Experimental Characterization of Flame Structure and Soot Volume Fraction of Premixed Kerosene Jet A-1 and Surrogate Flames
Abstract
:1. Introduction
2. Experimental Setup
2.1. Premixed Kerosene Flat Flame
2.2. TDLAS
2.3. GC
2.4. Laser Extinction
3. Results
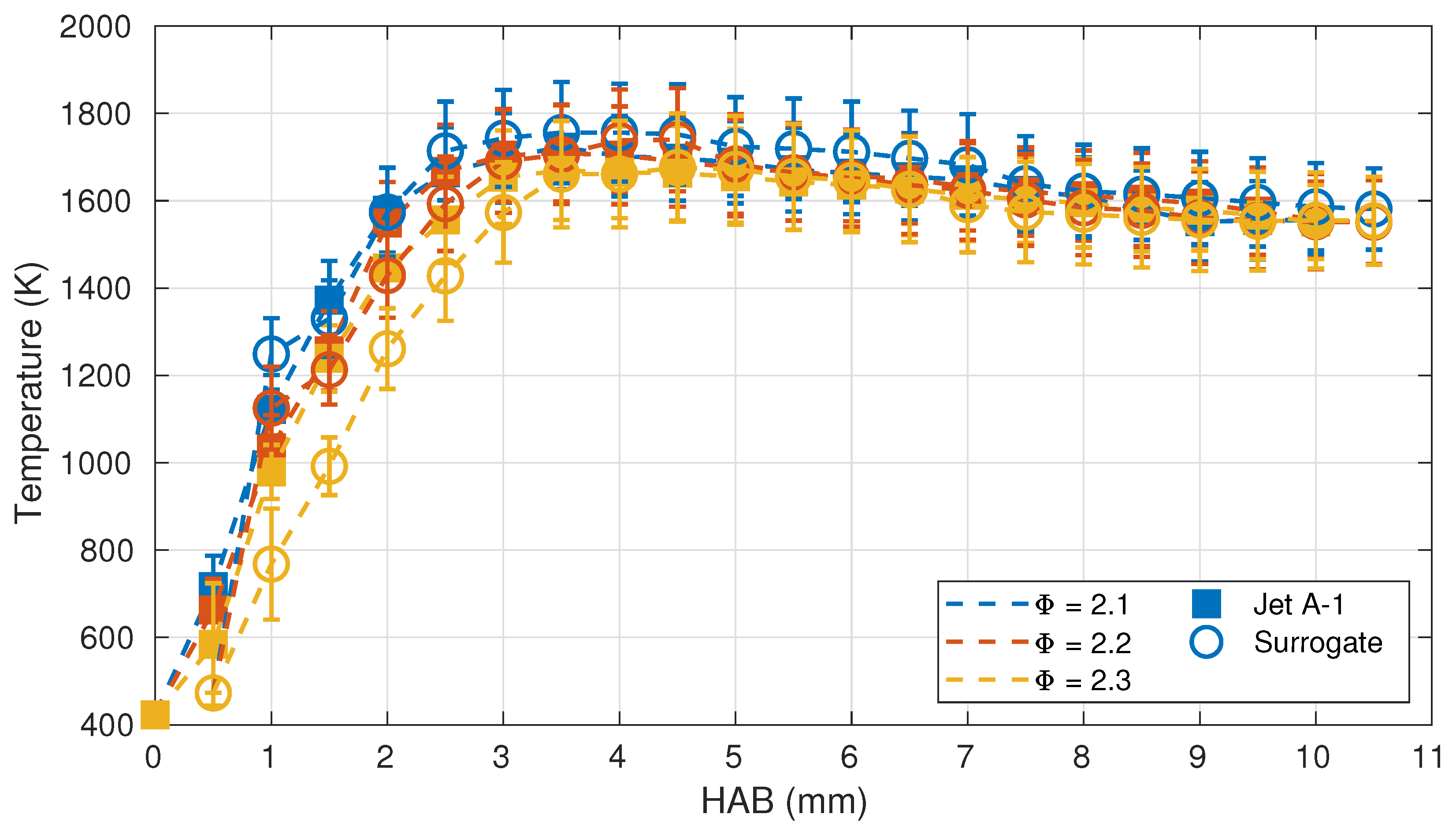
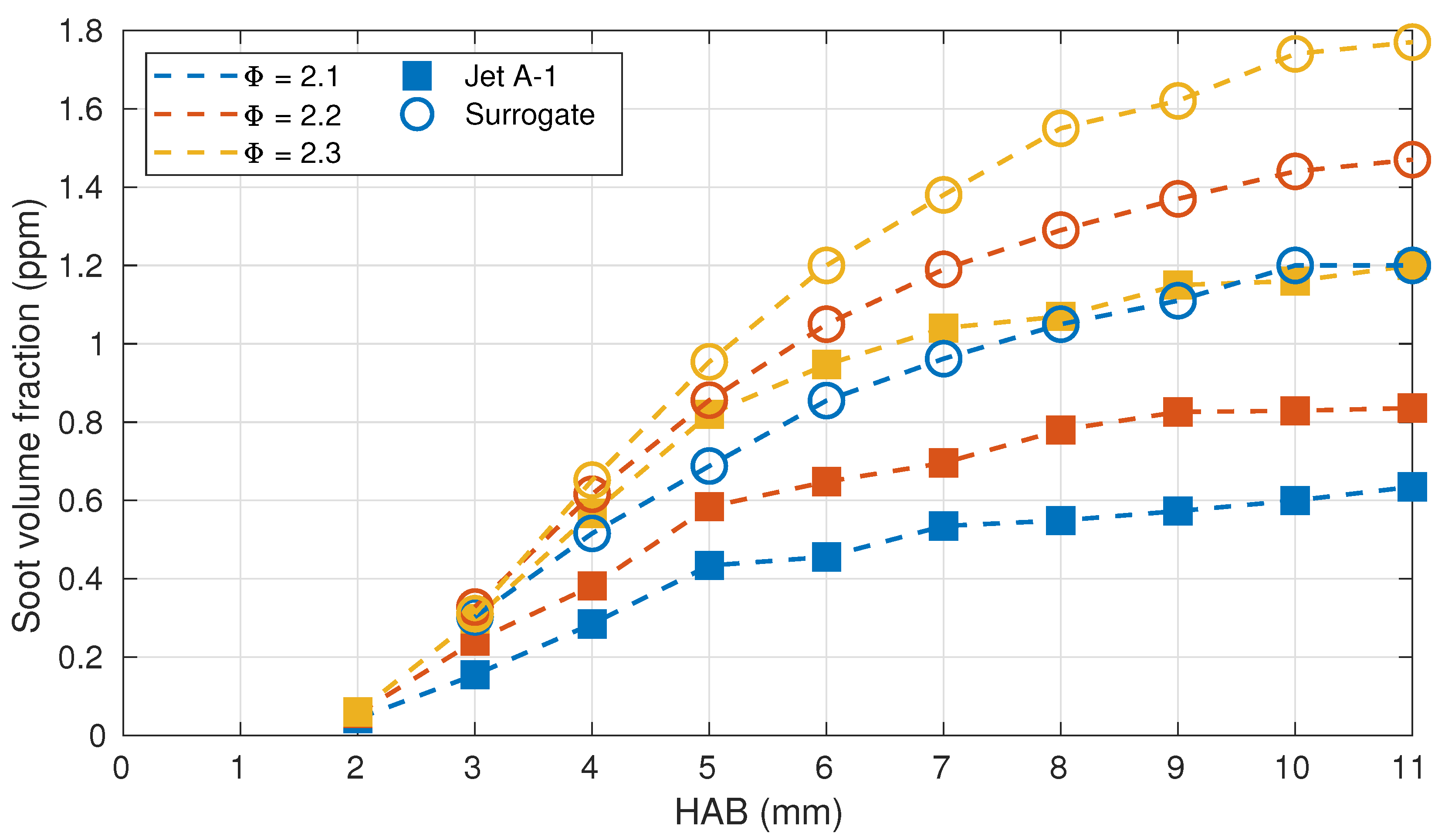
3.1. Flame Structure
3.2. Comparison with Numerical Calculations
- A reaction mechanism developed by Honnet et al. [12] for the trimethylbenzene and n-decane surrogate. By analogy with the name of the surrogate, this mechanism is referred to hereafter as the Aachen mechanism (120 species, 841 reactions).
- The mechanism developed by the University of California San Diego for JP10 [29]. It is hereafter referred to as the San Diego mechanism (61 species, 298 reactions).
- A mechanism developed by Kathrotia et al. [32] for kerosene surrogate mixtures of N-decane, cyclohexane, isooctane and toluene. Since this mechanism does not include all species necessary for the surrogate, a mixture of 42.67 vol.-% N-decane, 33.02 vol.-% isooctane and 24.31 vol.-% toluene was calculated based on Dooley et al. [33]. It is abbreviated here as DLR mechanism (189 species, 1327 reactions).
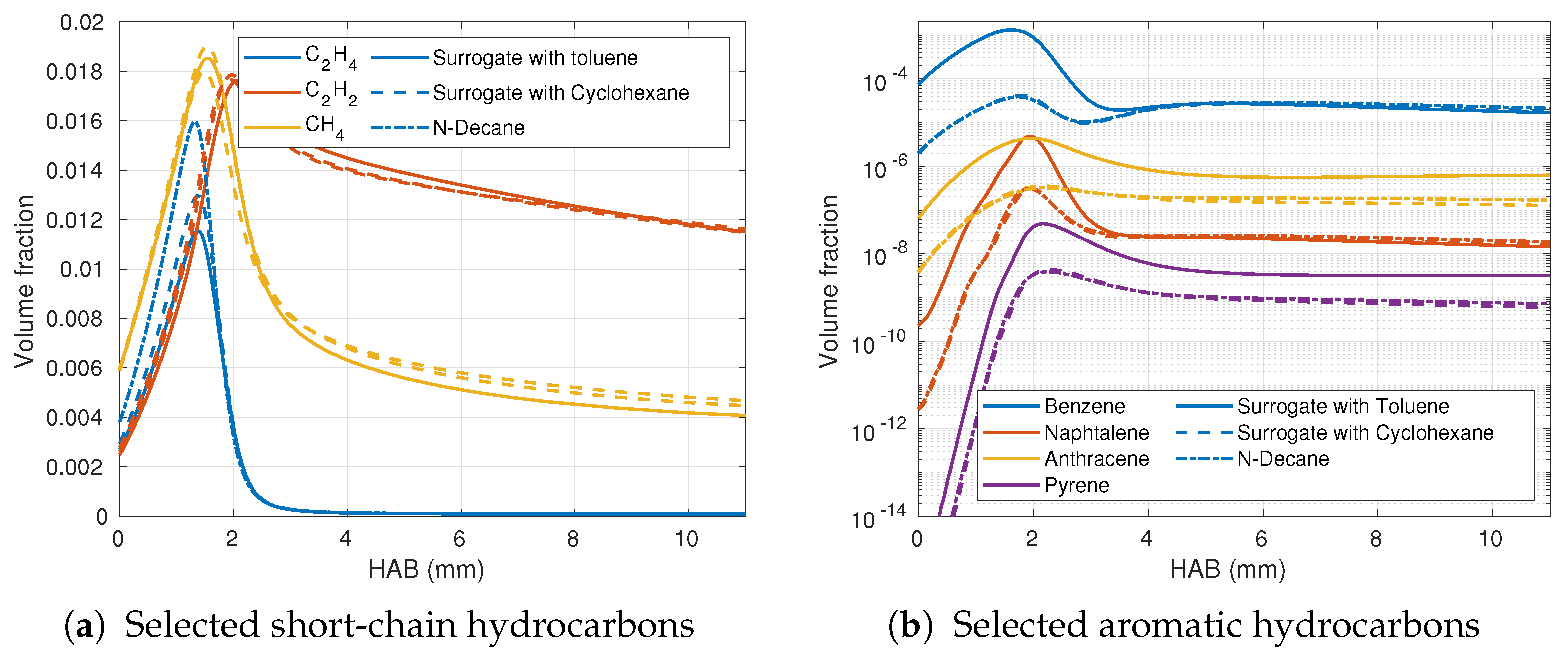
3.3. Numerical Variation of the Fuel Composition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AH | aromatic hydrocarbon |
| BAH | Bicyclic aromatic hydrocarbon |
| GC/MS | Gas chromatography–mass spectrometry |
| HAB | Height above the burner |
| MAH | Monocyclic aromatic hydrocarbon |
| PAH | Polycyclic aromatic hydrocarbon |
| TDLAS | Tunable Diode Laser Absorption Spectroscopy |
References
- Finlayson-Pitts, B.J.; Pitts, J.N. Tropospheric air pollution: Ozone, airborne toxics, polycyclic aromatic hydrocarbons, and particles. Science 1997, 276, 1045–1052. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Ambient Air Pollution: A Global Assessment of Exposure and Burden of Disease; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Bond, T.C.; Bergstrom, R.W. Light Absorption by Carbonaceous Particles: An Investigative Review. Aerosol Sci. Technol. 2006, 40, 27–67. [Google Scholar] [CrossRef]
- Iuliis, S.D.; Maffi, S.; Cignoli, F.; Zizak, G. Three-angle scattering/extinction versus TEM measurements on soot in premixed ethylene/air flame. Appl. Phys. B 2010, 102, 891–903. [Google Scholar] [CrossRef]
- Lin, H.; Gu, C.; Camacho, J.; Lin, B.; Shao, C.; Li, R.; Gu, H.; Guan, B.; Wang, H.; Huang, Z. Mobility size distributions of soot in premixed propene flames. Combust. Flame 2016, 172, 365–373. [Google Scholar] [CrossRef]
- Filippo, A.D.; Sgro, L.; Lanzuolo, G.; D’Alessio, A. Probe measurements and numerical model predictions of evolving size distributions in premixed flames. Combust. Flame 2009, 156, 1744–1754. [Google Scholar] [CrossRef]
- Saggese, C.; Singh, A.V.; Xue, X.; Chu, C.; Kholghy, M.R.; Zhang, T.; Camacho, J.; Giaccai, J.; Miller, J.H.; Thomson, M.J.; et al. The distillation curve and sooting propensity of a typical jet fuel. Fuel 2019, 235, 350–362. [Google Scholar] [CrossRef]
- Saffaripour, M.; Zabeti, P.; Kholghy, M.; Thomson, M.J. An Experimental Comparison of the Sooting Behavior of Synthetic Jet Fuels. Energy Fuels 2011, 25, 5584–5593. [Google Scholar] [CrossRef]
- Saffaripour, M.; Veshkini, A.; Kholghy, M.; Thomson, M.J. Experimental investigation and detailed modeling of soot aggregate formation and size distribution in laminar coflow diffusion flames of Jet A-1, a synthetic kerosene, and n-decane. Combust. Flame 2014, 161, 848–863. [Google Scholar] [CrossRef]
- Chong, S.T.; Raman, V.; Mueller, M.E.; Selvaraj, P.; Im, H.G. Effect of soot model, moment method, and chemical kinetics on soot formation in a model aircraft combustor. Proc. Combust. Inst. 2019, 37, 1065–1074. [Google Scholar] [CrossRef] [Green Version]
- Rachner, M. Die Stoffeigenschaften von Kerosin Jet A-1. DLR-Mitteilungen 98-01. 1998. Available online: https://elib.dlr.de/3185/ (accessed on 2 May 2021).
- Honnet, S.; Seshadri, K.; Niemann, U.; Peters, N. A surrogate fuel for kerosene. Proc. Combust. Inst. 2009, 32, 485–492. [Google Scholar] [CrossRef]
- Mairinger, G.; Frassoldati, A.; Cuoci, A.; Pelucchi, M.; Pucher, E.; Seshadri, K. Experimental and computational investigation of autoignition of jet fuels and surrogates in nonpremixed flows at elevated pressures. Proc. Combust. Inst. 2019, 37, 1605–1614. [Google Scholar] [CrossRef]
- Gehmlich, R.K.; Kuo, A.; Seshadri, K. Experimental investigations of the influence of pressure on critical extinction conditions of laminar nonpremixed flames burning condensed hydrocarbon fuels, jet fuels, and surrogates. Proc. Combust. Inst. 2015, 35, 937–943. [Google Scholar] [CrossRef]
- Vukadinovic, V.; Habisreuther, P.; Zarzalis, N. Influence of pressure and temperature on laminar burning velocity and Markstein number of kerosene Jet A-1: Experimental and numerical study. Fuel 2013, 111, 401–410. [Google Scholar] [CrossRef]
- Migliorini, F.; Deiuliis, S.; Cignoli, F.; Zizak, G. How “flat” is the rich premixed flame produced by your McKenna burner? Combust. Flame 2008, 153, 384–393. [Google Scholar] [CrossRef]
- Goldenstein, C.S.; Spearrin, R.M.; Jeffries, J.B.; Hanson, R.K. Infrared laser-absorption sensing for combustion gases. Prog. Energy Combust. Sci. 2017, 60, 132–176. [Google Scholar] [CrossRef] [Green Version]
- Bolshov, M.A.; Kuritsyn, Y.A.; Romanovskii, Y. Tunable diode laser spectroscopy as a technique for combustion diagnostics. Spectrochim. Acta Part B At. Spectrosc. 2015, 106, 45–66. [Google Scholar] [CrossRef]
- Sentko, M.M.; Schulz, S.; Stelzner, B.; Anderlohr, C.; Vicari, M.; Trimis, D. Determination of temperature and water-concentration in fuel-rich oxy-fuel methane flames applying TDLAS. Combust. Flame 2020, 214, 336–345. [Google Scholar] [CrossRef]
- Wagner, S.; Fisher, B.T.; Fleming, J.W.; Ebert, V. TDLAS-based in situ measurement of absolute acetylene concentrations in laminar 2D diffusion flames. Proc. Combust. Inst. 2009, 32, 839–846. [Google Scholar] [CrossRef]
- Wagner, S.; Klein, M.; Kathrotia, T.; Riedel, U.; Kissel, T.; Dreizler, A.; Ebert, V. In situ TDLAS measurement of absolute acetylene concentration profiles in a non-premixed laminar counter-flow flame. Appl. Phys. B 2012, 107, 585–589. [Google Scholar] [CrossRef] [Green Version]
- Teichert, H.; Fernholz, T.; Ebert, V. Simultaneous in situ measurement of CO, H2O, and gas temperatures in a full-sized coal-fired power plant by near-infrared diode lasers. Appl. Opt. 2003, 42, 2043. [Google Scholar] [CrossRef] [Green Version]
- Gordon, I.E.; Rothman, L.S.; Hill, C.; Kochanov, R.V.; Tan, Y.; Bernath, P.F.; Birk, M.; Boudon, V.; Campargue, A.; Chance, K.V.; et al. The HITRAN2016 molecular spectroscopic database. J. Quant. Spectrosc. Radiat. Transf. 2017, 203, 3–69. [Google Scholar] [CrossRef]
- Rothman, L.S.; Gordon, I.E.; Barber, R.J.; Dothe, H.; Gamache, R.R.; Goldman, A.; Perevalov, V.I.; Tashkun, S.A.; Tennyson, J. HITEMP, the high-temperature molecular spectroscopic database. J. Quant. Spectrosc. Radiat. Transf. 2010, 111, 2139–2150. [Google Scholar] [CrossRef]
- Sentko, M.; Schulz, S.; Stelzner, B.; Anderlohr, C.; Vicari, M.; Trimis, D. Experimental investigation of the pressure influence on flame structure of fuel-rich oxy-fuel methane flames for synthesis gas production. Fuel 2021, 286, 119377. [Google Scholar] [CrossRef]
- Sante, R.D. Laser extinction technique for measurements of carbon particles concentration during combustion. Opt. Lasers Eng. 2013, 51, 783–789. [Google Scholar] [CrossRef]
- Chang, H.c.; Charalampopoulos, T.T. Determination of the wavelength dependence of refractive indices of flame soot. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1990, 430, 577–591. [Google Scholar] [CrossRef]
- Goodwin, D.G.; Speth, R.L.; Moffat, H.K.; Weber, B.W. Cantera: An Object-oriented Software Toolkit for Chemical Kinetics, Thermodynamics, and Transport Processes. Version 2.4.0. 2018. Available online: https://zenodo.org/record/1174508 (accessed on 19 March 2020). [CrossRef]
- Chemical-Kinetic Mechanisms for Combustion Applications. San Diego Mechanism Web Page, Mechanical and Aerospace Engineering (Combustion Research), University of California at San Diego. Available online: http://combustion.ucsd.edu (accessed on 24 March 2020).
- Wang, H.; Xu, R.; Wang, K.; Bowman, C.T.; Hanson, R.K.; Davidson, D.F.; Brezinsky, K.; Egolfopoulos, F.N. A physics-based approach to modeling real-fuel combustion chemistry—I. Evidence from experiments, and thermodynamic, chemical kinetic and statistical considerations. Combust. Flame 2018, 193, 502–519. [Google Scholar] [CrossRef]
- Xu, R.; Wang, K.; Banerjee, S.; Shao, J.; Parise, T.; Zhu, Y.; Wang, S.; Movaghar, A.; Lee, D.J.; Zhao, R.; et al. A physics-based approach to modeling real-fuel combustion chemistry – II. Reaction kinetic models of jet and rocket fuels. Combust. Flame 2018, 193, 520–537. [Google Scholar] [CrossRef]
- Kathrotia, T.; Richter, S.; Naumann, C.; Slavinskaya, N.; Methling, T.; Braun-Unkhoff, M.; Riedel, U. Reaction Model Development for Synthetic Jet Fuels: Surrogate Fuels As a Flexible Tool to Predict Their Performance. In Proceedings of the ASME Turbo Expo 2018, Oslo, Norway, 11–15 June 2018. [Google Scholar] [CrossRef]
- Dooley, S.; Won, S.H.; Chaos, M.; Heyne, J.; Ju, Y.; Dryer, F.L.; Kumar, K.; Sung, C.J.; Wang, H.; Oehlschlaeger, M.A.; et al. A jet fuel surrogate formulated by real fuel properties. Combust. Flame 2010, 157, 2333–2339. [Google Scholar] [CrossRef]
- Ranzi, E.; Frassoldati, A.; Grana, R.; Cuoci, A.; Faravelli, T.; Kelley, A.P.; Law, C.K. Hierarchical and comparative kinetic modeling of laminar flame speeds of hydrocarbon and oxygenated fuels. Prog. Energy Combust. Sci. 2012, 38, 468–501. [Google Scholar] [CrossRef]
- Ranzi, E.; Frassoldati, A.; Stagni, A.; Pelucchi, M.; Cuoci, A.; Faravelli, T. Reduced Kinetic Schemes of Complex Reaction Systems: Fossil and Biomass-Derived Transportation Fuels. Int. J. Chem. Kinet. 2014, 46, 512–542. [Google Scholar] [CrossRef]
- Ranzi, E.; Cavallotti, C.; Cuoci, A.; Frassoldati, A.; Pelucchi, M.; Faravelli, T. New reaction classes in the kinetic modeling of low temperature oxidation of n-alkanes. Combust. Flame 2015, 162, 1679–1691. [Google Scholar] [CrossRef]
- Mitra, T.; Chu, C.; Naseri, A.; Thomson, M.J. Polycyclic aromatic hydrocarbon formation in a flame of the alkylated aromatic trimethylbenzene compared to those of the alkane dodecane. Combust. Flame 2021, 223, 495–510. [Google Scholar] [CrossRef]
- Wang, H. Formation of nascent soot and other condensed-phase materials in flames. Proc. Combust. Inst. 2011, 33, 41–67. [Google Scholar] [CrossRef]





| C/O | v (cm/s) | Jet A-1 (g/h) | O (slpm) | Ar (slpm) | |
|---|---|---|---|---|---|
| 2.1 | 0.7 | 6 | 76.29 | 1.45 | 5.47 |
| 2.2 | 0.73 | 6 | 79.81 | 1.45 | 5.46 |
| 2.3 | 0.77 | 6 | 83.32 | 1.45 | 5.45 |
| 2.1 | 0.7 | 5 | 64.93 | 1.21 | 4.56 |
| Parameter | Method | Result |
|---|---|---|
| Olefin content | ASTM D 1319 | <4 vol.-% |
| Aromatic content | 16.5 vol.-% | |
| Density (15 °C) | ASTM D 4052 | 800.8 kg/m |
| Sulfur Content | ASTM D 5453 (IP 336) | 600 mg/kg |
| Naphthalene Content | ASTM D 1840 | 1.5 vol.-% |
| Lower heating value | ASTM D 240 mod. | 43.24 MJ/kg |
| Carbon content | ASTM D 5291 | 85.0 m.-% |
| Hydrogen content | 13.1 m.-% | |
| MAH | ASTM D 6379 | 19.8 m.-% |
| BAH | 2.1 m.-% | |
| Total AH | 21.9 m.-% |
| (nm) | d (m) | d (m) | d (m) | l (mm) |
|---|---|---|---|---|
| 1345 | 1013 | 168 | 357 | 16 |
| 1392 | 1207 | 140 | 398 | 12 |
| 1535 | 1412 | 153 | 411 | 12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
von Langenthal, T.; Sentko, M.M.; Schulz, S.; Stelzner, B.; Trimis, D.; Zarzalis, N. Experimental Characterization of Flame Structure and Soot Volume Fraction of Premixed Kerosene Jet A-1 and Surrogate Flames. Appl. Sci. 2021, 11, 4796. https://doi.org/10.3390/app11114796
von Langenthal T, Sentko MM, Schulz S, Stelzner B, Trimis D, Zarzalis N. Experimental Characterization of Flame Structure and Soot Volume Fraction of Premixed Kerosene Jet A-1 and Surrogate Flames. Applied Sciences. 2021; 11(11):4796. https://doi.org/10.3390/app11114796
Chicago/Turabian Stylevon Langenthal, Thomas, Matthias Martin Sentko, Sebastian Schulz, Björn Stelzner, Dimosthenis Trimis, and Nikolaos Zarzalis. 2021. "Experimental Characterization of Flame Structure and Soot Volume Fraction of Premixed Kerosene Jet A-1 and Surrogate Flames" Applied Sciences 11, no. 11: 4796. https://doi.org/10.3390/app11114796
APA Stylevon Langenthal, T., Sentko, M. M., Schulz, S., Stelzner, B., Trimis, D., & Zarzalis, N. (2021). Experimental Characterization of Flame Structure and Soot Volume Fraction of Premixed Kerosene Jet A-1 and Surrogate Flames. Applied Sciences, 11(11), 4796. https://doi.org/10.3390/app11114796





