Tea Interaction with Cardiovascular Drugs. Curr. Pharm. Des. 2015, 21, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.E.; Shin, K.H.; Park, J.E.; Kim, M.G.; Yun, Y.M.; Choi, D.H.; Kwon, K.J.; Lee, J. Effect of green tea catechins on the pharmacokinetics of digoxin in humans. Drug Des. Dev. Ther. 2018, 12, 2139–2147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alemdaroglua, N.C.; Dietzb, U.; Wolfframc, S.; Spahn-Langguth, H.; Langguth, P. Influence of Green and Black Tea on Folic Acid Pharmacokinetics in Healthy Volunteers: Potential Risk of Diminished Folic Acid Bioavailability. Biopharm. Drug Dispos. 2008, 29, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.O.; Wang, C.C.; Chu, C.Y.; Chan, K.P.; Rogers, M.S.; Choy, K.W.; Pang, C.P. Pharmacokinetic studies of green tea catechins in maternal plasma and fetuses in rats. J. Pharm. Sci. 2006, 95, 1372–1381. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.R.; Wilt, V.M. Probable antagonism of warfarin by green tea. Ann. Pharmacother. 1999, 33, 426–428. [Google Scholar] [CrossRef]
- Wang, J.S.; Luo, H.; Wang, P.; Yu, J.; Huang, T.; Cox, S.; Gao, W. Validation of green tea polyphenol biomarkers in a phase II human intervention trial. Food Chem. Toxicol. 2008, 46, 232–240. [Google Scholar] [CrossRef] [Green Version]
- Bakhtiyari, S.; Zaherara, M.; Haghani, K.; Khatami, M.; Rashidinejad, A. The Phosphorylation of IRS1 S307 and Akt S473 Molecules in Insulin-Resistant C2C12 Cells Induced with Palmitate Is Influenced by Epigallocatechin Gallate from Green Tea. Lipids 2019, 54, 141–148. [Google Scholar] [CrossRef]
- Knop, J.; Misaka, S.; Singer, K.; Hoier, E.; Müller, F.; Glaeser, H.; König, J.; Fromm, M.F. Inhibitory effects of green tea and (−)-epigallocatechin gallate on transport by OATP1B1, OATP1B3, OCT1, OCT2, MATE1, MATE2-K and P-glycoprotein. PLoS ONE 2015, 10, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaston, T.E.; Mendrick, D.L.; Paine, M.F.; Roe, A.L.; Yeung, C.K. “Natural” is not synonymous with “Safe”: Toxicity of natural products alone and in combination with pharmaceutical agents. Regul. Toxicol. Pharmacol. 2020, 113, 104642. [Google Scholar] [CrossRef] [PubMed]
- Izzo, V.; Pietrocola, F.; Sica, V.; Durand, S.; Lachkar, S.; Enot, D.; Bravo-San Pedro, J.M.; Chery, A.; Esposito, S.; Raia, V.; et al. Metabolic interactions between cysteamine and epigallocatechin gallate. Cell Cycle 2017, 16, 271–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behbehani, J.M.; Irshad, M.; Shreaz, S.; Karched, M. Synergistic effects of tea polyphenol epigallocatechin 3-O-gallate and azole drugs against oral Candida isolates. J. De Mycol. Med. 2019, 29, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Misaka, S.; Abe, O.; Sato, H.; Ono, T.; Shikama, Y.; Onoue, S.; Yabe, H.; Kimura, J. Lack of pharmacokinetic interaction between fluvastatin and green tea in healthy volunteers. Eur. J. Clin. Pharmacol. 2018, 74, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Xu, Q.; Liu, L.; Zhang, S.; Huang, C.; Miao, L. Effect of Green Tea and (−)-epigallocatechin Gallate on the Pharmacokinetics of Rosuvastatin. Curr. Drug Metab. 2020, 21, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zhang, Q.; Yang, Y.; Xu, J.; Fan, A.; Yang, C.S.; Li, N.; Lu, Y.; Chen, J.; Zhao, D.; et al. Epigallocatechin-3-gallate decreases the transport and metabolism of simvastatin in rats. Xenobiotica 2017, 47, 86–92. [Google Scholar] [CrossRef]
- Misaka, S.; Kawabe, K.; Onoue, S.; Werba, J.P.; Giroli, M.; Watanabe, H.; Yamada, S. Green tea extract affects the cytochrome P450 3A activity and pharmacokinetics of simvastatin in rats. Drug Metab. Pharmacokinet. 2013, 28, 514–518. [Google Scholar] [CrossRef]
- Werba, J.P.; Giroli, M.; Cavalca, V.; Nava, M.C.; Tremoli, E.; Dal Bo, L. The Effect of Green Tea on Simvastatin Tolerability. Ann. Intern. Med. 2008, 149, 286–287. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Jaccob, A. Effects of Long-term Use of Polyphenols on the Absorption and Tissue Distribution of Orally Administered Metformin and Atenolol in Rats. J. Intercult. Ethnopharmacol. 2013, 2, 147. [Google Scholar] [CrossRef]
- Misaka, S.; Miyazaki, N.; Fukushima, T.; Yamada, S.; Kimura, J. Effects of green tea extract and (−)-epigallocatechin-3-gallate on pharmacokinetics of nadolol in rats. Phytomedicine 2013, 20, 1247–1250. [Google Scholar] [CrossRef] [PubMed]
- Abe, O.; Ono, T.; Sato, H.; Müller, F.; Ogata, H.; Miura, I.; Shikama, Y.; Yabe, H.; Onoue, S.; Fromm, M.F.; et al. Role of (−)-epigallocatechin gallate in the pharmacokinetic interaction between nadolol and green tea in healthy volunteers. Eur. J. Clin. Pharmacol. 2018, 74, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Misaka, S.; Abe, O.; Ono, T.; Ono, Y.; Ogata, H.; Miura, I.; Shikama, Y.; Fromm, M.F.; Yabe, H.; Shimomura, K. Effects of single green tea ingestion on pharmacokinetics of nadolol in healthy volunteers. Br. J. Clin. Pharmacol. 2020, 86, 2314–2318. [Google Scholar] [CrossRef] [PubMed]
- Donovan, J.L.; Chavin, K.D.; DeVane, C.L.; Taylor, R.M.; Wang, J.S.; Ruan, Y.; Markowitz, J.S. Green tea (Camellia sinensis) extract does not alter cytochrome P450 3A4 or 2D6 activity in healthy volunteers. Drug Metab. Dispos. 2004, 32, 906–908. [Google Scholar] [CrossRef] [Green Version]
- Chow, H.H.S.; Hakim, I.A.; Vining, D.R.; Crowell, J.A.; Cordova, C.A.; Chew, W.M.; Xu, M.J.; Hsu, C.H.; Ranger-Moore, J.; Alberts, D.S. Effects of repeated green tea catechin administration on human cytochrome P450 activity. Cancer Epidemiol. Biomark. Prev. 2006, 15, 2473–2476. [Google Scholar] [CrossRef] [Green Version]
- Jang, E.H.; Choi, J.Y.; Park, C.S.; Lee, S.K.; Kim, C.E.; Park, H.J.; Kang, J.S.; Lee, J.W.; Kang, J.H. Effects of green tea extract administration on the pharmacokinetics of clozapine in rats. J. Pharm. Pharmacol. 2005, 57, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, M.; Ariyoshi, N.; Kotani, A.; Ishii, I.; Nakamura, H.; Nakasa, H.; Ida, M.; Nakamura, H.; Kimura, N.; Kimura, M.; et al. Effects of continuous ingestion of green tea or grape seed extracts on the pharmacokinetics of midazolam. Drug Metab. Pharmacokinet. 2004, 19, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Ezzeldin, E.; Asiri, Y.A.; Iqbal, M. Effects of green tea extracts on the pharmacokinetics of quetiapine in rats. Evid. Based Complement. Altern. Med. 2015, 2015, 4–7. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, R.; Yang, T.; Wei, Y.; Yang, C.; Zhou, J.; Liu, Y.; Shi, S. Inhibition effect of epigallocatechin-3-gallate on the pharmacokinetics of calcineurin inhibitors, tacrolimus, and cyclosporine A, in rats. Expert Opin. Drug Metab. Toxicol. 2021, 17, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Misaka, S.; Ono, Y.; Uchida, A.; Ono, T.; Abe, O.; Ogata, H.; Sato, H.; Suzuki, M.; Onoue, S.; Shikama, Y.; et al. Impact of Green Tea Catechin Ingestion on the Pharmacokinetics of Lisinopril in Healthy Volunteers. Clin. Transl. Sci. 2020, 14, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.; Rodrigues, M.; Marques, A.; Falcão, A.; Alves, G. Influence of the dual combination of silymarin and (−)-epigallocatechin gallate, natural dietary flavonoids, on the pharmacokinetics of oxcarbazepine in rats. Food Chem. Toxicol. 2017, 106, 446–454. [Google Scholar] [CrossRef]
- Wang, Z.; Xue, Y.; Sun, H.; Zhang, Z.; Tang, Z.J.; Liu, S.B.; Cai, W.M. Effect of tea polyphenols on the oral and intravenous pharmacokinetics of ticagrelor in rats and its in vitro metabolism. J. Food Sci. 2020, 85, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Braal, C.L.; Hussaarts, K.G.A.M.; Seuren, L.; Oomen-de Hoop, E.; de Bruijn, P.; Buck, S.A.J.; Bos, M.E.M.M.; Thijs-Visser, M.F.; Zuetenhorst, H.J.M.; Mathijssen-van Stein, D.; et al. Influence of green tea consumption on endoxifen steady-state concentration in breast cancer patients treated with tamoxifen. Breast Cancer Res. Treat. 2020, 184, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.I.; Chan, S.W.; Wong, L.L.; Tan, M.L.; Liaw, S.Y. Are first-year healthcare undergraduates at an Asian university ready for interprofessional education? J. Interprofessional Care 2013, 27, 341–343. [Google Scholar] [CrossRef] [PubMed]
- Bedrood, Z.; Rameshrad, M.; Hosseinzadeh, H. Toxicological effects of Camellia sinensis (green tea): A review. Phytother. Res. 2018, 32, 1163–1180. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Chi, N.-N.; Liu, J.-D. Green tea extract for treatment of cancers: A systematic review protocol. Medicine 2019, 98, e15117. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Farzin, N.; Taheri, S.; Mahlouji, M.; Akbari, H.; Karamali, F.; Asemi, Z. The Effect of Dietary Supplements Containing Green Tea, Capsaicin and Ginger Extracts on Weight Loss and Metabolic Profiles in Overweight Women: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Ann. Nutr. Metab. 2017, 70, 277–285. [Google Scholar] [CrossRef]
- Hartley, L.; Flowers, N.; Holmes, J.; Clarke, A.; Stranges, S.; Hooper, L.; Rees, K. Green and black tea for the primary prevention of cardiovascular disease. In The Cochrane Collaboration; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013. [Google Scholar]
- Brown, A.L.; Lane, J.; Coverly, J.; Stocks, J.; Jackson, S.; Stephen, A.; Bluck, L.; Coward, A.; Hendrickx, H. Effects of dietary supplementation with the green tea polyphenol epigallocatechin-3-gallate on insulin resistance and associated metabolic risk factors: Randomized controlled trial. Br. J. Nutr. 2008, 101, 886–894. [Google Scholar] [CrossRef]
- Zheng, X.-X.; Xu, Y.-L.; Li, S.-H.; Hui, R.; Wu, Y.J.; Huang, X.H. Effects of green tea catechins with or without caffeine on glycemic control in adults: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2013, 97, 750–762. [Google Scholar] [CrossRef] [Green Version]
- Abe, S.K.; Saito, E.; Sawada, N.; Tsugane, S.; Ito, H.; Lin, Y.; Tamakoshi, A.; Sado, J.; Kitamura, Y.; Sugawara, Y.; et al. Green tea consumption and mortality in Japanese men and women: A pooled analysis of eight population-based cohort studies in Japan. Eur. J. Epidemiol. 2019, 34, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Zhang, Z.; Zheng, T.; Yang, Y.J.; Li, N.; Bai, M.; Peng, Y.; Zhang, J.; Li, Q.; Zhang, B. Association of green tea consumption with risk of coronary heart disease in Chinese population. Int. J. Cardiol. 2015, 179, 275–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jatuworapruk, K.; Srichairatanakool, S.; Ounjaijean, S.; Kasitanon, N.; Wangkaew, S.; Louthrenoo, W. Effects of Green Tea Extract on Serum Uric Acid and Urate Clearance in Healthy Individuals. J. Clin. Rheumatol. 2014, 20, 310–313. [Google Scholar] [CrossRef] [PubMed]
- Oh, C.-M.; Oh, I.-H.; Choe, B.-K.; Yoon, T.Y.; Choi, J.M.; Hwang, J. Consuming Green Tea at Least Twice Each Day Is Associated with Reduced Odds of Chronic Obstructive Lung Disease in Middle-Aged and Older Korean Adults. J. Nutr. 2018, 148, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Ferzli, G.; Patel, M.; Phrsai, N.; Brody, N. Reduction of facial redness with resveratrol added to topical product containing green tea polyphenols and caffeine. J. Drugs Dermatol. 2013, 12, 770–774. [Google Scholar] [PubMed]
- Rabinovich, L.; Kazlouskaya, V. Herbal sun protection agents: Human studies. Clin. Dermatol. 2018, 36, 369–375. [Google Scholar] [CrossRef]
- Ikeda, S.; Kanoya, Y.; Nagata, S. Effects of a foot bath containing green tea polyphenols on interdigital tinea pedis. Foot 2013, 23, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Chopra, A.; Thomas, B.S.; Sivaraman, K.; Prasad, H.K.; Kamath, S.U. Green Tea Intake as an Adjunct to Mechanical Periodontal Therapy for the Management of Mild to Moderate Chronic Periodontitis: A Randomized Controlled Clinical Trial. Oral. Health Prev. Dent. 2016, 14, 293–303. [Google Scholar] [PubMed]
- Yuan, X.; Long, Y.; Ji, Z.; Fu, T.; Yan, M.; Zhang, L.; Su, H.; Zhang, W.; Wen, X.; Pu, Z.; et al. Green Tea Liquid Consumption Alters the Human Intestinal and Oral Microbiome. Mol. Nutr. Food Res. 2018, 62, 1800178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meltzer, S.M.; Monk, B.J.; Tewari, K.S. Green tea catechins for treatment of external genital warts. Am. J. Obstet. Gynecol. 2009, 200, e1–e233. [Google Scholar] [CrossRef] [Green Version]
- U.S. Food and Drug Administration. FDA Briefing Document—Pharmacy Compounding Advisory Committee (PCAC) Meeting 20–21 November 2017; 2017. Available online: https://www.fda.gov/media/108743/download (accessed on 28 February 2021).
- Crighton, E.; Coghlan, M.L.; Farrington, R.; Hoban, C.L.; Power, M.W.P.; Nash, C.; Mullaney, I.; Byard, R.W.; Trengove, R.; Musgrave, I.F.; et al. Toxicological screening and DNA sequencing detects contamination and adulteration in regulated herbal medicines and supplements for diet, weight loss and cardiovascular health. J. Pharm. Biomed. Anal. 2019, 176, 112834. [Google Scholar] [CrossRef]
- Smith, T.; Gillespie, M.; Eckl, V.; Reynolds, C.M. Herbal Supplement Sales in US Increase by 9.4% in 2018. Record growth driven by sales of CBD, mushrooms, and immune-health products. HerbalGram 2019, 123, 62–73. [Google Scholar]
- Gottlieb, S.; U.S. Food and Drug Administration. Statement from FDA Commissioner Scott Gottlieb, M.D. On the Agency’s New Efforts to Strengthen Regulation of Dietary Supplements by Modernizing and Reforming FDA’s Oversight, 2019. Available online: https://www.fda.gov/news-events/press-announcements/statement-fda-commissioner-scott-gottlieb-md-agencys-new-efforts-strengthen-regulation-dietary (accessed on 28 February 2021).
- Geller, A.I.; Shehab, N.; Weidle, N.J.; Rose, K.O.; Weidle, N.J.; Budnitz, D.S. Emergency Department Visits for Adverse Events Related to Dietary Supplements. N. Engl. J. Med. 2015, 373, 1531–1540. [Google Scholar] [CrossRef] [PubMed]
- Czerwiński, A.; Liebers, D.; Nowak, J.; Wietebska, M. Regulacja Rynku Suplementów Diety. Czy Polska ma Szansę Zostać Europejskim Liderem? 2019. Available online: http://pie.net.pl/wp-content/uploads/2020/03/PIE-Poland_and_Supplements-PL.pdf (accessed on 28 February 2021).
- Austin, S.B.; Yu, K.; Liu, S.H.; Dong, F.; Tefft, N. Household expenditures on dietary supplements sold for weight loss, muscle building, and sexual function: Disproportionate burden by gender and income. Prev. Med. Rep. 2017, 6, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Polish Supreme Audit Office. Supreme Audit Office on the Authorization of Dietary Supplements 2017. Available online: https://www.nik.gov.pl/aktualnosci/nik-o-dopuszczaniu-do-obrotu-suplementow-diety.html (accessed on 28 February 2021).
- Long, R.; Drawbaugh, M.L.; Davis, C.M.; Goodlett, C.R.; Williams, J.R.; Roper, R.J. Usage of and attitudes about green tea extract and Epigallocathechin-3-gallate (EGCG) as a therapy in individuals with Down syndrome. Complement. Ther. Med. 2019, 45, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Vernieri, C.; Nichetti, F.; Raimondi, A.; Pusceddu, S.; Platania, M.; Berrino, F.; de Braud, F. Diet and supplements in cancer prevention and treatment: Clinical evidences and future perspectives. Crit. Rev. Oncol. Hematol. 2018, 123, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.-N.; Deng, G.; Mao, J.J. Practical Application of “About Herbs” Website: Herbs and Dietary Supplement Use in Oncology Settings. Cancer J. 2019, 25, 357–366. [Google Scholar] [CrossRef]
- Hu, J.; Webster, D.; Cao, J.; Shao, A. The safety of green tea and green tea extract consumption in adults—Results of a systematic review. Regulatory Toxicol. Pharmacol. 2018, 95, 412–433. [Google Scholar] [CrossRef] [PubMed]
- Oketch-Rabah, H.A.; Roe, A.L.; Rider, C.V.; Bonkovsky, H.L.; Giancaspro, G.I.; Navarro, V.; Paine, M.F.; Betz, J.M.; Marles, R.J.; Casper, S.; et al. United States Pharmacopeia (USP) comprehensive review of the hepatotoxicity of green tea extracts. Toxicol. Rep. 2020, 7, 386–402. [Google Scholar] [CrossRef]
- Dekant, W.; Fujii, K.; Shibata, E.; Morita, O.; Shimotoyodome, A. Safety assessment of green tea based beverages and dried green tea extracts as nutritional supplements. Toxicol. Lett. 2017, 277, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Zheng, E.X.; Rossi, S.; Fontana, R.J.; Vuppalanchi, R.; Hoofnagle, J.H.; Khan, I.; Navarro, V.J. Risk of Liver Injury Associated with Green Tea Extract in SLIMQUICK® Weight Loss Products: Results from the DILIN Prospective Study. Drug Saf. 2016, 39, 749–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saratale, R.G.; Lee, H.S.; Koo, Y.E.; Saratale, G.D.; Kim, Y.J.; Imm, J.Y.; Park, Y. Absorption kinetics of vitamin E nanoemulsion and green tea microstructures by intestinal in situ single perfusion technique in rats. Food Res. Int. 2018, 106, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Tea Association of the, U.S.A. Inc.: Tea Fact Sheet—2021. 2021. Available online: http://www.teausa.com/teausa/images/Tea_Fact_2021.pdf (accessed on 20 May 2021).
- Statista: Volume of Tea Consumption Worldwide from 2012 to 2025. 2021. Available online: https://www.statista.com/statistics/940102/global-tea-consumption/ (accessed on 20 May 2021).
- Saldaña-Mendoza, S.A.; Ascacio-Valdés, J.A.; Palacios-Ponce, A.S.; Contreras-Esquivel, J.C.; Rodríguez-Herrera, R.; Ruiz, H.A.; Martínez-Hernandez, J.L.; Sugathan, S.; Aguilar, C.N. Use of wastes from the tea and coffee industries for the production of cellulases using fungi isolated from the Western Ghats of India. Syst. Microbiol. Biomanuf. 2021, 1, 33–41. [Google Scholar] [CrossRef]
- STiR: Home Features: The Many Uses of Tea Waste. 2020. Available online: https://stir-tea-coffee.com/features/the-many-uses-of-tea-waste/ (accessed on 20 May 2021).
- Patel, S.S.; Beer, S.; Kearney, D.L.; Phillips, G.; Carter, B.A. Green tea extract: A potential cause of acute liver failure. WJG 2013, 19, 5174. [Google Scholar] [CrossRef] [PubMed]



| Fresh tea leaves | Fixation (Panning or steaming) | Shaping (rolling) | Drying | Green tea | ||
| Wilting | Bruising and partial oxidation | Fixation (Panning or baking) | Shaping (rolling) | Drying | Oolong tea | |
| Wilting | Bruising and full oxidation | Shaping (rolling) | Drying | Black tea |
| Therapeutic Group | ||
|---|---|---|
| Drug Name Route of Administration | Animal Studies | Clinical Studies |
| statins | ||
| fluvastatin iv | [GTE] no significant change in plasma concentrations [91] | |
| rosuvastatin po | [GTE] ↑AUC, [EGCG] ↑AUC [92] | [EGCG] ↓AUC, ↓Cmax, ↑CL [74] |
| simvastatin igas | [EGCG] ↑AUC, ↑t1/2, ↓CL [93] [GTE] ↑AUC, ↓t1/2 [94] | |
| simvastatin po | [GTE] ↑AUC, ↑Cmax [95] [GTE] ↑AUC, ↑Cmax [80] | |
| calcium channel blockers | ||
| amlodypine po | [EGCG] ↑AUC, ↑Cmax, ↑t1/2, ↓CL, ↓Tmax [76] | |
| diltiazem po | [EGCG] ↑AUC, ↑Cmax, ↓CL [78] | |
| nicardipine iv | [EGCG] no significant change in plasma concentrations [77] | |
| nicardipine po | [EGCG] ↑AUC, ↑Cmax [77] | |
| verapamil po | [EGCG] ↑AUC, ↑Cmax [23] | |
| β-blockers | ||
| atenolol po | [EGCG] no significant change in serum level and tissues distribution [96] | |
| nadolol igas | [GTE] ↑AUC, [EGCG] ↑AUC [97] | |
| nadolol po | [GTE] ↓AUC, ↓Cmax [98] [GTE] ↓AUC, ↓Cmax [99] [GTE] ↓AUC, ↓Cmax [75] | |
| psycholeptics | ||
| alprazolam po | [GTE] no significant change in alprazolam pharmacokinetics [100] | |
| buspirone po | [GTE] ↑AUC [101] | |
| clozapine po | [GTE] ↓AUC, ↓Cmax [102] | |
| midazolam iv | [GTE] ↓t1/2, ↑ke [103] | |
| midazolam po | [GTE] ↑AUC, ↑Cmax, ↓CL [103] | |
| quetiapine po | [GTE] ↓AUC, ↓Cmax [104] | |
| other | ||
| caffeine po | [GTE] no significant change in caffeine plasma concentration [101] | |
| cyclosporine A iv & po | [EGCG] ↑Cmax, ↑AUC (smaller dose 3~30 mg·kg−1) [EGCG] ↓Cmax, ↓AUC, ↑CL (higher dose 100 mg·kg−1) [105] | |
| dextromethorphan po | [GTE] no significant change in urine dextromethorphan levels [101] [GTE] no significant change in dextrometorfan metabolic ratio [100] | |
| digoxin po | [GTE] ↓AUC, ↓Cmax [81] | |
| lisinopril po | [GTE] ↓AUC, ↓Cmax, no significant change in tmax or CL, the amount of lisinopril excreted into urine over 24 h in the GTE phase was significantly reduced [106] | |
| losartan po | [GTE] no significant change in urine losartan levels [101] | |
| metformin po | [EGCG] ↑serum level, higher tissues distribution [96] | |
| oxcarbazepine ip | [EGCG] ↓AUC, ↓Cmax [107] | |
| ticagrelor iv | [GTE] no significant differences in the pharmacokinetic parameters [108] | |
| ticagrelor po | [GTE] ↓AUC, ↓Cmax, ↑CL, no difference in t1/2 [108] | |
| sildenafil po | [GTE] ↑AUC, ↑Cmax, ↑t1/2, ↓ ke [79] | |
| tacrolimus iv | [EGCG] ↓Cmax, ↓AUC, ↑CL [105] | |
| tacrolimus po | [EGCG] ↓Cmax, (regardless of the dose) ↓AUC, ↑CL (higher dose 100 mg·kg−1) [105] | |
| Tamoxifen po | [GTE] no significant differences in the pharmacokinetic parameters [109] | |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerbin-Koczorowska, M.; Waszyk-Nowaczyk, M.; Bakun, P.; Goslinski, T.; Koczorowski, T. Current View on Green Tea Catechins Formulations, Their Interactions with Selected Drugs, and Prospective Applications for Various Health Conditions. Appl. Sci. 2021, 11, 4905. https://doi.org/10.3390/app11114905
Cerbin-Koczorowska M, Waszyk-Nowaczyk M, Bakun P, Goslinski T, Koczorowski T. Current View on Green Tea Catechins Formulations, Their Interactions with Selected Drugs, and Prospective Applications for Various Health Conditions. Applied Sciences. 2021; 11(11):4905. https://doi.org/10.3390/app11114905
Chicago/Turabian StyleCerbin-Koczorowska, Magdalena, Magdalena Waszyk-Nowaczyk, Paweł Bakun, Tomasz Goslinski, and Tomasz Koczorowski. 2021. "Current View on Green Tea Catechins Formulations, Their Interactions with Selected Drugs, and Prospective Applications for Various Health Conditions" Applied Sciences 11, no. 11: 4905. https://doi.org/10.3390/app11114905
APA StyleCerbin-Koczorowska, M., Waszyk-Nowaczyk, M., Bakun, P., Goslinski, T., & Koczorowski, T. (2021). Current View on Green Tea Catechins Formulations, Their Interactions with Selected Drugs, and Prospective Applications for Various Health Conditions. Applied Sciences, 11(11), 4905. https://doi.org/10.3390/app11114905










