Effects of H2S Loading Rate on the Performance of Reactive Absorption with Electrochemical Oxidation
Abstract
:1. Introduction
2. Materials and Methods
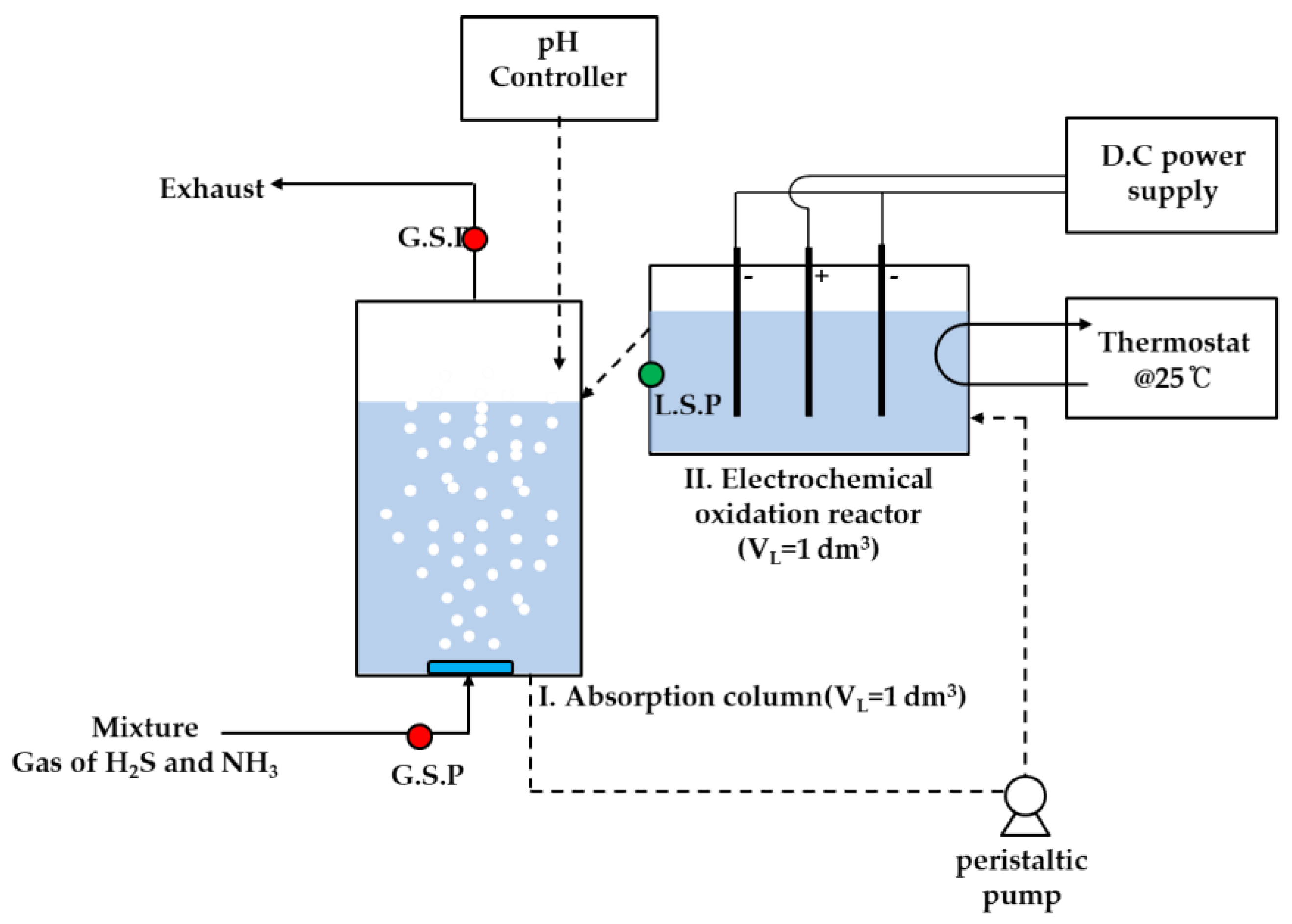
2.1. Experimental Setup
2.2. Analytical Methods
3. Model Development
3.1. Model Establishment
3.2. Numerical Analysis
4. Discussion
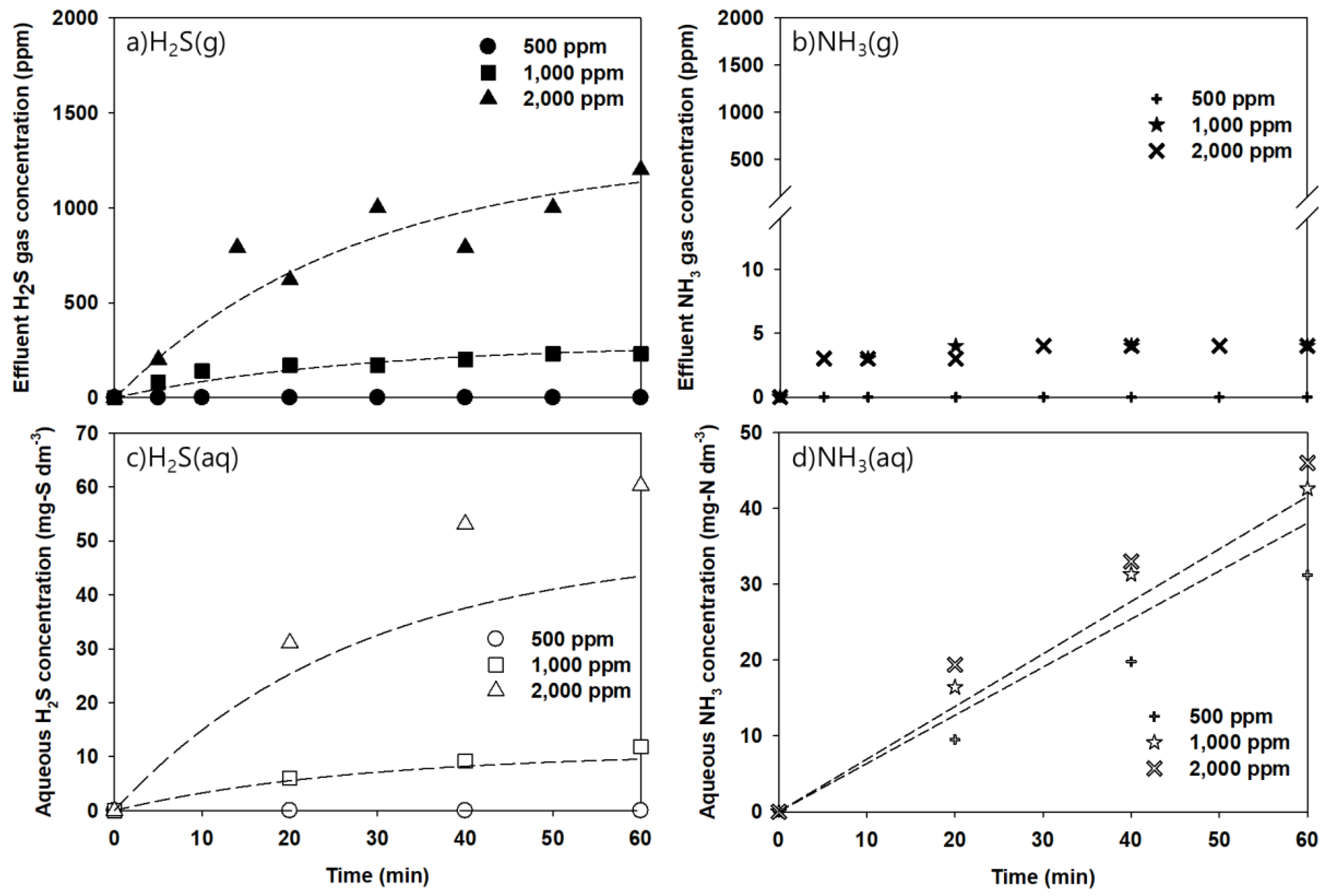
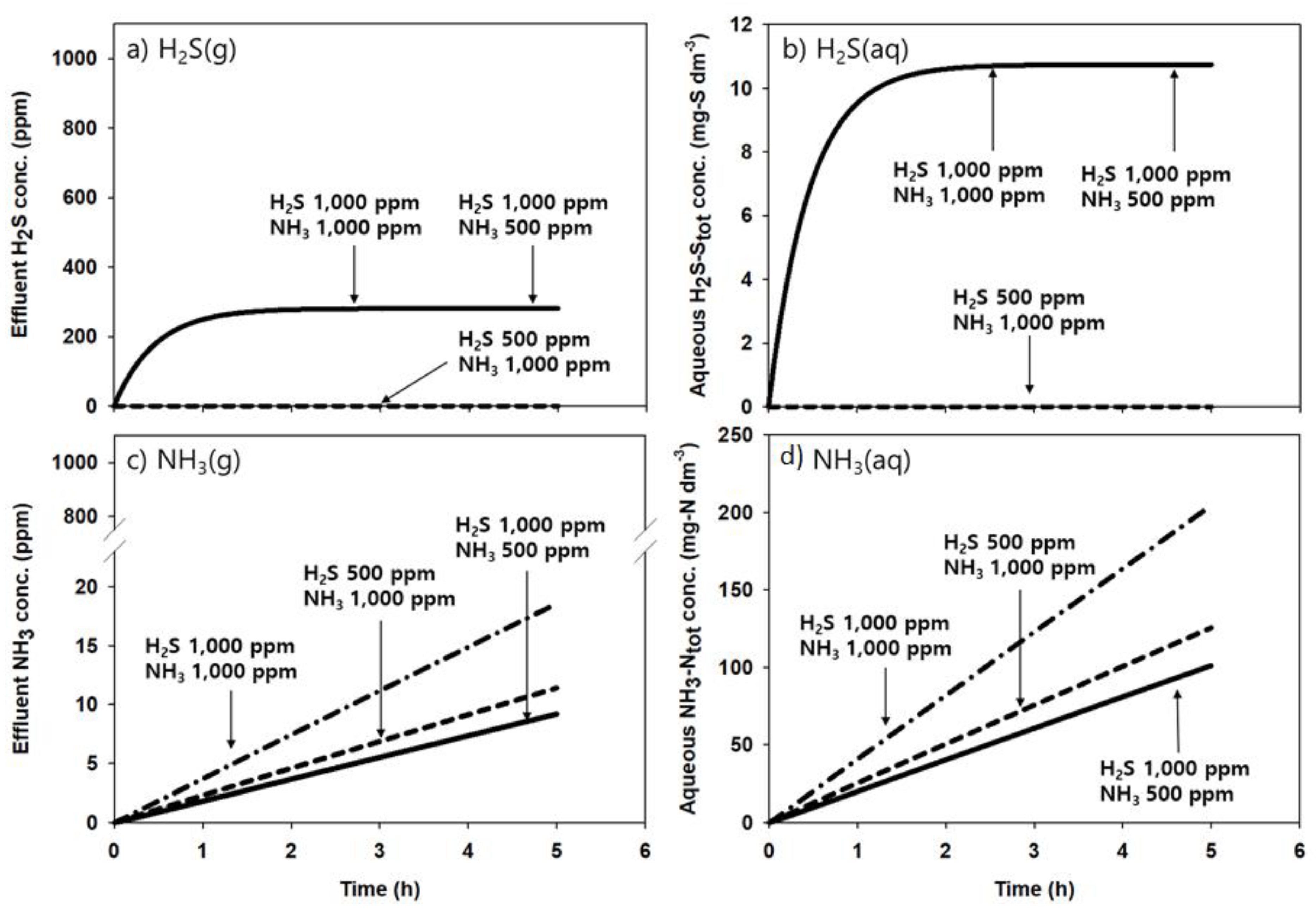
4.1. Model Validation
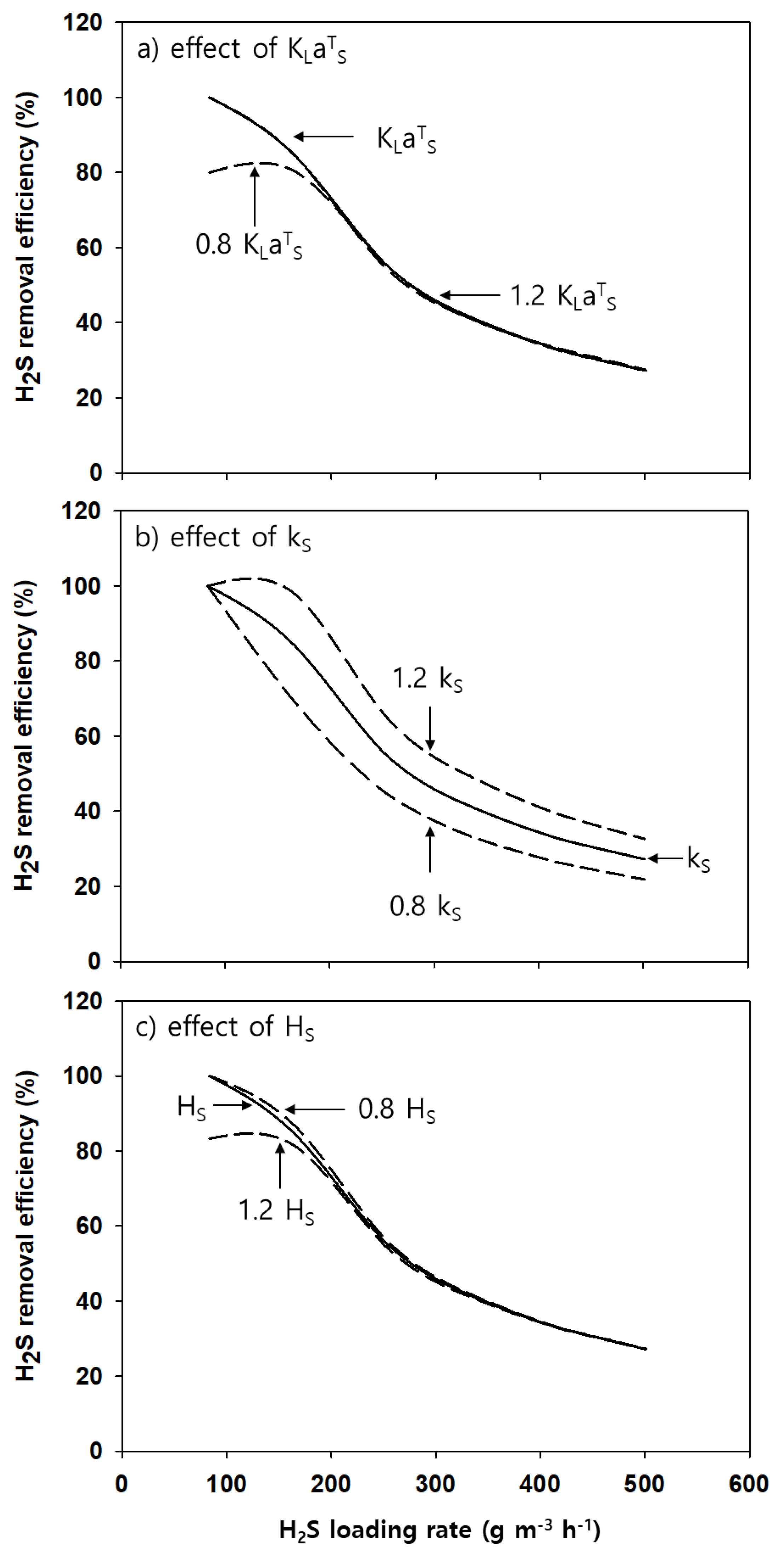
4.2. Performance Prediction
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| CLS | concentration of H2S in aqueous phase (mg dm−3) |
| CGS | concentration of H2S in gaseous phase (mg dm−3) |
| CLN | concentration of NH3 in aqueous phase (mg dm−3) |
| CGN | concentration of NH3 in gaseous phase (mg dm−3) |
| QG | gas flow rate (dm3 min−1) |
| VL | reactor volume (dm3) |
| VG | bubble volume (dm3) |
| KLaS | mass transfer coefficient of H2S (min–1) |
| KLaN | mass transfer coefficient of NH3 (min–1) |
| kS | zero-order kinetic constant H2S (mg dm−3 min−1) |
| kN | zero-order kinetic constant of NH3 (mg dm−3 min−1) |
| KI | retardation constant (mg dm−3) |
| HS | Henry’s law constant of H2S (dimensionless) |
| HN | Henry’s law constant of NH3 (dimensionless) |
References
- Qamaruz-Zaman, N.; Milke, M.W. VFA and ammonia from residential food waste as indicators of odor potential. Waste Manag. 2012, 32, 2426–2430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madhavaraj, L.; Lim, H.D.; Kim, K.M.; Kim, D.H.; Han, G.H. Influence of Sargassum horneri Mitigating odorous gas emissions from swine manure storage facilities. Sustainability 2020, 12, 7587. [Google Scholar] [CrossRef]
- Van der Heyden, C.; Demeyer, P.; Volcke, E. Mitigating emissions from pig and poultry housing facilities through air scrubbers and biofilters: State-of-the-art and perspectives. Biosyst. Eng. 2015, 134, 74–93. [Google Scholar] [CrossRef]
- He, P.; Du, W.; Xu, X.; Zhang, H.; Shao, L.; Lü, F. Effect of biochemical composition on odor emission potential of biowaste during aerobic biodegradation. Sci. Total Environ. 2020, 727, 138285. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, P.E.; Suffet, I.H. Understanding odorants associated with compost, biomass facilities, and the land application of biosolids. Water Sci. Technol. 2004, 49, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Wysocka, I.; Gębicki, J.; Namieśnik, J. Technologies for deodorization of malodorous gases. Environ. Sci. Pollut. Res. 2019, 26, 9409–9434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muthuraman, G.; Moon, I.S. A review on an electrochemically assisted-scrubbing process for environmental harmful pollutant’s destruction. J. Ind. Eng. Chem. 2012, 18, 1540–1550. [Google Scholar] [CrossRef]
- Oliveira, F.H.; Osugi, M.E.; Paschoal, F.M.; Profeti, D.; Olivi, P.; Zanoni, M.V.B. Electrochemical oxidation of an acid dye by active chlorine generated using Ti/Sn(1− x)IrxO2 electrodes. J. Appl. Electrochem. 2007, 37, 583–592. [Google Scholar] [CrossRef]
- Singh, S.; Lo, S.L.; Srivastava, V.C.; Hiwarkar, A.D. Comparative study of electrochemical oxidation for dye degradation: Parametric optimization and mechanism identification. J. Environ. Chem. Eng. 2016, 4, 2911–2921. [Google Scholar] [CrossRef]
- Chen, G. Electrochemical technologies in wastewater treatment. Sep. Purif. Techno. 2004, 38, 11–41. [Google Scholar] [CrossRef]
- Kang, J.H.; Yoon, Y.; Song, J. Simultaneous removal of hydrogen sulfide and ammonia using a combined system with absorption and electrochemical oxidation. J. Environ. Sci. Heal. A Tox. Hazard. Subst. Environ. Eng. 2019, 54, 1430–1440. [Google Scholar] [CrossRef] [PubMed]
- Koronaki, I.P.; Prentza, L.; Papaefthimiou, V. Modeling of CO2 capture via chemical absorption processes− an extensive literature review. Renew. Sustain. Energ. Rev. 2015, 50, 547–566. [Google Scholar] [CrossRef]
- Yildirim, Ö.; Kiss, A.A.; Hüser, N.; Leßmann, K.; Kenig, E.Y. Reactive absorption in chemical process industry: A review on current activities. Chem. Eng. J. 2012, 213, 371–391. [Google Scholar] [CrossRef]
- Kenig, E.Y.; Wiesner, U.; Gorak, A. Modeling of reactive absorption using the Maxwell−Stefan equations. Ind. Eng. Chem. Res. 1997, 36, 4325–4334. [Google Scholar] [CrossRef]
- Ghaemi, A.; Torab-Mostaedi, M.; Maragheh, M.G. Nonequilibrium dynamic modeling of simultaneous reactive absorption of gases. J. Taiwan Inst. Chem. Eng. 2011, 42, 173–179. [Google Scholar] [CrossRef]
- Kerr, C.P. A method for predicting the performance of packed columns operating with a reactive scrubbing liquid that control gaseous air pollutants. J. Air Waste Manag. Assoc. 2002, 52, 396–399. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.M.; Krishnamoorthi, V.; Mahmud, T. Modelling reactive absorption of CO2 in packed columns for post-combustion carbon capture applications. Chem. Eng. Res. Des. 2011, 89, 1600–1608. [Google Scholar] [CrossRef]
- Shaikh, A.A.; Abo-Ghander, N.S.; Binous, H. Modeling and parametric-sensitivity analysis of nonisothermal reactive absorption. Chem. Eng. Commun. 2017, 204, 310–320. [Google Scholar] [CrossRef]
- Azizi, M.; Biard, P.F.; Couvert, A.; Ben Amor, M.B. Competitive kinetics study of sulfide oxidation by chlorine using sulfite as reference compound. Chem. Eng. Res. Des. 2015, 94, 141–152. [Google Scholar] [CrossRef] [Green Version]
- Benjamin, M.M.; Lawler, D.F. Water Quality Engineering Physical Chemical Treatment Processes; Wiley: Hoboken, NJ, USA, 2014; p. 165. [Google Scholar]
- Martínez-Huitle, C.A.; Ferro, S. Electrochemical oxidation of organic pollutants for the wastewater treatment: Direct and indirect processes. Chem. Soc. Rev. 2006, 35, 1324–1340. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Symbol | Value | Unit |
|---|---|---|---|
| Gas-phase flow rate | QG | 2 1 | dm3 min−1 |
| Reactor volume | VL | 2 1 | dm3 |
| Bubble volume | VG | 0.08 2 | dm3 |
| Mass transfer coefficient of H2S | KLaS | 0.4 2 | min−1 |
| Mass transfer coefficient of NH3 | KLaN | 0.0007 2 | min−1 |
| Zero-order kinetic constant of H2S | kS | 1.0 2 | mg dm−3 min−1 |
| Zero-order kinetic constant of NH3 | kN | 0.3 2 | mg dm−3 min−1 |
| Retardation constant | KI | 0.001 2 | mg dm−3 |
| Henry’s law constant of H2S | HS | 0.4 3 | dimensionless |
| Henry’s law constant of NH3 | HN | 0.00695 3 | dimensionless |
| Parameter | Sensitivity Index | Rank | |
|---|---|---|---|
| −20% | +20% | ||
| KLaS | 0.2 | 5.2 | 2 |
| kS | 9.4 | 6.1 | 1 |
| HS | 0.7 | 2.9 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, J.; Song, J.; Ji, H.; Yun, S.; Kim, W.; Yoo, S. Effects of H2S Loading Rate on the Performance of Reactive Absorption with Electrochemical Oxidation. Appl. Sci. 2021, 11, 4867. https://doi.org/10.3390/app11114867
Kang J, Song J, Ji H, Yun S, Kim W, Yoo S. Effects of H2S Loading Rate on the Performance of Reactive Absorption with Electrochemical Oxidation. Applied Sciences. 2021; 11(11):4867. https://doi.org/10.3390/app11114867
Chicago/Turabian StyleKang, Jeonghee, Jihyeon Song, Hyonwook Ji, Sangleen Yun, Weonjae Kim, and Sungsoo Yoo. 2021. "Effects of H2S Loading Rate on the Performance of Reactive Absorption with Electrochemical Oxidation" Applied Sciences 11, no. 11: 4867. https://doi.org/10.3390/app11114867
APA StyleKang, J., Song, J., Ji, H., Yun, S., Kim, W., & Yoo, S. (2021). Effects of H2S Loading Rate on the Performance of Reactive Absorption with Electrochemical Oxidation. Applied Sciences, 11(11), 4867. https://doi.org/10.3390/app11114867






