Cellulose Recovery from Agri-Food Residues by Effective Cavitational Treatments
Abstract
1. Introduction
2. Acoustic and Hydrodynamic Cavitation: Highlights
2.1. Acoustic Cavitation
2.2. Hydrodynamic Cavitation
3. Biomass Treatments for Cellulose Recovery
3.1. Acoustic Cavitation (AC) as Effective Biomass Pretreatment
Acoustic Cavitation Coupled with Alternative Solvents
3.2. Biomass Pretreatment under Hydrodynamic Cavitation
- ABP-90 days of operations (without feedstock pretreatment)
- ABP-UAT-90 days of operations
- ABP-HCAT-90 days of operations
3.3. Cavitational Treatments in Comparison
4. Cavitation Processes at Industrial Level
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | Acoustic cavitation |
| ABP | agricultural biogas plant |
| Bmim | 1-butyl-3-methylimiazolium ion BPR: biogas production rate |
| CBY | cumulative biogas yield |
| C-HC | continuous hydrodynamic cavitation ChOAc: choline acetate |
| CSS | coffee silverskin DES: deep eutectic solvent Dmp: dimethylphosphate |
| DP | degree of polymerization EH: enzymatic hydrolysis |
| Emim | 1-ethyl-3-methylimiazolium ion |
| FE-SEM | field emission scanning electron microscope |
| FE-RS | Fenton’s reagent process |
| FTIR | fourier transform infrared spectroscopy GAE: gallic acid equivalent |
| Glu | glucose GS: garlic skin |
| GVL | γ-valerolactone |
| HBA | hydrogen bond acceptor |
| HBD | hydrogen bond donor |
| HC | hydrodynamic cavitation |
| HCE | Hydrodynamic cavitation and enzymatic method |
| HGS | p-hydroxyphenyl-guaiacyl-syringyl |
| HVED | high voltage electrical discharges |
| IL | ionic liquid |
| MAE | microwave assisted extraction |
| NADES | natural deep eutectic solvents OP: orifice plate |
| OPEFB | oil palm empty fruit bunch fibers |
| OPF | oil palm fronds |
| RapS | rapeseed straw |
| R-RS | row rice straw |
| RS | rice straw |
| RSM | response surface methodology |
| SB-HC | sequential batches hydrodynamic cavitation |
| SCB | sugarcane bagasse |
| SC-CO2 | supercritical CO2 |
| SC-HC | semi-continuous hydrodynamic cavitation |
| SCOD | soluble chemical oxygen demand |
| SEM | scanning electron microscopy |
| SPT | Substrate Preparation Tank |
| TBAH | tetra-butylammonium hydroxide |
| TDES | ternary deep eutectic solvents |
| TGA | thermogravimetric analysis |
| UAE | ultrasound-assisted extraction |
| U/F-RS | combined US/Fenton process on rice straw |
| U-RS | ultrasound pretreated rice straw |
| US | ultrasound |
| UAT | ultrasound-assisted treatment WS: wheat straw |
| XRD | X-ray diffraction |
| Xyl | xylose |
References
- Rafiaani, P.; Kuppens, T.; Van Dael, M.; Azadi, H.; Lebailly, P.; Van Passel, S. Social sustainability assessments in the biobased economy: Towards a systemic approach. Renew. Sustain. Energy Rev. 2018, 82, 1839–1853. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Williams, C.K.; Davison, B.H.; Britovsek, G.; Cairney, J.; Eckert, C.A.; Frederick, W.J., Jr.; Hallett, J.P.; Leak, D.J.; Liotta, C.L.; et al. The path forward for biofuels and biomaterials. Science 2006, 311, 484–489. [Google Scholar] [CrossRef]
- Flores, E.M.M.; Cravotto, G.; Bizzi, C.A.; Santos, D.; Iop, G.D. Ultrasound-assisted biomass valorization to industrial interesting products: State-of-the-art, perspectives and challenges. Ultrason. Sonochem. 2021, 72, 105455. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.K.; Xu, C.; Qin, W. Biological Pretreatment of Lignocellulosic Biomass for Biofuels and Bioproducts: An Overview. Waste Biomass Valor. 2019, 10, 235–251. [Google Scholar] [CrossRef]
- Dessie, W.; Luo, X.; Wang, M.; Feng, L.; Liao, Y.; Wang, Z.; Yong, Z.; Qin, Z. Current advances on waste biomass transformation into value-added products. Appl. Microbiol. Biotechnol. 2020, 104, 4757–4770. [Google Scholar] [CrossRef] [PubMed]
- Calcio Gaudino, E.; Cravotto, G.; Manzoli, M.; Tabasso, S. Sono- and mechanochemical processes in the catalytic conversion of biomass. Chem. Soc. Rev. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—The US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Chatel, G. Chemists around the World, Take Your Part in the Circular Economy! Chem. Eur. J. 2020, 26, 9665–9673. [Google Scholar] [CrossRef]
- Imbert, E. Food waste valorization options: Opportunities from the bioeconomy. De Gruyter Open 2017, 2, 195–204. [Google Scholar] [CrossRef]
- Kuisma, M.; Kahiluoto, H.; Havukainen, J.; Lehtonen, E.; Luoranen, M.; Myllymaa, T.; Grönroos, J.; Horttanainen, M. Understanding biorefining efficiency—The case of agrifood waste. Bioresour. Technol. 2013, 135, 588–597. [Google Scholar] [CrossRef]
- Tabasso, S.; Carnaroglio, D.; Calcio Gaudino, E.; Cravotto, G. Microwave, ultrasound and ball mill procedures for bio-waste valorisation. Green Chem. 2015, 17, 684–693. [Google Scholar] [CrossRef]
- Teigiserova, D.A.; Hamelin, L.; Thomsen, M. Review of high-value food waste and food residues biorefineries with focus on unavoidable wastes from processing. Resour. Conserv. Recycl. 2019, 149, 413–426. [Google Scholar] [CrossRef]
- Valentine, J.; Clifton-Brown, J.; Hastings, A.; Robson, P.; Allison, G.; Smith, P. Food vs. fuel: The use of land for lignocellulosic ‘next generation’ energy crops that minimize competition with primary food production. GCB Bioenergy 2012, 4, 1–19. [Google Scholar] [CrossRef]
- Gupta, P.K.; Raghunath, S.S.; Prasanna, D.V.; Venkat, P.; Shree, V.; Chithananthan, C.; Choudhary, S.; Surender, K.; Geetha, K. An Update on Overview of Cellulose, Its Structure and Applications. In Cellulose; Pascual, A.R., Ed.; Chapter 4; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Jagtap, S.S.; Bedekar, A.A.; Bhatia, R.K.; Patel, A.K.; Pant, D.; Banu, J.R.; Rao, C.V.; Kim, Y.-G.; Yang, Y.-H. Recent developments in pretreatment technologies on lignocellulosic biomass: Effect of key parameters, technological improvements, and challenges. Bioresour. Technol. 2020, 300, 122724–122736. [Google Scholar] [CrossRef]
- Galbe, M.; Wallberg, O. Pretreatment for biorefineries: A review of common methods for efficient utilisation of lignocellulosic materials. Biotechnol. Biofuels 2019, 12, 294–319. [Google Scholar] [CrossRef]
- Tocco, D.; Carucci, C.; Monduzzi, M.; Salis, A.; Sanjust, E. Recent Developments in the Delignification and Exploitation of Grass Lignocellulosic Biomass. ACS Sustain. Chem. Eng. 2021, 9, 2412–2432. [Google Scholar] [CrossRef]
- Ravindran, R.; Jaiswal, A.K. A comprehensive review on pretreatment strategy for lignocellulosic food industry waste: Challenges and opportunities. Bioresour. Technol. 2016, 199, 92–102. [Google Scholar] [CrossRef]
- Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. Emerging technologies for the pretreatment of lignocellulosic biomass. Bioresour. Technol. 2018, 262, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Tezcan, E.; Atıcı, O.G. A new method for recovery of cellulose from lignocellulosic bio-waste: Pile processing. Waste Manag. 2017, 70, 181–188. [Google Scholar] [CrossRef]
- Madadi, M.; Tu, Y.; Abbas, A. Pretreatment of Lignocelollusic Biomass Based on Improving Enzymatic Hydrolysis. Int. J. Appl. Sci. Biotechnol. 2017, 5, 1–11. [Google Scholar] [CrossRef]
- Chen, H.; Liu, J.; Chang, X.; Chen, D.; Xue, Y.; Liu, P.; Lin, H.; Han, S. A review on the pretreatment of lignocellulose for high-value chemicals. Fuel Process. Technol. 2017, 160, 196–206. [Google Scholar] [CrossRef]
- Alvira, P.; Tomás-Pejó, E.; Ballesteros, M.; Negro, M.J. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review. Bioresour. Technol. 2010, 101, 4851–4861. [Google Scholar] [CrossRef]
- Moreno, A.D.; Carbone, A.; Pavone, R.; Olsson, L.; Geijer, C. Evolutionary engineered Candida intermedia exhibits improved xylose utilization and robustness to lignocellulose-derived inhibitors and ethanol. Appl. Microbiol. Biotechnol. 2019, 103, 1405–1416. [Google Scholar] [CrossRef] [PubMed]
- Parawira, W.; Tekere, M. Biotechnological strategies to overcome inhibitors in lignocellulose hydrolysates for ethanol production: Review. Crit. Rev. Biotechnol. 2011, 31, 20–31. [Google Scholar] [CrossRef]
- Agbor, V.B.; Cicek, N.; Sparling, R.; Berlin, A.; Levin, D.B. Biomass pretreatment: Fundamentals toward application. Biotechnol. Adv. 2011, 29, 675–685. [Google Scholar] [CrossRef]
- Kumar, A.K.; Sharma, S. Recent updates on different methods of pretreatment of lignocellulosic feedstocks: A review. Bioresour. Bioprocess. 2017, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; He, Y.; Yu, X.; Banks, S.W.; Yang, Y.; Zhang, X.; Yu, Y.; Liu, R.; Bridgwater, A.V. Review of physicochemical properties and analytical characterization of lignocellulosic biomass. Renew. Sustain. Energy Rev. 2017, 76, 309–322. [Google Scholar] [CrossRef]
- Clark, J.H.; Deswarte, F.E.I.; Farmer, T.J. The integration of green chemistry into future biorefineries. Biofuels Bioprod. Biorefin. 2009, 3, 72–90. [Google Scholar] [CrossRef]
- Bussemaker, M.J.; Zhang, D. Effect of US on Lignocellulosic Biomass as a Pretreatment for Biorefinery and Biofuel Applications. Ind. Eng. Chem. Res. 2013, 52, 3563–3580. [Google Scholar] [CrossRef]
- Cintas, P.; Tagliapietra, S.; Caporaso, M.; Tabasso, S.; Cravotto, G. Enabling technologies built on a sonochemical platform: Challenges and opportunities. Ultrason. Sonochem. 2015, 25, 8–16. [Google Scholar] [CrossRef]
- Mason, T.J. Practical Sonochemistry: User’s Guide to Applications. In Chemistry and Chemical Engineering; Ellis Howood Ltd.: New York, NY, USA, 1992; p. 150. [Google Scholar]
- Mason, T.J.; Peters, D. Practical Sonochemistry: Power US Uses and Applications, 2nd ed.; Woodhead Publishing: Cambridge, UK, 2002; p. 166. [Google Scholar]
- Suslick, K.S. Sonoluminescence and Sonochemistry. In Encyclopedia of Physical Science and Technology, 3rd ed.; Meyers, R.A., Ed.; Academic Press, Inc.: San Diego, CA, USA, 2002; Volume 15, pp. 363–376. [Google Scholar]
- Cravotto, G.; Calcio Gaudino, E.; Cintas, P. On the mechanochemical activation by US. Chem. Soc. Rev. 2013, 42, 7521–7534. [Google Scholar] [CrossRef] [PubMed]
- Leighton, T.G. The Acoustic Bubble; Academic Press: London, UK, 1994. [Google Scholar]
- Calcio Gaudino, E.; Colletti, A.; Grillo, G.; Tabasso, S.; Cravotto, G. Emerging Processing Technologies for the Recovery of Valuable Bioactive Compounds from Potato Peels. Foods 2020, 9, 1598. [Google Scholar] [CrossRef]
- Grillo, G.; Boffa, L.; Talarico, S.; Solarino, R.; Binello, A.; Cavaglià, G.; Bensaid, S.; Telysheva, G.; Cravotto, G. Batch and Flow Ultrasound-Assisted Extraction of Grape Stalks: Process Intensification Design up to a Multi-Kilo Scale. Antioxidants 2020, 9, 730. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Kuna, E.; Behling, R.; Valange, S.; Chatel, G.; Colmenares, J.C. Sonocatalysis: A Potential Sustainable Pathway for the Valorization of Lignocellulosic Biomass and Derivatives. Top. Curr. Chem. Z 2017, 375, 41. [Google Scholar] [CrossRef] [PubMed]
- Chatel, G.; de Oliveira, V.K.; Jérôme, F. Sonochemistry: What potential for conversion of lignocellulosic biomass into platform chemicals? ChemSusChem 2014, 7, 2774–2787. [Google Scholar] [CrossRef]
- Sharma, A.; Gogate, P.R.; Mahulkar, A.; Pandit, A.B. Modeling of hydrodynamic cavitation reactors using orifice plates considering hydrodynamics and chemical reactions occurring in bubble. Chem. Eng. J. 2008, 143, 201–209. [Google Scholar] [CrossRef]
- Yan, Y.; Thorpe, R.B. Flow Regime Transitions Due to Cavitation in Flow through an Orifice. Int. J. Multiph. Flow 1990, 16, 1023–1045. [Google Scholar] [CrossRef]
- Shah, Y.T.; Pandit, A.B.; Moholkar, V.S. Cavitation Reaction Engineering, 1st ed.; Springer Science and Business Media: New York, NY, USA, 1999. [Google Scholar]
- Gogate, P.R.; Pandit, A.B. Engineering design method for cavitational reactors: I. Sonochemical reactors. AIChE J. 2000, 46, 372–379. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Lauberte, L.; Telysheva, G.; Cravotto, G.; Andersone, A.; Janceva, S.; Dizhbite, T.; Arshanitsa, A.; Jurkjane, V.; Vevere, L. Lignin—Derived antioxidants as value-added products obtained under cavitation treatments of the wheat straw processing for sugar production. J. Clean. Prod. 2021, 303, 126369. [Google Scholar] [CrossRef]
- Leea, I.; Ohb, Y.K.; Hanc, J.I. Design optimization of hydrodynamic cavitation for effectual lipid extraction from wet microalgae. J. Environ. Chem. Eng. 2019, 7, 102942. [Google Scholar] [CrossRef]
- Wu, Z.; Ferreira, D.F.; Crudo, D.; Bosco, V.; Stevanato, L.; Costale, A.; Cravotto, G. Plant and Biomass Extraction and Valorisation under Hydrodynamic Cavitation. Processes 2019, 7, 965. [Google Scholar] [CrossRef]
- Grillo, G.; Boffa, L.; Binello, A.; Mantegna, S.; Cravotto, G.; Chemat, F.; Dizhbite, T.; Lauberte, L.; Telysheva, G. Cocoa bean shell waste valorisation; extraction from lab to pilot-scale cavitational reactors. Food Res. Int. 2019, 115, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.-Q.; You, T.-T.; Wang, W.; Xu, F.; Sun, R.-C. Synergistic benefits of ionic liquid and alkaline pretreatments of poplar wood. Part 2: Characterization of lignin and hemicelluloses. Bioresour. Technol. 2013, 136, 345–350. [Google Scholar] [CrossRef]
- Baruah, J.; Nath, B.K.; Sharma, R.; Kumar, S.; Deka, R.C.; Baruah, D.C.; Kalita, E. Recent Trends in the Pretreatment of Lignocellulosic Biomass for Value-Added Products. Front. Energy Res. 2018, 6, 141. [Google Scholar] [CrossRef]
- Chandel, A.K.; Gonçalves, B.C.M.; Strap, J.L.; da Silva, S.S. Biodelignification of lignocellulose substrates: An intrinsic and sustainable pretreatment strategy for clean energy production. Crit. Rev. Biotechnol. 2015, 35, 281–293. [Google Scholar] [CrossRef]
- Jin, M.; Dale, B.E. AFEXTM Pretreatment-Based Biorefinery Technologies. In Handbook of Biorefinery Research and Technology; Park, J.M., Ed.; Springer: Dordrecht, The Netherlands, 2018; pp. 1–16. [Google Scholar]
- Ji, Q.; Yu, X.; Yagoub, A.E.-G.A.; Chen, L.; Zhou, C. Efficient Removal of Lignin from Vegetable Wastes by Ultrasonic and Microwave-Assisted Treatment with Ternary Deep Eutectic Solvent. Ind. Crop. Prod. 2020, 149, 112357. [Google Scholar] [CrossRef]
- Moodley, P.; Gueguim Kana, E.B. Development of a Steam or Microwave-Assisted Sequential Salt-Alkali Pretreatment for Lignocellulosic Waste: Effect on Delignification and Enzymatic Hydrolysis. Energy Convers. Manag. 2017, 148, 801–808. [Google Scholar] [CrossRef]
- Sorn, V.; Chang, K.-L.; Phitsuwan, P.; Ratanakhanokchai, K.; Dong, C.-D. Effect of microwave-assisted ionic liquid/acidic ionic liquid pretreatment on the morphology, structure, and enhanced delignification of rice straw. Bioresour. Technol. 2019, 293, 121929. [Google Scholar] [CrossRef]
- Duque, A.; Manzanares, P.; Ballesteros, M. Extrusion as a Pretreatment for Lignocellulosic Biomass: Fundamentals and Applications. Renew. Energy 2017, 114, 1427–1441. [Google Scholar] [CrossRef]
- Yu, X.; Gouyo, T.; Grimi, N.; Bals, O.; Vorobiev, E. Pulsed electric field pretreatment of rapeseed green biomass (stems) to enhance pressing and extractives recovery. Bioresour. Technol. 2016, 199, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Lee, Y.Y.; Kim, T.H. A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour. Technol. 2016, 199, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, D.; Ummalyma, S.B.; Okram, A.K.; Pandey, A.; Sankar, M.; Sukumaran, R.K. Effect of dilute acid pretreatment of wild rice grass (Zizania latifolia) from Loktak Lake for enzymatic hydrolysis. Bioresour. Technol. 2018, 253, 252–255. [Google Scholar] [CrossRef] [PubMed]
- Rasid, N.S.A.; Zainol, M.M.; Amin, N.A.S. Pretreatment of agroindustry waste by ozonolysis for synthesis of biorefinery products. In Refining Biomass Residues for Sustainable Energy and Bioproducts; Kumar, R.P., Gnansounou, E., Raman, J.K., Baskar, G., Eds.; Chapter 14; Academic Press: Cambridge, MA, USA, 2020; pp. 303–336. [Google Scholar]
- Salapa, I.; Katsimpouras, C.; Topakas, E.; Sidiras, D. Organosolv pretreatment of wheat straw for efficient ethanol production using various solvents. Biomass Bioenergy 2017, 100, 10–16. [Google Scholar] [CrossRef]
- Wang, F.-L.; Li, S.; Sun, Y.-X.; Han, H.-Y.; Zhang, B.-X.; Hu, B.-Z.; Gao, Y.-F.; Hu, X.-M. Ionic liquids as efficient pretreatment solvents for lignocellulosic biomass. RSC Adv. 2017, 7, 47990–47998. [Google Scholar] [CrossRef]
- Ximenes, E.; Farinas, C.S.; Kim, Y.; Ladisch, M.R. Hydrothermal Pretreatment of Lignocellulosic Biomass for Bioethanol Production. In Hydrothermal Processing in Biorefineries; Ruiz, H., Hedegaard Thomsen, M., Trajano, H., Eds.; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Wang, W.; Yang, B.; Li, W.; Zhou, Q.; Liu, C.; Zheng, C. Effects of steam explosion pretreatment on the bioactive components and characteristics of rapeseed and rapeseed products. LWT Food Sci. Technol. 2021, 143, 111172–111181. [Google Scholar] [CrossRef]
- Chundawat, S.P.S.; Pal, R.K.; Zhao, C.; Campbell, T.; Teymouri, F.; Videto, J.; Nielson, C.; Wieferich, B.; Sousa, L.; Dale, B.E.; et al. Ammonia Fiber Expansion (AFEX) Pretreatment of Lignocellulosic Biomass. J. Vis. Exp. 2020, 2020. [Google Scholar] [CrossRef]
- Carneiro, T.F.; Timko, M.; Prado, J.M.; Berni, M. Biomass Pretreatment with Carbon Dioxide. In Biomass Fractionation Technologies for a Lignocellulosic Feedstock Based Biorefinery; Mussatto, S.I., Ed.; Chapter 17; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Bhatia, S.K.; Joo, H.-S.; Yang, Y.-H. Biowaste-to-bioenergy using biological methods—A mini-review. Energy Convers. Manag. 2018, 177, 640–660. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Kim, S.-H.; Yoon, J.-J.; Yang, Y.-H. Current status and strategies for second generation biofuel production using microbial systems. Energy Convers. Manag. 2017, 148, 1142–1156. [Google Scholar] [CrossRef]
- Sun, S.; Sun, S.; Cao, X.; Sun, R. The role of pretreatment in improving the enzymatic hydrolysis of lignocellulosic materials. Bioresour. Technol. 2016, 199, 49–58. [Google Scholar] [CrossRef]
- Kim, H.J.; Chang, J.H.; Jeong, B.-Y.; Lee, J.H. Comparison of Milling Modes as a Pretreatment Method for Cellulosic Biofuel Production. J. Clean. Energy Technol. 2013, 1, 45–48. [Google Scholar] [CrossRef]
- Moodley, P.; Kana, E.B.G. Microwave-assisted inorganic salt pretreatment of sugarcane leaf waste: Effect on physiochemical structure and enzymatic saccharification. Bioresour. Technol. 2017, 235, 35–42. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, H.; Liu, D.; Zhao, X. Pretreatment of lignocellulosic biomass for efficient enzymatic saccharification of cellulose. In Lignocellulosic Biomass to Liquid Biofuels; Yousuf, A., Pirozzi, D., Sannino, F., Eds.; Chapter 2; Academic Press: Cambridge, MA, USA, 2020; pp. 17–65. [Google Scholar]
- Alayoubi, R.; Mehmood, N.; Husson, E.; Kouzayha, A.; Tabcheh, M.; Chaveriat, L.; Sarazin, C.; Gosselin, I. Low temperature ionic liquid pretreatment of lignocellulosic biomass to enhance bioethanol yield. Renew. Energy 2020, 145, 1808–1816. [Google Scholar] [CrossRef]
- Taherzadeh, M.J.; Karimi, K. Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: A review. Int. J. Mol. Sci. 2008, 9, 1621–1651. [Google Scholar] [CrossRef] [PubMed]
- Iskalieva, A.; Yimmou, B.M.; Gogate, P.R.; Horvath, M.; Horvath, P.G.; Csoka, L. Cavitation assisted delignification of wheat straw: A review. Ultrason. Sonochem. 2012, 19, 984–993. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Bharadwaja, S.T.P.; Yadav, P.K.; Moholkar, V.S.; Goyal, A. Mechanistic Investigation in US-Assisted (Alkaline) Delignification of Parthenium hysterophorus Biomass. Ind. Eng. Chem. Res. 2014, 53, 14241–14252. [Google Scholar] [CrossRef]
- He, Z.; Wang, Z.; Zhao, Z.; Yi, S.; Mu, J.; Wang, X. Influence of US pretreatment on wood physiochemical structure. Ultrason. Sonochem. 2017, 34, 136–141. [Google Scholar] [CrossRef]
- Brahim, M.; El Kantar, S.; Boussetta, N.; Grimi, N.; Brosse, N.; Vorobiev, E. Delignification of rapeseed straw using innovative chemo-physical Pretreatments. Biomass Bioenergy 2016, 95, 92–98. [Google Scholar] [CrossRef]
- Brahim, M.; Checa Fernandez, B.L.; Regnier, O.; Boussetta, N.; Grimi, N.; Sarazin, C.; Husson, E.; Vorobiev, E.; Brosse, N. Impact of USs and high voltage electrical discharges on physico-chemical properties of rapeseed straw’s lignin and pulps. Bioresour. Technol. 2017, 237, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Brahim, M.; Boussetta, N.; Grimi, N.; Vorobiev, E.; Brosse, N. Innovative physically-assisted soda fractionation of rapeseed hulls for better recovery of biopolymers. RSC Adv. 2016, 6, 19833–19842. [Google Scholar] [CrossRef]
- Garcia, A.; Alriols, M.G.; Llano-Ponte, R.; Labidi, J. US-assisted fractionation of the lignocellulosic material. Bioresour. Technol. 2011, 102, 6326–6330. [Google Scholar] [CrossRef] [PubMed]
- Subhedar, P.B.; Gogate, P.R. Alkaline and US assisted alkaline pretreatment for intensification of delignification process from sustainable raw-material. Ultrason. Sonochem. 2014, 21, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Yunus, R.; Salleh, S.F.; Abdullah, N.; Biak, D.R.A. Effect of ultrasonic pretreatment on low temperature acid hydrolysis of oil palm empty fruit bunch. Bioresour. Technol. 2010, 101, 9792–9796. [Google Scholar] [CrossRef]
- Kandasamy, M.; Hamawand, I.; Bowtell, L.; Seneweera, S.; Chakrabarty, S.; Yusaf, T.; Shakoor, Z.; Algayyim, S.; Eberhard, F. Investigation of Ethanol Production Potential from Lignocellulosic Material without Enzymatic Hydrolysis Using the US Technique. Energies 2017, 10, 62. [Google Scholar] [CrossRef]
- Guang, Y.; Jianlong, W. Ultrasound combined with dilute acid pretreatment of grass for improvement of fermentative hydrogen production. Bioresour. Technol. 2019, 275, 10–18. [Google Scholar]
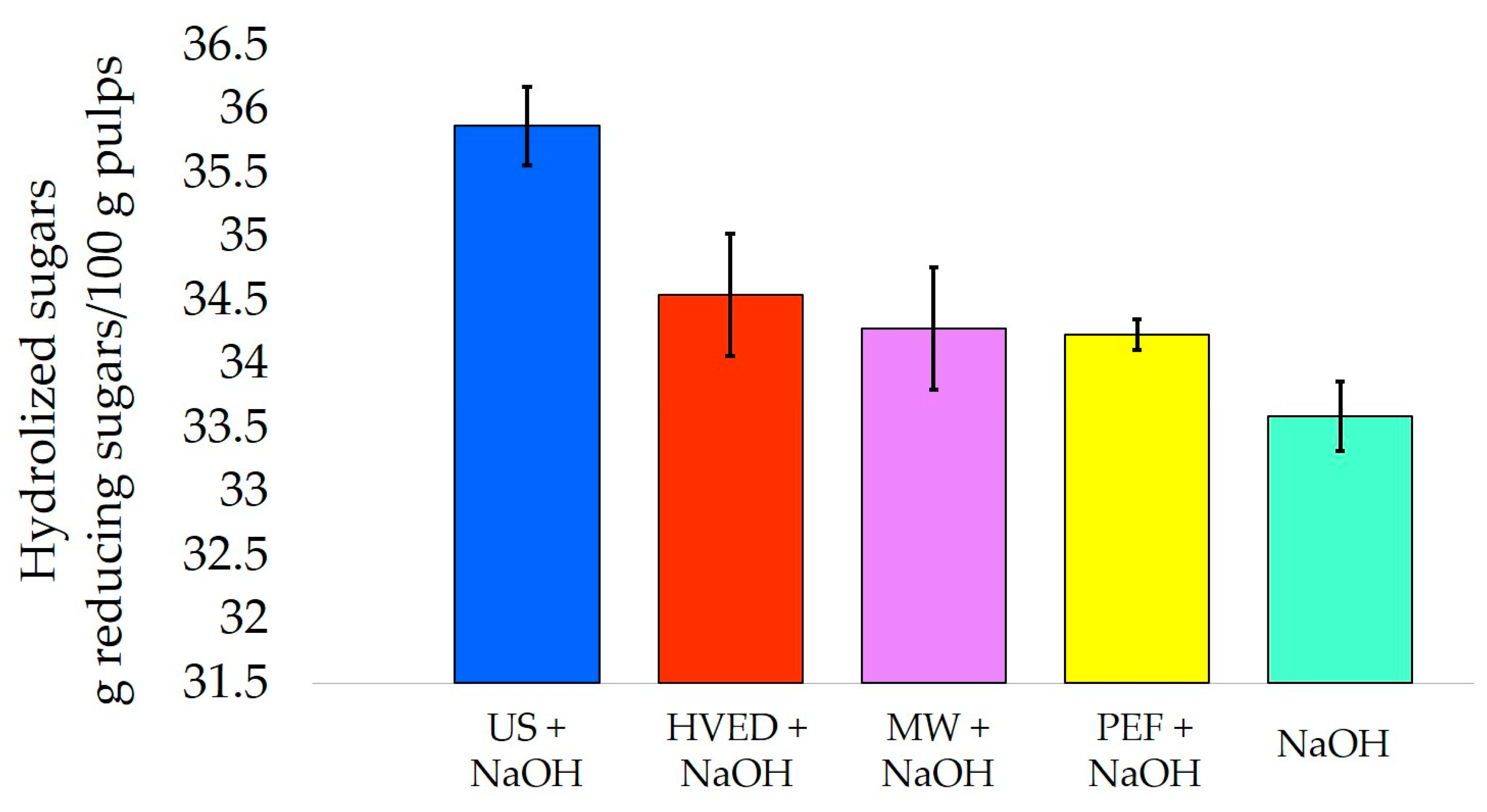
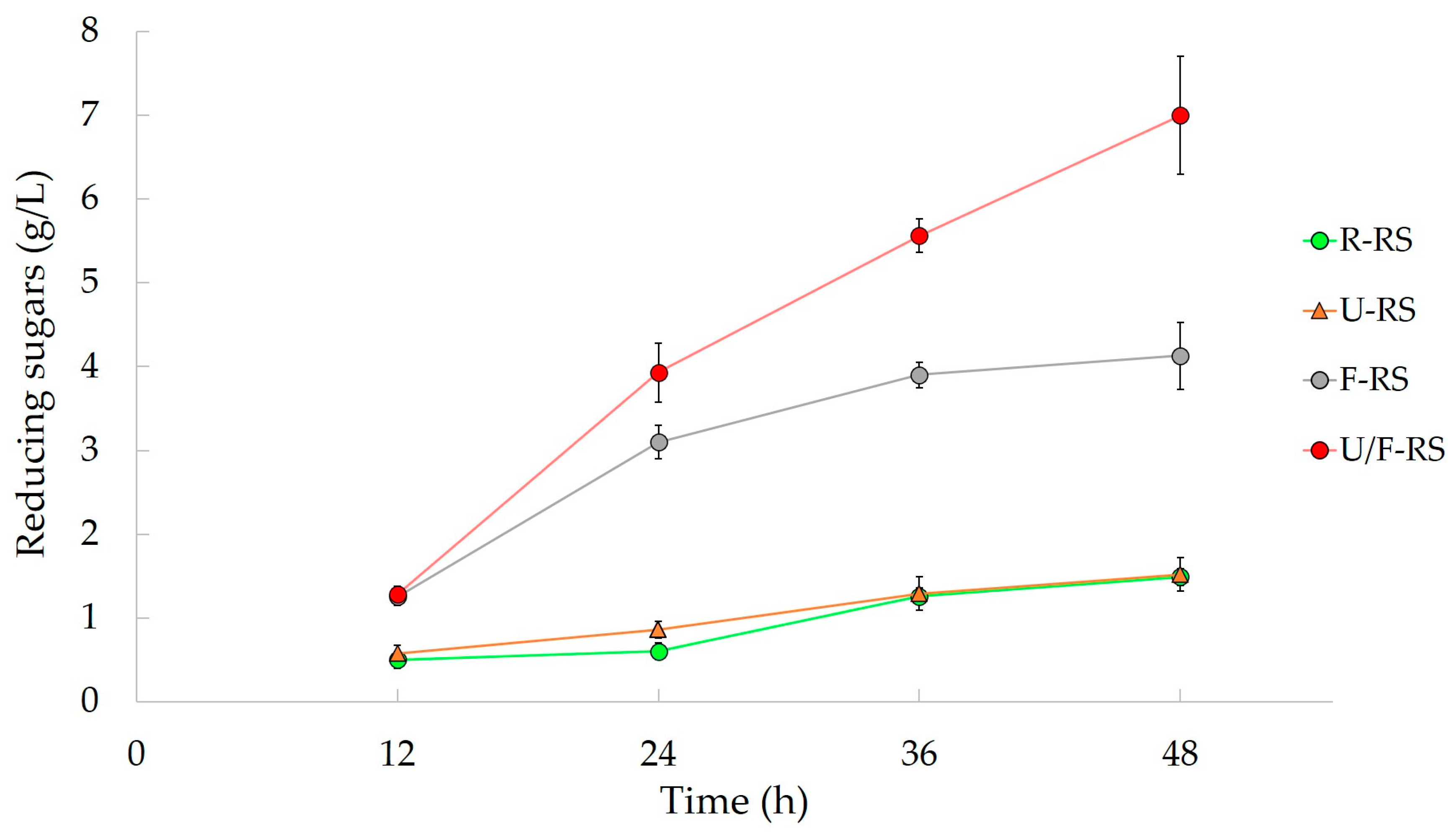
- Xiong, Z.-Y.; Qin, Y.-H.; Ma, J.-Y.; Yang, L.; Wu, Z.-K.; Wang, T.-L.; Wang, W.-G.; Wang, C.-W. Pretreatment of rice straw by US-assisted Fenton process. Bioresour. Technol. 2017, 227, 408–411. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Dai, X.; Zhou, S.-L.; Gan, Y.-Y.; Xiong, Z.-Y.; Qin, Y.-H.; Ma, J.; Yang, L.; Wu, Z.-K.; Wang, T.-L.; et al. US-assisted alkaline pretreatment for enhancing the enzymatic hydrolysis of rice straw by using the heat energy dissipated from ultrasonication. Bioresour. Technol. 2017, 241, 70–74. [Google Scholar] [CrossRef] [PubMed]
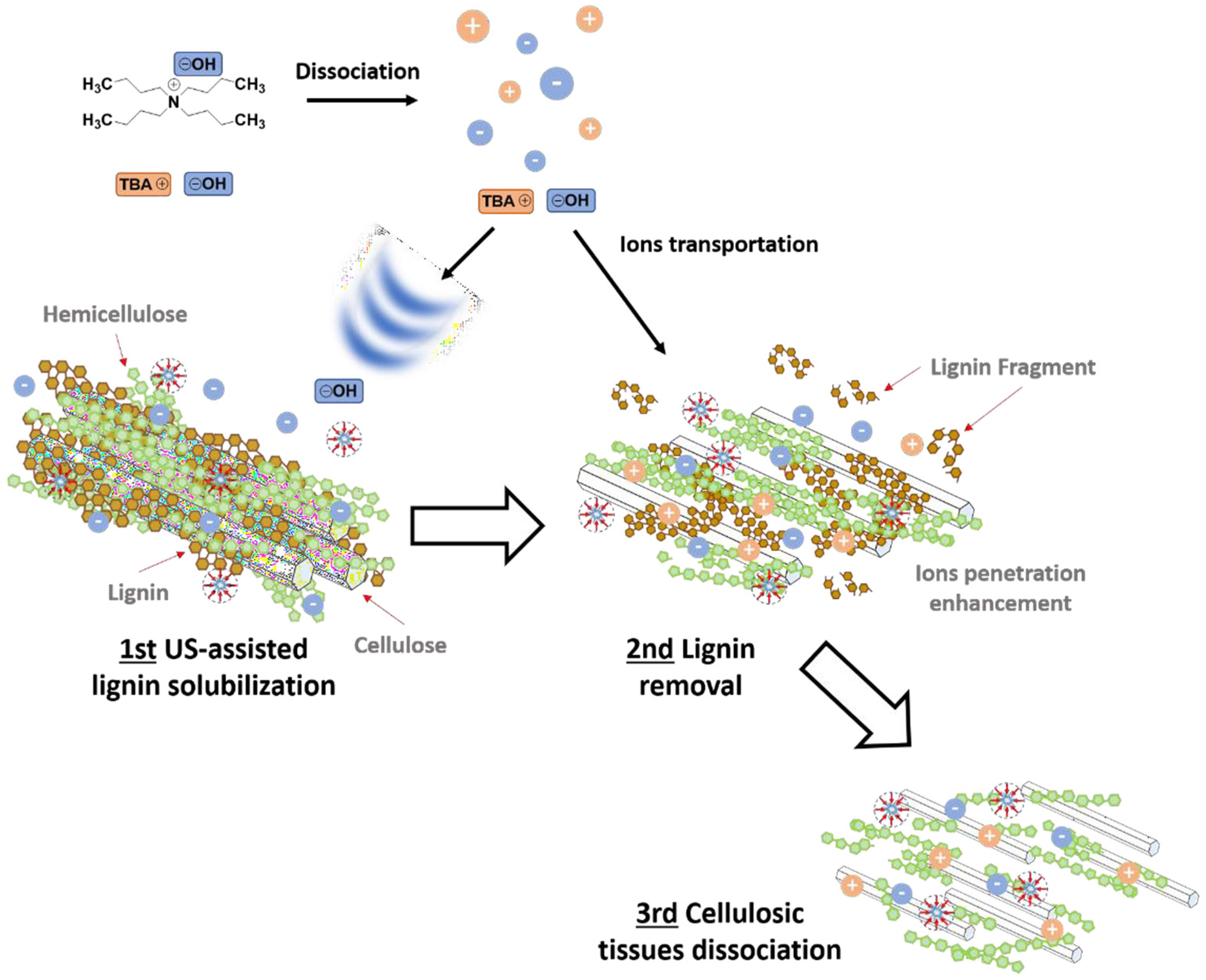
- Zhong, H.; Jia, C.; Wei, P. Enhanced saccharification of wheat straw with the application ofultrasonic-assisted quaternary ammonium hydroxide pretreatment. Process Biochem. 2017, 53, 180–187. [Google Scholar] [CrossRef]
- Ivetić, D.Ž.; Omorjan, R.P.; Đorđević, T.R.; Antov, M.G. The Impact of US Pretreatment on the Enzymatic Hydrolysis of Cellulose from Sugar Beet Shreds: Modeling of the Experimental Results. Environ. Prog. Sustain. 2017, 36, 1164–1172. [Google Scholar] [CrossRef]
- Niglio, S.; Procentese, A.; Russo, M.E.; Sannia, G.; Marzocchella, A. US-assisted Dilute Acid Pretreatment of Coffee Silverskin for Biorefinery Applications. Chem. Eng. Trans. 2017, 57, 109–114. [Google Scholar]
- Niglio, S.; Procentese, A.; Russo, M.E.; Sannia, G.; Marzocchella, A. Combined pretreatments of coffee silverskin to enhance fermentable sugar yield. Biomass Convers. Biorefinery 2020, 10, 1237–1249. [Google Scholar] [CrossRef]
- Conde, T.; Mussatto, S.I. Isolation of polyphenols from spent coffee grounds and silverskin by mild hydrothermal pretreatment. Prep. Biochem. Biotechnol. 2016, 46, 406–409. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, R.; Binod, P.; Mathew, A.K.; Abraham, A.; Gnansounou, E.; Ummalyma, S.B.; Thomas, L.; Pandey, A. Development of a novel US-assisted alkali pretreatment strategy for the production of bioethanol and xylanases from chili post-harvest residue. Bioresour. Technol. 2017, 242, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-X.; Li, M.-F.; Yu, P.; Fan, Y.-M.; Shou, J.-N.; Sun, R.-C. Valorization of bamboo by γ-valerolactone/acid/water to produce digestible cellulose, degraded sugars and lignin. Bioresour. Technol. 2017, 230, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Yi, J.; Tong, D.; Hu, C. Production of γ-valerolactone via selective catalytic conversion of hemicellulose in pubescens without addition of external hydrogen. Green Chem. 2016, 18, 848–857. [Google Scholar] [CrossRef]
- Tabasso, S.; Grillo, G.; Carnaroglio, D.; Calcio Gaudino, E.; Cravotto, G. Microwave-Assisted γ-Valerolactone Production for Biomass Lignin Extraction: A Cascade Protocol. Molecules 2016, 21, 413. [Google Scholar] [CrossRef]
- Zhang, P.; Dong, S.-J.; Ma, H.-H.; Zhang, B.-X.; Wang, Y.-F.; Hu, X.-M. Fractionation of corn stover into cellulose, hemicellulose and lignin using a series of ionic liquids. Ind. Crop. Prod. 2015, 76, 688–696. [Google Scholar] [CrossRef]
- Sharma, V.; Nargotra, P.; Kumar Bajaj, B.K. Ultrasound and surfactant assisted ionic liquid pretreatment of sugarcane bagasse for enhancing saccharification using enzymes from an ionic liquid tolerant Aspergillus assiutensis VS34. Bioresour. Technol. 2019, 285, 121319. [Google Scholar] [CrossRef]
- Ninomiya, K.; Ohta, A.; Omote, S.; Ogino, C.; Takahashi, K.; Shimizu, N. Combined use of completely bio-derived cholinium ionic liquids and US irradiation for the pretreatment of lignocellulosic material to enhance enzymatic saccharification. Chem. Eng. J. 2013, 215–216, 811–818. [Google Scholar] [CrossRef]
- Ninomiya, K.; Kohori, A.; Tatsumi, M.; Osawa, K.; Endo, T.; Kakuchi, R.; Ogino, C.; Shimizu, N.; Takahashi, K. Ionic liquid/US pretreatment and in situ enzymatic saccharification of bagasse using biocompatible cholinium ionic liquid. Bioresour. Technol. 2015, 176, 169–174. [Google Scholar] [CrossRef]
- Yang, F.; Li, L.; Li, Q.; Tan, W.; Liu, W.; Xian, M. Enhancement of enzymatic in situ saccharification of cellulose in aqueous-ionic liquid media by ultrasonic intensification. Carbohyd. Polym. 2010, 81, 311–316. [Google Scholar] [CrossRef]
- Brandt, A.; Gräsvik, J.; Hallett, J.P.; Welton, T. Deconstruction of lignocellulosic biomass with ionic liquids. Green Chem. 2013, 15, 550–583. [Google Scholar] [CrossRef]
- Onga, V.Z.; Wua, T.Y.; Leea, C.B.T.L.; Cheonga, N.W.R.; Shakc, K.P.Y. Sequential ultrasonication and deep eutectic solvent pretreatment to remove lignin and recover xylose from oil palm fronds. Ultrason. Sonochem. 2019, 58, 104598. [Google Scholar] [CrossRef]
- Ji, Q.; Yu, X.; Yagoub, A.E.A.; Chen, L.; Fakayode, O.A.; Zhou, C. Synergism of sweeping frequency ultrasound and deep eutectic solvents pretreatment for fractionation of sugarcane bagasse and enhancing enzymatic hydrolysis. Ultrason Sonochem. 2021, 73, 105470. [Google Scholar] [CrossRef]
- Calcio Gaudino, E.; Tabasso, S.; Grillo, G.; Jashina, L.; Telysheva, G.; Cravotto, G. Wheat straw lignin extraction with bio-based solvents using enabling technologies. Comptes Rendus Chim. 2018, 21, 563–571. [Google Scholar] [CrossRef]
- Desikan, R.; Thangavelu, K.; Uthandi, S. Hydrodynamic Cavitation—A Promising Technology for Biomass Pretreatment. Int. J. Environ. Sci. Nat. Res. 2019, 19, 84–88. [Google Scholar] [CrossRef]
- Kim, I.; Lee, I.; Jeon, S.H.; Hwang, T.; Han, J.-I. Hydrodynamic cavitation as a novel pretreatment approach for bioethanol production from reed. Bioresour. Technol. 2015, 192, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Hilares, R.T.; César dos Santos, J.C.; Ahmed, M.A.; Jeon, S.H.; Silvério da Silva, S.; Han, J.-I. Hydrodynamic cavitation-assisted alkaline pretreatment as a new approach for sugarcane bagasse biorefineries. Bioresour. Technol. 2016, 214, 609–614. [Google Scholar] [CrossRef]
- Hilares, R.T.; Faria de Almeida, G.; Ahmed, M.A.; Antunes, F.A.F.; Silvério da Silva, S.; Han, J.-I.; César dos Santos, J. Hydrodynamic cavitation as an efficient pretreatment method for lignocellulosic biomass: A parametric study. Bioresour. Technol. 2017, 235, 301–308. [Google Scholar] [CrossRef]
- Hilares, R.T.; Kamoei, D.V.; Ahmed, M.A.; Silvério da Silva, S.; Han, J.-I.; César dos Santos, J. A new approach for bioethanol production from sugarcane bagasse using hydrodynamic cavitation assisted-pretreatment and column reactors. Ultrason. Sonochem. 2018, 43, 219–226. [Google Scholar] [CrossRef]
- Nakashima, K.; Ebi, Y.; Shibasaki-Kitakawa, N.; Soyama, H.; Yonemoto, T. Hydrodynamic Cavitation Reactor for Efficient Pretreatment of Lignocellulosic Biomass. Ind. Eng. Chem. Res. 2016, 55, 1866–1871. [Google Scholar] [CrossRef]
- Tao, Y.; Cai, J.; Huai, X.; Liu, B.; Guo, Z. Application of hydrodynamic cavitation into wastewater treatment: A review. Chem. Eng. Technol. 2016, 39, 1363–1376. [Google Scholar] [CrossRef]
- Badve, M.P.; Gogate, P.R.; Pandit, A.B.; Csoka, L. Hydrodynamic cavitation as a novel approach for delignification of wheat straw for paper manufacturing. Ultrason. Sonochem. 2014, 21, 162–168. [Google Scholar] [CrossRef]
- Crudo, D.; Bosco, V.; Cavaglià, G.; Grillo, G.; Mantegna, S.; Cravotto, G. Biodiesel production process intensification using a rotor-stator type generator of hydrodynamic cavitation. Ultrason. Sonochem. 2016, 33, 220–225. [Google Scholar] [CrossRef]
- Patil, P.N.; Gogate, P.R.; Csoka, L.; Dregelyi-Kiss, A.; Horvath, M. Intensification of biogas production using pretreatment based on hydrodynamic cavitation. Ultrason. Sonochem. 2016, 30, 79–86. [Google Scholar] [CrossRef]
- Garutia, M.; Langoneb, M.; Fabbria, C.; Piccininia, S. Monitoring of full-scale hydrodynamic cavitation pretreatment in agricultural biogas plant. Bioresour. Technol. 2018, 247, 599–609. [Google Scholar] [CrossRef]
- Zieliński, M.; Dębowski, M.; Kisielewska, M.; Nowicka, A.; Rokicka, M.; Szwarc, K. Cavitation-based pretreatment strategies to enhance biogas production in a small-scale agricultural biogas plant. Energy Sustain. Dev. 2019, 49, 21–26. [Google Scholar] [CrossRef]
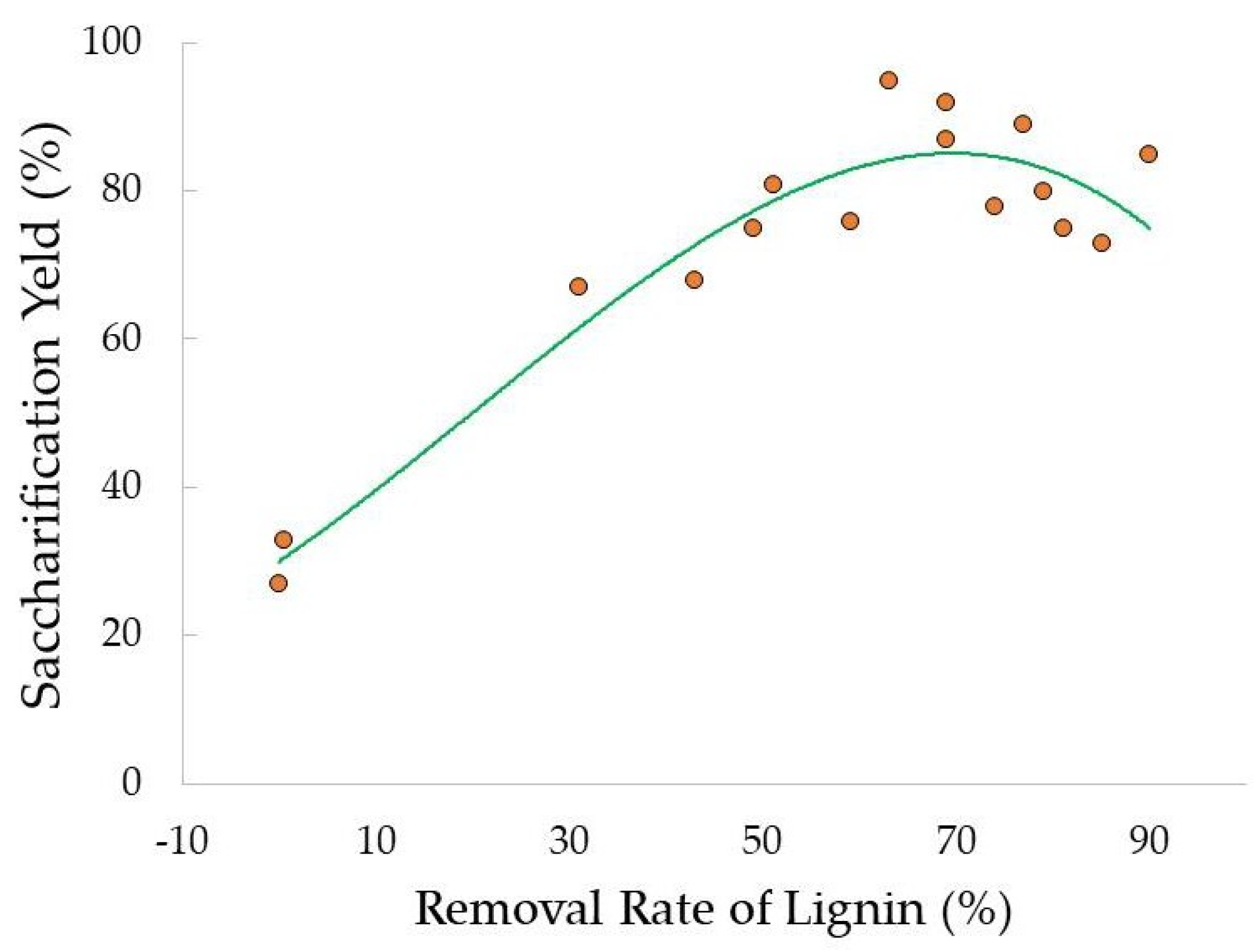
- Thangavelu, K.; Desikan, R.; Taran, O.P.; Uthandi, S. Delignifcation of corncob via combined hydrodynamic cavitation and enzymatic pretreatment: Process optimization by response surface methodology. Biotechnol. Biofuels 2018, 11, 203. [Google Scholar] [CrossRef]
- Hilaresa, R.T.; Dionízio, R.M.; Prado, C.A.; Ahmed, M.A.; da Silva, S.S.; Santos, J.C. Pretreatment of sugarcane bagasse using hydrodynamic cavitation technology: Semi-continuous and continuous process. Bioresour. Technol. 2019, 290, 121777. [Google Scholar] [CrossRef] [PubMed]
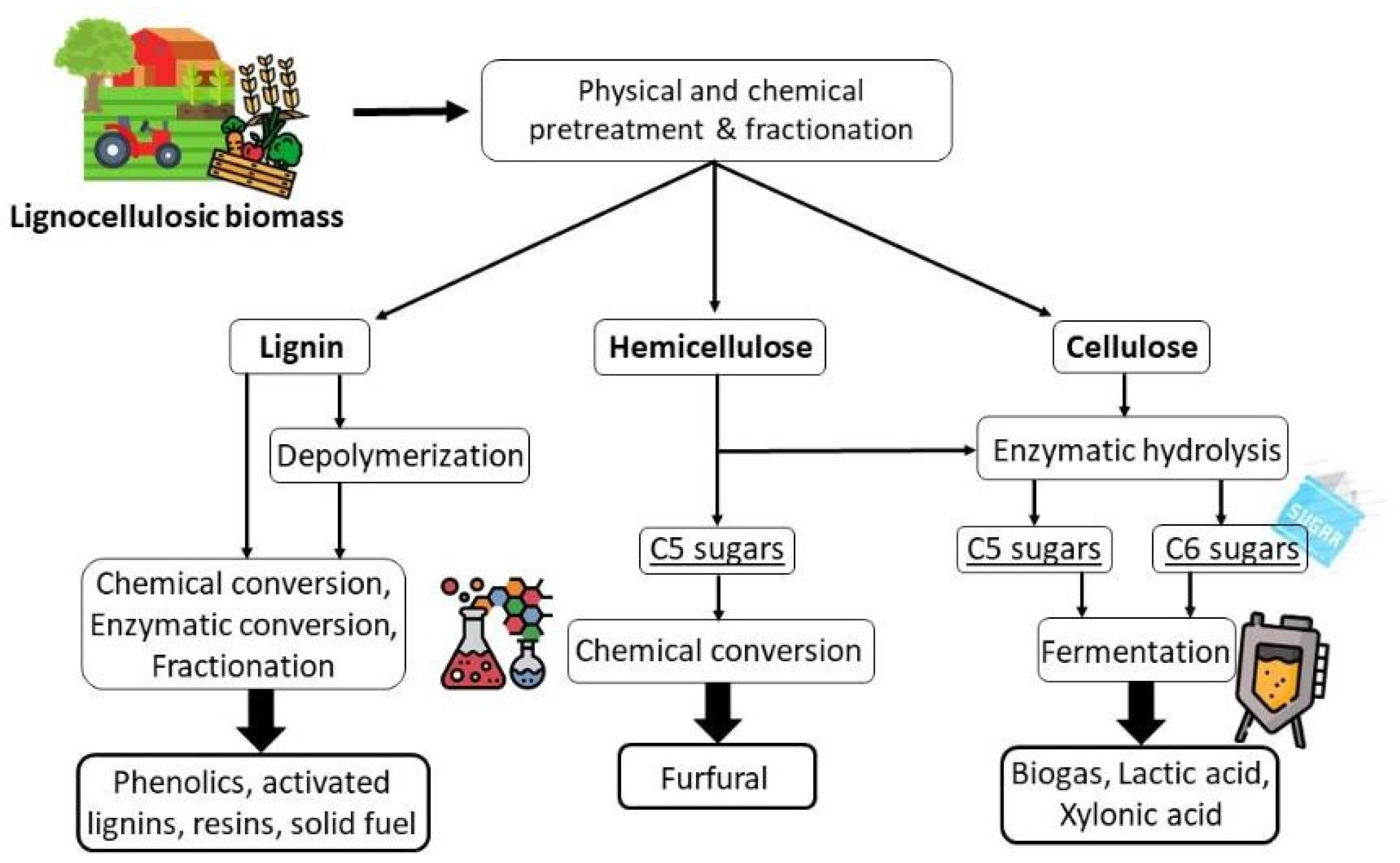
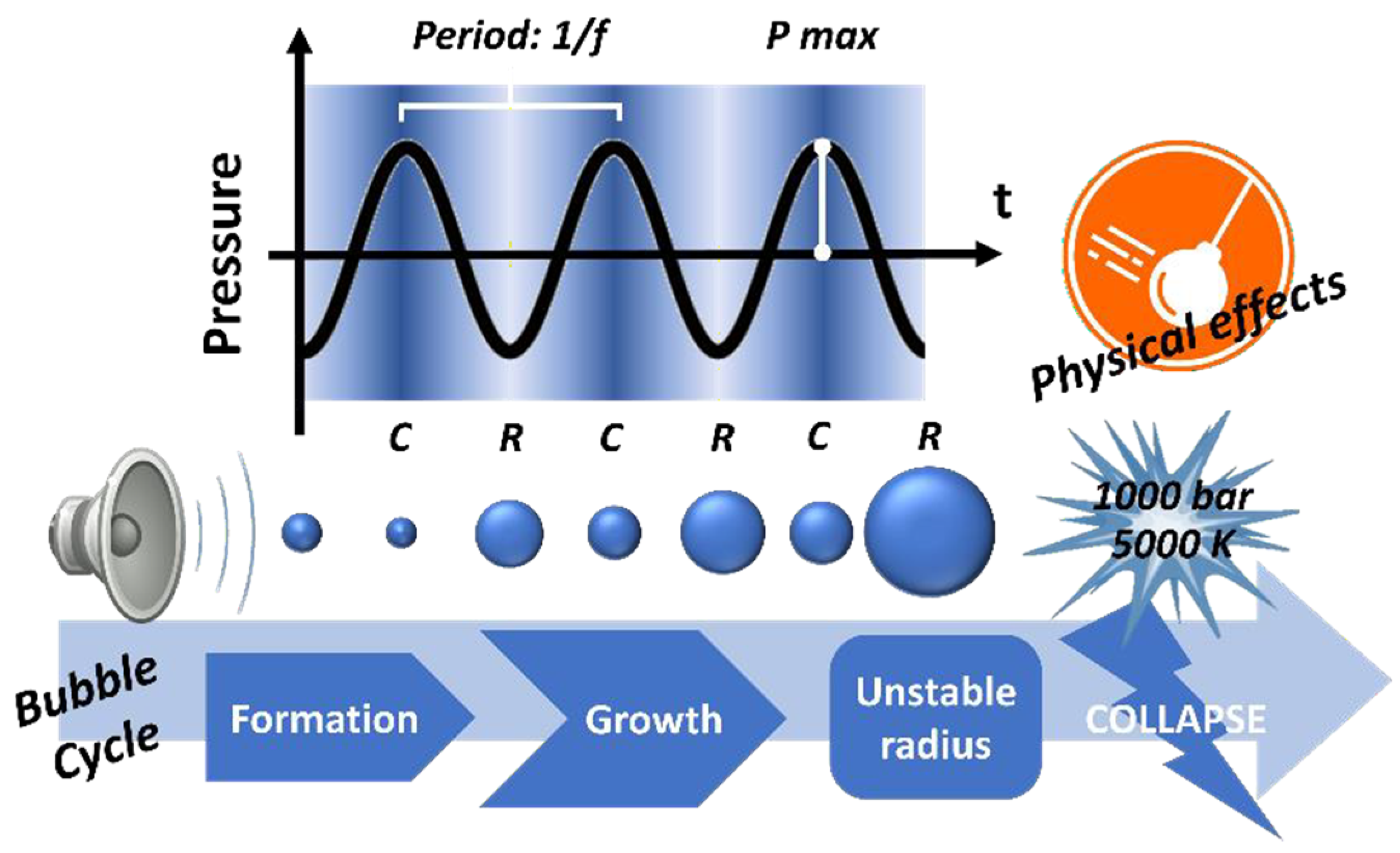
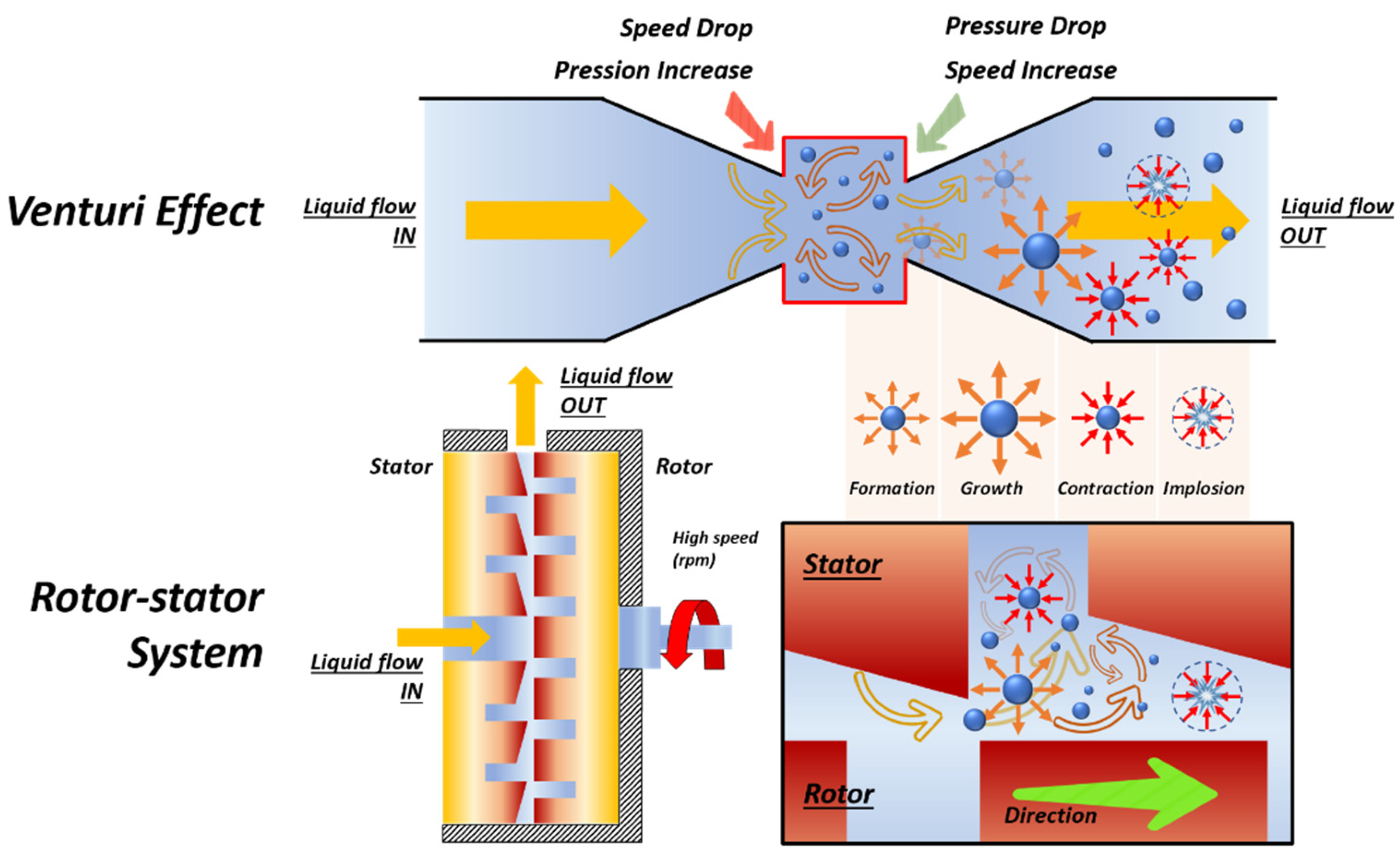
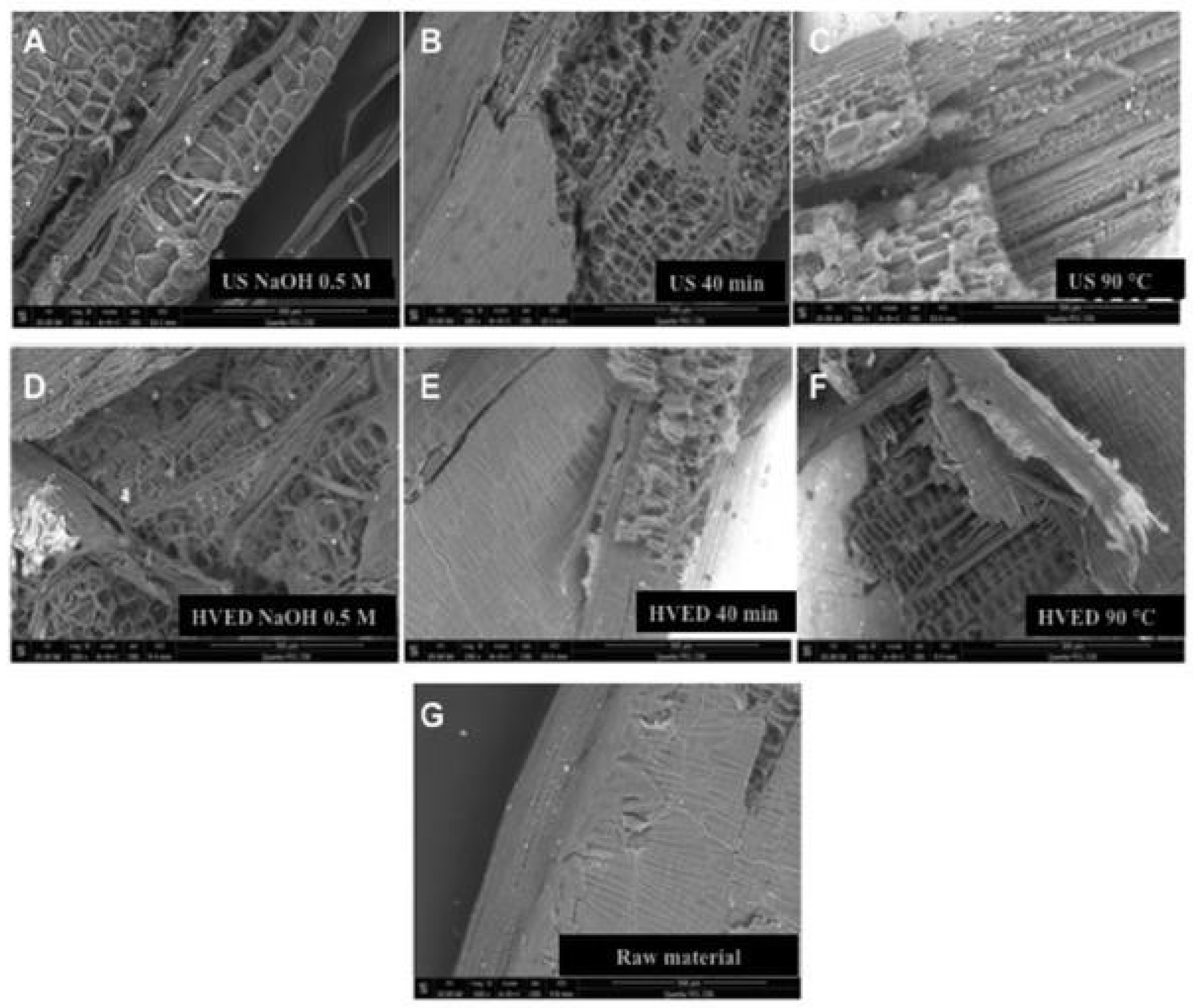




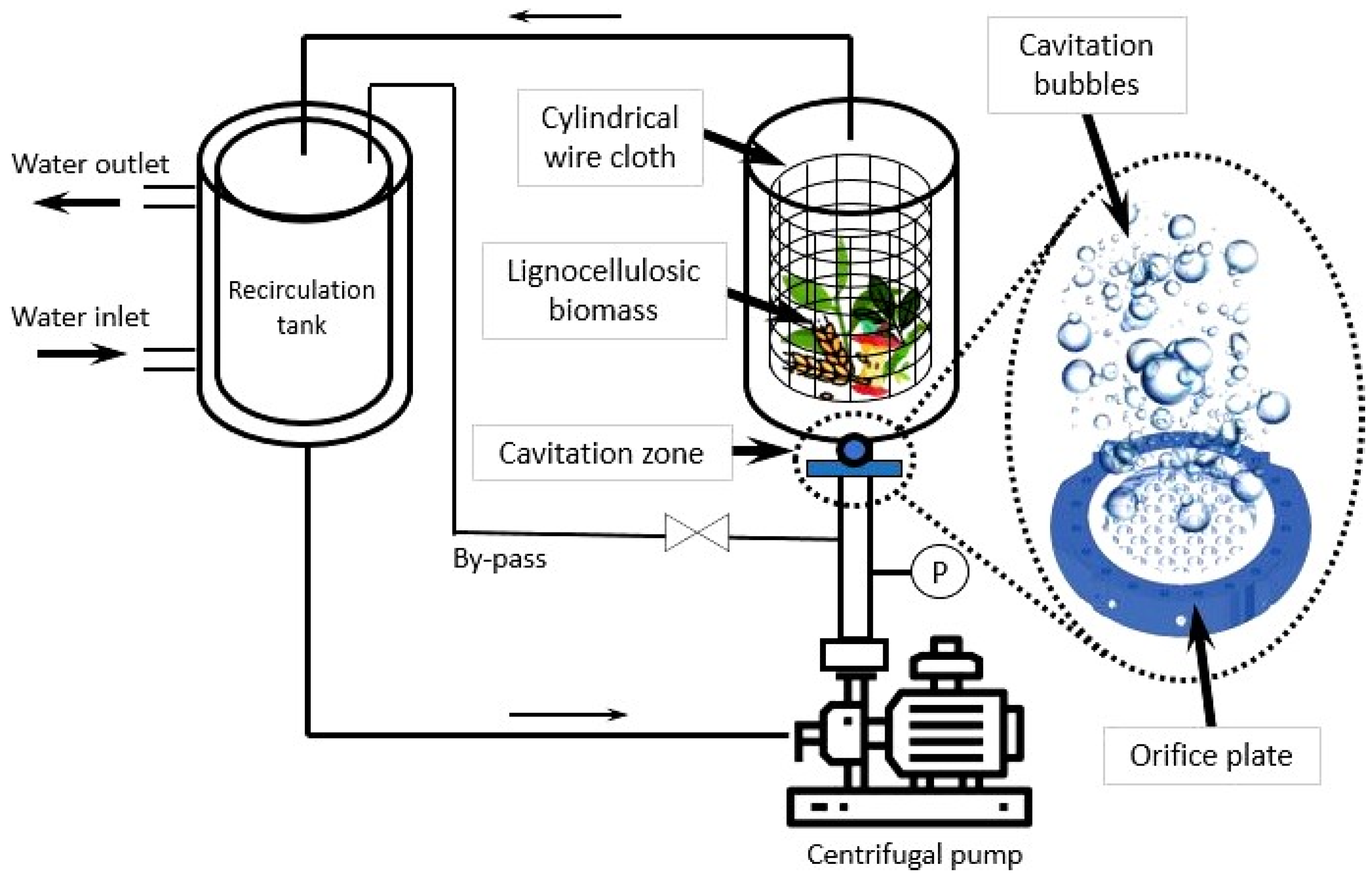
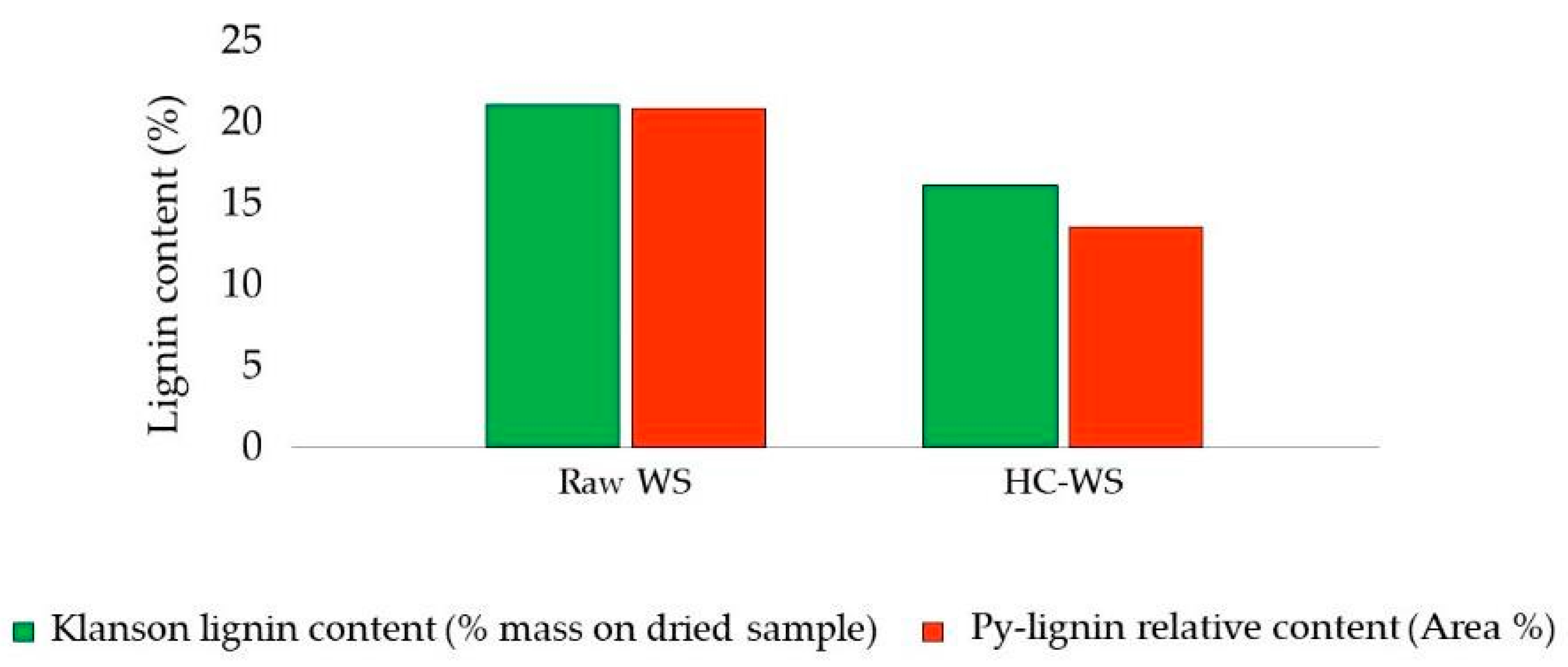
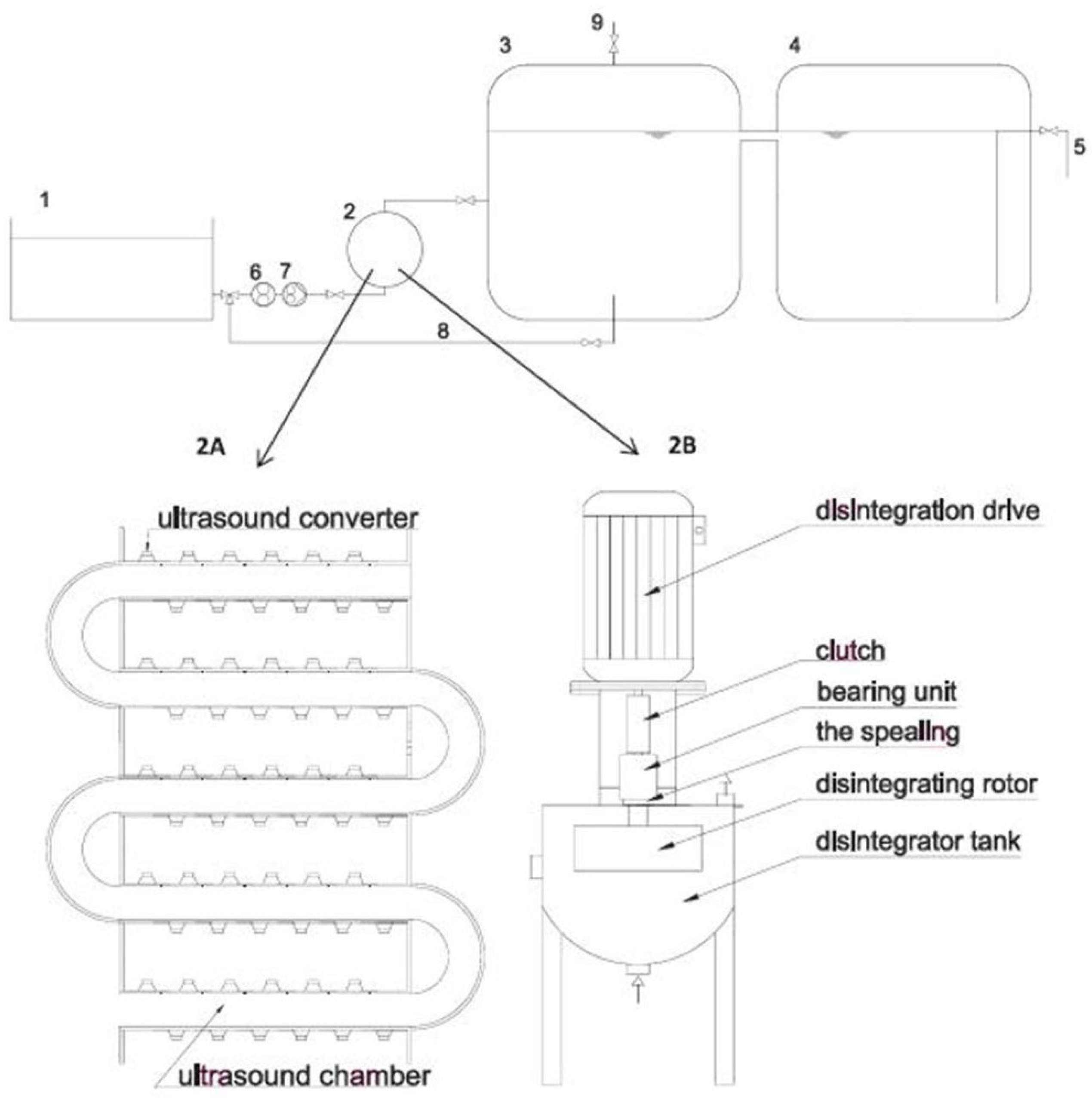



| Pretreatment for Cellulose Recovery | Processes | Possible Biomass Alterations | Remarks |
|---|---|---|---|
| Milling | Increases the pore size and the accessible specific surface area | High energy consumption | |
| Physical | Decrease the cellulose crystallinity | ||
| Irradiation | Decrease the polymerization degrees of cellulose | Cannot remove lignin and hemicellulose | |
| Steam Explosion | Solubilizes hemicelluloses, alters the structure of lignin | Harsh environment High equipment cost and inhibitors generation | |
| Hydrothermal | Solubilizes hemicelluloses recovering sugars | High equipment cost | |
| Ammonia fiber explosion | Decreases the polymerization degrees of cellulose | High cost Not effective for biomass with high lignin content | |
| Physicochemical and Chemical | SC-CO2 | Increases the accessible specific surface area | High pressure requirements Easy CO2 recovery after extraction |
| Organosolv process | High delignification Separation and recovery of high- quality cellulose | High price of organic solvents | |
| Alkali | Decreases the cellulose crystallinity | High pollution and high chemical recovery cost | |
| Acid | Decreases the polymerization degrees of cellulose Hydrolysis of hemicelluloses | Rapid treatment rate | |
| Oxidative | High delignification Hydrolysis of hemicelluloses | High cost of bleaching agent | |
| High delignification | Long pretreatment period | ||
| Biological | Fungi | Degrades lignin and hemicelluloses | Low energy requirement |
| Decreases the polymerization degree of cellulose |
| US Parameters | Delignification (%) | Rate Constant k (min−1) |
|---|---|---|
| Power (W) | ||
| 60 | 42.77 | 0.0081 |
| 70 | 50.21 | 0.0106 |
| 80 | 60 | 0.0138 |
| 90 | 73.26 | 0.0197 |
| 100 | 80.16 | 0.0239 |
| 110 | 82.01 | 0.0259 |
| Duty Cycle (%) | ||
| 40 | 63.15 | 0.0148 |
| 50 | 69.97 | 0.0178 |
| 60 | 75.59 | 0.0213 |
| 70 | 79.94 | 0.0242 |
| 80 | 80.92 | 0.0246 |
| Agri-Food Residue | Pretreatment Process | Conditions | Results (g of Glu/J) |
|---|---|---|---|
| Sugarcane bagasse | NaOH-HCAT | NaOH (1.9% w/v) 45 min | 6.43 × 10−5 |
| NaOH-UAT | 2.61 × 10−5 | ||
| Reed | NaOH-HCAT | NaOH (3.0% w/v) 40 min | 6.51 × 10−5 |
| NaOH-UAT | 2.58 × 10−5 | ||
| Corn stover | SP-HCAT | NaCO3 (0.4 mol/L) H2O2 (0.6 mol/L) 60 min | 2.24 × 10−5 |
| SP-UAT | 0.11 × 10−5 |
| Cavitational Pretreatment for Cellulose Recovery | Possible Biomass Alterations | Advantages | Remarks |
|---|---|---|---|
| Acoustic cavitation [3,6] |
|
|
|
| Hydrodynamic cavitation [3,112] |
|
|
|
| Biomass | Cavitation Technology | Chemical Conditions | Added Value Products | Process yield (Conventional Reference) | Ref. |
|---|---|---|---|---|---|
| Carrot grass | US-probe | alkaline | Cellulose recovery a | 90% (45%) | [78] |
| Eucalyptus wood | US-bath | alkaline | Biochar | +10–20% (respect to raw) | [79] |
| Rapeseed Straw (RapS) | US-probe | alkaline | Cellulose hydrolysis | 41.60% | [80] |
| Rapeseed Straw (RapS) | US-probe | organosolv | Lignin recovery | high polymerization degree | [81] |
| Rapeseed Hulls | US-probe | alkaline | Cellulose hydrolysis | 36% (33.5%) | [82] |
| Olive tree pruning | US-bath | acid | Cellulose recovery a | 15.87% (6.03%) | [83] |
| Newspaper waste slurry | US-probe | alkaline | Cellulose recovery a | ca. 80% (40%) | [84] |
| Oil palm empty fruit bunch (OPEFB) | US-probe | acid | Xylose | 58% (22%) | [85] |
| Sugarcane bagasse (SCB) | US-probe | acid | Bio-ethanol | 820 mg/mL | [86] |
| US-probe | acid + alkaline | Bio-ethanol | 911 mg/mL | [86] | |
| US-probe | IL | Fermentable sugars | 177 mg/g biomass | [100] | |
| US-probe | IL + surfactant | Fermentable sugars | 255 mg/g biomass | [100] | |
| HC (venturi) | alkaline | Cellulose recovery—Cellulose hydrolysis | 60–97.2% (67%) | [110] | |
| HC (venturi) | alkaline | Cellulose—Hemicellulose hydrolysis | 93–95% | [111] | |
| HC (venturi) | alkaline + H2O2 | Cellulose recovery a—Fermentable sugars—Bio-ethanol | 27.2–95.4%—0.49 g/g biomass | [112] | |
| HC (venturi), batch | alkaline + H2O2 | Cellulose recovery a—Glu—Xyl | 44%—42 g/100 g (12.1 g/100 g)—20 g/100 g (5.7 g/100 g) | [121] | |
| HC (venturi), semi-cont. | alkaline + H2O2 | Cellulose recovery a—Glu—Xyl | 48%—35 g/100 g (12.1 g/100 g)–23 g/100 g (5.7 g/100 g) | [121] | |
| HC (venturi), continuous | alkaline + H2O2 | Glu—Xyl | 46 g/100 g (12.1 g/100 g)–32 g/100 g (5,7 g/100 g) | [121] | |
| US-probe | ChOAc | Cellulose—Hemicellulose hydrolysis | 85–100% | [102] | |
| Grass | US-probe | acid | Hydrogen | 84.4 mL (54.9 mL) | [87] |
| Rice Straw | US-probe | Fenton Reagent | Fermentable sugars | 7 g/L (4.6 g/L) | [88] |
| US-probe | alkaline | Fermentable sugars | 2.91 g/L (0.85 g/L) | [89] | |
| Wheat straw | US-probe | TBAOH | Cellulose hydrolysis—Delignification | 92.4 % (23.1% untreated)—70% | [90] |
| US-bath | NaDES | Cellulose recovery a—Antiox. activity | 29.81%—0.2713 mg/mL IC50 | [107] | |
| US-bath | GVL | Cellulose accessibility—Polyphenols from lignin | Total pore volume 10.2 mm3/g—25 mg GAE/100 mL | [107] | |
| HC (rotor/stator) | alkaline | Increase in paper tensile strength | ca. 55% | [115] | |
| HC (rotor/stator) | alkaline | Biogas | 172.3 mL/g total solids (31.8 mL/g total solids untreated) | [117] | |
| HC (rotor/stator) | alkaline | Cellulose recovery a | 25% | [47] | |
| US | alkaline | Cellulose recovery a | 30–45% | [47] | |
| US-bath b | - | Biogas | 19.8 m3/d (15.9 m3/d) | [119] | |
| HC (not specified) | - | Biogas | 18.5 m3/d (15.9 m3/d) | [119] | |
| Sugar beet shreds | US-probe | - | Cellulose recovery—Cellulose hydrolysis | 90%—780 mg/g cellulose (211 mg/g cellulose) | [91] |
| Coffee silverskin (CSS) | US-probe | acid | Fermentable sugars | 0.190 g/g (0.103 g/g) | [92] |
| US -probe | alkaline | Fermentable sugars—Polyphenols | 24 g/L (21 g/L)—25 mg GAE/g CSS (23.1 mg GAE/g CSS) | [93] | |
| Chili post-harvest residue | US | alkaline | Fermentable sugars—Bio-ethanol | 0.44 g/g—1.94% GC area | [95] |
| Bamboo components | US | Acid—GVL | Cellulose hydrolysis | 90% (12%) | [96] |
| Powdered bamboo waste | US-probe | ChOAc | Cellulose hydrolysis | 92% (55%) | [101] |
| Corn stover | US | IL | Lignin recovery—Cellulose hydrolysis | 60%–98% | [99] |
| HC (Venuri) | Na2H3CO6 | Fermentable sugars | 3 mg/mL | [113] | |
| US-probe | Na2H3CO6 | Fermentable sugars | 2 mg/mL | [113] | |
| Microcrystalline cellulose | US-bath | IL | Cellulose hydrolysis | 95.5% (42.8%) | [103] |
| Oil palm fronds (OPF) | US-cup horn | NaDES | Cellulose recovery a | 36.42% (14% NaDES only) | [105] |
| Garlic skin (GS) | US + MW | NaDES | Cellulose recovery a | 90,14% (76.10%) | [106] |
| Reed | HC (orifice plate) | alkaline | Delignification—Bio-ethanol | 85%—510 mg/g cellulose | [109] |
| Agriculture biomass mix c | HC (rotor/stator) | - | Bio-methane | +14% (respect to raw) | [118] |
| Corncob | HC (orifice plate)/Enzymes | - | Cellulose recovery a | 47.40% | [120] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verdini, F.; Calcio Gaudino, E.; Grillo, G.; Tabasso, S.; Cravotto, G. Cellulose Recovery from Agri-Food Residues by Effective Cavitational Treatments. Appl. Sci. 2021, 11, 4693. https://doi.org/10.3390/app11104693
Verdini F, Calcio Gaudino E, Grillo G, Tabasso S, Cravotto G. Cellulose Recovery from Agri-Food Residues by Effective Cavitational Treatments. Applied Sciences. 2021; 11(10):4693. https://doi.org/10.3390/app11104693
Chicago/Turabian StyleVerdini, Federico, Emanuela Calcio Gaudino, Giorgio Grillo, Silvia Tabasso, and Giancarlo Cravotto. 2021. "Cellulose Recovery from Agri-Food Residues by Effective Cavitational Treatments" Applied Sciences 11, no. 10: 4693. https://doi.org/10.3390/app11104693
APA StyleVerdini, F., Calcio Gaudino, E., Grillo, G., Tabasso, S., & Cravotto, G. (2021). Cellulose Recovery from Agri-Food Residues by Effective Cavitational Treatments. Applied Sciences, 11(10), 4693. https://doi.org/10.3390/app11104693










