Synthesis of Amorphous Conjugated Copolymers Based on Dithienosilole-Benzothiadiazole Dicarboxylic Imide with Tuned Optical Band Gaps and High Thermal Stability
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Measurements
2.3. Polymers Synthesis
3. Results and Discussion
3.1. Polymer Synthesis
3.2. Molecular Weights and Yield of the Polymers
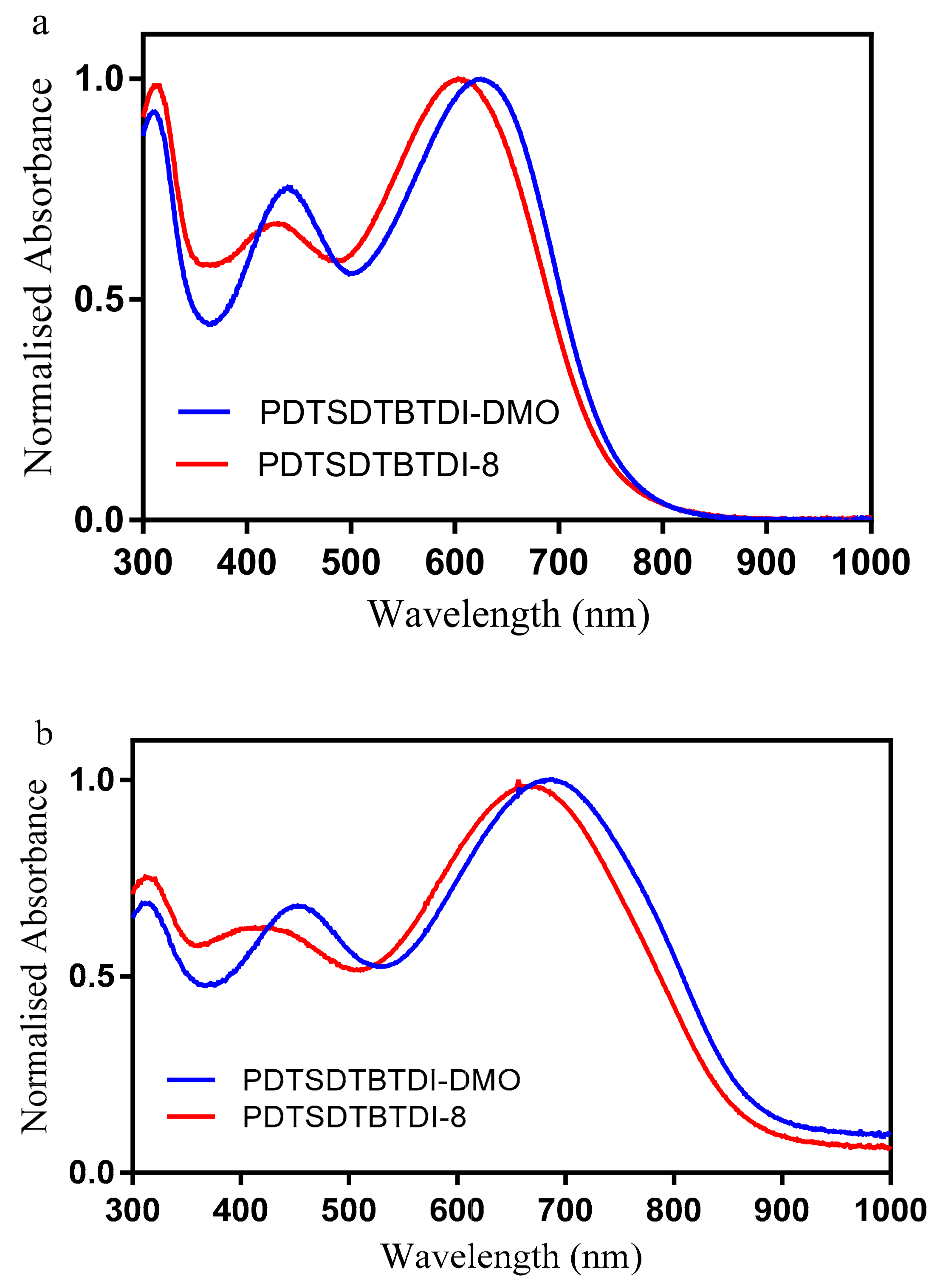
3.3. Optical Properties of the Polymers
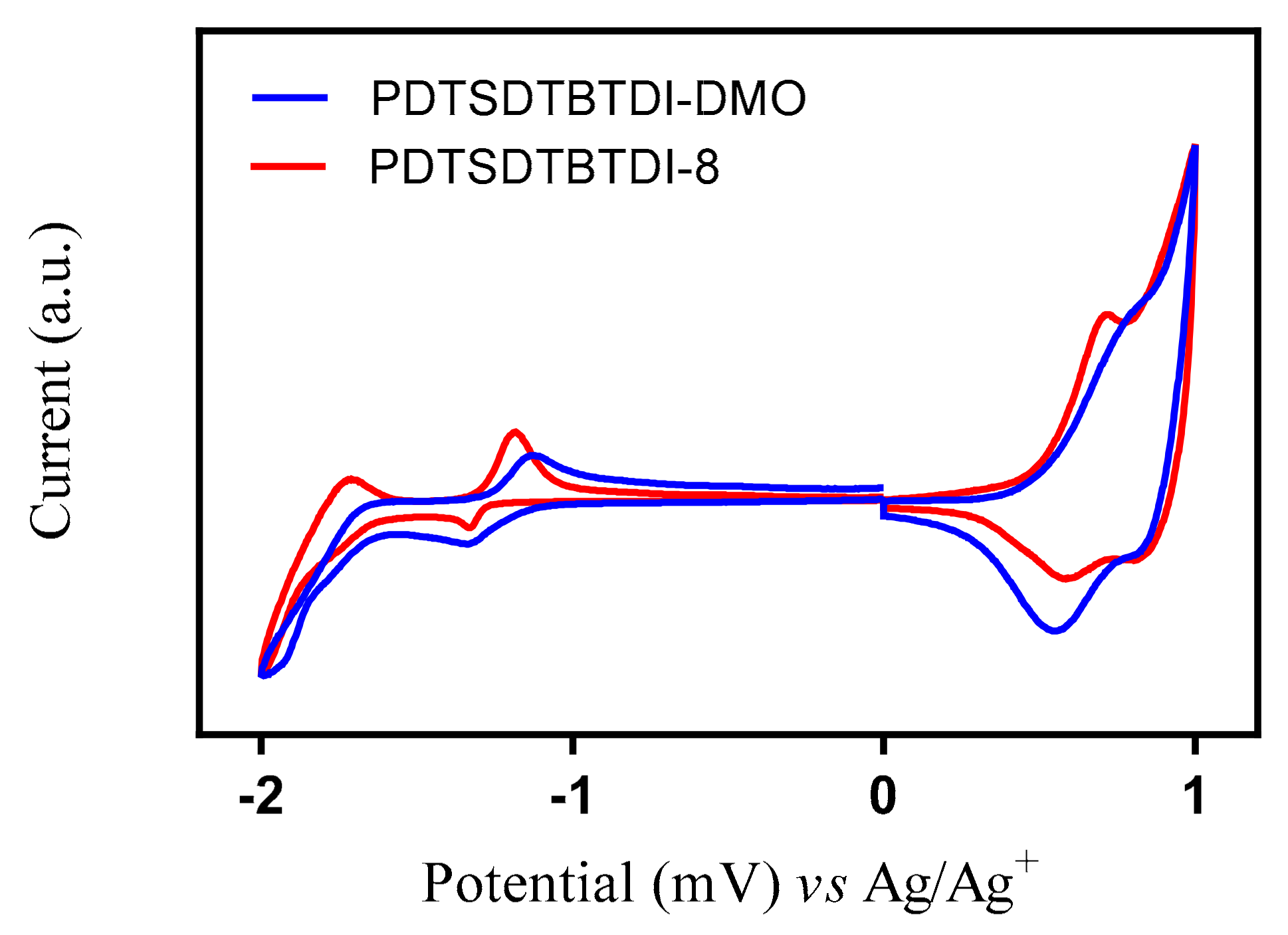
3.4. Electrochemical Properties of the Polymers
3.5. Thermal Properties of the Polymers
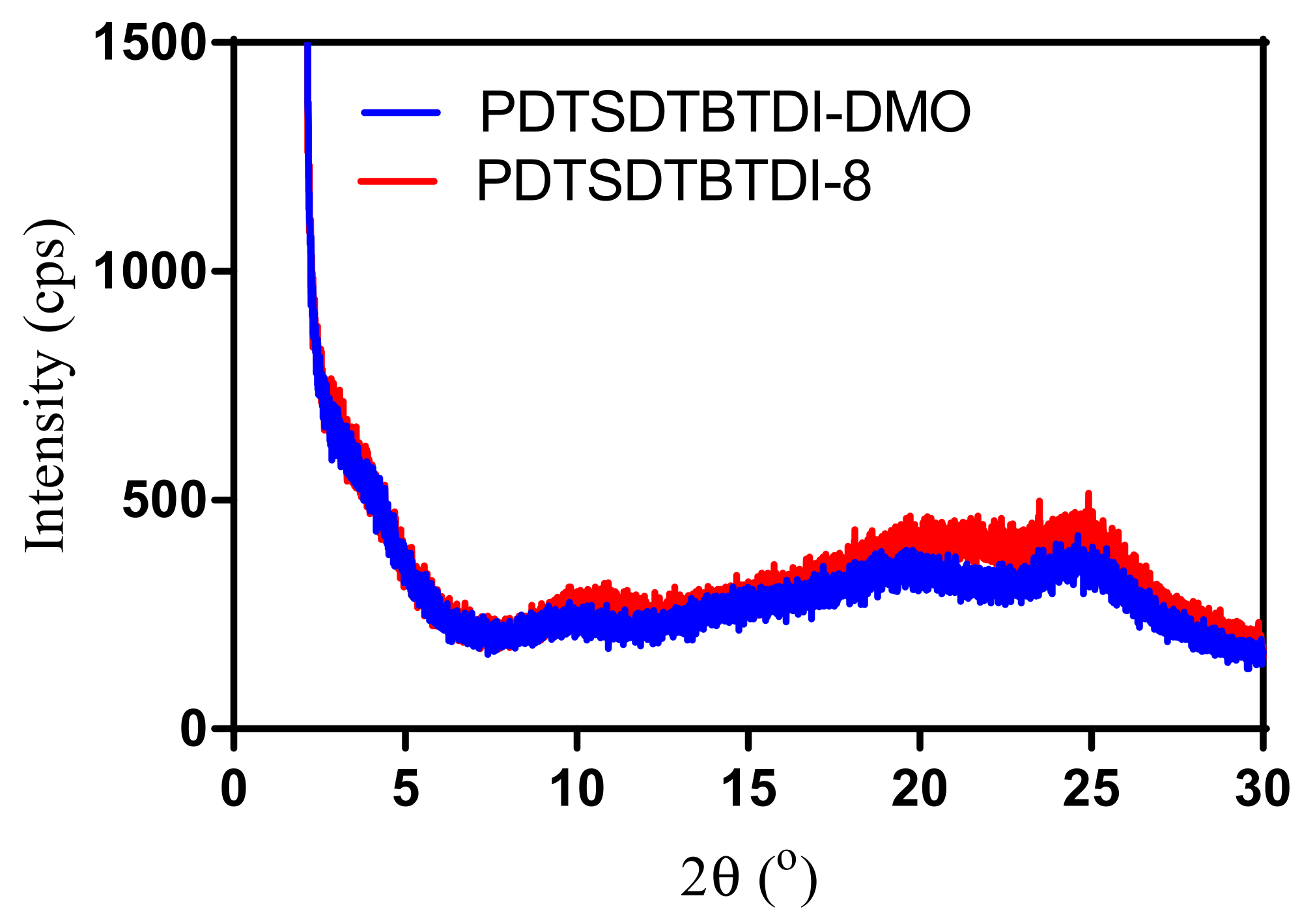
3.6. Powder X-ray Diffraction of the Polymers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, C.; Lee, S.; Kim, G.; Lee, W.; Kim, B.J. Recent advances, design guidelines, and prospects of all-polymer solar cells. Chem. Rev. 2019, 119, 8028–8086. [Google Scholar] [CrossRef] [PubMed]
- Murad, A.; Iraqi, A.; Aziz, S.; Abdullah, S.; Brza, M. Conducting polymers foroptoelectronic devices andorganic solar cells: A review. Polymers 2020, 12, 2627. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Bo, Z.; Geng, Y.; Wang, X.; Wang, L.; Ma, Y.; Hou, J.; Hu, W.; Pei, J.; Dong, H.; et al. Study on optoelectronic polymers: An overview and outlook. Acta Polym. Sin. 2019, 50, 988–1046. [Google Scholar]
- Huo, L.; Hou, J.; Zhang, S.; Chen, H.-Y.; Yang, Y. A polybenzo[1,2-b:4,5-b’]dithiophene Derivative with Deep HOMO Level and its application in high-performance polymer solar cells. Angew. Chem. Int. Ed. 2010, 49, 1500–1503. [Google Scholar] [CrossRef]
- Price, S.C.; Stuart, A.C.; Yang, L.; Zhou, H.; You, W. Fluorine Substituted conjugated polymer of medium band gap yields 7% efficiency in polymer−fullerene solar cells. J. Am. Chem. Soc. 2011, 133, 4625–4631. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Yu, L. A new class of semiconducting polymers for bulk heterojunction solar cells with exceptionally high performance. Acc. Chem. Res. 2010, 43, 1227–1236. [Google Scholar] [CrossRef] [PubMed]
- Cabanetos, C.; El Labban, A.; Bartelt, J.A.; Douglas, J.D.; Mateker, W.R.; Fréchet, J.M.J.; McGehee, M.D.; Beaujuge, P.M. Linear side chains in Benzo[1,2-b:4,5-b’]dithiophene Thieno[3,4-c]pyrrole-4,6-dione polymers direct self-assembly and solar cell performance. J. Am. Chem. Soc. 2013, 135, 4656–4659. [Google Scholar] [CrossRef]
- Liang, Y.; Xu, Z.; Xia, J.; Tsai, S.T.; Wu, Y.; Li, G.; Ray, C.; Yu, L. For the bright future-bulk heterojunction polymer solar cells with power conversion efficiency of 7.4%. Adv. Mater. 2010, 22, E135–E138. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, L.; You, W. Rational Design of high performance conjugated polymers for organic solar cells. Macromolecules 2012, 45, 607–632. [Google Scholar] [CrossRef]
- Lu, L.; Zheng, T.; Wu, Q.; Schneider, A.M.; Zhao, D.; Yu, L. Recent advances in bulk heterojunction polymer solar cells. Chem. Rev. 2015, 115, 12666–12731. [Google Scholar] [CrossRef]
- Zhang, Q.T.; Tour, J.M. Low optical band gap polythiophenes by an alternating donor/acceptor repeat unit strategy. J. Am. Chem. Soc. 1997, 119, 5065–5066. [Google Scholar] [CrossRef]
- Mei, J.; Bao, Z. Side chain engineering in solution-processable conjugated polymers. Chem. Mater. 2014, 26, 604–615. [Google Scholar] [CrossRef]
- Jhuo, H.; Yeh, P.; Liao, S.; Li, Y.; Cheng, Y.; Chen, S. Review on the recent progress in low band gap conjugated polymers for bulk hetero-junction polymer solar cells. J. Chin. Chem. Soc. 2014, 61, 115–126. [Google Scholar] [CrossRef]
- Zheng, B.; Huo, L.; Li, Y. Benzodithiophenedione-based polymers: Recentadvances in organic photovoltaics. NPG Asia Mater. 2020, 12, 3. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, C.; Pang, S.; Li, N.; Brabec, C.J.; Duan, C.; Huang, F.; Cao, Y. Ternary all-polymer solar cells with 8.5% power conversion efficiency and excellent thermal stability. Front. Chem. 2020, 8, 302. [Google Scholar] [CrossRef]
- El Karkri, A.; El Malki, Z.; Bouachrine, M.; Serein-Spirau, F.; Sotiropoulos, J. Characterization and simulation study of organicsolar cells based on donor–acceptor (D–π–A)molecular materials. RSC Adv. 2020, 10, 18816–18823. [Google Scholar] [CrossRef]
- Weng, K.; Ye, L.; Zhu, L.; Xu, J.; Zhou, J.; Feng, X.; Lu, G.; Tan, S.; Liu, F.; Sun, Y. Optimised active layer morphology toward efficient and polymer batch insensitive organic solar cells. Nat. Commun. 2020, 11, 2855. [Google Scholar] [CrossRef]
- Chen, H.Y.; Hou, J.; Hayden, A.E.; Yang, H.; Houk, K.; Yang, Y. Silicon atom substitution enhances interchain packing in a thiophene-based polymer system. Adv. Mater. 2010, 22, 371–375. [Google Scholar] [CrossRef]
- Hou, J.; Chen, H.-Y.; Zhang, S.; Li, G.; Yang, Y. Synthesis, Characterization, and photovoltaic properties of a low band gap polymer based on silole-containing polythiophenes and 2,1,3-benzothiadiazole. J. Am. Chem. Soc. 2008, 130, 16144–16145. [Google Scholar] [CrossRef]
- Coffin, R.C.; Peet, J.; Rogers, J.; Bazan, G.C. Streamlined microwave-assisted preparation of narrow-bandgap conjugated polymers for high-performance bulk heterojunction solar cells. Nat. Chem. 2009, 1, 657–661. [Google Scholar] [CrossRef]
- Huo, L.; Chen, H.-Y.; Hou, J.; Chen, T.L.; Yang, Y. Low band gap dithieno[3,2-b:2′,3′-d]silole-containing polymers, synthesis, characterisation and photovoltaic application. Chem. Commun. 2009, 37, 5570–5572. [Google Scholar] [CrossRef] [PubMed]
- Moulé, A.J.; Tsami, A.; Bünnagel, T.W.; Forster, M.; Kronenberg, N.M.; Scharber, M.; Koppe, M.; Morana, M.; Brabec, C.J.; Meerholz, K. Two novel cyclopentadithiophene-based alternating copolymers as potential donor components for high-efficiency bulk-heterojunction-type solar cells. Chem. Mater. 2008, 20, 4045–4050. [Google Scholar] [CrossRef]
- Chu, T.-Y.; Lu, J.; Beaupré, S.; Zhang, Y.; Pouliot, J.-R.; Wakim, S.; Zhou, J.; Leclerc, M.; Li, Z.; Ding, J. Bulk heterojunction solar cells using thieno[3,4-c]pyrrole-4,6-dione and dithieno[3,2-b:2′,3′-d]silole copolymer with a power conversion efficiency of 7.3%. J. Am. Chem. Soc. 2011, 133, 4250–4253. [Google Scholar] [CrossRef] [PubMed]
- Chu, T.Y.; Lu, J.; Beaupré, S.; Zhang, Y.; Pouliot, J.R.; Zhou, J.; Najari, A.; Leclerc, M.; Tao, Y. Effects of the molecular weight and the side-chain length on the photovoltaic performance of dithienosilole/thienopyrrolodione copolymers. Adv. Funct. Mater. 2012, 22, 2345–2351. [Google Scholar] [CrossRef]
- Guo, X.; Xin, H.; Kim, F.S.; Liyanage, A.D.; Jenekhe, S.A.; Watson, M.D. Thieno[3,4-c]pyrrole-4,6-dione-based donor−acceptor conjugated polymers for solar cells. Macromolecules 2010, 44, 269–277. [Google Scholar] [CrossRef]
- Li, Z.; Tsang, S.W.; Du, X.; Scoles, L.; Robertson, G.; Zhang, Y.; Toll, F.; Tao, Y.; Lu, J.; Ding, J. alternating copolymers of cyclopenta [2, 1-b; 3, 4-b′] dithiophene and Thieno [3, 4-c] pyrrole-4, 6-dione for high-performance polymer solar cells. Adv. Funct. Mater. 2011, 21, 3331–3336. [Google Scholar] [CrossRef]
- Zhang, M.; Fan, H.; Guo, X.; He, Y.; Zhang, Z.; Min, J.; Zhang, J.; Zhao, G.; Zhan, X.; Li, Y. Synthesis and photovoltaic properties of bithiazole-based donor-acceptor copolymers. Macromolecules 2010, 43, 5706–5712. [Google Scholar] [CrossRef]
- Zhang, Z.-G.; Min, J.; Zhang, S.; Zhang, J.; Zhang, M.; Li, Y. Alkyl chain engineering on a dithieno [3, 2-b: 2′, 3′-d] silole-alt-dithienylthiazolo [5, 4-d] thiazole copolymer toward high performance bulk heterojunction solar cells. Chem. Commun. 2011, 47, 9474–9476. [Google Scholar] [CrossRef]
- Cui, C.; Fan, X.; Zhang, M.; Zhang, J.; Min, J.; Li, Y. AD–A copolymer of dithienosilole and a new acceptor unit of naphtho [2, 3-c] thiophene-4, 9-dione for efficient polymer solar cells. Chem. Commun. 2011, 47, 11345–11347. [Google Scholar] [CrossRef]
- Min, J.; Zhang, Z.-G.; Zhang, S.; Zhang, M.; Zhang, J.; Li, Y. Synthesis and photovoltaic properties of D–A copolymers based on dithienosilole and benzotriazole. Macromolecules 2011, 44, 7632–7638. [Google Scholar] [CrossRef]
- Ponomarenko, S.A.; Muzafarov, A.M.; Borshchev, O.V.; Vodopyanov, E.A.; Demchenko, N.V.; Myakushev, V.D. Synthesis of bithiophenesilane dendrimer of the first generation. Russ. Chem. Bull. 2005, 54, 684–690. [Google Scholar] [CrossRef]
- Wen, L.; Rasmussen, S.C. Synthesis and structural characterisation of 2,5-dihalo-3,4-dinitrothiophenes. J. Chem. Crystallogr. 2007, 37, 387–398. [Google Scholar] [CrossRef]
- Schwiderski, R.L.; Rasmussen, S.C. Synthesis and characterization of thieno[3,4-b]pyrazine-based terthienyls: Tunable precursors for low band gap conjugated materials. J. Org. Chem. 2013, 78, 5453–5462. [Google Scholar] [CrossRef] [PubMed]
- Hailu, H.; Gebru, B.A.; Admassie, S.; Mammo, W.; Raju, V.J.; Chebude, Y. Variable denticity of a multidentate terthiophene derivative towards Ni (II) and Zn (II)–structural studies. Bull. Chem. Soc. Ethiop. 2011, 25, 221–231. [Google Scholar] [CrossRef]
- Delgado, M.R.; Hernandez, V.; Navarrete, J.L.; Tanaka, S.; Yamashita, Y. Combined spectroscopic and theoretical study of narrow band gap heterocyclic co-oligomers containing alternating aromatic donor and o-quinoid acceptor units. J. Phys. Chem. 2004, 108, 2516–2526. [Google Scholar] [CrossRef]
- Wang, L.; Cai, D.; Zheng, Q.; Tang, C.; Chen, S.; Yin, Z. Low band gap polymers incorporating a dicarboxylic imide-derived acceptor moiety for efficient polymer solar cells. ACS Macro Lett. 2013, 2, 605–608. [Google Scholar] [CrossRef]
- Nielsen, C.B.; Ashraf, R.S.; Treat, N.D.; Schroeder, B.C.; Donaghey, J.E.; White, A.J.; Stingelin, N.; McCulloch, I. 2,1,3-Benzothiadiazole-5,6-Dicarboxylic Imide—A versatile building block for additive- and annealing-free processing of organic solar cells with efficiencies exceeding 8%. Adv. Mater. 2015, 27, 948–953. [Google Scholar] [CrossRef]
- Lan, L.; Chen, Z.; Li, Y.; Ying, L.; Huang, F.; Cao, Y. Donor–acceptor conjugated polymers based on cyclic imide substituted quinoxaline or dibenzo[a,c]phenazine for polymer solar cells. Polym. Chem. 2015, 43, 7558–7569. [Google Scholar] [CrossRef]
- Matsueda, Y.; Xu, S.; Negishi, E. A novel highly enantio- and diastereoselective synthesis of vitamin E side-chain. Tetrahedron Lett. 2015, 56, 3346–3348. [Google Scholar] [CrossRef]
- Thomson, A.; O’Connor, S.; Knuckley, B.; Causey, C.P. Design, synthesis, and in vitro evaluation of an activity-based protein profiling (ABPP) probe targeting agmatine deiminases. Bioorg. Med. Chem. 2014, 22, 4602–4608. [Google Scholar] [CrossRef]
- Yue, W.; Zhao, Y.; Shao, S.; Tian, H.; Xie, Z.; Geng, Y.; Wang, F. Novel NIR-absorbing conjugated polymers for efficient polymer solar cells: Effect of alkyl chain length on device performance. J. Mater. Chem. 2009, 19, 2199–2206. [Google Scholar] [CrossRef]
- Murad, A.R.; Iraqi, A.; Aziz, S.B.; Abdullah, S.N.; Abdulwahid, R.T.; Hussen, S.A. Optical, electrochemical, thermal, and structural properties of synthesised fluorene/dibenzosilole-benzothiadiazole dicarboxylic imide alternating organic copolymers for photovoltaic applications. Coatings 2020, 10, 1147. [Google Scholar] [CrossRef]
- Murad, A.R.; Iraqi, A.; Aziz, S.B.; Hi, H.; Abdullah, S.N.; Brza, M.A.; Abdulwahid, R.T. Influence of fluorine substitution on the optical, thermal, electrochemical and structural properties of carbazole-benzothiadiazole dicarboxylic imide alternate copolymers. Polymers 2020, 12, 2910. [Google Scholar] [CrossRef] [PubMed]
- Murad, A.R.; Iraqi, A.; Aziz, S.B.; Almeataq, M.S.; Abdullah, S.N.; Brza, M.A. Characteristics of low band gap copolymers containing anthracene-benzothiadiazole dicarboxylic imide: Synthesis, optical, electrochemical, thermal and structural studies. Polymers 2021, 13, 62. [Google Scholar]
- Murad, A.R.; Iraqi, A.; Aziz, S.B.; Abdullah, S.N.; Brza, M.A.; Saeed, S.R.; Abdulwahid, R.T. Fabrication of alternating copolymers based on cyclopentadithiophene-benzothiadiazole dicarboxylic imide with reduced optical band gap: Synthesis, optical, electrochemical, thermal, and structural properties. Polymers 2021, 13, 63. [Google Scholar]
- Khor, E.; Ng, S.C.; Li, H.C.; Chai, S. Selective functionalization of 2,2’-bithiophenes. Heterocycles 1991, 32, 1805–1812. [Google Scholar] [CrossRef]
- Letizia, J.A.; Salata, M.R.; Tribout, C.M.; Facchetti, A.; Ratner, M.A.; Marks, T.J. N-Channel polymers by design: Optimising the interplay of solubilising substituents, crystal packing, and field-effect transistor characteristics in polymeric bithiophene-imide semiconductors. J. Am. Chem. Soc. 2008, 130, 9679–9694. [Google Scholar] [CrossRef]
- Beaujuge, P.M.; Tsao, H.N.; Hansen, M.R.; Amb, C.M.; Risko, C.; Subbiah, J.; Choudhury, K.R.; Mavrinskiy, A.; Pisula, W.; Brédas, J.-L. Synthetic principles directing charge transport in low-band-gap dithienosilole–benzothiadiazole copolymers. J. Am. Chem. Soc. 2012, 134, 8944–8957. [Google Scholar] [CrossRef]
- Medlej, H.; Awada, H.; Abbas, M.; Wantz, G.; Bousquet, A.; Grelet, E.; Hariri, K.; Hamieh, T.; Hiorns, R.C.; Dagron-Lartigau, C. Effect of spacer insertion in a commonly used dithienosilole/benzothiadiazole-based low band gap copolymer for polymer solar cells. Eur. Polym. J. 2013, 49, 4176–4188. [Google Scholar] [CrossRef]
- Eslami, H.; Gharibi, A.; Müller-Plathe, F. Mechanisms of nucleation and solid–solid-phase transitions in Triblock Janus Assemblies. J. Chem. Theory Comput. 2021, 17, 3. [Google Scholar] [CrossRef]
- Liu, C.; Cai, W.; Guan, X.; Duan, C.; Xue, Q.; Ying, L.; Huang, F.; Cao, Y. Synthesis of donor-acceptor copolymers based on anthracene derivatives for polymer solar cells. Polym. Chem. 2013, 4, 3949–3958. [Google Scholar] [CrossRef]
- Cartwright, L.; Taylor, L.J.; Yi, H.; Iraqi, A.; Zhang, Y.; Scarratt, N.W.; Wang, T.; Lidzey, D.G. Triisopropylsilylacetylene-functionalised anthracene-alt-benzothiadiazole copolymers for application in bulk heterojunction solar cells. RSC Adv. 2015, 5, 101607–101615. [Google Scholar] [CrossRef]
- Cartwright, L.; Neal, T.J.; Rutland, N.J.; Iraqi, A. Anthracene-thieno[3,4-c]pyrrole-4,6-dione based donor–acceptor conjugated copolymers for applications in optoelectronic devices. Polym. Adv. Technol. 2016, 27, 525–531. [Google Scholar] [CrossRef]
- Cartwright, L.; Iraqi, A.; Zhang, Y.; Wang, T.; Lidzey, D.G. Impact of fluorine substitution upon the photovoltaic properties of benzothiadiazole-fluorene alternate copolymers. RSC Adv. 2015, 5, 46386–46394. [Google Scholar] [CrossRef]
- Cartwright, L.; Yi, H.; Iraqi, A. Effect of fluorination pattern and extent on the properties of PCDTBT derivatives. New J. Chem. 2016, 40, 1655–1662. [Google Scholar] [CrossRef]
- Murad, R.; Iraqi, A.; Aziz, S.B.; Abdullah, S.N.; Abdulwahid, R.T. Synthesis, optical, thermal and structural characteristics of novel thermocleavable polymers based on phthalate esters. Polymers 2020, 12, 2791. [Google Scholar] [CrossRef]


| Polymer | % Yield | Toluene Fraction | Chloroform Fraction | ||||
|---|---|---|---|---|---|---|---|
| Mn (g mol−1) | Mw (g mol−1) | PDI | Mn (g mol−1) | Mw (g mol−1) | PDI | ||
| PDTSDTBTDI-DMO | 92 | 6200 | 20,600 | 3.2 | 14,600 | 79,900 | 5.4 |
| PDTSDTBTDI-8 | 57 | 5700 | 14,000 | 2.4 | |||
| Polymer | ε (M−1 cm−1) | Solution | Film | ||
|---|---|---|---|---|---|
| λmax (nm) | λmax (nm) | λonset (nm) | Eg (eV) | ||
| PDTSDTBTDI-DMO | 32,300 | 627 | 689 | 873 | 1.42 |
| PDTSDTBTDI-8 | 23,400 | 613 | 670 | 863 | 1.43 |
| Polymer | Td (°C) | Eox0 (V) | HOMO (eV) | Ered0 (V) | LUMO (eV) | Eg (elec) (eV) |
|---|---|---|---|---|---|---|
| PDTSDTBTDI-DMO | 357 | 0.50 | −5.21 | 1.15 | −3.56 | 1.65 |
| PDTSDTBTDI-8 | 394 | 0.52 | −5.23 | 1.26 | −3.45 | 1.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murad, A.R.; Dannoun, E.M.A.; Aziz, S.B.; Iraqi, A.; Abdullah, S.N.; Nofal, M.M.; Abdullah, R.M. Synthesis of Amorphous Conjugated Copolymers Based on Dithienosilole-Benzothiadiazole Dicarboxylic Imide with Tuned Optical Band Gaps and High Thermal Stability. Appl. Sci. 2021, 11, 4866. https://doi.org/10.3390/app11114866
Murad AR, Dannoun EMA, Aziz SB, Iraqi A, Abdullah SN, Nofal MM, Abdullah RM. Synthesis of Amorphous Conjugated Copolymers Based on Dithienosilole-Benzothiadiazole Dicarboxylic Imide with Tuned Optical Band Gaps and High Thermal Stability. Applied Sciences. 2021; 11(11):4866. https://doi.org/10.3390/app11114866
Chicago/Turabian StyleMurad, Ary R., Elham M. A. Dannoun, Shujahadeen B. Aziz, Ahmed Iraqi, Sozan N. Abdullah, Muaffaq M. Nofal, and Ranjdar M. Abdullah. 2021. "Synthesis of Amorphous Conjugated Copolymers Based on Dithienosilole-Benzothiadiazole Dicarboxylic Imide with Tuned Optical Band Gaps and High Thermal Stability" Applied Sciences 11, no. 11: 4866. https://doi.org/10.3390/app11114866
APA StyleMurad, A. R., Dannoun, E. M. A., Aziz, S. B., Iraqi, A., Abdullah, S. N., Nofal, M. M., & Abdullah, R. M. (2021). Synthesis of Amorphous Conjugated Copolymers Based on Dithienosilole-Benzothiadiazole Dicarboxylic Imide with Tuned Optical Band Gaps and High Thermal Stability. Applied Sciences, 11(11), 4866. https://doi.org/10.3390/app11114866









