Involvement of Antioxidant Defenses and NF-κB/ERK Signaling in Anti-Inflammatory Effects of Pterostilbene, a Natural Analogue of Resveratrol
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. MTT Assay and Morphology of RAW Cells
2.3. Quantification of NO Production
2.4. Western Blotting Study
2.5. Confocal Microscopy Assay
2.6. Native Polyacrylamide Gel Electrophoresis (NATIVE-PAGE)
2.7. Statistical Analyses
3. Results
3.1. PTE on Viability and Morphology in RAW 264.7 Cells
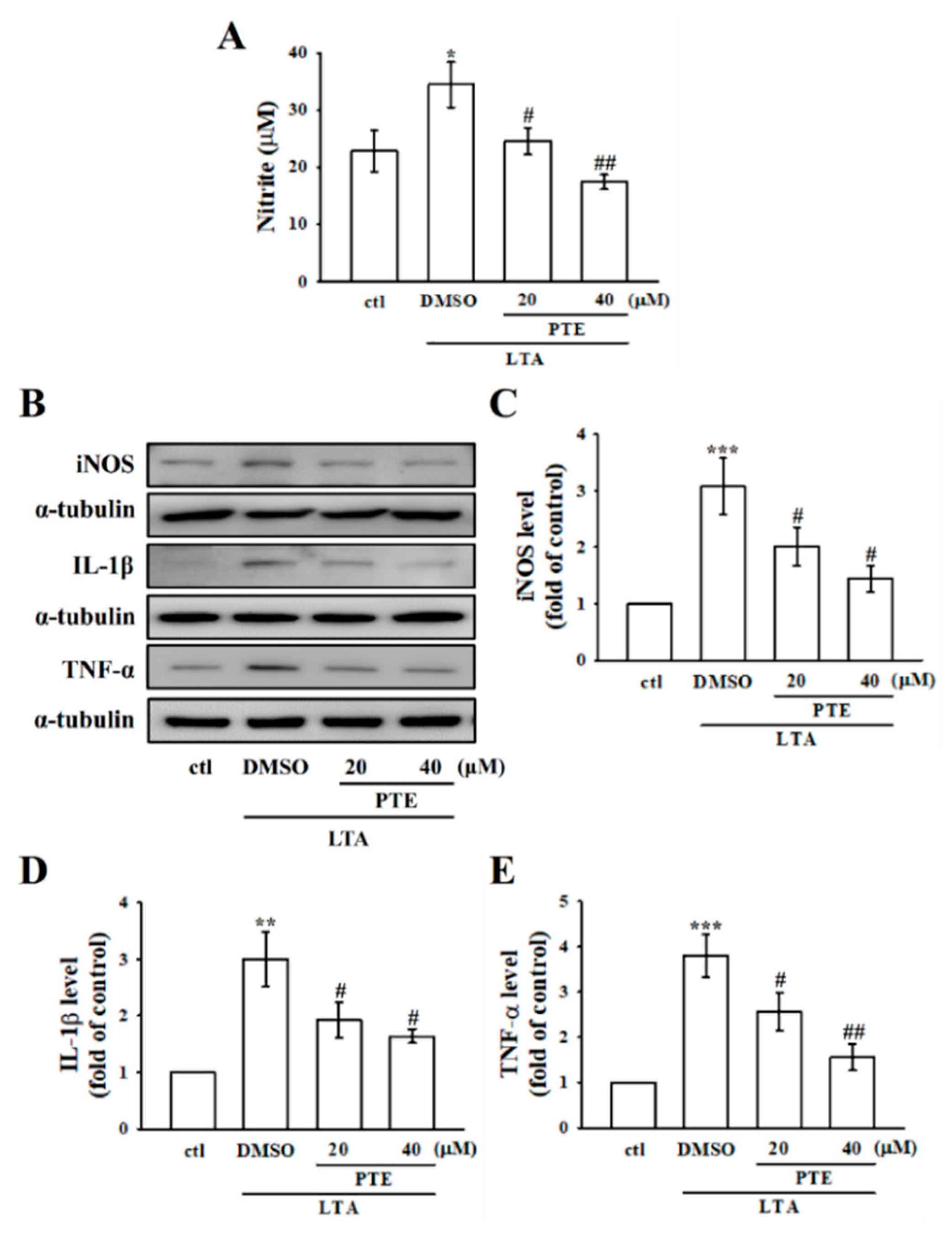
3.2. PTE Inhibited LTA-Induced NO Production by Tempering iNOS in RAW Cells
3.3. PTE Inhibited LTA-Induced Inflammatory IL-1β and TNF-α Expression
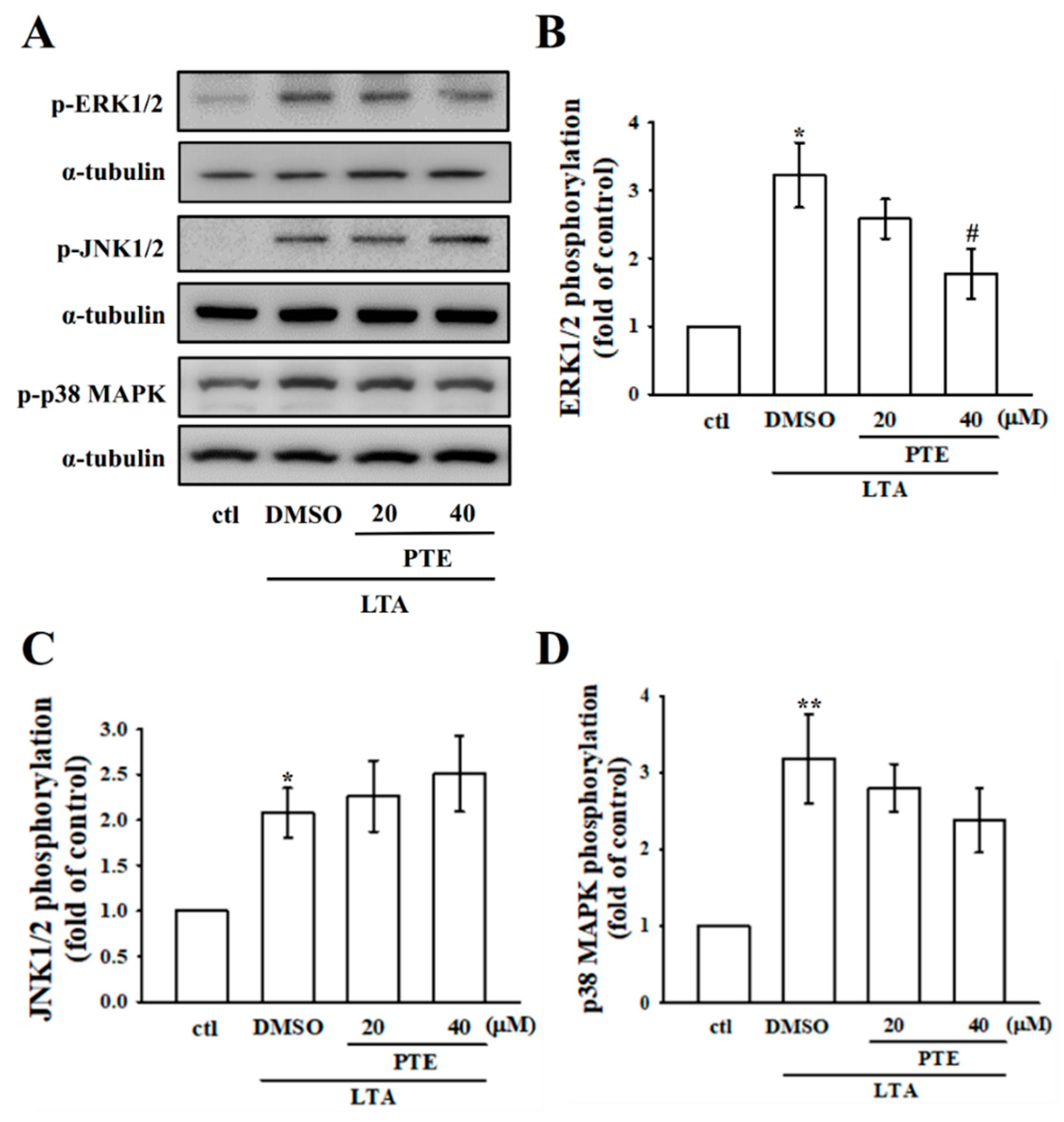
3.4. PTE Inhibits ERK, but Not JNK and p38 MAPK Phosphorylation
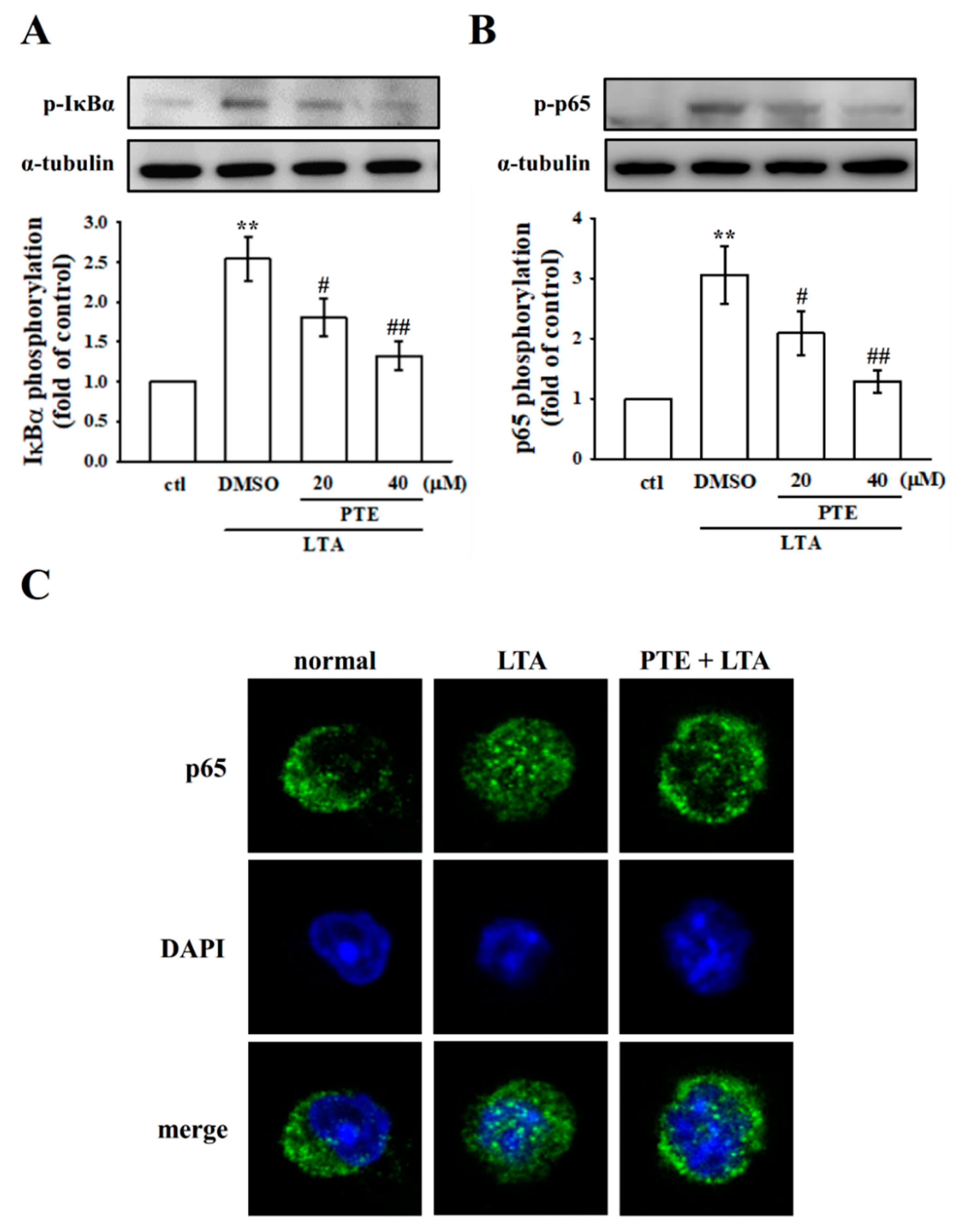
3.5. PTE Inhibited LTA-Induced NF-κB Signaling Pathway
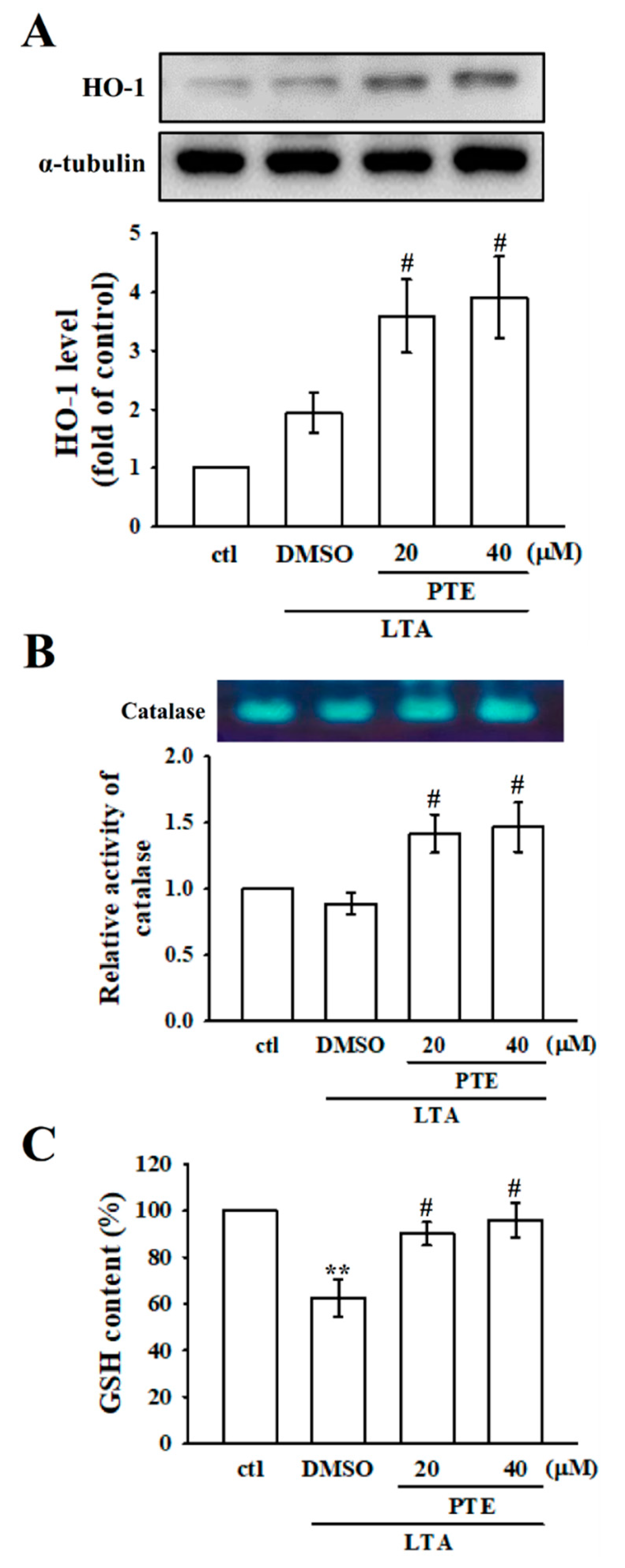
3.6. Effects of PTE on Antioxidant Defenses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goldsby, R.A.; Kindt, T.J.; Osborne, B.A. Kuby Immunology, 4th ed.; W. H. Freeman and Company Ed: New York, NY, USA, 2000; pp. 303–327. [Google Scholar]
- Shao, J.; Li, Y.; Wang, Z.; Xiao, M.; Yin, P.; Lu, Y.; Qian, X.; Xu, Y.; Liu, J. 7b, A novel naphthalimide derivative, exhibited anti-inflammatory effects via targeted-inhibiting TAK1 following down-regulation of ERK1/2- and p38 MAPK-mediated activation of NF-κB in LPS-stimulated RAW264.7 macrophages. Int. Immunopharmacol. 2013, 17, 216–228. [Google Scholar] [CrossRef] [PubMed]
- Aruoma, O.I. Free radicals oxidative stress, and antioxidants in human health and disease. J. Am. Oil Chem. Soc. 1998, 75, 199–212. [Google Scholar] [CrossRef]
- Möller, B.; Villiger, P.M. Inhibition of IL-1, IL-6, and TNF-alpha in immune-mediated inflammatory diseases. Springer Semin. Immunopathol. 2006, 27, 391–408. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.E.; Dahle, M.K.; McDonald, M.; Foster, S.J.; Aasen, A.O.; Thiemermann, C. Peptidoglycan and lipoteichoic acid in gram-positive bacterial sepsis: Receptors, signal transduction, biological effects, and synergism. Shock 2003, 20, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Leemans, J.C.; Heikens, M.; van Kessel, K.P.; Florquin, S.; van der Poll, T. Lipoteichoic acid and peptidoglycan from Staphylococcus aureus synergistically induce neutrophil influx into the lungs of mice. Clin. Diagn. Lab. Immunol. 2003, 10, 950–953. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, E.M.; Beidelschies, M.A.; Tatro, J.M.; Goldberg, V.M.; Hise, A.G. Bacterial pathogen-associated molecular patterns stimulate biological activity of orthopaedic wear particles by activating cognate Toll-like receptors. J. Biol. Chem. 2010, 285, 32378–32384. [Google Scholar] [CrossRef]
- Chang, H.C.; Lin, K.H.; Tai, Y.T.; Chen, J.T.; Chen, R.M. Lipoteichoic acid-induced TNF-α and IL-6 gene expressions and oxidative stress production in macrophages are suppressed by ketamine through downregulating toll-like receptor 2-mediated activation of ERK1/2 and NFκB. Shock 2010, 33, 485–492. [Google Scholar] [CrossRef]
- Otterbein, L.E.; Bach, F.H.; Alam, J.; Soares, M.; Tao Lu, H.; Wysk, M.; Davis, R.J.; Flavell, R.A.; Choi, A.M. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat. Med. 2000, 6, 422–428. [Google Scholar] [CrossRef]
- Sim, H.; Choo, S.; Kim, J.; Baek, M.C.; Bae, J.S. Fisetin Suppresses Pulmonary Inflammatory Responses through Heme Oxygenase-1 Mediated Downregulation of Inducible Nitric Oxide Synthase. J. Med. Food 2020, 23, 1163–1168. [Google Scholar] [CrossRef]
- Pae, H.O.; Kim, E.C.; Chung, H.T. Integrative survival response evoked by heme oxygenase-1 and heme metabolites. J. Clin. Biochem. Nutr. 2008, 42, 197–203. [Google Scholar] [CrossRef]
- Salah, N.; Miller, N.J.; Paganga, G.; Tijburg, L.; Bolwell, G.P.; Rice-Evans, C. Polyphenolic flavanols as scavengers of aqueous phase radicals and as chain-breaking antioxidants. Arch. Biochem. Biophys. 1995, 322, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.M.; Andrade, P.B.; Valentão, P.; Ferreres, F.; Seabra, R.M.; Ferreira, M.A. Quince (Cydonia oblonga Miller) fruit (pulp, peel, and seed) and jam: Antioxidant activity. J. Agric. Food Chem. 2004, 52, 4705–4712. [Google Scholar] [CrossRef] [PubMed]
- Estrela, J.M.; Ortega, A.; Mena, S.; Rodriguez, M.L.; Asensi, M. Pterostilbene: Biomedical applications. Crit. Rev. Clin. Lab. Sci. 2013, 50, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Remsberg, C.M.; Yáñez, J.A.; Ohgami, Y.; Vega-Villa, K.R.; Rimando, A.M.; Davies, N.M. Pharmacometrics of pterostilbene: Preclinical pharmacokinetics and metabolism, anticancer, antiinflammatory, antioxidant and analgesic activity. Phytother. Res. 2008, 22, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ruzhi, D.; Hua, X.; Zhang, L.; Lu, F.; Coursey, T.G.; Pflugfelder, S.C.; Li, D.Q. Blueberry Component Pterostilbene Protects Corneal Epithelial Cells from Inflammation via Anti-oxidative Pathway. Sci. Rep. 2016, 6, 19408. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, G.; Song, W.; Tan, X.; Guo, Y.; Zhou, B.; Jing, H.; Zhao, S.; Chen, L. Pterostilbene protects vascular endothelial cells against oxidized low-density lipoprotein-induced apoptosis in vitro and in vivo. Apoptosis 2012, 17, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Chiou, Y.S.; Tsai, M.L.; Nagabhushanam, K.; Wang, Y.J.; Wu, C.H.; Ho, C.T.; Pan, M.H. Pterostilbene is more potent than resveratrol in preventing azoxymethane (AOM)-induced colon tumorigenesis via activation of the NF-E2-related factor 2 (Nrf2)-mediated antioxidant signaling pathway. J. Agric. Food Chem. 2011, 59, 2725–2733. [Google Scholar] [CrossRef]
- Bhakkiyalakshmi, E.; Sireesh, D.; Sakthivadivel, M.; Sivasubramanian, S.; Gunasekaran, P.; Ramkumar, K.M. Anti-hyperlipidemic and anti-peroxidative role of pterostilbene via Nrf2 signaling in experimental diabetes. Eur. J. Pharmacol. 2016, 777, 9–16. [Google Scholar] [CrossRef]
- Mori, S.; Kishi, S.; Honoki, K.; Fujiwara-Tani, R.; Moriguchi, T.; Sasaki, T.; Fujii, K.; Tsukamoto, S.; Fujii, H.; Kido, A.; et al. Anti-Stem Cell Property of Pterostilbene in Gastrointestinal Cancer Cells. Int. J. Mol. Sci. 2020, 21, 9347. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Fang, H.; Yu, H.; Zhao, Y.; Shen, J.; Zhou, C.; Jin, Y. Pterostilbene inhibits deoxynivalenol-induced oxidative stress and inflammatory response in bovine mammary epithelial cells. Toxicon 2021, 189, 10–18. [Google Scholar] [CrossRef]
- Woodbury, W.; Spencer, A.K.; Stahman, M.A. An improved procedure using ferricyanide for detecting catalase isozymes. Anal. Biochem. 1971, 44, 301–305. [Google Scholar] [CrossRef]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Stark, G.R. NFκB-dependent signaling pathways. Exp. Hematol. 2002, 30, 285–296. [Google Scholar] [CrossRef]
- Nagao, K.; Jinnouchi, T.; Kai, S.; Yanagita, T. Pterostilbene, a dimethylated analog of resveratrol, promotes energy metabolism in obese rats. J. Nutr. Biochem. 2017, 43, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.J.; Kuo, H.C.; Cheng, L.H.; Lee, Y.H.; Chang, W.T.; Wang, B.J.; Wang, Y.J.; Cheng, H.C. Apoptotic and nonapoptotic activities of pterostilbene against cancer. Int. J. Mol. Sci. 2018, 19, 287. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Lin, K.C.; Hsia, C.W.; Hsia, C.H.; Chen, T.Y.; Bhavan, P.S.; Sheu, J.R.; Hou, S.M. The Antithrombotic Agent Pterostilbene Interferes with Integrin αIIbβ3-Mediated Inside-Out and Outside-In Signals in Human Platelets. Int. J. Mol. Sci. 2021, 22, 3643. [Google Scholar] [CrossRef] [PubMed]
- Aktan, F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 2004, 75, 639–653. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.; Hänsch, H.; Bancroft, G.; Ehlers, S. T-cell-independent granuloma formation in response to Mycobacterium avium: Role of tumour necrosis factor-alpha and interferon-gamma. Immunology 1997, 92, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Takahama, U.; Yamamoto, A.; Hirota, S.; Oniki, T. Quercetin-dependent reduction of salivary nitrite to nitric oxide under acidic conditions and interaction between quercetin and ascorbic acid during the reduction. J. Agric. Food Chem. 2003, 51, 6014–6020. [Google Scholar] [CrossRef]
- Huang, G.C.; Chow, J.M.; Shen, S.C.; Yang, L.Y.; Lin, C.W.; Chen, Y.C. Wogonin but not Nor-wogonin inhibits lipopolysaccharide and lipoteichoic acid-induced iNOS gene expression and NO production in macrophages. Int. Immunopharmacol. 2007, 7, 1054–1063. [Google Scholar] [CrossRef]
- Kang, O.H.; Lee, J.H.; Kwon, D.Y. Apigenin inhibits release of inflammatory mediators by blocking the NF-κB activation pathways in the HMC-1 cells. Immunopharmacol. Immunotoxicol. 2011, 33, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Park, Y.C.; Kim, J.H.; Kim, H.R.; Yu, T.; Byeon, S.E.; Unsworth, L.D.; Lee, J.; Cho, J.Y. Nanostructured, self-assembling peptide K5 blocks TNF-α and PGE2 production by suppression of the AP-1/p38 pathway. Mediat. Inflamm. 2012, 2012, 489810. [Google Scholar] [CrossRef] [PubMed]
- Bates, M.E.; Green, V.L.; Bertics, P.J. ERK1 and ERK2 activation by chemotactic factors in human eosinophils is interleukin 5-dependent and contributes to leukotriene C(4) biosynthesis. J. Biol. Chem. 2000, 275, 10968–10975. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Venegas, G.; Bando-Campos, C.G. The flavonoids luteolin and quercetagetin inhibit lipoteichoic acid actions on H9c2 cardiomyocytes. Int. Immunopharmacol. 2010, 10, 1003–1009. [Google Scholar] [CrossRef]
- Liu, F.L.; Chuang, C.Y.; Tai, Y.T.; Tang, H.L.; Chen, T.G.; Chen, T.L.; Chen, R.M. Lipoteichoic acid induces surfactant protein-A biosynthesis in human alveolar type II epithelial cells through activating the MEK1/2-ERK1/2-NF-κB pathway. Respir. Res. 2012, 13, 88. [Google Scholar] [CrossRef]
- Chuang, C.Y.; Chen, T.L.; Chen, R.M. Molecular mechanisms of lipopolysaccharide-caused induction of surfactant protein-A gene expression in human alveolar epithelial A549 cells. Toxicol. Lett. 2009, 191, 132–139. [Google Scholar] [CrossRef]
- Yu, Y.; Shen, Q.; Lai, Y.; Park, S.Y.; Ou, X.; Lin, D.; Jin, M.; Zhang, W. Anti-inflammatory Effects of Curcumin in Microglial Cells. Front. Pharmacol. 2018, 9, 386. [Google Scholar] [CrossRef]
- Park, B.G.; Yoo, C.I.; Kim, H.T.; Kwon, C.H.; Kim, Y.K. Role of mitogen-activated protein kinases in hydrogen peroxide-induced cell death in osteoblastic cells. Toxicology 2005, 215, 115–125. [Google Scholar] [CrossRef]
- Tomczyk, M.; Kraszewska, I.; Dulak, J.; Jazwa-Kusior, A. Modulation of the monocyte/macrophage system in heart failure by targeting heme oxygenase-1. Vascul. Pharmacol. 2019, 112, 79–90. [Google Scholar] [CrossRef]
- Sun, G.Y.; Chen, Z.; Jasmer, K.J.; Chuang, D.Y.; Gu, Z.; Hannink, M.; Simonyi, A. Quercetin attenuates inflammatory responses in BV-2 microglial cells: Role of MAPKs on the Nrf2 pathway and induction of heme oxygenase-1. PLoS ONE 2015, 10, e0141509. [Google Scholar] [CrossRef]
- McDermott, J.H. Antioxidant nutrients: Current dietary recommendations and research update. J. Am. Pharm. Assoc. 2000, 40, 785–799. [Google Scholar] [CrossRef]
- Lin, X.; Bai, D.; Wei, Z.; Zhang, Y.; Huang, Y.; Deng, H.; Huang, X. Curcumin attenuates oxidative stress in RAW264.7 cells by increasing the activity of antioxidant enzymes and activating the Nrf2-Keap1 pathway. PLoS ONE 2019, 14, e0216711. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Cabrera, M.C.; Salvador-Pascual, A.; Cabo, H.; Ferrando, B.; Viña, J. Redox modulation of mitochondriogenesis in exercise. Does antioxidant supplementation blunt the benefits of exercise training? Free Radic. Biol. Med. 2015, 86, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Deponte, M. The incomplete glutathione puzzle: Just guessing at numbers and figures? Antioxid. Redox Signal. 2017, 27, 1130–1161. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Li, F.F.; Hong, L.; Yan, X.F.; Tan, G.L.; He, J.S.; Dong, X.W.; Bao, M.J.; Xie, Q.M. Protective effects of liquiritin apioside on cigarette smoke-induced lung epithelial cell injury. Fundam. Clin. Pharmacol. 2012, 26, 473–483. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jayakumar, T.; Wu, M.-P.; Sheu, J.-R.; Hsia, C.-W.; Bhavan, P.S.; Manubolu, M.; Chung, C.-L.; Hsia, C.-H. Involvement of Antioxidant Defenses and NF-κB/ERK Signaling in Anti-Inflammatory Effects of Pterostilbene, a Natural Analogue of Resveratrol. Appl. Sci. 2021, 11, 4666. https://doi.org/10.3390/app11104666
Jayakumar T, Wu M-P, Sheu J-R, Hsia C-W, Bhavan PS, Manubolu M, Chung C-L, Hsia C-H. Involvement of Antioxidant Defenses and NF-κB/ERK Signaling in Anti-Inflammatory Effects of Pterostilbene, a Natural Analogue of Resveratrol. Applied Sciences. 2021; 11(10):4666. https://doi.org/10.3390/app11104666
Chicago/Turabian StyleJayakumar, Thanasekaran, Ming-Ping Wu, Joen-Rong Sheu, Chih-Wei Hsia, Periyakali Saravana Bhavan, Manjunath Manubolu, Chi-Li Chung, and Chih-Hsuan Hsia. 2021. "Involvement of Antioxidant Defenses and NF-κB/ERK Signaling in Anti-Inflammatory Effects of Pterostilbene, a Natural Analogue of Resveratrol" Applied Sciences 11, no. 10: 4666. https://doi.org/10.3390/app11104666
APA StyleJayakumar, T., Wu, M.-P., Sheu, J.-R., Hsia, C.-W., Bhavan, P. S., Manubolu, M., Chung, C.-L., & Hsia, C.-H. (2021). Involvement of Antioxidant Defenses and NF-κB/ERK Signaling in Anti-Inflammatory Effects of Pterostilbene, a Natural Analogue of Resveratrol. Applied Sciences, 11(10), 4666. https://doi.org/10.3390/app11104666







