An In Vitro Evaluation of the Biocidal Effect of Oregano and Cloves’ Volatile Compounds against Microorganisms Colonizing an Oil Painting—A Pioneer Study
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
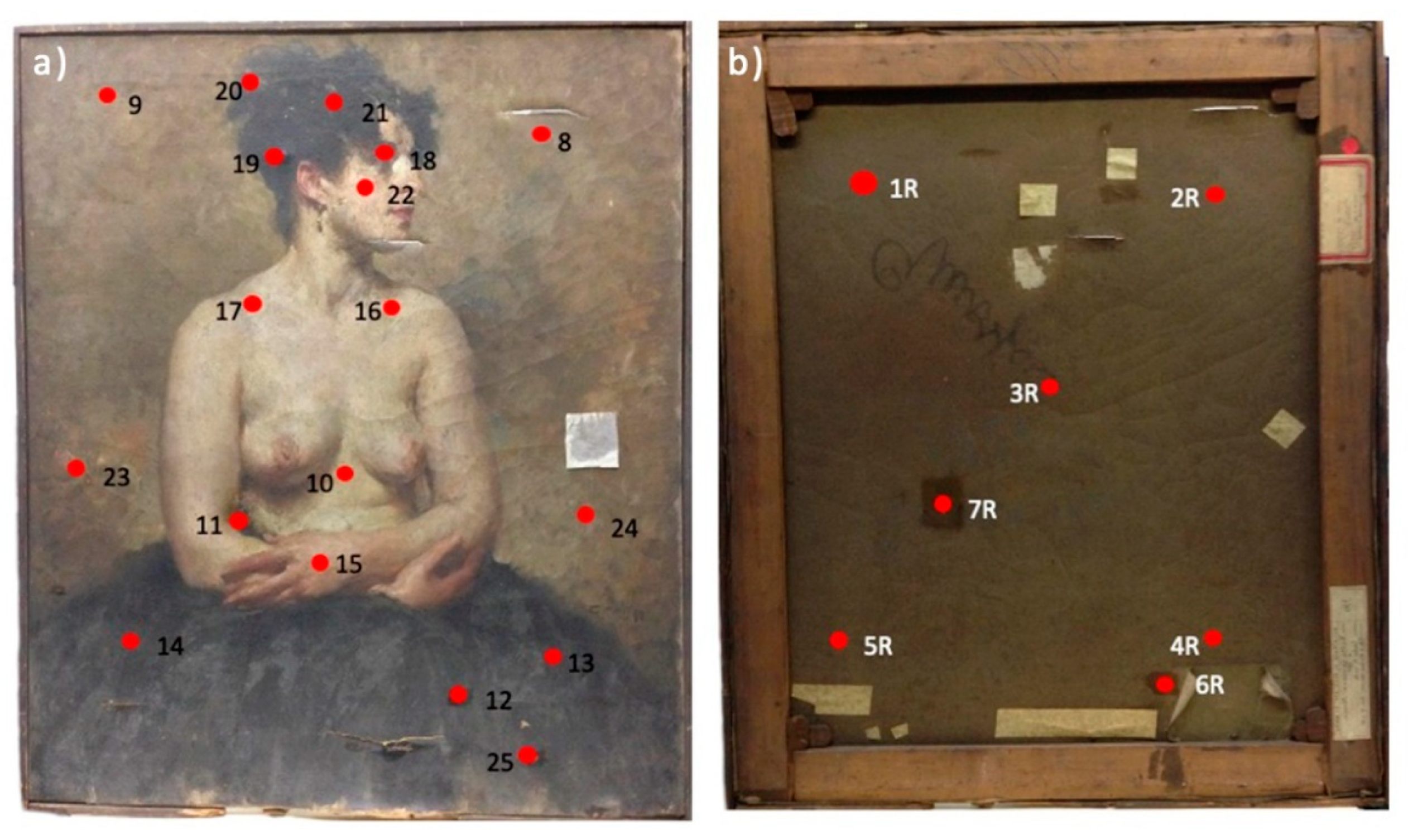
2.1. Artwork Description and Conservation State
2.2. Sampling Method
2.3. Isolation and Identification of Microbial Community (Fungi and Bacteria)
2.4. Essential Oils
2.4.1. Contact Test with Essential Oils
2.4.2. Contactless Test with Essential Oils
3. Results and Discussion
3.1. Isolation and Morphological Characterization of Microbial Community (Fungi and Bacteria)
3.2. Molecular Characterization of Microbial Community (Fungi and Bacteria)
3.3. Assessment for Antifungal and Antibacterial Activity of EOs in Contact Tests
3.4. Assessment for Antifungal and Antibacterial Activity in the Contactless Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Casiglia, S.; Bruno, M.; Senatore, F. Activity against Microorganisms Affecting Cellulosic Objects of the Volatile Constituents of Leonotis nepetaefolia from Nicaragua. Nat. Prod. Commun. 2014, 9, 1637–1639. [Google Scholar] [CrossRef]
- Barresi, G.; Di Carlo, E.; Trapani, M.R.; Parisi, M.G.; Chille, C.; Mule, M.F.; Cammarata, M.; Palla, F. Marine organisms as source of bioactive molecules applied in restoration projects. Herit. Sci. 2015, 3, 15–18. [Google Scholar] [CrossRef]
- Fidanza, M.R.; Caneva, G. Natural biocides for the conservation of stone cultural heritage: A review. J. Cult. Herit. 2019, 38, 271–286. [Google Scholar] [CrossRef]
- Raut, J.S.; Karuppayil, S.M. A status review on the medicinal properties of essential oils. Ind. Crop. Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- Zoubi, Y.E.; Farah, A.; Zaroual, H.; El Ouali, A. Antimicrobial activity of Lavandula stoechas phenolic extracts against pathogenic bacteria isolated from a hospital in Morocco. Vegetos Int. J. Plant Res. 2020, 33, 703–711. [Google Scholar] [CrossRef]
- Ríos, J.L.; Recio, M.C. Medicinal plants and antimicrobial activity. J. Ethnopharmacol. 2005, 100, 80–84. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial Activity of Some Essential Oils-Present Status and Future Perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef]
- Predoi, D.; Iconaru, S.L.; Buton, N.; Badea, M.L.; Măruţescu, L. Antimicrobial Activity of New Materials Based on Lavender and Basil Essential Oils and Hydroxyapatite. Nanomaterials 2018, 8, 291. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, Z.; Huang, Q.; Zhang, D. Antifungal activity of several essential oils and major components against wood-rot fungi. Ind. Crop. Prod. 2017, 108, 278–285. [Google Scholar] [CrossRef]
- Romano, I.; Granata, G.; Poli, A.; Finore, I.; Napoli, E.; Geraci, C. Inhibition of bacterial growth on marble stone of 18th century by treatment of nanoencapsulated essential oils. Int. Biodeterior. Biodegrad. 2020, 148, 104909. [Google Scholar] [CrossRef]
- Rotolo, V.; Barresi, G.; Di Carlo, E.; Giordano, A.; Lombardo, G.; Crimi, E.; Costa, E.; Bruno, M.; Palla, F. Plant extracts as green potential strategies to control the biodeterioration of cultural heritage. Int. J. Conserv. Sci. 2016, 7, 839–846. [Google Scholar]
- Jugreet, B.S.; Suroowan, S.; Rengasamy, R.K.; Mahomoodally, M.F. Chemistry, bioactivities, mode of action and industrial applications of essential oils. Trends Food Sci. Technol. 2020, 101, 89–105. [Google Scholar] [CrossRef]
- Campanella, L.; Angeloni, R.; Cibin, F.; Dell’Aglio, E.; Grimaldi, F.; Reale, R.; Vitali, M. Capsulated essential oil in gel spheres for the protection of cellulosic cultural heritage. Nat. Prod. Res. 2019, 1–8. [Google Scholar] [CrossRef]
- Čabalová, I.; Češek, B.; Mikala, O.; Gojný, J.; Kačík, F.; Tribulová, T. The influence of selected efficient compounds of essential oils for paper protection. J. Cult. Herit. 2019, 37, 148–154. [Google Scholar] [CrossRef]
- Borrego, S.; Valdés, O.; Vivar, I.; Lavin, P.; Guiamet, P.; Battistoni, P.; De Saravia, S.G.; Borges, P. Essential Oils of Plants as Biocides against Microorganisms Isolated from Cuban and Argentine Documentary Heritage. ISRN Microbiol. 2012, 2012, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Matusiak, K.; MacHnowski, W.; Wrzosek, H.; Polak, J.; Rajkowska, K.; Śmigielski, K.; Kunicka-Styczyńska, A.; Gutarowska, B. Application of Cinnamomum zeylanicum essential oil in vapour phase for heritage textiles disinfection. Int. Biodeterior. Biodegrad. 2018, 131, 88–96. [Google Scholar] [CrossRef]
- Geweely, N.; Afifi, H.; Ibrahim, D.; Soliman, M. Inhibitory Effect of Essential Oils on Growth and Physiological Activity of Deteriorated Fungal Species Isolated from Three Archeological Objects, Saqqara excavation, Egypt. Geomicrobiol. J. 2020, 37, 520–533. [Google Scholar] [CrossRef]
- Stupar, M.; Grbić, M.L.; Džamić, A.; Unković, N.; Ristić, M.; Jelikić, A.; Vukojević, J. Antifungal activity of selected essential oils and biocide benzalkonium chloride against the fungi isolated from cultural heritage objects. S. Afr. J. Bot. 2014, 93, 118–124. [Google Scholar] [CrossRef]
- Di Vito, M.; Bellardi, M.G.; Violi, M. Gli oli essenziali entrano nei musei. Natural 2018, 1, 69–73. [Google Scholar]
- Elsayed, Y.; Shabana, Y. The effect of some essential oils on Aspergillus niger and Alternaria alternata infestation in archaeological oil paintings. Mediterr. Archaeol. Archaeom. 2018, 18, 71–87. [Google Scholar]
- Garti, N.; Yaghmur, A.; Leser, M.E.; Clement, V.; Watzke, H.J. Improved Oil Solubilization in Oil/Water Food Grade Microemulsions in the Presence of Polyols and Ethanol. J. Agric. Food Chem. 2001, 49, 2552–2562. [Google Scholar] [CrossRef]
- Tokuoka, Y.; Uchiyama, H.; Abe, M.; Christian, S.D. Solubilization of Some Synthetic Perfumes by Anionic-Nonionic Mixed Surfactant Systems. 1. Langmuir 1995, 11, 725–729. [Google Scholar] [CrossRef]
- Okpalanozie, O.E.; Adebusoye, S.A.; Troiano, F.; Polo, A.; Cappitelli, F.; Ilori, M. O Evaluating the microbiological risk to a contemporary Nigerian painting: Molecular and biodegradative studies. Int. Biodeter. Biodegr. 2016, 114, 184–192. [Google Scholar] [CrossRef]
- Troiano, F.; Polo, A.; Villa, F.; Cappitelli, F. Assessing the microbiological risk to stored sixteenth century parchment manuscripts: A holistic approach based on molecular and environmental studies. Biofouling 2014, 30, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, E.; Lari, M.; Gigli, E.; De Bellis, G.; Caramelli, D. Ancient DNA studies: New perspectives on old samples. Genet. Sel. Evol. 2012, 44, 21. [Google Scholar] [CrossRef]
- Manter, D.K.; Vivanco, J.M. Use of the ITS primers, ITS1F and ITS4, to characterize fungal abundance and diversity in mixed-template samples by qPCR and length heterogeneity analysis. J. Microbiol. Methods 2007, 71, 7–14. [Google Scholar] [CrossRef]
- Available online: https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 9 November 2020).
- Available online: http://rdp.cme.msu.edu/classifier/classifier.jsp (accessed on 9 November 2020).
- MycoBank. Available online: http://www.mycobank.org (accessed on 9 November 2020).
- Bonaduce, I.; Duce, C.; Lluveras-Tenorio, A.; Lee, J.; Ormsby, B.; Burnstock, A.; Berg, K.J.V.D. Conservation Issues of Modern Oil Paintings: A Molecular Model on Paint Curing. Accounts Chem. Res. 2019, 52, 3397–3406. [Google Scholar] [CrossRef]
- Poyatos, F.; Morales, F.; Nicholson, A.W.; Giordano, A. Physiology of biodeterioration on canvas paintings. J. Cell. Physiol. 2018, 233, 2741–2751. [Google Scholar] [CrossRef]
- Kakakhel, M.A.; Wu, F.; Gu, J.-D.; Feng, H.; Shah, K.; Wang, W. Controlling biodeterioration of cultural heritage objects with biocides: A review. Int. Biodeterior. Biodegrad. 2019, 143, 104721. [Google Scholar] [CrossRef]
- Leyva-López, N.; Gutiérrez-Grijalva, E.P.; Vazquez-Olivo, G.; Heredia, J.B. Essential Oils of Oregano: Biological Activity beyond Their Antimicrobial Properties. Molecules 2017, 22, 989. [Google Scholar] [CrossRef]
- Pramod, K.; Ansari, S.H.; Ali, J. Eugenol: A Natural Compound with Versatile Pharmacological Actions. Nat. Prod. Commun. 2010, 5, 1999–2006. [Google Scholar] [CrossRef] [PubMed]
- Mauriello, E.; Ferrari, G.; Donsì, F. Effect of formulation on properties, stability, carvacrol release and antimicrobial activity of carvacrol emulsions. Colloids Surf. B Biointerfaces 2021, 197, 111424. [Google Scholar] [CrossRef] [PubMed]
- Klarić, M. Šegvić; Kosalec, I.; Mastelić, J.; Piecková, E.; Pepeljnak, S. Antifungal activity of thyme (Thymus vulgaris L.) essential oil and thymol against moulds from damp dwellings. Lett. Appl. Microbiol. 2007, 44, 36–42. [Google Scholar] [CrossRef]
- Marchese, A.; Barbieri, R.; Coppo, E.; Orhan, I.E.; Daglia, M.; Nabavi, S.M.; Izadi, M.; Abdollahi, M.; Ajami, M. Antimicrobial activity of eugenol and essential oils containing eugenol: A mechanistic viewpoint. Crit. Rev. Microbiol. 2017, 43, 668–689. [Google Scholar] [CrossRef] [PubMed]
- Capodicasa, S.; Fedi, S.; Porcelli, A.M.; Zannoni, D. The microbial community dwelling on a biodeteriorated 16th century painting. Int. Biodeterior. Biodegrad. 2010, 64, 727–733. [Google Scholar] [CrossRef]
- Pasquarella, C.; Balocco, C.; Saccani, E.; Capobianco, E.; Viani, I.; Veronesi, L.; Pavani, F.; Pasquariello, G.; Rotolo, V.; Palla, F.; et al. Biological and microclimatic monitoring for conservation of cultural heritage: A case study at the De Rossi room of the Palatina library in Parma. Aerobiologia 2019, 36, 105–111. [Google Scholar] [CrossRef]
- Montanari, M.; Iotti, M.; Innocenti, G. Isolamento, identificazione e attività cellulosolitica di microrganismi fungini associati al biodeterioramento di un dipinto su tela del XIXesimo secolo. Micol. Ital. 2009, 3, 19–24. [Google Scholar]
- Lech, T. Evaluation of a Parchment Document, the 13th Century Incorporation Charter for the City of Krakow, Poland, for Microbial Hazards. Appl. Environ. Microbiol. 2016, 82, 2620–2631. [Google Scholar] [CrossRef]
- Kumar, S.; Priyanka; Kumar, U. Microbial Biotechnology Approaches to Monuments of Cultural Heritage; Springer Science and Business Media LLC: Singapore, 2020; pp. 1–12. [Google Scholar]
- Orehek, J.; Dogsa, I.; Tomšič, M.; Jamnik, A.; Kočar, D.; Stopar, D. Structural investigation of carboxymethyl cellulose biodeterioration by Bacillus subtilis subsp. subtilis NCIB 3610. Int. Biodeterior. Biodegrad. 2013, 77, 10–17. [Google Scholar] [CrossRef]
- Fouda, A.; Abdel-Maksoud, G.; Abdel-Rahman, M.A.; Salem, S.S.; Hassan, S.E.-D.; El-Sadany, M.A.-H. Eco-friendly approach utilizing green synthesized nanoparticles for paper conservation against microbes involved in biodeterioration of archaeological manuscript. Int. Biodeterior. Biodegrad. 2019, 142, 160–169. [Google Scholar] [CrossRef]
- Sakr, A.A.; Ghaly, M.F.; Edwards, H.G.M.; Ali, M.F.; Abdel-Haliem, M.E.F. Involvement of Streptomyces in the Deterioration of Cultural Heritage Materials Through Biomineralization and Bio-Pigment Production Pathways: A Review. Geomicrobiol. J. 2020, 37, 653–662. [Google Scholar] [CrossRef]
- Mazzoli, R.; Giuffrida, M.G.; Pessione, E. Back to the past: Find the guilty bug—Microorganisms involved in the biodeterioration of archeological and historical artifacts. Appl. Microbiol. Biotechnol. 2018, 102, 6393–6407. [Google Scholar] [CrossRef] [PubMed]
- Sáez-Nieto, J.; Medina-Pascual, M.; Carrasco, G.; Garrido, N.; Fernandez-Torres, M.; Villalón, P.; Valdezate, S. Paenibacillus spp. isolated from human and environmental samples in Spain: Detection of 11 new species. New Microbes New Infect. 2017, 19, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Chu, C.; Zhang, M.; He, L.; Qin, L.; Li, X.; Yuan, M. Different Cell Wall-Degradation Ability Leads to Tissue-Specificity between Xanthomonas oryzae pv. oryzae and Xanthomonas oryzae pv. oryzicola. Pathogens 2020, 9, 187. [Google Scholar] [CrossRef] [PubMed]
- Zhgun, A.A.; Avdanina, D.; Shumikhin, K.; Simonenko, N.; Lyubavskaya, E.; Volkov, I.; Ivanov, V. Detection of potential biodeterioration risks for tempera painting in 16th century exhibits from State Tretyakov Gallery. PLoS ONE 2020, 15, e0230591. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Zhang, S.; Mei, R.; Zhang, Y.; Hashmi, M.Z.; Liu, J.; Lin, H.; Ding, L.; Sun, F. Resuscitation of viable but non-culturable bacteria to enhance the cellulose-degrading capability of bacterial community in composting. Microb. Biotechnol. 2018, 11, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Marjakangas, J.M.; Lakaniemi, A.-M.; Koskinen, P.E.; Chang, J.-S.; Puhakka, J.A. Lipid production by eukaryotic microorganisms isolated from palm oil mill effluent. Biochem. Eng. J. 2015, 99, 48–54. [Google Scholar] [CrossRef]
- Wang, H.; Kuang, S.; Lang, Q.; Yu, W. Effects of Aged Oil Sludge on Soil Physicochemical Properties and Fungal Diversity Revealed by High-Throughput Sequencing Analysis. Archaea 2018, 2018, 1–8. [Google Scholar] [CrossRef]
- Polo, A.; Cappitelli, F.; Villa, F.; Pinzari, F. Biological invasion in the indoor environment: The spread of Eurotium halophilicum on library materials. Int. Biodeterior. Biodegrad. 2017, 118, 34–44. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, J.; Liu, J.; Wang, R.; Liu, L.; Wang, F.; Yuan, H. The composition of accessory enzymes of Penicillium chrysogenum P33 revealed by secretome and synergistic effects with commercial cellulase on lignocellulose hydrolysis. Bioresour. Technol. 2018, 257, 54–61. [Google Scholar] [CrossRef]
- Tsang, C.-C.; Tang, J.Y.M.; Chan, K.-F.; Lee, C.-Y.; Chan, J.; Ngan, A.H.Y.; Cheung, M.; Lau, E.C.L.; Li, X.; Ng, R.H.Y.; et al. Diversity of phenotypically non-dermatophyte, non-Aspergillus filamentous fungi causing nail infections: Importance of accurate identification and antifungal susceptibility testing. Emerg. Microbes Infect. 2019, 8, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Kurowski, G.; Vogt, O.; Ogonowski, J. Paint-degrading microorganisms. Czas. Tech. 2017, 2017, 81–92. [Google Scholar] [CrossRef][Green Version]
- Veneranda, M.; Blanco-Zubiaguirre, L.; Roselli, G.; Di Girolami, G.; Castro, K.; Madariaga, J.M. Evaluating the exploitability of several essential oils constituents as a novel biological treatment against cultural heritage biocolonization. Microchem. J. 2018, 138, 1–6. [Google Scholar] [CrossRef]
- Navarrete, C.; Martínez, J.L. Non-conventional yeasts as superior production platforms for sustainable fermentation based bio-manufacturing processes. AIMS Environ. Sci. 2020, 7, 289–305. [Google Scholar] [CrossRef]
- Borrego, S.; Gómez de Saravia, S.; Valdés, O.; Vivar, I.; Battistoni, P.; Guiamet, P. Biocidal activity of two essential oils on fungi that cause biodeterioration of paper documents. Int. J. Conserv. Sci. 2016, 7, 369–380. [Google Scholar]
- Paster, N.; Juven, B.J.; Shaaya, E.; Menasherov, M.; Nitzan, R.; Weisslowicz, H.; Ravid, U. Inhibitory effect of oregano and thyme essential oils on moulds and foodborne bacteria. Lett. Appl. Microbiol. 1990, 11, 33–37. [Google Scholar] [CrossRef]
| CONTACT TEST | ||||
|---|---|---|---|---|
| BACTERIA | ISOLATES | TAXA | OREGANO | CLOVE |
| Ic | Bacillus subtilis subsp. subtilis | 1.0 ± 0.2 cm | 0.4 ± 0.1 cm | |
| IIa | Xanthomonadaceae | 1.1 ± 0.2 cm | 0.4 ± 0.1 cm | |
| IIIa | Streptomyces sp. | 1.0 ± 0.1 cm | 0.5 ± 0.1 cm | |
| IVa | NI | 1 ± 0 cm | 0.3 ± 0.2 cm | |
| Va | NI | 1.3 ± 0.2 cm | 0.4 ± 0.1 cm | |
| VIa | Stenotrophomonas | 0.9 ± 0.1 cm | 0.4 ± 0.1 cm | |
| VIIa | Pseudomonas psychrotolerans | 0.3 ± 0.1 cm | 0.2 ± 0 cm | |
| VIIIa | Xanthomonadaceae | 1 ± 0 cm | 0.3 ± 0.2cm | |
| IXa | Cellulosimicrobium cellulans | 0.9 ± 0.1 cm | 0.5 ± 0 cm | |
| Xa | Penibacillaceae | 1.4 ± 0.1 cm | 0.4 ± 0.2 cm | |
| XIa | NI | 1.3 ± 0.2 cm | 0.7 ± 0.1 cm | |
| XIIa | Paenibacillus sp. | 1.2 ± 0 cm | 0.9 ± 0.2 cm | |
| XIIIa | Bacillus simplex | 2 ± 0 cm | 0.6 ± 0.2 cm | |
| FUNGI | Ia | Penicillium chrysogenum | ++ | + |
| IIc | NI | + | + | |
| IIIa | Penicillium chrysogenum | ++ | ++ | |
| IVb | Cephalotheca foveolata | ++ | ++ | |
| Va | Aspergillus sp. | + | + | |
| VIa | Cephalotheca foveolata | ++ | ++ | |
| VIIa | Cladosporium parahalotolerans | + | + | |
| VIIIa | NI | ++ | + | |
| IXa | Cephalotheca foveolata | ++ | ++ | |
| Xa | Aspergillus versicolor | ++ | ++ | |
| XIa | NI | ++ | ++ | |
| XIIa | NI | - | - | |
| XIIIa | Penicillium chrysogenum | ++ | ++ | |
| XIVa | Trichocomaceae | + | + | |
| XVa | Chaetomiaceae | ++ | ++ | |
| XVIa | Penicillium chrysogenum | ++ | ++ | |
| XVIIa | Cephalotheca foveolata | ++ | ++ | |
| XVIIIa | NI | + | + | |
| XIXa | Phaeosphaeriaceae | + | + | |
| XXa | Penicillium sp. | + | + | |
| XXIb | NI | + | + | |
| XXIIb | NI | + | + | |
| XXIIIb | NI | ++ | + | |
| XXIVa | NI | ++ | + |
| CONTACTLESS TEST | |||
|---|---|---|---|
| BACTERIA | ISOLATES | TAXA | OREGANO |
| Ic | Bacillus subtilis subsp. subtilis | 1.7 ± 0.3 cm | |
| IIa | Xanthomonadaceae | Total inhibition | |
| IIIa | Streptomyces | 1.6 ± 0.4 cm | |
| IVa | NI | 1.6 ± 0.4 cm | |
| Va | NI | 1.8 ± 0.2 cm | |
| VIa | Stenotrophomonas | 1.6 ± 0.2 cm | |
| VIIa | Pseudomonas psychrotolerans | 1.5 ± 0.5 cm | |
| VIIIa | Xanthomonadaceae | 1.1 ± 0.4 cm | |
| IXa | Cellulosimicrobium cellulans | Total inhibition | |
| Xa | Penibacillaceae | 1.9 ± 0.1 cm | |
| XIa | NI | 1.6 ± 0.2 cm | |
| XIIa | Paenibacillus sp. | Total inhibition | |
| XIIIa | Bacillus simplex | 2 ± 0 cm | |
| FUNGI | Ia | Penicillium chrysogenum | ++ |
| IIc | NI | + | |
| IIIa | Penicillium chrysogenum | +++ | |
| IVb | Cephalotheca foveolata | ++ | |
| Va | Aspergillus sp. | +++ | |
| VIa | Cephalotheca foveolata | ++ | |
| VIIa | Cladosporium parahalotolerans | + | |
| VIIIa | NI | +++ | |
| IXa | Cephalotheca foveolata | +++ | |
| Xa | Aspergillus versicolor | +++ | |
| XIa | NI | ++ | |
| XIIa | NI | + | |
| XIIIa | Penicillium chrysogenum | +++ | |
| XIVa | Trichocomaceae | + | |
| XVa | Chaetomiaceae | ++ | |
| XVIa | Penicillium chrysogenum | ++ | |
| XVIIa | Cephalotheca foveolata | +++ | |
| XVIIIa | NI | ++ | |
| XIXa | Phaeosphaeriaceae sp. | + | |
| XXa | Penicillium sp. | + | |
| XXIb | NI | +++ | |
| XXIIb | NI | + | |
| XXIIIb | NI | + | |
| XXIVa | NI | +++ | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gatti, L.; Troiano, F.; Vacchini, V.; Cappitelli, F.; Balloi, A. An In Vitro Evaluation of the Biocidal Effect of Oregano and Cloves’ Volatile Compounds against Microorganisms Colonizing an Oil Painting—A Pioneer Study. Appl. Sci. 2021, 11, 78. https://doi.org/10.3390/app11010078
Gatti L, Troiano F, Vacchini V, Cappitelli F, Balloi A. An In Vitro Evaluation of the Biocidal Effect of Oregano and Cloves’ Volatile Compounds against Microorganisms Colonizing an Oil Painting—A Pioneer Study. Applied Sciences. 2021; 11(1):78. https://doi.org/10.3390/app11010078
Chicago/Turabian StyleGatti, Lucrezia, Federica Troiano, Violetta Vacchini, Francesca Cappitelli, and Annalisa Balloi. 2021. "An In Vitro Evaluation of the Biocidal Effect of Oregano and Cloves’ Volatile Compounds against Microorganisms Colonizing an Oil Painting—A Pioneer Study" Applied Sciences 11, no. 1: 78. https://doi.org/10.3390/app11010078
APA StyleGatti, L., Troiano, F., Vacchini, V., Cappitelli, F., & Balloi, A. (2021). An In Vitro Evaluation of the Biocidal Effect of Oregano and Cloves’ Volatile Compounds against Microorganisms Colonizing an Oil Painting—A Pioneer Study. Applied Sciences, 11(1), 78. https://doi.org/10.3390/app11010078






