Abstract
Diatoms are unicellular eukaryotic microalgae widely distributed in aquatic environments, possessing a porous silica cell wall known as frustule. Diatom frustules are considered as a sustainable source for several industrial applications because of their high biocompatibility and the easiness of surface functionalisation, which make frustules suitable for regenerative medicine and as drug carriers. Frustules are made of hydrated silica, and can be extracted and purified both from living and fossil diatoms using acid treatments or high temperatures. Biosilica frustules have proved to be suitable for biomedical applications, but, unfortunately, they are not officially recognised as safe by governmental food and medical agencies yet. In the present review, we highlight the frustule formation process, the most common purification techniques, as well as advantages and bottlenecks related to the employment of diatom-derived silica for medical purposes, suggesting possible solutions for a large-scale biosilica production.
1. Introduction
Diatoms are an extremely diverse group of algae, comprising more than 100,000 different species [1]. They are able to colonise a large plethora of aquatic environments, and play a significant role on a global scale in the biogeochemical cycles of carbon and silicon in the water column. Two diatom species, Thalassiosira pseudonana and Phaeodactylum tricornutum, have been employed as model species for studies of gene expression and regulation, since they were the first species for which the whole genome was fully sequenced [2,3]. Subsequently, genomes have been sequenced from a number of diatoms possessing specific metabolic or physiological features, such as oleaginous (Fistulifera solaris), psicrophylic (Fragilariopsis cylindrus), araphid (Synedra acus subsp. radians), oceanic (Thalassiosira oceanica), biofilm-forming (Seminavis robusta), and heterotrophic (Nitzschia sp.) species [4,5,6,7,8,9]. Apart from their ecological role, diatoms are also suitable for several biotechnological applications. They can be cultured in the laboratory under sterile conditions and controlled temperatures, light irradiance and nutrient concentrations in order to achieve faster growth rates and to promote the accumulation of specialty products. Diatoms have been employed during the last decades for the production of metabolites exhibiting different biological activities and used as sources for cosmetic ingredients [10], food or feed supplements [11,12,13], fertilizers [14], and sorbents or accumulators for the bioremediation of aquatic environments [15,16]. Microalgae other than diatoms, especially freshwater green algae, also exhibit a great potential in one or more of the abovementioned fields of research.
The true distinctive feature that makes diatoms more suitable than other taxa for biotechnological purposes, is the high proportion of amorphous silica within their cell wall. This natural source of silicon has already shown several advantages, such as its high surface area and biocompatibility, and can be employed for various research fields, especially for biomedical applications after in vitro or in vivo treatments [17]. Diatom-derived silica is also available in huge amounts in aquatic benthic environments, as a consequence of the sedimentation of dead diatom cells.
Currently, diatom biosilica is considered as a suitable biomaterial for metal removal from aquatic environments, as a catalyst support, in optical devices, as a microsensor, and other kinds of applications [18,19]. Since its presence on the market as a device for aquatic remediation and as food-grade products is a pledge of its effectiveness in these fields, the present review is mainly focused on evaluating the potential of diatom biosilica for biomedical applications.
Diatom biosilica is actually exploited, indeed, for its potential as a drug carrier [20] and as a scaffold for bone tissue regeneration [21]. Biosilica-based processes can be considered as low-cost and environmentally friendly alternatives to processes based on artificial structures. While the production of synthetic materials requires the implementation of specific protocols, biosilica carries the advantage of triggering natural and sophisticated structure formation. For example, the employment of diatom-derived biosilica for the development of optical sensors may turn out to be, in the future, more attractive than using synthetic crystals, since it allows control and manipulation of light in a cost-effective way [22]. Biotemplated-based silica can be synthesized by rapid environmentally sustainable methods (solvent-free procedures), thus avoiding the use of hazardous chemicals, and allowing a good control of condensation rates [23].
In the last years, the effectiveness of living or fossil diatom-derived silica for biomedical applications (drug loadings, bone tissue regeneration) has been largely investigated by various research groups, and at least four recent reviews clearly summarize the most relevant studies [18,24,25,26]. In the present review, we pinpoint the major advantages and bottlenecks related to the employment of diatom silica sources. To this aim, we list examples of diatom-based systems that revealed satisfactory results in the laboratory and might be suitable for scale-up in industrial applications, and the few drawbacks that hinder the use of diatom-derived biosilica as a medical device. We critically compared the effectiveness of diatoms silica sources for drug delivery with respect to other biological and non-biological sources. This review also describes the process of frustule formation, the main techniques of silicon purification, and possible solutions to pave the way to silicon production on an industrial scale.
2. Silicon Capture from the Environment, Transport and Storage, Frustule Formation
In contrast with nitrogen and phosphorus metabolisms, silicon uptake in diatoms is linked with aerobic respiration rather than being strictly related to photosynthesis [27]. Silicon is mainly found in aquatic environments as Si(OH)4, and is usually transformed into solid SiO2 or other siliceous composites in the presence of organic substances, through condensation reactions [28]. It can enter cells by diffusion across their membranes; nevertheless, at very low concentrations of silicic acid in the surrounding environment, the cells activate silicon transporters that facilitate the uptake [29]. The genes coding for silicic acid transporters (SITs) were isolated and characterized from the marine diatom Cylindrotheca fusiformis several years ago [30,31] and, recently, in the freshwater species Synedra ulna subsp. danica [32]. The SITs specifically transport silicic and germanic acids through the lipid bilayer [33]. Studies performed on C. fusiformis revealed that ten transmembrane segments allow the passage of silicic acid through the lipid bilayer membrane, and chemical recognition is likely based on amino acids (Figure 1). The extent of frustule silicification depends on the rate of silicon uptake that is driven, in turn, by both the availability of the substrate and the expression levels of the SIT genes [30]. Five distinct clades have been identified for SIT genes in diatoms. The presence of genes from distinct clades within the same diatom species is likely to reflect different responses to changes in silicon availability and environmental conditions [34].
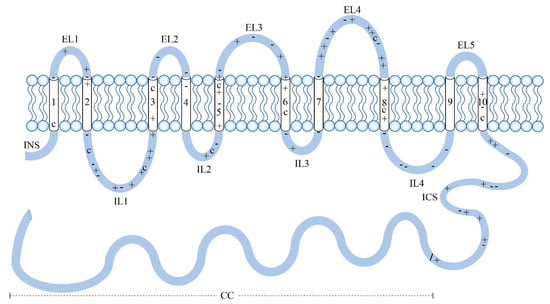
Figure 1.
Silicic acid transporters (SITs) in Cylindrotheca fusiformis. SITs contain 10 transmembrane segments (white cylinders), which allow the passage of silicic acid through the lipid bilayer membrane; an intracellular amino-terminal segment (INS); and an intracellular carboxy segment (ICS) connected to a coiled-coil motif (CC), which may play a role in the interactions with other proteins. Pluses (+) and minuses (-) indicate the position of positively and negatively charged amino acids, respectively. A major role in silicon uptake is likely played by cysteine residues (C) because of their sulfhydryl blocking agents. Figure redrawn from [31].
Intracellular pools of silicic acid can reach concentrations well beyond the saturation limit (2 mM), and this is likely due to complexation by organic compounds that prevents polymerization. The extent of the internal pools is both species-specific [35] and dependent upon environmental conditions [36]. When silicon deposition and uptake are imbalanced and internal pool tends to increase, a concentration gradient determines the efflux of the “unbound” silicon fraction outside the cell membrane [37].
The diatom cell wall has a Petri dish-like structure, with an upper part, known as epitheca, overlapping the smaller lower part, named hypotheca (Figure 2). Both epitheca and hypotheca consist, in turn, of valves and siliceous girdle bands, which confer an ordered structure to the frustule. Diatom frustule is a composite made of biogenic silica, carbohydrates and glycoproteins [31].
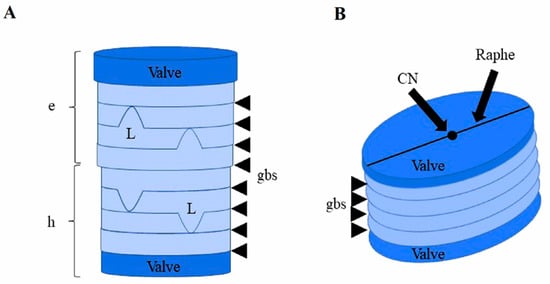
Figure 2.
Schematic representation of the cell wall structure of a centric diatom. (A) Dark-blue disks represent valves. The upper and larger part is the epytheca (e), the lower and narrower one is the hypotheca (h). Girdle bands (gbs) are indicated by arrows. Ligulas (L) are the bell-shaped structures within girdle bands. (B) Diagram of a pennate diatom showing the central nodule (CN) and the raphe on the upper valve. Figure from [38].
Frustule formation occurs through the polymerization of silicic acid in specific compartments, namely, the silica deposition vesicles (SDV), associated to the membrane silicalemma [39,40]. Silicalemma contributes to silicification through both the regulation and recognition of membrane-associated compounds, as well as the formation of a suitable microenvironment for polymerization.
SDVs in diatoms are formed inside the plasma membrane during cell division [41]. During valve formation, the SDVs rapidly expand and their movement is driven by the cytoskeleton [42].
The following several frustule-associated proteins are involved in the formation of the diatom cell wall: frustulins, pleuralins, cingulins, silacidins and silaffins. Aside from proteins, long-chain polyamines, which are constituents of diatom biosilica, are likely involved in silica biogenesis [43].
The following three main levels of cell wall structure organization have been found: (1) microscale, the largest one that determines the outline shape of the valve or girdle band; (2) the mesoscale, at which organized substructures are formed within the SDV; and (3) the nanoscale, which comprises the first products of polymerization and generates different frustule structures/textures of nanometric dimensions ([38] and references therein).
3. Diatom Biosilica Sources
Diatom-derived silica can be obtained either from living cultures or fossil diatoms (diatomite, e.g., chalky deposits of skeletal remains). The energy required for diatom growth is sustained by either led-based (i.e., low energy demanding) artificial light or sunlight. Furthermore, the nutrients required for algal growth, such as nitrates, phosphates, silicates, vitamins, and some trace elements, can be purchased for a relatively cheap price or even obtained from wastewaters. To avoid both the costs of artificial illumination and the seasonal variability of sunlight, cells can also be grown heterotrophically [44,45,46,47], although organic substrates are to be supplied in this case. However, only a small number of species are able to grow in the dark [48,49], and organic compounds can promote bacterial growth leading to culture contaminations and to a decrease in cell growth. Biosilica is obtained after cell dewatering (i.e., centrifugation or filtration of the whole culture), followed by a purification process that is usually based on treatments with strong acids and/or high temperatures (see below). Besides, the limited motility of diatoms (due to the lack of flagella) and the “heavy” cell wall (due to the presence of a high silicon amount) enhance the spontaneous sinking of cells, limiting the volume to harvest and, thus, costs of biomass collection.
Diatoms generally exhibit fast growth rates and high lipid and biomass productivities, [50] which can be further enhanced by tuning growth conditions [51,52], making diatoms promising candidates for mass culturing. However, to the best of our knowledge, no diatom-based industrial plants (i.e., indoor or outdoor systems of algal culturing) are focusing on biosilica production as their main activity. Follow-up studies are thus required to lay the foundations for the industrial production of silica-based biomaterials.
The most abundant source of biosilica that does not foresee the induction of living cultures is diatomite, which can be easily crushed into a fine powder to become a marketable product, namely, diatomaceous earth (DE). Diatomite is made of frustules of dead diatom cells, usually found in benthic environments. The harvesting of fossil frustules, which are naturally present in benthic environments, is cost-effective and makes diatomite a promising starter for the industrial production of biosilica. However, the composition of DE is variable and the purity is often lower than that of living culture-derived frustules. The quality and abundance of these impurities vary upon environmental and aging conditions [18]. DE, generally made of ca. 80–90% of silicon and of clay minerals [53], is used as a raw material for different kinds of applications, such as agricultural fertiliser, sorbent for pollutants, and filler in plastics and paints to improve the strength of construction materials. In addition, DE is also employed to filter impurities and as an abrasive agent in cleaning and polishing products.
4. Frustule Cleaning/Purification: Main Techniques and Technical Issues
Frustules can be thus purified from both living culture-derived algal biomass and diatomite stocks. The impurities of diatom frustules mainly consist of organic matters adhered to their surface [54]. In the case of diatomite samples, impurities are present in larger amounts, and can vary in relation to the local environment and aging conditions of these natural stocks [18]. Diatomite impurities typically contain also clay and metallic oxides, such as aluminium and ferric oxides [55]. Before cleaning procedures, diatomite particles usually undergo a first step of pulverization, in which micrometric powder is grinded to nanoparticles by mechanical crushing and sonication. However, apart from a few exceptions, most studies report purification protocols based on raw material derived from living cultures rather than diatomite, which is currently the only diatomic silica-based marketable product.
Organic impurities can be removed from the silica frustule by either a chemical pre-treatment with acids or other oxidative agents, or by exposing the frustules to high temperatures. Some studies, aimed at assessing the efficacy of preliminary hydrochloric acid treatments for organic mass removal, showed that acid concentration greatly influenced both the removal rate of impurities and the state of preservation of the frustule shape, with strong acidic pre-treatments causing frustule erosion [56]. Potassium permanganate can be also used to pre-treat frustules for organic compound removal [57,58]. However, this procedure is essentially limited to remove impurities outside the frustule, and pre-treatments with acidic solutions are usually applied (even if they are not mandatory) when purification protocols do not foresee acid-based cleaning procedures, such as baking-based purifications [59]. Some preliminary oxidations with acid solutions do not exclude the employment of both acids and high temperatures. Treatment of diatom frustules with sodium permanganate and oxalic acid, for example, is followed by perchloric acid treatments at 100 °C [57].
Baking (i.e., strong heating of silica cell walls) of diatom frustules at 400–800 °C is the simplest and least expensive method to remove organic components. However, high-temperature treatments can alter diatom architecture and pore size [60]. Oxygen plasma etching, a procedure consisting of the removal of impurities using ionised gases, was found to be effective to preserve the frustule structure, with a negligible loss of material and without shape alterations [61,62].
The most commonly used procedure for the removal of organic matter and the purification of diatom biosilica is, however, an oxidative washing treatment. Some protocols require the use of 30% [54,63,64,65,66,67] or 15% [68] hydrogen peroxide solutions.
The most common washing solvents used in acid-based treatments of diatom frustules are sulphuric [69,70] and nitric [68,71] acids. Sulphuric acid treatment is rapid (10–30 min) and revealed successful even on small amounts of biosilica [55]. Despite the rapidity of this strong acid-based method, cleaning procedures are time-consuming, since several washes with distilled/deionised water are required for a complete acid removal. However, the effect of acid strength needs to be evaluated in each case, since silica nanostructures can be damaged by the action of acids. For example, frustules from poorly silicified diatom species can be dissolved in strong acid cleaning solutions [70].
To improve the efficiency of biosilica purification, Wang and co-workers [72] set up a vacuum cleaning method in which all the cleaning steps, which are cell extraction, acid treatment and washing, are carried out on polytetrafluoroethylene (PTFE) filter cloths, thus decreasing the processing time. This allows the recycling of the sulphuric acid used for cleaning, decreasing the amount of both the reagent needed for purification and the liquid wastes. The main drawback of the vacuum cleaning method is that it depends on the mechanical properties of the raw material, and cannot be applied on poorly silicified diatoms.
Some purification methods combine the use of both sulphuric acid and hydrogen peroxide in a strong oxidizing agent (2 M H2SO4, 10% H2O2) called Piranha solution [26,73]. The purification process is relatively fast, while post-treatment washes can be time-consuming. The removal of Piranha solution requires, indeed, an overnight treatment with HCl (5 M, 80 °C) and two further washes with distilled water to eliminate the HCl residuals [20]. The main treatments for frustule separations, the tested diatom silica sources, and the main bottlenecks of each cleaning technique are summarized in Table 1.

Table 1.
Pre-treatments and treatments for diatom frustule cleaning and their main advantages and drawbacks.
5. Silica for Biomedical Applications: Advantages
The main benefits of biosilica for biomedical purposes are as follows: plasticity of frustules for functionalization, biocompatibility, possibility of genetic transformation of living cultures for protein immobilization, and high availability of silica-derived diatoms. The biosilica derived from diatoms requires cheap synthesis processes [26], and is also characterised by chemical inertness, low or null toxicity, thermal stability and high availability [18]. Silica has been widely investigated in drug delivery systems because of its high robustness and versatility compared to other materials [74], and frustules derived from both living cultures and diatomite particles have successfully been employed as drug carriers [73,75].
5.1. Surface Functionalization for Drug Loading and for Biosensing Chips for Biomedical Applications
Frustule functionalization consists of modifying its surface to enable the formation of stable covalent bonds with proteins or DNA [26,76], by introducing chemically reactive species functioning as cross-linkers. This step is crucial to improve the quality of the resulting material for specific applications. Chemical modification of biosilica can be critical, for example, to regulate the kinetics of drug release, and the high surface-to-volume ratio makes this raw material particularly suitable for drug delivery. Diatom frustules are characterized by precise and species-specific cell morphologies, and both the size and shape can highly differ among distinct diatom taxa. It has been estimated that the surface area ranges between 1.4 and 51 m2 g−1 [77,78,79,80]. The size and the architecture of the pores are likely to influence drug release [75].
Drug release in biosilica-based systems is usually characterized by the following two phases: a first phase of fast release, due to the detachment of drug molecules weakly bound to the frustule surface, and a slow releasing phase, due to drug delivery from the internal pore structure of diatom frustules [81]. Chemical modifications of diatom-derived biosilica allow their use as a carrier of both soluble and insoluble drugs.
The effectiveness of DEs as delivery systems for the drugs gentamicin (soluble) and indomethacin (insoluble) was demonstrated in previous studies [67], in which DE was modified with a self-assembling monolayer (SAM) including organosilanes and phosphonic acids, thus rendering the diatom frustules hydrophilic or hydrophobic, respectively, before drug loading. A sustained release of indomethacin, which has been exploited as a model drug for silica-based devices, was also demonstrated with DE particles functionalised by dopamine-modified iron oxide nanoparticles (DOPA/Fe3O4 nanoparticles). Diatom-derived silica was employed, in this case, as a magnetically guided micro-carrier for drug delivery, since dopamine amino groups on the diatom surface allow the attachment of targeting biomolecules [78]. Another kind of functionalization can be obtained by combining the frustule with graphene oxide (GO) sheets through covalent bindings. These nano-hybrid composites are suitable drug microcarriers. GO sheets enhanced, indeed, drug-surface interactions, improving the kinetics of drug release [82].
Silica functionalization was also used to counteract cancer progression, through the delivery of water-insoluble antitumor drugs. A recent study showed that DE particles coated with vitamin B12 allowed better delivery of cisplatin and 5-fluorouracil (5-FU), two anticancer agents effective against colorectal cancer cells [83]. Silicon nanoparticles (SiNPs) were also functionalized with 5-FU and the chemopreventive agent curcumin, and then encapsulated into acid-resistant microspheres to show the effectiveness of oral administration of these chemotherapeutics against colorectal cancer [84].
DE particles were also used as a solid drug-carrier in phospholipid suspensions for new oral formulations of non-anticancer water-insoluble drugs, such as the anticonvulsive carbamazepine [85].
While the abovementioned applications of biosilica were all based on the employment of fossil sources, other studies were focused on culture-derived biosilica. Functionalised frustules of the diatom Nitzschia palea have been successfully exploited as carriers for the antibacterial complex tyrosine-Zn(II); zinc ions covalently bounded to the frustule surface showed, indeed, a toxic effect on bacteria, thus reducing their concentration [86]. Esfandyari et al. [87] exploited the potential of Chaetoceros sp. frustules to detect circulating tumour cells. Diatoms were magnetized with iron oxide nanoparticles, and then conjugated with the monoclonal antibody Trastuzumab; this system was effective in selectively targeting and separating breast cancer cells, SKBR3 cells (HER2 positive cells), from HER2-negative cells under a magnetic field. The optical properties of these diatoms allowed to detect this specific binding ability by fluorescence microscopy, thanks to the optical properties of the silica.
Similar studies on antibody-functionalized nanoparticles deriving from living cultures were already performed more than ten years ago, and they exploited the potential of two modified centric diatoms as photoluminescent biosensors. Functionalization of Coscinodiscus wailesii frustules was one of the pioneer studies highlighting antigen recognition from antibodies that had been covalently bound to frustules [88]. Gale and co-workers [89] succeeded in transforming Cyclotella sp. frustules with the model rabbit IgG antibody, showing a correlation between the photoluminescence associated with the frustule/antibody complex and the antigen (goat anti-rabbit IgG) concentration. The main types of diatom silica functionalization are summarised in Table 2.

Table 2.
Sources, type of functionalization and biomedical applications of diatom-derived biosilica.
5.2. Biocompatibility
Diatom-derived biosilica has several advantages compared to other porous materials, in terms of high compatibility with biological systems [18,26]. Biocompatibility tests were performed on various tumour cells, and some significant examples are reported below. An ATP-based luminescent assay aimed at detecting the short-time (6–24 h) detrimental effects on cells showed that DE particles had very low toxicity on the following three colon cancer cell lines: Caco-2, HT-29, and HCT-116 [94]. The effect of amino-modified DE nanoparticles on human lung epidermoid carcinoma cells (H1355) was evaluated by the MTT (3-(4,5-dimethythiazol-2-yl)-2,5-diphenyl tetrazolium bromide) assay. Different concentrations of diatom particles were tested for 24, 48 and 72 h, and the results showed very low cytotoxicity against the abovementioned tumour cells. This feature made functionalized DE particles useful carriers to transport small interfering ribonucleic acid (SiRNA) inside human lung epidermoid carcinoma cells (H1355), silencing gene expression [93]. Biocompatibility was also assessed on bone cells, such as normal human dermal fibroblasts (NDHS) and Saos-2 osteoblasts, by the functionalization of Thalassiosira weissflogii frustules with 3-mercaptopropyl-trimethoxysilane (MPTMS). The mercapto-coated biosilica successfully stimulated the growth of both cell lines, even more than bare cells [90].
The biological compatibility of silica-derived diatoms was also assessed in studies aimed at targeting the antiapoptotic factor B-cell lymphoma/leukemia 2 (Bcl2) with small interfering RNA (siRNA). Specifically, the amino groups of silanized silica particles were complexed with siRNA to downregulate the expression of tumour-associated genes. The target line was the A20 murine lymphoma, and no differences in cytotoxicity between the functionalised frustules and controls (e.g., untreated cells) were observed by applying the following three different methodologies: MTT, Cell-Titer GLO and propidium iodide assays [92].
Biocompatibility between functionalized DE particles and breast cancer cells (lines MCF-7 and MDA-MB-231) has also been proven. In this case, amino-modified particles were further improved by PEGylation (i.e., diatom-coating with polyethylene glycol) and cell-penetrating peptide (CPP) bioconjugation, to promote cell internalization through physical and biological changes in the silicon source. The biological compatibility was also evaluated with a luminescent cell viability assay based on the adenosine triphosphate concentration, and the results showed that the cytotoxicity of biosilica that underwent a double modification with PEG and CCP was lower than that of the bare material, as well as that of diatoms that had been amino-modified only [95].
Most cytotoxicity assays mentioned above were performed on short timescales. The effect of longer exposure times (21 days) was assessed on human embryonic kidney cells (HEK-293) and MDA-MB-231 breast cancer cells exposed to syntherized (e.g., fused at high temperature) diatoms. Biocompatibility was tested through viability assays with the dye Calcein-AM (its fluorescence intensity depends on the activity of cellular esterases, and thus of viable cells), and the results confirmed that natural silicon is not toxic. This suggested that fused diatom frustules could be a suitable alternative for synthetic bone graft substitutes [96]. In order to foresee the effects of long-term exposure of silica-based devices on biological systems, Terracciano and co-workers [97] investigated the in vivo impact of diatomite particles on the model organism Hydra vulgaris. Untreated specimens and animals exposed to bare frustules and to diatom nanoparticles modified with the cell-penetrating peptide [(aminooxy)acetyl]-Lys-(Arg)9 (to enhance cellular uptake) were monitored for 14 days, and no detrimental effects in terms of growth rates and apoptosis were observed in all conditions.
In our opinion, further studies on living organisms are mandatory to definitely ascertain the lack of toxicity of biosilica, especially in the perspective of concrete biomedical applications for drug loading and as scaffolds for bone regeneration.
5.3. Employment of Genetically Engineered Diatom Frustules for Protein Immobilization
Diatom particles can be considered as useful scaffolds for enzyme immobilization that could enhance protein properties. Genetic engineering represents a viable alternative to in vitro immobilization systems, as it does not require protein purification and is carried out under physiological conditions [74]. Since silaffins and cingulins are involved in silica condensation becoming part of diatom frustules, the fusion of an exogenous protein to these frustule-associated proteins can result in the strong binding of exogenous proteins to the silica cell wall.
Transformation of diatom genomes with recombinant genes is a useful tool to allow the fusion between enzymes and cell wall proteins. This technology is mentioned in a recent study as living diatom silica immobilization (LiDSI), and has been mostly performed on the model species T. pseudonana [98,99]. To our knowledge, the pioneer studies focused on enzymes immobilised on diatom biosilica were aimed at inserting and blocking the bacterial enzyme hydroxylaminobenzene mutase (HabB) on the silaffin tpSil3 of T. pseudonana frustule [98]. Aside from the potential of this specific genome modification, this study paved the way for the genetic manipulation of diatom species to enhance protein immobilization on frustules for biomedical purposes.
The genome of T. pseudonana has been recently modified with the insertion of exogenous genes encoding the fusion of two enzymes, glucose oxidase and horseradish peroxidase, with cell wall proteins, enabling a regioselective functionalization, and suggesting that silica morphology could influence the effectiveness of the enzymes reactivity [99]. The frustule of this species has been also antibody-functionalised, in order to test its effectiveness in binding large and small antigen molecules [100].
5.4. Availability of Biosilica Feedstocks
In contrast with other synthetic materials, diatom biosilica is already available in huge amounts as diatomite. Moreover, diatom-derived silica feedstock could be easily obtained by culturing these microalgae in open ponds or enclosed systems, and separating them from the organic matter after culture dewatering.
6. Silica for Biomedical Applications: Bottlenecks
Among the weak aspects of the production of biosilica-based devices, it is worth mentioning that diatom frustules require strong treatments that are usually based on toxic and/or dangerous chemicals [26,70,73,101]. Furthermore, the accuracy of the purification protocols should to be the highest possible in case the biosilica-based material is to be used for biomedical purposes. Besides, experiments based on living culture manipulations are often carried out under axenic conditions [87,89], which requires very careful maintenance to keep the strains bacteria-free and, in case of contamination, highly meticulous protocols to remove bacteria need also to be applied [102].
Another drawback of using biosilica for biomedical purposes is the low degradation rate of this material. Although biosilica is known to be less stable than crystalline silica and its dissolution rate can be further enhanced by specific physico-chemical manipulations [103], it can still persist for long periods within organs with limited blood supply [104], which may lead to detrimental health consequences. Some authors do not consider biosilica degradation as a real problem, since the silicic acid (i.e., the main product of silica degradation) is naturally found in human tissues and can be easily excreted from the kidneys [105,106].
However, the employment of diatom-based biosilica for biomedical purposes has not been approved yet by the Food and Drug Administration (FDA), and neither by other safety governmental agencies. The approval of diatom-based biosilica for biomedical purposes is mandatory to follow up all the studies which foresee the development of biosilica-based devices for biomedical purposes. The FDA considers amorphous silica less toxic than crystalline forms and silicates as generally recognized as safe (GRAS) materials [57,107], and established that they can be included in oral delivery ingredients in amounts up to 1500 mg per day [103]. Some diatom-derived sources of silicon can be treated, indeed, to reach the food grade and can be used for nutraceutical purposes. Currently, diatomite of certain “purity levels” is considered as a food additive and is permitted as animal feed (https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=573.340, accessed date: 22 March 2021), as inert carrier or anticaking agent, and as additive for pharmaceutical preparations. Unfortunately, the use of diatomite for biomedical applications has not been approved yet.
The recognition of diatomite as a suitable biomaterial for medical and pharmaceutical purposes by the FDA is, indeed, mandatory to pave the way to industrial applications of diatomite-based materials, which have proven to be successful for drug delivery [74], through genetic engineering [108,109] and for regenerative medicine [26].
7. Future Perspectives
In the present review, we report studies from different research groups aimed at assessing the effectiveness of diatom-derived silica for biomedical applications. Previous studies clearly demonstrated that diatom biosilica could be suitable as a drug carrier, as a scaffold for regenerative medicine, and for in vivo enzyme immobilization. We summarized the main benefits and disadvantages associated with the use of this biomaterial, and, in our opinion, the advantages outweigh the drawbacks. Future research should ascertain long-term compatibility of biosilica with biological systems, and should search for cost-effective and environmentally friendly techniques for biosilica production.
The employment of silica-based diatom cell walls for biotechnological applications implies that naturally abundant fossil sources (e.g., diatomite) can be considered as a viable and cost-efficient alternative to synthetic silica, which requires time-consuming and hazardous methods for its production. Overall, fossil biosilica sources can be used as heavy metal sorbents, food-grade additives, fertilizers or biosensors.
In contrast, living diatom-derived biosilica appears to be more suitable than biosilica of fossil origin for biomedical applications such as drug carriers. Specifically, drugs need to be released from the carrier at constant and known rates, and for this purpose the size, shape, and porosity of biosilica particles should vary as little as possible. Biosilica derived from monospecific cultures has, indeed, a regular and predictable structure compared to fossil sources that typically encompass a given morphological diversity, leading to heterogeneity in the frustule size, shape and porosity. A recent device for biosilica production from living cultures has been patented [110] and paves the way to the exploitation of diatoms.
With respect to other biological silica sources, such as sponges and Radiolaria, diatoms can be easily cultured in the laboratory, and are ubiquitous and highly abundant in the marine environment. Moreover, silica structures from diatoms generally have smaller dimensions than protozoan shells and sponge spicules, which can reach 2 mm in length [74]. The reduced particle size is, for us, advantageous in drug delivery systems to enhance degradability, and for avoiding or limiting pulverization pre-treatments. In our opinion, treatments for the depigmentation and elimination of collagen (in which spicules are enveloped) can render the process of sponge-derived silica even more difficult than the typical techniques for frustule purification [111,112]. Other non-biological silica sources, such as fly ash derived from power plants, may be used in lieu of fossil diatom-derived sources. Similarly to diatomite, this alternative source is disposable and abundant in the natural environment, and its effectiveness as a raw material for carbon sequestration [113] and as a biofertilizer [114] has already been demonstrated. Nevertheless, in our opinion, the high heterogeneity of fly ash (structural features vary according to the power plant and the combustion processes), and the presence of metals and other impurities makes it more suitable for bioremediation and land fertilization purposes, rather than biomedical applications.
To improve the economic viability of massive diatom culturing for biosilica production, other fractions of algal biomass could also be exploited. Diatoms can be used, indeed, as photosynthetic biorefineries [115] for a number of industrial processes, and the exploitation of both the inorganic and organic fractions of the algal biomass can contribute to minimise waste production. A possible route for the complete exploitation of diatom biomass is shown in Figure 3. The extraction of the biochemical components from microalgal biomass does not affect the structure and the integrity of silica frustules, which can remain unaltered also in the presence of acidic conditions. Massive diatom culturing can lead to the combined production of biosilica for biomedical applications, and highly valuable organic compounds such as PUFAs and carotenoids.
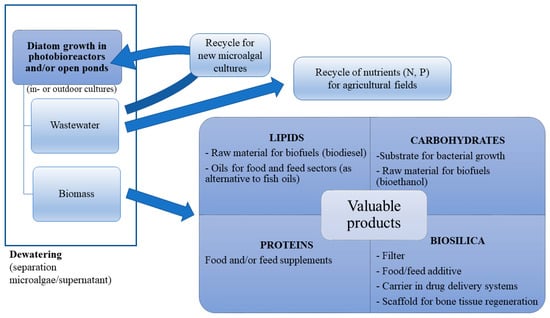
Figure 3.
Schematic representation of a hypothetic diatom-based biorefinery for the whole exploitation of microalgal mass. Diatoms are cultured in open ponds or photobioreactors; after harvesting, the remaining water is still rich in nutrients and can be partially reused as medium for new culture inocula or serve as agricultural fertiliser. Four major valuable products, lipids, proteins, carbohydrates, and biosilica can be obtained from the harvested biomass.
In summary, we believe that further studies are required to exclude any acute and long-term toxicity of diatom biosilica—as suggested by Castillo and Vallet-Regi [116]—and this will pave the way for clinical trials of biosilica transplantation. However, assessing the best processes to minimize costs and wastes for the concomitant production of biosilica and highly valuable products is mandatory to lay the foundations for this new industrial application.
Author Contributions
A.S. conceptualization and original draft preparation, I.O. support in draft preparation, figure preparation, support in literature research, S.B. and L.B. editing and critical review, G.R. editing and supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
The authors did not need any signed informed consent.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful to F. Palumbo and M. Perna (SZN, Marine Biotechnology Department) and G. Lanzotti (SZN, Research Infrastructures for MArine biological Resources, RIMAR) for graphical support. Thanks are due also to Adrianna Ianora for editing and reviewing the English language.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mann, D.G.; Vanormelingen, P. An inordinate fondness? The number, distributions, and origins of diatom species. J. Eukaryot. Microbiol. 2013, 60, 414–420. [Google Scholar] [CrossRef]
- Armbrust, E.V.; Berges, J.A.; Bowler, C.; Green, B.R.; Martinez, D.; Putnam, N.H.; Zhou, S.G.; Allen, A.E.; Apt, K.E.; Bechner, M.; et al. The genome of the diatom Thalassiosira pseudonana: Ecology, evolution, and metabolism. Science 2004, 306, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Bowler, C.; Allen, A.E.; Badger, J.H.; Grimwood, J.; Jabbari, K.; Kuo, A.; Maheswari, U.; Martens, C.; Maumus, F.; Otillar, R.P.; et al. The Phaeodactylum genome reveals the evolutionary history of diatom genomes. Nature 2008, 456, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Lommer, M.; Specht, M.; Roy, A.S.; Kraemer, L.; Andreson, R.; Gutowska, M.A.; Wolf, J.; Bergner, S.V.; Schilhabel, M.B.; Klostermeier, U.C.; et al. Genome and low-iron response of an oceanic diatom adapted to chronic iron limitation. Genome Biol. 2012, 13. [Google Scholar] [CrossRef] [PubMed]
- Galachyants, Y.P.; Zakharova, Y.R.; Petrova, D.P.; Morozov, A.A.; Sidorov, I.A.; Marchenkov, A.M.; Logacheva, M.D.; Markelov, M.L.; Khabudaev, K.V.; Likhoshway, Y.V.; et al. Sequencing of the complete genome of an araphid pennate diatom Synedra acus subsp. radians from Lake Baikal. Dokl. Biochem. Biophys. 2015, 461, 84–88. [Google Scholar] [CrossRef]
- Tanaka, T.; Maeda, Y.; Veluchamy, A.; Tanaka, M.; Abida, H.; Marechal, E.; Bowler, C.; Muto, M.; Sunaga, Y.; Tanaka, M.; et al. Oil accumulation by the oleaginous diatom Fistulifera solaris as revealed by the genome and transcriptome. Plant Cell 2015, 27, 162–176. [Google Scholar] [CrossRef]
- Mock, T.; Otillar, R.P.; Strauss, J.; McMullan, M.; Paajanen, P.; Schmutz, J.; Salamov, A.; Sanges, R.; Toseland, A.; Ward, B.J.; et al. Evolutionary genomics of the cold-adapted diatom Fragilariopsis cylindrus. Nature 2017, 541, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Osuna-Cruz, C.M.; Bilcke, G.; Vancaester, E.; De Decker, S.; Bones, A.M.; Winge, P.; Poulsen, N.; Bulankova, P.; Verhelst, B.; Audoor, S.; et al. The Seminavis robusta genome provides insights into the evolutionary adaptations of benthic diatoms. Nat. Commun. 2020, 11, 3320. [Google Scholar] [CrossRef]
- Pendergrass, A.; Roberts, W.; Ruck, E.C.; Lewis, J.A.; Alverson, A.J. The genome of a nonphotosynthetic diatom provides insights into the metabolic shift to heterotrophy and constraints on the loss of photosynthesis. BioRxiv 2020. [Google Scholar] [CrossRef]
- Mourelle, M.; Gómez, C.; Legido, J. The Potential Use of Marine Microalgae and Cyanobacteria in Cosmetics and Thalassotherapy. Cosmetics 2017, 4, 46. [Google Scholar] [CrossRef]
- Cui, Y.; Thomas-Hall, S.R.; Schenk, P.M. Phaeodactylum tricornutum microalgae as a rich source of omega-3 oil: Progress in lipid induction techniques towards industry adoption. Food Chem. 2019, 297, 124937. [Google Scholar] [CrossRef]
- Pudney, A.; Gandini, C.; Economou, C.K.; Smith, R.; Goddard, P.; Napier, J.A.; Spicer, A.; Sayanova, O. Multifunctionalizing the marine diatom Phaeodactylum tricornutum for sustainable co-production of omega-3 long chain polyunsaturated fatty acids and recombinant phytase. Sci. Rep. 2019, 9, 11444. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.; Melchor-Martinez, E.M.; Saxena, A.; Kapoor, N.; Singh, K.J.; Saldarriaga-Hernandez, S.; Parra-Saldivar, R.; Iqbal, H.M.N. Therapeutic attributes and applied aspects of biological macromolecules (polypeptides, fucoxanthin, sterols, fatty acids, polysaccharides, and polyphenols) from diatoms—A review. Int. J. Biol. Macromol. 2021, 171, 398–413. [Google Scholar] [CrossRef]
- Grzesik, M.; Romanowska-Duda, Z.; Piotrowski, K.; Janas, R. Diatoms (Bacillariophyceae) as an effective base of a new generation of organic fertilizers. Przemys Chem. 2015, 94, 391–396. [Google Scholar] [CrossRef]
- Hedayatkhah, A.; Cretoiu, M.S.; Emtiazi, G.; Stal, L.J.; Bolhuis, H. Bioremediation of chromium contaminated water by diatoms with concomitant lipid accumulation for biofuel production. J. Environ. Manag. 2018, 227, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Mojiri, A.; Baharlooeian, M.; Zahed, M.A. The Potential of Chaetoceros muelleri in Bioremediation of Antibiotics: Performance and Optimization. Int. J. Environ. Res. Public Health 2021, 18, 977. [Google Scholar] [CrossRef]
- Lomora, M.; Shumate, D.; Rahman, A.A.; Pandit, A. Therapeutic applications of phytoplankton, with an emphasis on diatoms and coccolithophores. Adv. Ther. 2019, 2, 1800099. [Google Scholar] [CrossRef]
- Uthappa, U.T.; Brahmkhatri, V.; Sriram, G.; Jung, H.Y.; Yu, J.; Kurkuri, N.; Aminabhavi, T.M.; Altalhi, T.; Neelgund, G.M.; Kurkuri, M.D. Nature engineered diatom biosilica as drug delivery systems. J. Control. Release 2018, 281, 70–83. [Google Scholar] [CrossRef]
- Uthappa, U.T.; Sriram, G.; Arvind, O.R.; Kumar, S.; Ho Young, J.; Neelgund, G.M.; Losic, D.; Kurkuri, M.D. Engineering MIL-100(Fe) on 3D porous natural diatoms as a versatile high performing platform for controlled isoniazid drug release, Fenton’s catalysis for malachite green dye degradation and environmental adsorbents for Pb2+ removal and dyes. Appl. Surf. Sci. 2020, 528, 146974. [Google Scholar] [CrossRef]
- Ruggiero, I.; Terracciano, M.; Martucci, N.M.; De Stefano, L.; Migliaccio, N.; Tate, R.; Rendina, I.; Arcari, P.; Lamberti, A.; Rea, I. Diatomite silica nanoparticles for drug delivery. Nanoscale Res. Lett. 2014, 9, 7. [Google Scholar] [CrossRef]
- Dalgic, A.D.; Atila, D.; Karatas, A.; Tezcaner, A.; Keskin, D. Diatom shell incorporated PHBV/PCL-pullulan co-electrospun scaffold for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 100, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Squire, K.; Li, E.; LeDuff, P.; Rorrer, G.L.; Tang, S.; Chen, B.; McKay, C.P.; Navarro-Gonzalez, R.; Wang, A.X. Chemical and Biological Sensing Using Diatom Photonic Crystal Biosilica With In-Situ Growth Plasmonic Nanoparticles. IEEE Trans. Nanobiosci. 2016, 15, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Albert, K.; Huang, X.C.; Hsu, H.Y. Bio-templated silica composites for next-generation biomedical applications. Adv. Colloid. Interface Sci. 2017, 249, 272–289. [Google Scholar] [CrossRef] [PubMed]
- Terracciano, M.; De Stefano, L.; Rea, I. Diatoms green nanotechnology for biosilica-based drug delivery systems. Pharmaceutics 2018, 10, 242. [Google Scholar] [CrossRef] [PubMed]
- Delasoie, J.; Zobi, F. Natural diatom biosilica as microshuttles in drug delivery systems. Pharmaceutics 2019, 11, 537. [Google Scholar] [CrossRef]
- Tramontano, C.; Chianese, G.; Terracciano, M.; de Stefano, L.; Rea, I. Nanostructured biosilica of diatoms: From water world to biomedical applications. Appl. Sci. 2020, 10, 6811. [Google Scholar] [CrossRef]
- Lewin, J.C. Silicon metabolism in diatoms. III. Respiration and silicon uptake in Navicula pelliculosa. J. Gen. Physiol. 1955, 39, 1–10. [Google Scholar] [CrossRef]
- Annenkov, V.V.; Danilovtseva, E.N.; Pal’shin, V.A.; Ol’ga, N.V.; Zelinskiy, S.N.; Krishnan, U.M. Silicic acid condensation under the influence of water-soluble polymers: From biology to new materials. RSC Adv. 2017, 7, 20995–21027. [Google Scholar] [CrossRef]
- Thamatrakoln, K.; Hildebrand, M. Silicon uptake in diatoms revisited: A model for saturable and nonsaturable uptake kinetics and the role of silicon transporters. Plant Physiol. 2008, 146, 1397–1407. [Google Scholar] [CrossRef]
- Hildebrand, M.; Dahlin, K.; Volcani, B.E. Characterization of a silicon transporter gene family in Cylindrotheca fusiformis: Sequences, expression analysis, and identification of homologs in other diatoms. Mol. Gen. Genet. 1998, 260, 480–486. [Google Scholar] [CrossRef]
- Hildebrand, M. Biological processing of nanostructured silica in diatoms. Prog. Org. Coat. 2003, 47, 256–266. [Google Scholar] [CrossRef]
- Marchenkov, A.M.; Petrova, D.P.; Morozov, A.A.; Zakharova, Y.R.; Grachev, M.A.; Bondar, A.A. A family of silicon transporter structural genes in a pennate diatom Synedra ulna subsp. danica (Kutz.) Skabitsch. PLoS ONE 2018, 13, e0203161. [Google Scholar] [CrossRef]
- Hildebrand, M.; Volcani, B.E.; Gassmann, W.; Schroeder, J.I. A gene family of silicon transporters. Nature 1997, 385, 688–689. [Google Scholar] [CrossRef]
- Durkin, C.A.; Koester, J.A.; Bender, S.J.; Armbrust, E.V. The evolution of silicon transporters in diatoms. J. Phycol. 2016, 52, 716–731. [Google Scholar] [CrossRef]
- Brzezinski, M.A.; Conley, D.J. Silicon deposition during the cell-cycle of Thalassiosira weissflogii (Bacillariophyceae) determined using dual Rhodamine-123 and propidium iodide staining. J. Phycol. 1994, 30, 45–55. [Google Scholar] [CrossRef]
- Taylor, N.J. Silica incorporation in the diatom Cosinodiscus granii as affected by light intensity. Br. Phycol. J. 1985, 20, 365–374. [Google Scholar] [CrossRef]
- Martin-Jezequel, V.; Hildebrand, M.; Brzezinski, M.A. Silicon metabolism in diatoms: Implications for growth. J. Phycol. 2000, 36, 821–840. [Google Scholar] [CrossRef]
- Hildebrand, M. Diatoms, Biomineralization Processes, and Genomics. Chem. Rev. 2008, 108, 4855–4874. [Google Scholar] [CrossRef]
- Crawford, R.; Schmid, A.M. Ultrastructure of silica deposition. In Biomineralization in Lower Plants and Animals; Leadbeater, B.S., Riding, R., Eds.; Oxford University Press: Oxford, UK, 1986; Volume 30, pp. 291–314. [Google Scholar]
- Schmidt, A.-M. Aspects of morphogenesis and function of diatom cell walls with implications for taxonomy. Protoplasma 1994, 181, 43–60. [Google Scholar] [CrossRef]
- Li, C.W.; Volcani, B.E. Aspects of silicification in wall morphogenesis of diatoms. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1984, 304, 519–528. [Google Scholar] [CrossRef]
- Tesson, B.; Hildebrand, M. Extensive and intimate association of the cytoskeleton with forming silica in diatoms: Control over patterning on the meso- and micro-scale. PLoS ONE 2010, 5. [Google Scholar] [CrossRef]
- Sumper, M.; Kroger, N. Silica formation in diatoms: The function of long-chain polyamines and silaffins. J. Mater. Chem. 2004, 14, 2059–2065. [Google Scholar] [CrossRef]
- Pahl, S.L.; Lewis, D.M.; Chen, F.; King, K.D. Heterotrophic growth and nutritional aspects of the diatom Cyclotella cryptica (Bacillariophyceae): Effect of some environmental factors. J. Biosci. Bioeng. 2010, 109, 235–239. [Google Scholar] [CrossRef]
- Pahl, S.L.; Lewis, D.M.; Chen, F.; King, K.D. Growth dynamics and the proximate biochemical composition and fatty acid profile of the heterotrophically grown diatom Cyclotella Cryptica. J. Appl. Phycol. 2010, 22, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Karmakar, R.; Das, B.; Diba, F.; Razu, M.H. Recent advances in microalgal biotechnology. In Heterotrophic Growth of Micro Algae; Jin, L., Zheng, S., Henri, G., Eds.; OMICS Group eBooks: Foster City, CA, USA, 2016; pp. 1–18. [Google Scholar]
- Mao, X.M.; Chen, S.H.Y.; Lu, X.; Yu, J.F.; Liu, B. High silicate concentration facilitates fucoxanthin and eicosapentaenoic acid (EPA) production under heterotrophic condition in the marine diatom Nitzschia laevis. Algal Res. Biomass Biofuels Bioprod. 2020, 52. [Google Scholar] [CrossRef]
- Chen, G.Q.; Chen, F. Growing phototrophic cells without light. Biotechnol. Lett. 2006, 28, 607–616. [Google Scholar] [CrossRef]
- Morales-Sanchez, D.; Martinez-Rodriguez, O.A.; Kyndt, J.; Martinez, A. Heterotrophic growth of microalgae: Metabolic aspects. World J. Microbiol. Biotechnol. 2015, 31, 1–9. [Google Scholar] [CrossRef]
- D’Ippolito, G.; Sardo, A.; Paris, D.; Vella, F.M.; Adelfi, M.G.; Botte, P.; Gallo, C.; Fontana, A. Potential of lipid metabolism in marine diatoms for biofuel production. Biotechnol. Biofuels 2015, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Botte, P.; D’Ippolito, G.; Gallo, C.; Sardo, A.; Fontana, A. Combined exploitation of CO2 and nutrient replenishment for increasing biomass and lipid productivity of the marine diatoms Thalassiosira weissflogii and Cyclotella cryptica. J. Appl. Phycol. 2017, 30, 243–251. [Google Scholar] [CrossRef]
- Orefice, I.; Musella, M.; Smerilli, A.; Sansone, C.; Chandrasekaran, R.; Corato, F.; Brunet, C. Role of nutrient concentrations and water movement on diatom’s productivity in culture. Sci. Rep. 2019, 9, 1479. [Google Scholar] [CrossRef]
- Lutyński, M.; Sakiewicz, P.; Lutyńska, S. Characterization of diatomaceous earth and halloysite resources of poland. Minerals 2019, 9, 670. [Google Scholar] [CrossRef]
- Qin, T.; Gutu, T.; Jiao, J.; Chang, C.H.; Rorrer, G.L. Photoluminescence of silica nanostructures from bioreactor culture of marine diatom Nitzschia frustulum. J. Nanosci. Nanotechnol. 2008, 8, 2392–2398. [Google Scholar] [CrossRef]
- Wang, Y.; Cai, J.; Jiang, Y.G.; Jiang, X.G.; Zhang, D.Y. Preparation of biosilica structures from frustules of diatoms and their applications: Current state and perspectives. Appl. Microbiol. Biotechnol. 2013, 97, 453–460. [Google Scholar] [CrossRef]
- Qi, Y.R.; Wang, X.; Cheng, J.J. Preparation and characteristics of biosilica derived from marine diatom biomass of Nitzschia closterium and Thalassiosira. Chin. J. Oceanol. Limnol. 2017, 35, 668–680. [Google Scholar] [CrossRef]
- Horn, M.G.; Robinson, R.S.; Rynearson, T.A.; Sigman, D.M. Nitrogen isotopic relationship between diatom-bound and bulk organic matter of cultured polar diatoms. Paleoceanography 2011, 26. [Google Scholar] [CrossRef]
- Mejia, L.M.; Isensee, K.; Mendez-Vicente, A.; Pisonero, J.; Shimizu, N.; Gonzalez, C.; Monteleone, B.; Stoll, H. B content and Si/C ratios from cultured diatoms (Thalassiosira pseudonana and Thalassiosira weissflogii): Relationship to seawater pH and diatom carbon acquisition. Geochim. Cosmochim. Acta 2013, 123, 322–337. [Google Scholar] [CrossRef]
- Jiang, W.K.; Luo, S.P.; Liu, P.W.; Deng, X.Y.; Jing, Y.; Bai, C.Y.; Li, J.B. Purification of biosilica from living diatoms by a two-step acid cleaning and baking method. J. Appl. Phycol. 2014, 26, 1511–1518. [Google Scholar] [CrossRef]
- Umemura, K.; Noguchi, Y.; Ichinose, T.; Hirose, Y.; Kuroda, R.; Mayama, S. Diatom Cells Grown and Baked on a Functionalized Mica Surface. J. Biol. Phys. 2008, 34, 189–196. [Google Scholar] [CrossRef]
- Watanabe, T.; Kodama, Y.; Mayama, S. Application of a novel cleaning method using low-temperature plasma on tidal flat diatoms with heterovalvy or delicate frustule structure. Proc. Acad. Nat. Sci. Phila. 2010, 160, 83–87. [Google Scholar] [CrossRef]
- Saad, E.M.; Pickering, R.A.; Shoji, K.; Hossain, M.I.; Glover, T.G.; Krause, J.W.; Tang, Y.Z. Effect of cleaning methods on the dissolution of diatom frustules. Mar. Chem. 2020, 224. [Google Scholar] [CrossRef]
- Jeffryes, C.; Solanki, R.; Rangineni, Y.; Wang, W.; Chang, C.H.; Rorrer, G.L. Electroluminescence and photoluminescence from nanostructured diatom frustules containing metabolically inserted germanium. Adv. Mater. 2008, 20, 2633–2637. [Google Scholar] [CrossRef]
- Townley, H.E.; Parker, A.R.; White-Cooper, H. Exploitation of diatom frustules for nanotechnology: Tethering active biomolecules. Adv. Funct. Mater. 2008, 18, 369–374. [Google Scholar] [CrossRef]
- Abramson, L.; Wirick, S.; Lee, C.; Jacobsen, C.; Brandes, J.A. The use of soft X-ray spectromicroscopy to investigate the distribution and composition of organic matter in a diatom frustule and a biomimetic analog. Deep Sea Res. Part II Top. Stud. Oceanogr. 2009, 56, 1369–1380. [Google Scholar] [CrossRef]
- Lin, K.C.; Kunduru, V.; Bothara, M.; Rege, K.; Prasad, S.; Ramakrishna, B.L. Biogenic nanoporous silica-based sensor for enhanced electrochemical detection of cardiovascular biomarkers proteins. Biosens. Bioelectron. 2010, 25, 2336–2342. [Google Scholar] [CrossRef] [PubMed]
- Bariana, M.; Aw, M.S.; Kurkuri, M.; Losic, D. Tuning drug loading and release properties of diatom silica microparticles by surface modifications. Int. J. Pharm. 2013, 443, 230–241. [Google Scholar] [CrossRef]
- Van Eynde, E.; Lenaerts, B.; Tytgat, T.; Verbruggen, S.W.; Hauchecorne, B.; Blust, R.; Lenaerts, S. Effect of pretreatment and temperature on the properties of Pinnularia biosilica frustules. RSC Adv. 2014, 4, 56200–56206. [Google Scholar] [CrossRef]
- Lettieri, S.; Setaro, A.; De Stefano, L.; De Stefano, M.; Maddalena, P. The gas-detection properties of light-emitting diatoms. Adv. Funct. Mater. 2008, 18, 1257–1264. [Google Scholar] [CrossRef]
- De Stefano, L.; Rendina, I.; De Stefano, M.; Bismuto, A.; Maddalena, P. Marine diatoms as optical chemical sensors. Appl. Phys. Lett. 2005, 87. [Google Scholar] [CrossRef]
- De Stefano, L.; Rotiroti, L.; De Stefano, M.; Lamberti, A.; Lettieri, S.; Setaro, A.; Maddalena, P. Marine diatoms as optical biosensors. Biosens. Bioelectron. 2009, 24, 1580–1584. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, D.Y.; Cai, J.; Pan, J.F.; Chen, M.L.; Li, A.B.; Jiang, Y.G. Biosilica structures obtained from Nitzschia, Ditylum, Skeletonema, and Coscinodiscus diatom by a filtration-aided acid cleaning method. Appl. Microbiol. Biotechnol. 2012, 95, 1165–1178. [Google Scholar] [CrossRef]
- Delalat, B.; Sheppard, V.C.; Rasi Ghaemi, S.; Rao, S.; Prestidge, C.A.; McPhee, G.; Rogers, M.L.; Donoghue, J.F.; Pillay, V.; Johns, T.G.; et al. Targeted drug delivery using genetically engineered diatom biosilica. Nat. Commun. 2015, 6, 8791. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, M.A.A.; Pack, S.P. Biomimetic and bioinspired silicifications: Recent advances for biomaterial design and applications. Acta Biomater. 2021, 120, 38–56. [Google Scholar] [CrossRef] [PubMed]
- Vasani, R.B.; Losic, D.; Cavallaro, A.; Voelcker, N.H. Fabrication of stimulus-responsive diatom biosilica microcapsules for antibiotic drug delivery. J. Mater. Chem. B 2015, 3, 4325–4329. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Hanini, A.; Shah, A.; Patel, D.; Patel, S.; Bhatt, P.; Pathak, V.Y. Surface modification of nanoparticles for targeted drug delivery. In Surface Modification of Nanoparticles for Targeted Drug Delivery; Pathak, V.Y., Ed.; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Bao, Z.; Weatherspoon, M.R.; Shian, S.; Cai, Y.; Graham, P.D.; Allan, S.M.; Ahmad, G.; Dickerson, M.B.; Church, B.C.; Kang, Z.; et al. Chemical reduction of three-dimensional silica micro-assemblies into microporous silicon replicas. Nature 2007, 446, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Losic, D.; Yu, Y.; Aw, M.S.; Simovic, S.; Thierry, B.; Addai-Mensah, J. Surface functionalisation of diatoms with dopamine modified iron-oxide nanoparticles: Toward magnetically guided drug microcarriers with biologically derived morphologies. Chem. Commun. 2010, 46, 6323–6325. [Google Scholar] [CrossRef]
- Jantschke, A.; Fischer, C.; Hensel, R.; Braun, H.G.; Brunner, E. Directed assembly of nanoparticles to isolated diatom valves using the non-wetting characteristics after pyrolysis. Nanoscale 2014, 6, 11637–11645. [Google Scholar] [CrossRef]
- Diab, M.; Mokari, T. Bioinspired hierarchical porous structures for engineering advanced functional inorganic materials. Adv. Mater. 2018, 30, e1706349. [Google Scholar] [CrossRef]
- Aw, M.S.; Simovic, S.; Yu, Y.; Addai-Mensah, J.; Losic, D. Porous silica microshells from diatoms as biocarrier for drug delivery applications. Powder Technol. 2012, 223, 52–58. [Google Scholar] [CrossRef]
- Kumeria, T.; Bariana, M.; Altalhi, T.; Kurkuri, M.; Gibson, C.T.; Yang, W.; Losic, D. Graphene oxide decorated diatom silica particles as new nano-hybrids: Towards smart natural drug microcarriers. J. Mater. Chem. B 2013, 1, 6302–6311. [Google Scholar] [CrossRef]
- Delasoie, J.; Rossier, J.; Haeni, L.; Rothen-Rutishauser, B.; Zobi, F. Slow-targeted release of a ruthenium anticancer agent from vitamin B12 functionalized marine diatom microalgae. Dalton Trans. 2018, 47, 17221–17232. [Google Scholar] [CrossRef]
- Maher, S.; Santos, A.; Kumeria, T.; Kaur, G.; Lambert, M.; Forward, P.; Evdokiou, A.; Losic, D. Multifunctional microspherical magnetic and pH responsive carriers for combination anticancer therapy engineered by droplet-based microfluidics. J. Mater. Chem. B 2017, 5, 4097–4109. [Google Scholar] [CrossRef] [PubMed]
- Milovic, M.; Simovic, S.; Losic, D.; Dashevskiy, A.; Ibric, S. Solid self-emulsifying phospholipid suspension (SSEPS) with diatom as a drug carrier. Eur. J. Pharm. Sci. 2014, 63, 226–232. [Google Scholar] [CrossRef]
- Singh, R.; Khan, M.J.; Rane, J.; Gajbhiye, A.; Vinayak, V.; Joshi, K.B. Biofabrication of Diatom Surface by Tyrosine-Metal Complexes: Smart Microcontainers to Inhibit Bacterial Growth. Chemistryselect 2020, 5, 3091–3097. [Google Scholar] [CrossRef]
- Esfandyari, J.; Shojaedin-Givi, B.; Hashemzadeh, H.; Mozafari-Nia, M.; Vaezi, Z.; Naderi-Manesh, H. Capture and detection of rare cancer cells in blood by intrinsic fluorescence of a novel functionalized diatom. Photodiagn. Photodyn. Ther. 2020, 30, 101753. [Google Scholar] [CrossRef] [PubMed]
- De Stefano, L.; Lamberti, A.; Rotiroti, L.; De Stefano, M. Interfacing the nanostructured biosilica microshells of the marine diatom Coscinodiscus wailesii with biological matter. Acta Biomater. 2008, 4, 126–130. [Google Scholar] [CrossRef]
- Gale, D.K.; Gutu, T.; Jiao, J.; Chang, C.-H.; Rorrer, G.L. Photoluminescence Detection of Biomolecules by Antibody-Functionalized Diatom Biosilica. Adv. Funct. Mater. 2009, 19, 926–933. [Google Scholar] [CrossRef]
- Cicco, S.R.; Vona, D.; Gristina, R.; Sardella, E.; Ragni, R.; Lo Presti, M.; Farinola, G.M. Biosilica from Living Diatoms: Investigations on Biocompatibility of Bare and Chemically Modified Thalassiosira weissflogii Silica Shells. Bioengineering 2016, 3, 35. [Google Scholar] [CrossRef]
- Aw, M.S.; Bariana, M.; Yu, Y.; Addai-Mensah, J.; Losic, D. Surface-functionalized diatom microcapsules for drug delivery of water-insoluble drugs. J. Biomater. Appl. 2013, 28, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Martucci, N.M.; Migliaccio, N.; Ruggiero, I.; Albano, F.; Cali, G.; Romano, S.; Terracciano, M.; Rea, I.; Arcari, P.; Lamberti, A. Nanoparticle-based strategy for personalized B-cell lymphoma therapy. Int. J. Nanomed. 2016, 11, 6089–6101. [Google Scholar] [CrossRef]
- Rea, I.; Martucci, N.M.; De Stefano, L.; Ruggiero, I.; Terracciano, M.; Dardano, P.; Migliaccio, N.; Arcari, P.; Tate, R.; Rendina, I.; et al. Diatomite biosilica nanocarriers for siRNA transport inside cancer cells. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 3393–3403. [Google Scholar] [CrossRef]
- Zhang, H.; Shahbazi, M.A.; Makila, E.M.; da Silva, T.H.; Reis, R.L.; Salonen, J.J.; Hirvonen, J.T.; Santos, H.A. Diatom silica microparticles for sustained release and permeation enhancement following oral delivery of prednisone and mesalamine. Biomaterials 2013, 34, 9210–9219. [Google Scholar] [CrossRef] [PubMed]
- Terracciano, M.; Shahbazi, M.A.; Correia, A.; Rea, I.; Lamberti, A.; De Stefano, L.; Santos, H.A. Surface bioengineering of diatomite based nanovectors for efficient intracellular uptake and drug delivery. Nanoscale 2015, 7, 20063–20074. [Google Scholar] [CrossRef]
- Amoda, A.; Borkiewicz, L.; Rivero-Müller, A.; Alam, P. Sintered nanoporous biosilica diatom frustules as high efficiency cell-growth and bone-mineralisation platforms. Mater. Today Commun. 2020, 24, 100923. [Google Scholar] [CrossRef]
- Terracciano, M.; De Stefano, L.; Tortiglione, C.; Tino, A.; Rea, I. In Vivo Toxicity Assessment of Hybrid Diatomite Nanovectors Using Hydra vulgaris as a Model System. Adv. Biosyst. 2019, 3, e1800247. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, N.; Berne, C.; Spain, J.; Kroger, N. Silica immobilization of an enzyme through genetic engineering of the diatom Thalassiosira pseudonana. Angew. Chem. Int. Ed. 2007, 46, 1843–1846. [Google Scholar] [CrossRef]
- Kumari, E.; Görlich, S.; Poulsen, N.; Kröger, N. Genetically Programmed Regioselective Immobilization of Enzymes in Biosilica Microparticles. Adv. Funct. Mater. 2020, 30, 2000442. [Google Scholar] [CrossRef]
- Ford, N.R.; Hecht, K.A.; Hu, D.H.; Orr, G.; Xiong, Y.J.; Squier, T.C.; Rorrer, G.L.; Roesijadi, G. Antigen Binding and Site-Directed Labeling of Biosilica-Immobilized Fusion Proteins Expressed in Diatoms. ACS Synth. Biol. 2016, 5, 193–199. [Google Scholar] [CrossRef]
- Vona, D.; Urbano, L.; Bonifacio, M.A.; De Giglio, E.; Cometa, S.; Mattioli-Belmonte, M.; Palumbo, F.; Ragni, R.; Cicco, S.R.; Farinola, G.M. Data from two different culture conditions of Thalassiosira weissflogii diatom and from cleaning procedures for obtaining monodisperse nanostructured biosilica. Data Brief 2016, 8, 312–319. [Google Scholar] [CrossRef]
- Saxena, A.; Tiwari, A.; Kaushik, R.; Iqbal, H.M.N.; Parra-Saldivar, R. Diatoms recovery from wastewater: Overview from an ecological and economic perspective. J. Water Process Eng. 2021, 39. [Google Scholar] [CrossRef]
- Diab, R.; Canilho, N.; Pavel, I.A.; Haffner, F.B.; Girardon, M.; Pasc, A. Silica-based systems for oral delivery of drugs, macromolecules and cells. Adv. Colloid Interface Sci. 2017, 249, 346–362. [Google Scholar] [CrossRef]
- Cauda, V.; Schlossbauer, A.; Bein, T. Bio-degradation study of colloidal mesoporous silica nanoparticles: Effect of surface functionalization with organo-silanes and poly(ethylene glycol). Microporous Mesoporous Mater. 2010, 132, 60–71. [Google Scholar] [CrossRef]
- Anderson, S.H.C.; Elliott, H.; Wallis, D.J.; Canham, L.T.; Powell, J.J. Dissolution of different forms of partially porous silicon wafers under simulated physiological conditions. Phys. Status Solidi A Appl. Mater. Sci. 2003, 197, 331–335. [Google Scholar] [CrossRef]
- Popplewell, J.F.; King, S.J.; Day, J.P.; Ackrill, P.; Fifield, L.K.; Cresswell, R.G.; Di Tada, M.L.; Liu, K. Kinetics of uptake and elimination of silicic acid by a human subject: A novel application of Si-32 and accelerator mass spectrometry. J. Inorg. Biochem. 1998, 69, 177–180. [Google Scholar] [CrossRef]
- Zhang, Y.; Hsu, B.Y.W.; Ren, C.L.; Li, X.; Wang, J. Silica-based nanocapsules: Synthesis, structure control and biomedical applications. Chem. Soc. Rev. 2015, 44, 315–335. [Google Scholar] [CrossRef]
- Wallace, A.K.; Chanut, N.; Voigt, C.A. Silica Nanostructures Produced Using Diatom Peptides with Designed Post-Translational Modifications. Adv. Funct. Mater. 2020, 30. [Google Scholar] [CrossRef]
- Rogato, A.; De Tommasi, E. Physical, Chemical, and Genetic Techniques for Diatom Frustule Modification: Applications in Nanotechnology. Appl. Sci. 2020, 10, 8738. [Google Scholar] [CrossRef]
- Sun, Z.; Zhou, Z.; Li, J.; Feng, C.; Chen, X.; Zhang, Y. Industrialized System for Producing Bio-Silicon, Has Centrifugal Shaft That is Connected to Barrel Structure of Inner Barrel, and Flocculation Tank Whose Outlet is Connected to Algae-Liquid Separation Tank. China Patent CN110616139-A, 27 December 2019. CN110616139-B, 28 August 2020. [Google Scholar]
- Kaya, M.; Bilican, I.; Mujtaba, M.; Sargin, I.; Haskoylu, M.E.; Oner, E.T.; Zheng, K.; Boccaccini, A.R.; Cansaran-Duman, D.; Onses, M.S.; et al. Sponge-derived natural bioactive glass microspheres with self-assembled surface channel arrays opening into a hollow core for bone tissue and controlled drug release applications. Chem. Eng. J. 2021, 407. [Google Scholar] [CrossRef]
- Granito, R.N.; Custodio, M.R.; Renno, A.C.M. Natural marine sponges for bone tissue engineering: The state of art and future perspectives. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1717–1727. [Google Scholar] [CrossRef]
- Miricioiu, M.G.; Niculescu, V.C.; Filote, C.; Raboaca, M.S.; Nechifor, G. Coal Fly Ash Derived Silica Nanomaterial for MMMs-Application in CO2/CH4 Separation. Membranes 2021, 11, 78. [Google Scholar] [CrossRef]
- Miricioiu, M.G.; Niculescu, V.C. Fly ash, from recycling to potential raw material for mesoporous silica synthesis. Nanomaterials 2020, 10, 474. [Google Scholar] [CrossRef]
- Jeffryes, C.; Agathos, S.N.; Rorrer, G. Biogenic nanomaterials from photosynthetic microorganisms. Curr. Opin. Biotechnol. 2015, 33, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Castillo, R.R.; Vallet-Regi, M. Functional mesoporous silica nanocomposites: Biomedical applications and biosafety. Int. J. Mol. Sci. 2019, 20, 929. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).