Supplementation of Ex-Situ Biofloc to Improve Growth Performance and Enhance Nutritional Values of the Pacific White Shrimp Rearing at Low Salinity Conditions
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Ex-Situ Biofloc Production
2.2. Supplementation of Ex-Situ Biofloc to Shrimp Grow-Out Culture at Low Salinity Condition
2.3. Growth-Related Gene Expression Profiles in Shrimp Using Quantitative Realtime PCR
2.4. Assessment of Nutritional Properties in Feeds and Shrimp
2.4.1. Proximate and Trace Mineral Analyses
2.4.2. Determination of Amino Acid Profiles in Feeds and Shrimp Using Gas Chromatography-Mass Spectrometry (GC-MS)
2.4.3. Determination of Fatty Acid Analysis in Feeds and Shrimp Using GC-MS
2.5. Statistical Analysis
3. Results
3.1. Supplementation of Ex-Situ Biofloc in Shrimp Grow-Out Culture at Low Salinity Conditions
3.1.1. Water Quality Parameters
3.1.2. Shrimp Growth Performance
3.2. Nutritional Analysis between Commercial Pellet Diet and Ex-Situ Biofloc
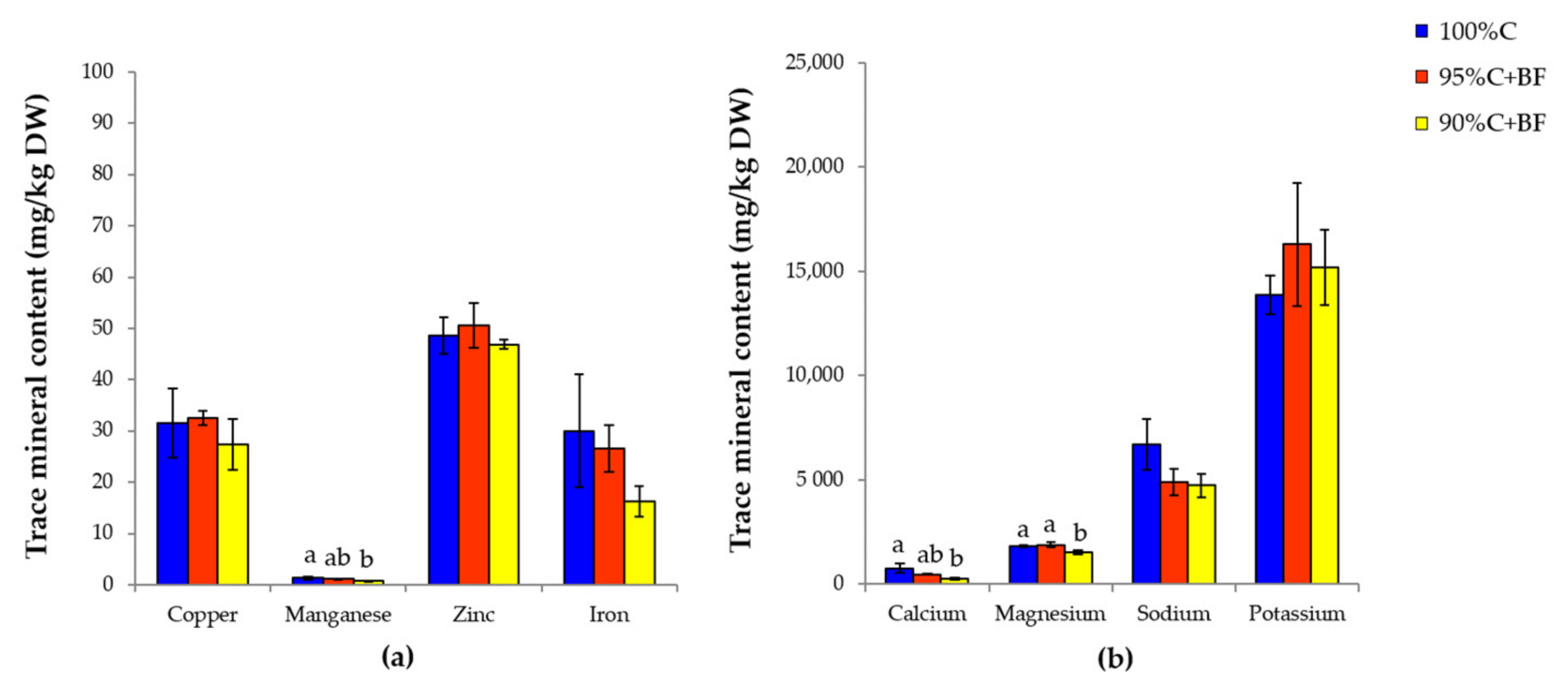
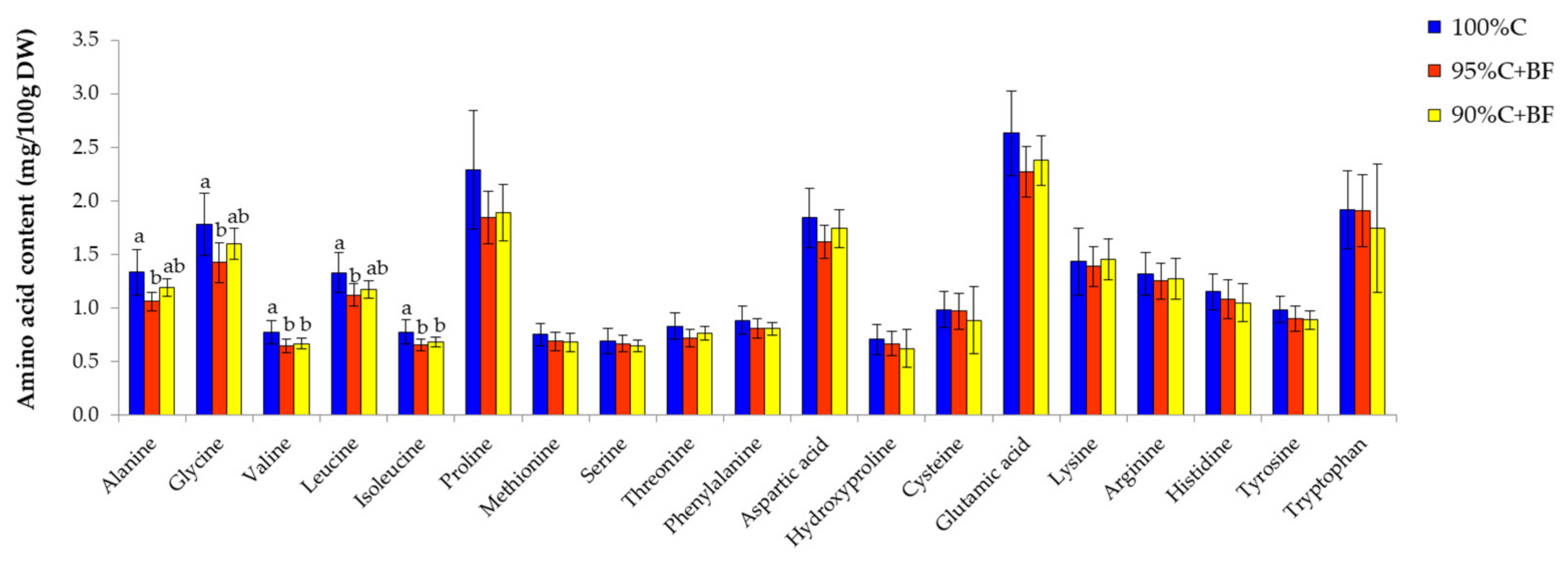
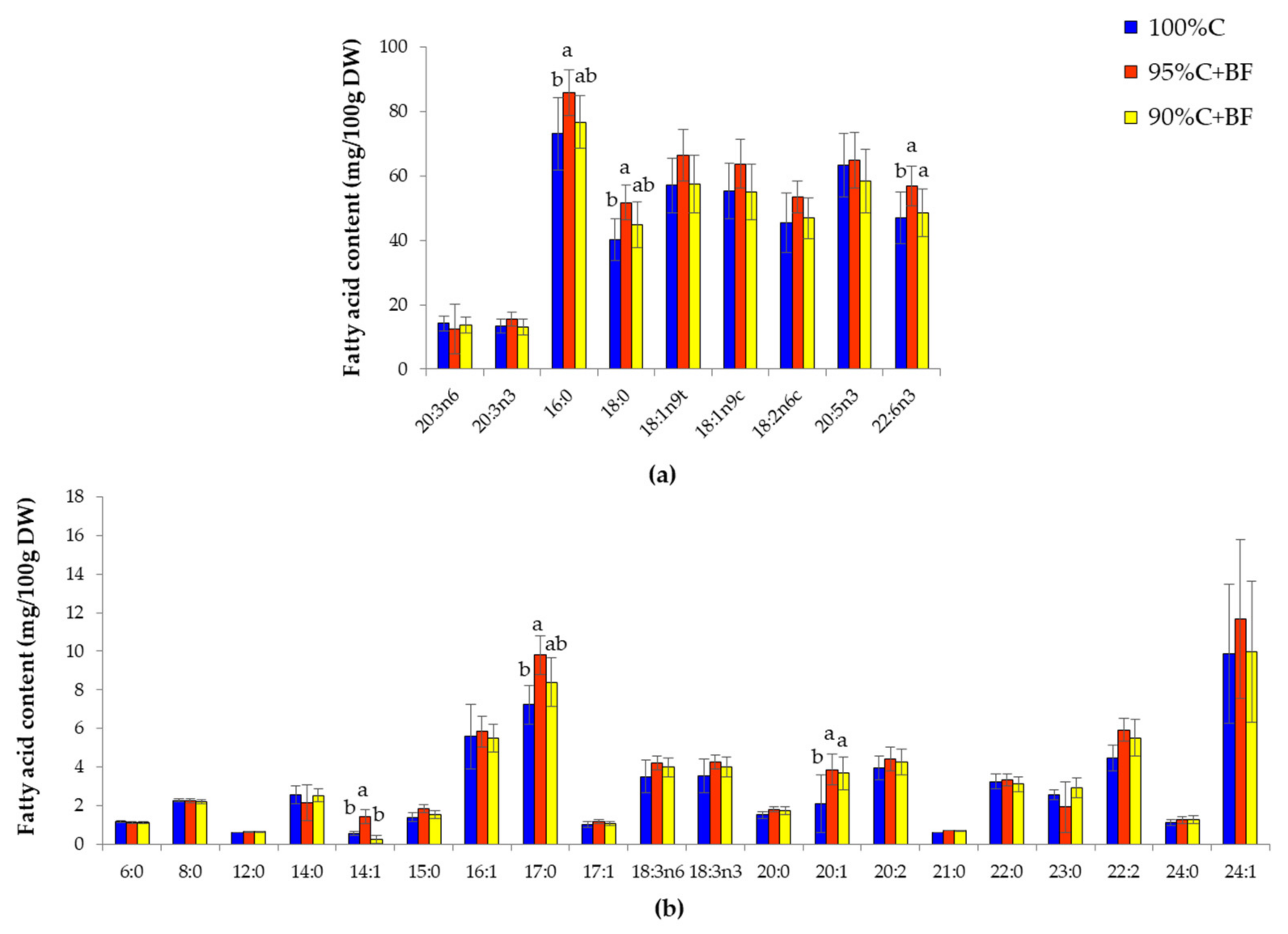
3.3. Nutritional Analysis in Shrimp under Different Feeding Regime
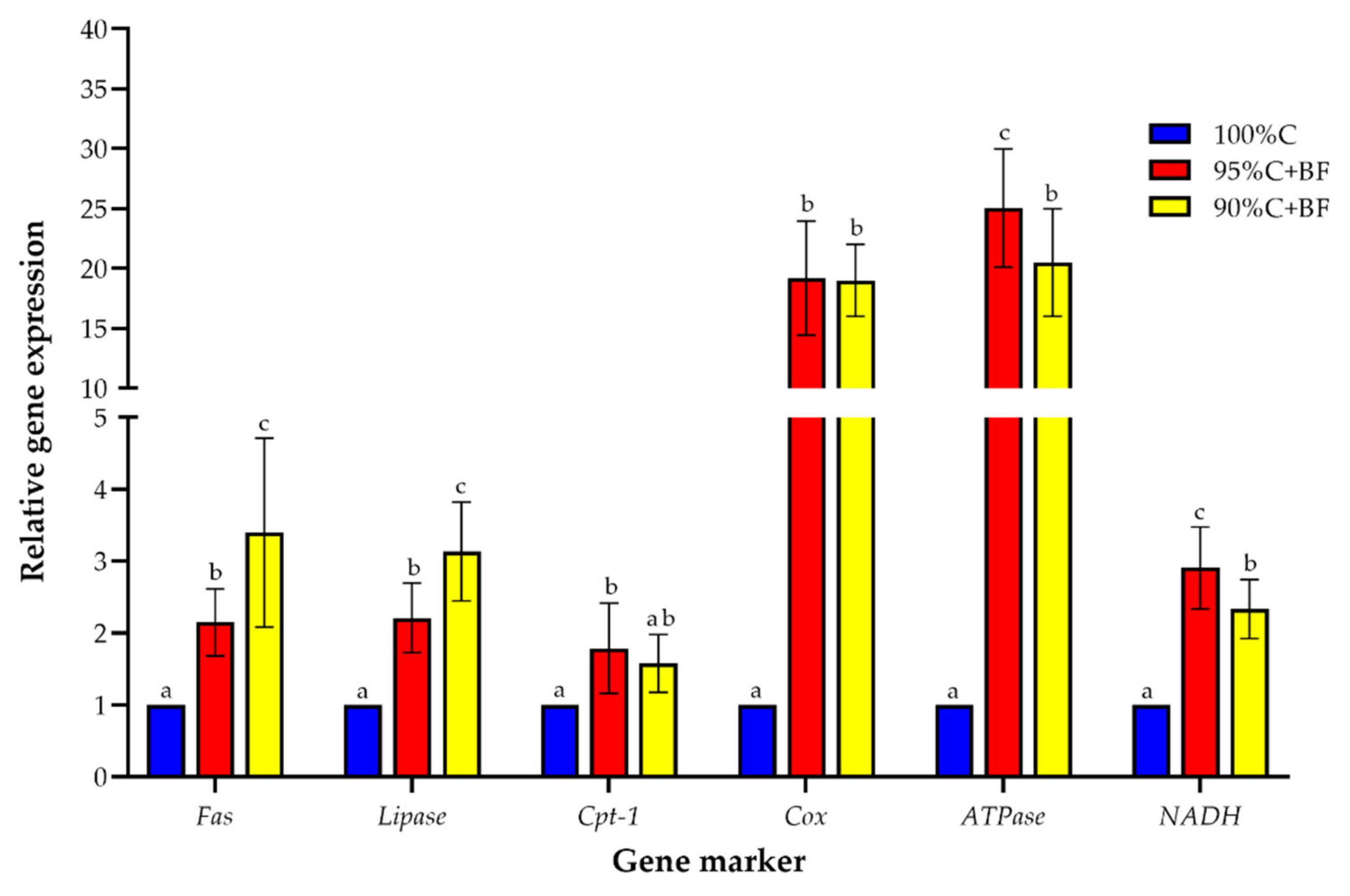
3.4. Gene Expression Analysis
4. Discussion
4.1. Effect of Ex-Situ Biofloc on Water Quality Maintenance
4.2. Effects of Ex-Situ Biofloc as Feed Supplement to Improve Shrimp Growth Performance
4.3. Effects of Ex-Situ Biofloc as Feed Supplement to Enhance Shrimp Nutritional
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Avnimelech, Y. Bio-filters: The need for a new comprehensive approach. Aquac. Eng. 2006, 34, 172–178. [Google Scholar] [CrossRef]
- Burford, M.A.; Thompson, P.J.; McIntosh, R.P.; Bauman, R.H.; Pearson, D.C. The contribution of flocculated material to shrimp (Litopenaeus vannamei) nutrition in a high-intensity, zero-exchange system. Aquaculture 2004, 232, 525–537. [Google Scholar] [CrossRef]
- Avnimelech, Y.; Ritvo, G. Shrimp and fish pond soils: Processes and management. Aquaculture 2003, 220, 549–567. [Google Scholar] [CrossRef]
- Avnimelech, Y. Biofloc Technology: A Practical Guide Book; The World Aquaculture Society: Technion, Israel, 2009. [Google Scholar]
- Moriarty, D.J. Disease control in shrimp aquaculture with probiotic bacteria. In Proceedings of the 8th International Symposium in Microbial Ecology, Halifax, NS, Canada, 9–14 August 1998; pp. 237–243. [Google Scholar]
- Defoirdt, T.; Boon, N.; Sorgeloos, P.; Verstraete, W.; Bossier, P. Alternatives to antibiotics to control bacterial infections: Luminescent vibriosis in aquaculture as an example. Trends Biotechnol. 2007, 25, 472–479. [Google Scholar] [CrossRef]
- Han, S.; Wang, B.; Liu, Q. Effects of ammonia and nitrite accumulation on the survival and growth performance of white shrimp Litopenaeus vannamei. Invertebr. Surviv. J. 2017, 14, 221–232. [Google Scholar]
- Tacon, A.G.J.; Jory, D.E.; Nunes, A.J.P. Shrimp feed management: Issues and perspectives. In On-Farm Feeding and Feed Management in Aquaculture; Hasan, M.R., New, M.B., Eds.; FAO: Rome, Italy, 2013; pp. 481–488. [Google Scholar]
- Thitamadee, S.; Prachumwat, A.; Srisala, J.; Jaroenlak, P.; Salachan, P.V.; Sritunyalucksana, K.; Flegel, T.W.; Itsathitphaisarn, O. Review of current disease threats for cultivated penaeid shrimp in Asia. Aquaculture 2016, 452, 69–87. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2016. Contributing to Food Security and Nutrition for All. Available online: www.fao.org/3/a-i5555e.pdf (accessed on 8 February 2019).
- Crab, R.; Defoirdt, T.; Bossier, P.; Verstraete, W. Biofloc technology in aquaculture: Beneficial effects and future challenges. Aquaculture 2012, 356–357, 351–356. [Google Scholar] [CrossRef]
- De Schryver, P.; Crab, R.; Defoirdt, T.; Boon, N.; Verstraete, W. The basics of bio-flocs technology: The added value for aquaculture. Aquaculture 2008, 277, 125–137. [Google Scholar] [CrossRef]
- Xu, W.; Xu, Y.; Su, H.; Hu, X.; Yang, K.; Wen, G.; Cao, Y. Characteristics of ammonia removal and nitrifying microbial communities in a hybrid biofloc-RAS for intensive Litopenaeus vannamei culture: A pilot-scale study. Water 2020, 12, 3000. [Google Scholar] [CrossRef]
- Suantika, G.; Situmorang, M.L.; Kurniawan, J.B.; Pratiwi, S.A.; Aditiawati, P.; Astuti, D.I.; Azizah, F.F.N.; Djohan, Y.A.; Zuhri, U.; Simatupang, T.M. Development of a zero water discharge (ZWD)—Recirculating aquaculture system (RAS) hybrid system for super intensive white shrimp (Litopenaeus vannamei) culture under low salinity conditions and its industrial trial in commercial shrimp urban farming in Gresik, East Java, Indonesia. Aquac. Eng. 2018, 82, 12–24. [Google Scholar] [CrossRef]
- Emerenciano, M.G.C.; Martínez- Córdova, L.R.; Martínez-Porchas, M.; Miranda-Baeza, A. Biofloc Technology (BFT): A tool for water quality management in aquaculture, water quality. IntechOpen 2017. [Google Scholar] [CrossRef]
- Arantes, R.; Schveitzer, R.; Magnotti, C.; Lapa, K.R.; Vinatea, L. A comparison between water exchange and settling tank as a method for suspended solids management in intensive biofloc technology systems: Effects on shrimp (Litopenaeus vannamei) performance, water quality and water use. Aquac. Res. 2017, 48, 1478–1490. [Google Scholar] [CrossRef]
- Brito, L.O.; Arana, L.A.V.; Soares, R.B.; Severi, W.; Miranda, R.H.; da Silva, S.M.B.C.; Coimbra, M.R.M.; Gálvez, A.O. Water quality, phytoplankton composition and growth of Litopenaeus vannamei (Boone) in an integrated biofloc system with Gracilariabirdiae (Greville) and Gracilaria domingensis (Kützing). Aquac. Int. 2014, 22, 1649–1664. [Google Scholar] [CrossRef]
- Seraspe, E.B.; Ticar, B.F.; Formacion, M.J.; Pahila, I.G.; de la Peña, M.R.; Amar, E.C. Antibacterial properties of the microalgae Chaetoceros calcitrans. Asian Fish. Sci. 2012, 25, 343–356. [Google Scholar]
- Vidal, J.M.A.; Pessoa, M.N.D.C.; Santos, F.L.D.; Mendes, P.P.; Mendes, M.S. Probiotic potential of Bacillus cereus against Vibrio spp. in post-larvae shrimps. Rev. Caatinga 2018, 31, 495–503. [Google Scholar] [CrossRef]
- Kumar, V.; Wille, M.; Lourenço, T.M.; Bossier, P. Biofloc-Based enhanced survival of Litopenaeus vannamei upon AHPND-causing Vibrio parahaemolyticus challenge is partially mediated by reduced expression of its virulence genes. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef]
- Martínez-Córdova, L.R.; Emerenciano, M.; Miranda-Baeza, A.; Martínez-Porchas, M. Microbial-based systems for aquaculture of fish and shrimp: An updated review. Rev. Aquac. 2015, 7, 131–148. [Google Scholar] [CrossRef]
- Jiménez-Ordaz, F.J.; Cadena-Roa, M.A.; Pacheco-Vega, J.M.; Rojas-Contreras, M.; Tovar-Ramírez, D.; Arce-Amezquita, P.M. Microalgae and probiotic bacteria as biofloc inducers in a hyper-intensive Pacific white shrimp (Penaeus vannamei) culture. Lat. Am. J. Aquat. Res. 2021, 49. [Google Scholar] [CrossRef]
- Anand, P.S.S.; Kohli, M.P.S.; Kumar, S.; Sundaray, J.K.; Roy, S.D.; Venkateshwarlu, G.; Sinha, A.; Pailan, G.H. Effect of dietary supplementation of biofloc on growth performance and digestive enzyme activities in Penaeus monodon. Aquaculture 2014, 418–419, 108–115. [Google Scholar] [CrossRef]
- Shyne Anand, P.S.; Kumar, S.; Kohli, M.P.S.; Sundaray, J.K.; Sinha, A.; Pailan, G.H.; Dam Roy, S. Dietary biofloc supplementation in black tiger shrimp, Penaeus monodon: Effects on immunity, antioxidant and metabolic enzyme activities. Aquac. Res. 2017, 48, 4512–4523. [Google Scholar] [CrossRef]
- Lee, C.; Kim, S.; Lim, S.-J.; Lee, K.-J. Supplemental effects of biofloc powder on growth performance, innate immunity, and disease resistance of Pacific white shrimp Litopenaeus vannamei. Fish. Aquat. Sci. 2017, 20, 15. [Google Scholar] [CrossRef]
- APHA. Standard Method for the Examination of the Water and Wastewater, 22nd ed.; Public Health Association: Washington, DC, USA, 1998. [Google Scholar]
- Xie, S.; Liu, Y.; Tian, L.; Niu, J.; Tan, B. Low dietary fish meal induced endoplasmic reticulum stress and impaired phospholipids metabolism in juvenile pacific white shrimp, Litopenaeus vannamei. Front. Physiol. 2020, 11, 1024. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhang, X.; Yu, Y.; Li, F.; Xiang, J. RNA-Seq reveals the dynamic and diverse features of digestive enzymes during early development of Pacific white shrimp Litopenaeus vannamei. Comp. Biochem. Physiol. Part D Genom. Proteom. 2014, 11, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Lage, L.P.A.; Plagnes-Juan, E.; Putrino, S.M.; Baron, F.; Weissman, D.; Guyonvarch, A.; Brugger, R.; Nunes, A.J.P.; Panserat, S. Ontogenesis of metabolic gene expression in whiteleg shrimp (Litopenaeus vannamei): New molecular tools for programming in the future. Aquaculture 2017, 479, 142–149. [Google Scholar] [CrossRef]
- Ning, P.; Zheng, Z.; Aweya, J.J.; Yao, D.; Li, S.; Ma, H.; Wang, F.; Zhang, Y. Litopenaeus vannamei notch affects lipopolysaccharides induced reactive oxygen species. Dev. Comp. Immunol. 2018, 81, 74–82. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Hai, T.N.; Tao, T.T.; Khoa, T.N.D.; Khanh, L.V.; Anh, N.T.N. Nursery of the black tiger shrimp Penaeus monodon postlarvae in a biofloc system with different carbon sources. Oceanogr. Fish Open Access J 2020, 11, 555821. [Google Scholar] [CrossRef]
- Kirchman, D.L. The uptake of inorganic nutrients by heterotrophic bacteria. Microb. Ecol. 1994, 28, 255–271. [Google Scholar] [CrossRef]
- Amin, S.A.; Parker, M.S.; Armbrust, E.V. Interactions between diatoms and bacteria. Microbiol. Mol. Biol. Rev. 2012, 76, 667–684. [Google Scholar] [CrossRef]
- Delgadillo-Mirquez, L.; Lopes, F.; Taidi, B.; Pareau, D. Nitrogen and phosphate removal from wastewater with a mixed microalgae and bacteria culture. Biotechnol. Rep. 2016, 11, 18–26. [Google Scholar] [CrossRef]
- Tocher, D.R.; Betancor, M.B.; Sprague, M.; Olsen, R.E.; Napier, J.A. Omega-3 long-chain polyunsaturated fatty acids, EPA and DHA: Bridging the gap between supply and demand. Nutrients 2019, 11, 89. [Google Scholar] [CrossRef]
- Shurson, G.C.; Kerr, B.J.; Hanson, A.R. Evaluating the quality of feed fats and oils and their effects on pig growth performance. J. Anim. Sci. Biotechnol. 2015, 6, 10. [Google Scholar] [CrossRef]
- Innis, S.M. Palmitic Acid in Early Human Development. Crit. Rev. Food Sci. Nutr. 2016, 56, 1952–1959. [Google Scholar] [CrossRef]
- Glencross, B.D. Exploring the nutritional demand for essential fatty acids by aquaculture species. Rev. Aquac. 2009, 1, 71–124. [Google Scholar] [CrossRef]
- Li, P.; Wu, G. Roles of dietary glycine, proline, and hydroxyproline in collagen synthesis and animal growth. Amino Acids 2018, 50, 29–38. [Google Scholar] [CrossRef]
- Li, X.; Rezaei, R.; Li, P.; Wu, G. Composition of amino acids in feed ingredients for animal diets. Amino Acids 2011, 40, 1159–1168. [Google Scholar] [CrossRef]
- Becker, W. Microalgae in Human and Animal Nutrition; Richmond, A., Ed.; Blackwell Science: Hoboken, NJ, USA, 2004. [Google Scholar]
- Fox, J.M.; Zimba, P.V. Chapter 8—Minerals and trace elements in microalgae. In Microalgae in Health and Disease Prevention; Levine, I.A., Fleurence, J., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 177–193. [Google Scholar] [CrossRef]
- Natrah, F.M.I.; Bossier, P.; Sorgeloos, P.; Yusoff, F.M.; Defoirdt, T. Significance of microalgal–bacterial interactions for aquaculture. Rev. Aquac. 2014, 6, 48–61. [Google Scholar] [CrossRef]
- Naorbe, M.C.; Garibay, S.; Serrano, A. Simultaneous replacement of protein, vitamins and minerals with Chaetoceros calcitrans paste in the diet of the black tiger shrimp (Penaeus monodon) larvae. ABAH Bioflux 2015, 7, 28–36. [Google Scholar]
- ICAR—Central Institute of Brackishwater Aquaculture. Applications of Mineral in Shrimp Culture Systems; CIBA: Chennai, India, 2016. [Google Scholar]
- Cheng, K.-M.; Hu, C.-Q.; Liu, Y.-N.; Zheng, S.-X.; Qi, X.-J. Effects of dietary calcium, phosphorus and calcium/phosphorus ratio on the growth and tissue mineralization of Litopenaeus vannamei reared in low-salinity water. Aquaculture 2006, 251, 472–483. [Google Scholar] [CrossRef]
- Davis, D.A.; Boyd, C.E.; Rouse, D.B.; Saoud, I.P. Effects of Potassium, Magnesium and age on growth and survival of Litopenaeus vannamei post-larvae reared in inland low salinity well waters in west alabama. J. World Aquac. Soc. 2005, 36, 416–419. [Google Scholar] [CrossRef]
- Roy, L.A.; Davis, D.A.; Saoud, I.P.; Henry, R.P. Effects of varying levels of aqueous potassium and magnesium on survival, growth, and respiration of the Pacific white shrimp, Litopenaeus vannamei, reared in low salinity waters. Aquaculture 2007, 262, 461–469. [Google Scholar] [CrossRef]
- Frassinetti, S.; Bronzetti, G.; Caltavuturo, L.; Cini, M.; Croce, C.D. The role of zinc in life: A review. J. Environ. Pathol. Toxicol. Oncol. 2006, 25, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Maret, W. Zinc biochemistry: From a single zinc enzyme to a key element of life. Adv. Nutr. 2013, 4, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Xu, F.; Zhou, Q.; Regan, M.K.; Betancor, M.B.; Tocher, D.R.; Sun, M.; Meng, F.; Jiao, L.; Jin, M. Dietary organic zinc promotes growth, immune response and antioxidant capacity by modulating zinc signaling in juvenile Pacific white shrimp (Litopenaeus vannamei). Aquac. Rep. 2021, 19, 100638. [Google Scholar] [CrossRef]
- Lin, S.; Lin, X.; Yang, Y.; Li, F.; Luo, L. Comparison of chelated zinc and zinc sulfate as zinc sources for growth and immune response of shrimp (Litopenaeus vannamei). Aquaculture, 2003; 406–407, 79–84. [Google Scholar] [CrossRef]
- Galbraith, E.D.; Le Mézo, P.; Solanes Hernandez, G.; Bianchi, D.; Kroodsma, D. Growth limitation of marine fish by low iron availability in the open ocean. Front. Mar. Sci. 2019, 6. [Google Scholar] [CrossRef]
- Boyd, C.E. Health & Welfare: The Importance of Iron in Aquaculture Systems. Glob. Aquac. 2016. Available online: https://www.aquaculturealliance.org/advocate/the-importance-of-iron-in-aquaculture-systems/ (accessed on 4 March 2020).
- Adhikari, S.; Naqvi, A.A.; Pani, K.C.; Pillai, B.R.; Jena, J.K.; Sarangi, N. Effect of manganese and iron on growth and feeding of juvenile giant river prawn, Macrobrachium rosenbergii (De-Man). J. World Aquac. Soc. 2007, 38, 161–168. [Google Scholar] [CrossRef]
- Huner, J.V.; Kowalczuk, J.G.; Avault, J.W., Jr. Calcium and magnesium levels in the intermolt (C4) carapaces of the three species of freshwater crawfish (Cambaridae: Decapoda). Comp. Biochem. Physiol. Part A Physiol. 1976, 55, 183–185. [Google Scholar] [CrossRef]
- Galkanda-Arachchige, H.S.C.; Roy, L.A.; Davis, D.A. The effects of magnesium concentration in low-salinity water on growth of Pacific white shrimp (Litopenaeus vannamei). Aquac. Res. 2021, 52, 589–597. [Google Scholar] [CrossRef]
- Pirahanchi, Y.; Sharma, S. Lipase; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Ziaei-Nejad, S.; Rezaei, M.H.; Takami, G.A.; Lovett, D.L.; Mirvaghefi, A.-R.; Shakouri, M. The effect of Bacillus spp. bacteria used as probiotics on digestive enzyme activity, survival and growth in the Indian white shrimp Fenneropenaeus indicus. Aquaculture 2006, 252, 516–524. [Google Scholar] [CrossRef]
- Zokaeifar, H.; Balcázar, J.L.; Saad, C.R.; Kamarudin, M.S.; Sijam, K.; Arshad, A.; Nejat, N. Effects of Bacillus subtilis on the growth performance, digestive enzymes, immune gene expression and disease resistance of white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 2012, 33, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Bernal, M.G.; Marrero, R.M.; Campa-Córdova, Á.I.; Mazón-Suástegui, J.M. Probiotic effect of Streptomyces strains alone or in combination with Bacillus and Lactobacillus in juveniles of the white shrimp Litopenaeus vannamei. Aquac. Int. 2017, 25, 927–939. [Google Scholar] [CrossRef]
- Miandare, H.K.; Mirghaed, A.T.; Hosseini, M.; Mazloumi, N.; Zargar, A.; Nazari, S. Dietary Immunogen® modulated digestive enzyme activity and immune gene expression in Litopenaeus vannamei post larvae. Fish Shellfish Immunol. 2017, 70, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Sargent, J.R.; Tocher, D.R.; Bell, J.G. Fish Nutrition; Halver, J.E., Hardey, R.W., Eds.; Academic Press: Cambridge, MA, USA, 2002. [Google Scholar]
- Turkmen, S.; Perera, E.; Zamorano, M.J.; Simó-Mirabet, P.; Xu, H.; Pérez-Sánchez, J.; Izquierdo, M. Effects of dietary lipid composition and fatty acid desaturase 2 expression in broodstock gilthead sea bream on lipid metabolism-related genes and methylation of the fads2 gene promoter in their offspring. Int. J. Mol. Sci. 2019, 20, 6250. [Google Scholar] [CrossRef]
- Wanders, R.J. Peroxisomes, lipid metabolism, and peroxisomal disorders. Mol. Genet. Metab. 2004, 83, 16–27. [Google Scholar] [CrossRef]
- Caldow, M.K.; Ham, D.J.; Trieu, J.; Chung, J.D.; Lynch, G.S.; Koopman, R. Glycine protects muscle cells from wasting in vitro via mTORC1 signaling. Front. Nutr. 2019, 6. [Google Scholar] [CrossRef]
- Xie, S.-W.; Tian, L.-X.; Jin, Y.; Yang, H.-J.; Liang, G.-Y.; Liu, Y.-J. Effect of glycine supplementation on growth performance, body composition and salinity stress of juvenile Pacific white shrimp, Litopenaeus vannamei fed low fishmeal diet. Aquaculture 2014, 418–419, 159–164. [Google Scholar] [CrossRef]
- Jäger, R.; Zaragoza, J.; Purpura, M.; Iametti, S.; Marengo, M.; Tinsley, G.M.; Anzalone, A.J.; Oliver, J.M.; Fiore, W.; Biffi, A.; et al. Probiotic administration increases amino acid absorption from plant protein: A placebo-controlled, randomized, double-blind, multicenter, crossover study. Probiotics Antimicrob. Proteins 2020, 12, 1330–1339. [Google Scholar] [CrossRef]
- Suantika, G.; Situmorang, M.L.; Saputra, F.I.; Putri, S.L.E.; Putri, S.P.; Aditiawati, P.; Fukusaki, E. Metabolite profiling of whiteleg shrimp Litopenaeus vannamei from super-intensive culture in closed aquaculture systems: A recirculating aquaculture system and a hybrid zero water discharge-recirculating aquaculture system. Metabolomics 2020, 16, 49. [Google Scholar] [CrossRef]
- Jónasdóttir, S.H. Fatty acid profiles and production in marine phytoplankton. Mar. Drugs 2019, 17, 151. [Google Scholar] [CrossRef]
- Ahmmed, M.K.; Ahmmed, F.; Tian, H.; Carne, A.; Bekhit, A.E.-D. Marine omega-3 (n-3) phospholipids: A comprehensive review of their properties, sources, bioavailability, and relation to brain health. Compr. Rev. Food Sci. Food Saf. 2020, 19, 64–123. [Google Scholar] [CrossRef]
- Durmus, M. Fish oil for human health: Omega-3 fatty acid profiles of marine seafood species. Food Sci. Technol. 2019, 39, 454–461. [Google Scholar] [CrossRef]
- Saini, R.K.; Song, M.-H.; Rengasamy, K.R.R.; Ko, E.-Y.; Keum, Y.-S. Red shrimp are a rich source of nutritionally vital lipophilic compounds: A comparative study among edible flesh and processing waste. Foods 2020, 9, 1179. [Google Scholar] [CrossRef]
- Pereira, H.; Barreira, L.; Figueiredo, F.; Custódio, L.; Vizetto-Duarte, C.; Polo, C.; Rešek, E.; Engelen, A.; Varela, J. Polyunsaturated Fatty acids of marine macroalgae: Potential for nutritional and pharmaceutical applications. Mar. Drugs 2012, 10, 1920–1935. [Google Scholar] [CrossRef]
- Yang, Z.-H.; Emma-Okon, B.; Remaley, A.T. Dietary marine-derived long-chain monounsaturated fatty acids and cardiovascular disease risk: A mini review. Lipids Health Dis. 2016, 15, 201. [Google Scholar] [CrossRef]
- Senarath, S.; Yoshinaga, K.; Nagai, T.; Yoshida, A.; Beppu, F.; Gotoh, N. Differential effect of cis-eicosenoic acid positional isomers on adipogenesis and lipid accumulation in 3T3-L1 cells. Eur. J. Lipid Sci. Technol. 2018, 120, 1700512. [Google Scholar] [CrossRef]
- Foysal, M.J.; Fotedar, R.; Siddik, M.A.B.; Tay, A. Lactobacillus acidophilus and L. plantarum improve health status, modulate gut microbiota and innate immune response of marron (Cherax cainii). Sci. Rep. 2020, 10, 5916. [Google Scholar] [CrossRef]
- Ley, R.E.; Hamady, M.; Lozupone, C.; Turnbaugh, P.J.; Ramey, R.R.; Bircher, J.S.; Schlegel, M.L.; Tucker, T.A.; Schrenzel, M.D.; Knight, R.; et al. Evolution of mammals and their gut microbes. Science 2008, 320, 1647–1651. [Google Scholar] [CrossRef]
- Uengwetwanit, T.; Uawisetwathana, U.; Arayamethakorn, S.; Khudet, J.; Chaiyapechara, S.; Karoonuthaisiri, N.; Rungrassamee, W. Multi-omics analysis to examine microbiota, host gene expression and metabolites in the intestine of black tiger shrimp (Penaeus monodon) with different growth performance. PeerJ 2020, 8, e9646. [Google Scholar] [CrossRef]
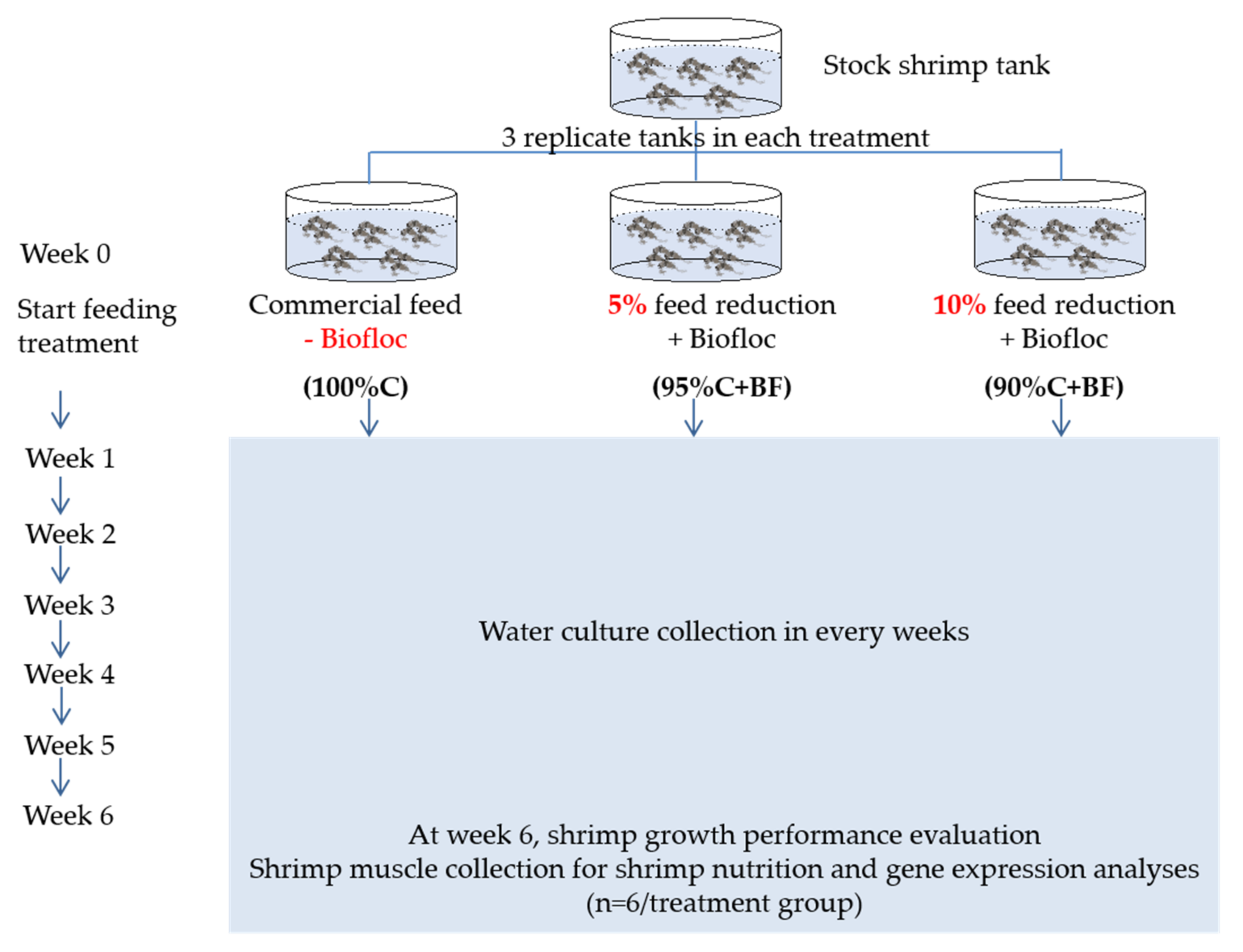
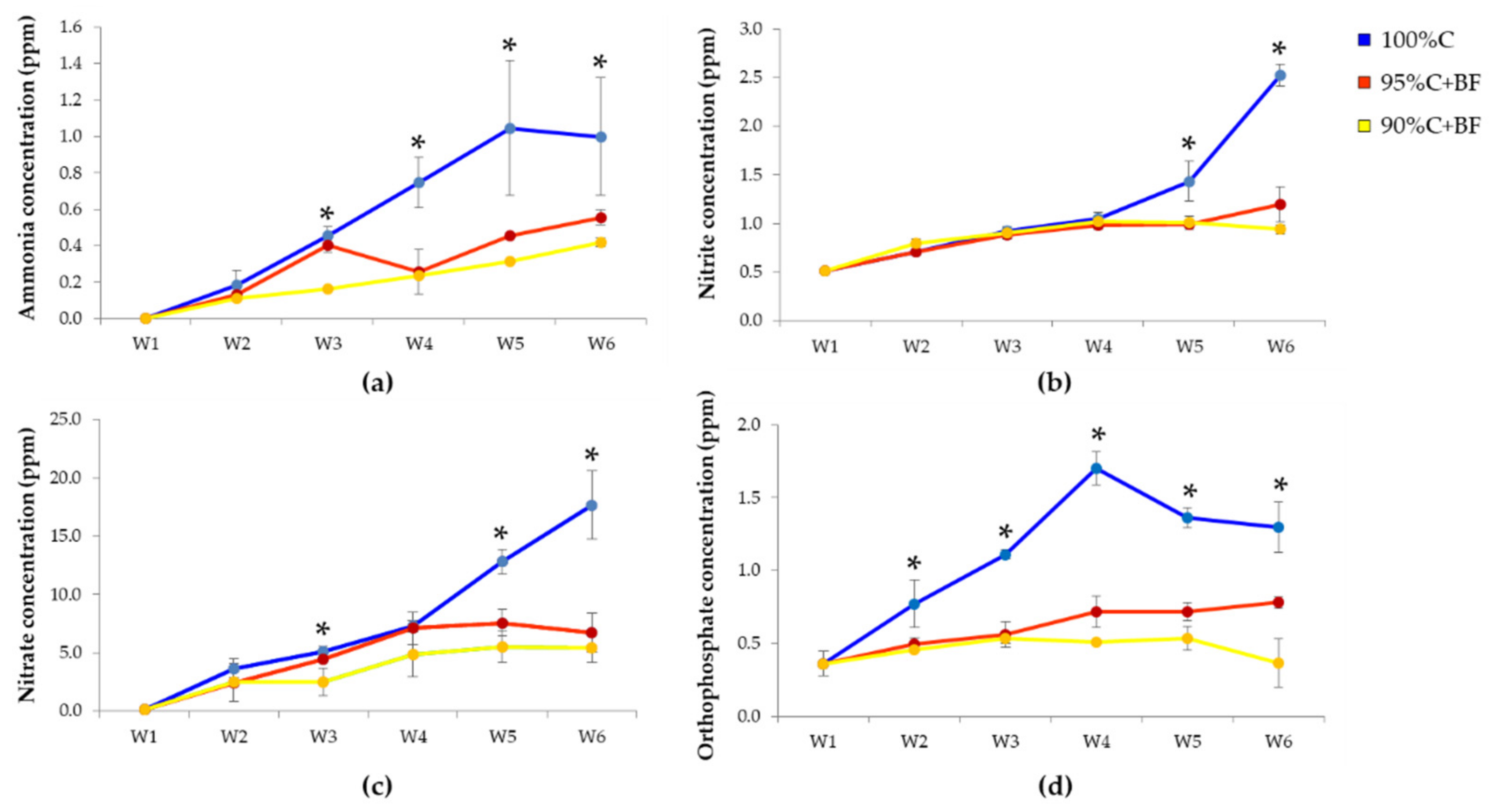
| Parameter | Optimum Range | 100%C | 95%C+BF | 90%C+BF |
|---|---|---|---|---|
| Temperature (°C) | 28–32 | 27.4–30.3 | 27.8–30.8 | 27.8–31.0 |
| Salinity (ppt) | 0.5–35 | 11.0–15.7 | 10.7–16.0 | 11.7–16.7 |
| DO (mg/L) | 5.0–9.0 | 5.8–6.4 | 6.0–6.4 | 5.9–6.5 |
| pH | 7.0–8.3 | 7.2–7.5 | 7.3–7.5 | 7.3–7.5 |
| Alkalinity (CaCO3/L) | 50–150 | 50.0–70.7 | 50.0–83.3 | 53.3–80.0 |
| Biofloc volume (mL/L) | 2.0–15.0 | 0.6–0.7 | 3.8–4.4 | 3.4–3.8 |
| Shrimp Growth Performance | 100%C | 95%C+BF | 90%C+BF |
|---|---|---|---|
| Survival (%) | 53.33 ± 5.94 a | 83.81 ± 3.30 b | 85.71 ± 7.56 b |
| Gained weight (g) | 0.86 ± 0.15 a | 1.97 ± 0.14 b | 1.89 ± 0.29 b |
| Gained length (cm) | 4.57 ± 0.52 a | 6.82 ± 0.13 b | 6.92 ± 0.22 b |
| Specific Growth Rate (SGR) (%/day) | 6.59 ± 0.50 a | 8.31 ± 0.28 b | 8.45 ± 0.71 b |
| Biomass (g/m3) | 246.81 ± 67.17 a | 851.63 ± 86.79 b | 826.55 ± 44.00 b |
| Parameter | Unit | Commercial Pellet (C) | Ex-Situ Biofloc (BF) | Fold Change (C/BF) | Reference Method |
|---|---|---|---|---|---|
| Ash | g/100 g | 11.61 | 48.46 | 0.24 | [a] |
| Carbohydrate | g/100 g | 33.92 | 16.99 | 2.00 | [b] |
| Energy | Kcal/100 g | 334.84 | 126.81 | 2.64 | [b] |
| Crude fat | g/100 g | 3.92 | 0.33 | 11.88 | [c] |
| Moisture | g/100 g | 9.58 | 20.25 | 0.47 | [d] |
| Protein | g/100 g | 40.97 | 13.97 | 2.93 | [e] |
| Mineral (mg/kg) | Commercial Pellet (C) | Ex-Situ Biofloc (BF) | Fold Change (C/BF) |
|---|---|---|---|
| Copper | 50.150 | 5.908 | 8.49 |
| Manganese | 30.680 | 12.020 | 2.55 |
| Zinc | 50.340 | 184.000 | 0.27 |
| Iron | 477.00 | 1857.00 | 0.26 |
| Calcium | 2064 | 3824 | 0.54 |
| Magnesium | 1928 | 25,084 | 0.08 |
| Sodium | 3898 | 129,067 | 0.03 |
| Potassium | 9956 | 7758 | 1.28 |
| Amino Acids (g/100 g DW) | Commercial Pellet (C) | Ex Situ-Biofloc (BF) | Fold Change (C/BF) |
|---|---|---|---|
| Alanine | 2.29 ± 0.04 a | 0.73 ± 0.06 b | 3.14 |
| Glycine | 2.95 ± 0.09 a | 0.50 ± 0.09 b | 5.90 |
| Valine | 1.76 ± 0.06 a | 0.52 ± 0.02 b | 3.38 |
| Leucine | 2.68 ± 0.09 a | 0.64 ± 0.02 b | 4.19 |
| Isoleucine | 1.55 ± 0.06 a | 0.48 ± 0.01 b | 3.23 |
| Proline | 4.63 ± 0.12 a | 0.51 ± 0.04 b | 9.08 |
| Methionine | 0.69 ± 0.01 a | 0.23 ± 0.01 b | 3.00 |
| Serine | 1.80 ± 0.13 a | 0.29 ± 0.09 b | 6.21 |
| Threonine | 1.68 ± 0.12 a | 0.34 ± 0.08 b | 4.94 |
| Phenylalanine | 1.55 ± 0.06 a | 0.46 ± 0.02 b | 3.37 |
| Aspartic acid | 2.63 ± 0.20 a | 0.77 ± 0.27 b | 3.42 |
| Hydroxyproline | 0.77 ± 0.01 a | 0.144 ± 0.0004 b | 5.35 |
| Cysteine | 0.54 ± 0.04 a | 0.053 ± 0.003 b | 10.19 |
| Glutamic acid | 5.22 ± 0.24 a | 1.44 ± 0.39 b | 3.63 |
| Lysine | 1.19 ± 0.02 a | 0.34 ± 0.10 b | 3.50 |
| Arginine | 2.89± 0.06 a | 0.660 ± 0.126 b | 4.38 |
| Histidine | 2.61 ± 0.01 a | 0.415 ± 0.011 b | 6.29 |
| Tyrosine | 1.27 ± 0.05 a | 0.44 ± 0.03 b | 2.89 |
| Tryptophan | 1.03 ± 0.03 a | 0.170 ± 0.0003 b | 6.06 |
| Fatty Acid (mg/100 g DW) | Symbol | Commercial Pellet (C) | Ex-Situ Biofloc (BF) | Fold Change (C/BF) |
|---|---|---|---|---|
| Methyl Hexanoate | 6:0 | 1.23 ± 0.14 a | 1.08 ± 0.07 a | 1.14 |
| Methyl Octanoate | 8:0 | 2.03 ± 0.01 a | 2.04 ± 0.04 a | 0.99 |
| Methyl Decanoate | 10:0 | ND | ND | ND |
| Methyl Undecanoate | 11:0 | ND | ND | ND |
| Methyl Laurate | 12:0 | 2.70 ± 0.45 a | 0.91 ± 0.10 b | 2.98 |
| Methyl Tridecanoate | 13:0 | ND | ND | ND |
| Methyl Myristate | 14:0 | 36.39 ± 7.85 a | 9.44 ± 2.43 b | 3.86 |
| Myristoleic Acid Methyl Ester | 14:1 | 1.21 ± 0.27 b | 3.20 ± 0.81 a | 0.38 |
| Methyl Pentadecanoate | 15:0 | 6.55 ± 1.42 a | 2.90 ± 0.68 b | 2.25 |
| Cis-10-Pentadecanoic Acid Methyl Ester | 15:1 | 61.17 ± 45.90 a | 90.38 ± 53.40 a | 0.68 |
| Methyl Palmitate | 16:0 | 215.46 ± 30.88 a | 91.74 ± 53.35 b | 2.35 |
| Methyl Pamitoleate | 16:1 | 48.82 ± 11.91 b | 95.06 ± 25.59 a | 0.51 |
| Methyl Heptadecanoate | 17:0 | 16.63 ± 4.51 a | 3.76 ± 0.92 b | 4.42 |
| Cis-10-Heptadecanoic Acid Methyl Ester | 17:1 | 3.64 ± 0.77 a | 2.13 ± 0.57 b | 1.71 |
| Methyl Stearate | 18:0 | 79.60 ± 25.93 a | 8.17 ± 2.06 b | 9.75 |
| Trans-9-Elaidic Methyl Ester | 18:1n9t | 67.15 ± 15.97 a | 5.59 ± 1.48 b | 12.01 |
| Cis-9-Oleic Acid Methyl ester | 18:1n9c | 158.45 ± 16.28 a | 13.55 ± 3.52 b | 11.69 |
| Linolelaidic Acid Methyl Ester | 18:2n6t | ND | ND | ND |
| Methyl Linoleate | 18:2n6c | 14.05 ± 4.95 a | 4.18 ± 3.99 b | 3.36 |
| Gamma-Linolenic Acid Methyl Ester | 18:3n6 | 42.49 ± 10.24 a | 1.34 ± 0.13 b | 31.62 |
| Methyl Linolenate | 18:3n3 | 42.93 ± 10.35 a | 7.29 ± 1.81 b | 5.89 |
| Methyl Arachidate | 20:0 | 6.40 ± 1.18 a | 1.94 ± 0.30 b | 3.31 |
| Methyl cis-11-Eicosanoate | 20:1 | 18.46 ± 3.50 a | 0.76 ± 0.06 b | 24.22 |
| Cis-11,14-Eicosadienoic Acid Methyl Ester | 20:2 | 5.61 ± 1.07 a | 0.87 ± 0.08 b | 6.45 |
| Methyl Heneicosanoate | 21:0 | 1.36 ± 0.12 a | 0.71 ± 0.04 b | 1.92 |
| Cis-8,11,14-Eicosatrienoic Acid Methyl Ester | 20:3n6 | 3.03 ± 0.46 b | 22.43 ± 6.12 a | 0.14 |
| Cis-11,14,17-Eicosatrienoic Acid Methyl Ester | 20:3n3 | 15.54 ± 3.10 a | 21.77 ± 6.02 a | 0.71 |
| Methyl Cis-5,8,11,14-Eicosatetraenoic Acid Methyl Ester | 20:4n6 | 2.56 ± 0.43 a | 1.73 ± 0.26 b | 1.48 |
| Methyl Cis-5,8,11,14,17-Eicosapentaenoic Acid Methyl Ester | 20:5n3 | 43.44 ± 9.43 a | 29.29 ± 7.89 a | 1.48 |
| Methyl Behenate | 22:0 | 2.44 ± 0.35 a | 1.90 ± 0.30 a | 1.28 |
| Methyl Erucate | 22:1n9 | 14.03 ± 4.67 | ND | ND |
| Methyl Tricosanoate | 23:0 | 0.84 ± 0.09 a | 0.75 ± 0.11 a | 1.12 |
| Cis-13,16-Docosadienoic Acid Methyl Ester | 22:2 | 11.44 ± 2.85 | ND | ND |
| Methyl Lignocerate | 24:0 | 4.45 ± 0.78 a | 2.86 ± 0.53 b | 1.55 |
| Cis-4,7,10,13,16,19-Docosahexaenoic Acid Methyl Ester | 22:6n3 | 69.92 ± 14.57 a | 3.12 ± 0.74 b | 22.38 |
| Methyl Nervonate | 24:1 | 3.77 ± 1.14 a | 0.94 ± 0.37 b | 4.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uawisetwathana, U.; Situmorang, M.L.; Arayamethakorn, S.; Haniswita; Suantika, G.; Panya, A.; Karoonuthaisiri, N.; Rungrassamee, W. Supplementation of Ex-Situ Biofloc to Improve Growth Performance and Enhance Nutritional Values of the Pacific White Shrimp Rearing at Low Salinity Conditions. Appl. Sci. 2021, 11, 4598. https://doi.org/10.3390/app11104598
Uawisetwathana U, Situmorang ML, Arayamethakorn S, Haniswita, Suantika G, Panya A, Karoonuthaisiri N, Rungrassamee W. Supplementation of Ex-Situ Biofloc to Improve Growth Performance and Enhance Nutritional Values of the Pacific White Shrimp Rearing at Low Salinity Conditions. Applied Sciences. 2021; 11(10):4598. https://doi.org/10.3390/app11104598
Chicago/Turabian StyleUawisetwathana, Umaporn, Magdalena Lenny Situmorang, Sopacha Arayamethakorn, Haniswita, Gede Suantika, Atikorn Panya, Nitsara Karoonuthaisiri, and Wanilada Rungrassamee. 2021. "Supplementation of Ex-Situ Biofloc to Improve Growth Performance and Enhance Nutritional Values of the Pacific White Shrimp Rearing at Low Salinity Conditions" Applied Sciences 11, no. 10: 4598. https://doi.org/10.3390/app11104598
APA StyleUawisetwathana, U., Situmorang, M. L., Arayamethakorn, S., Haniswita, Suantika, G., Panya, A., Karoonuthaisiri, N., & Rungrassamee, W. (2021). Supplementation of Ex-Situ Biofloc to Improve Growth Performance and Enhance Nutritional Values of the Pacific White Shrimp Rearing at Low Salinity Conditions. Applied Sciences, 11(10), 4598. https://doi.org/10.3390/app11104598







