Optimization of Lignin Recovery from the Pre-Hydrolysate of Kraft-Based Dissolving Pulp Production Processes
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
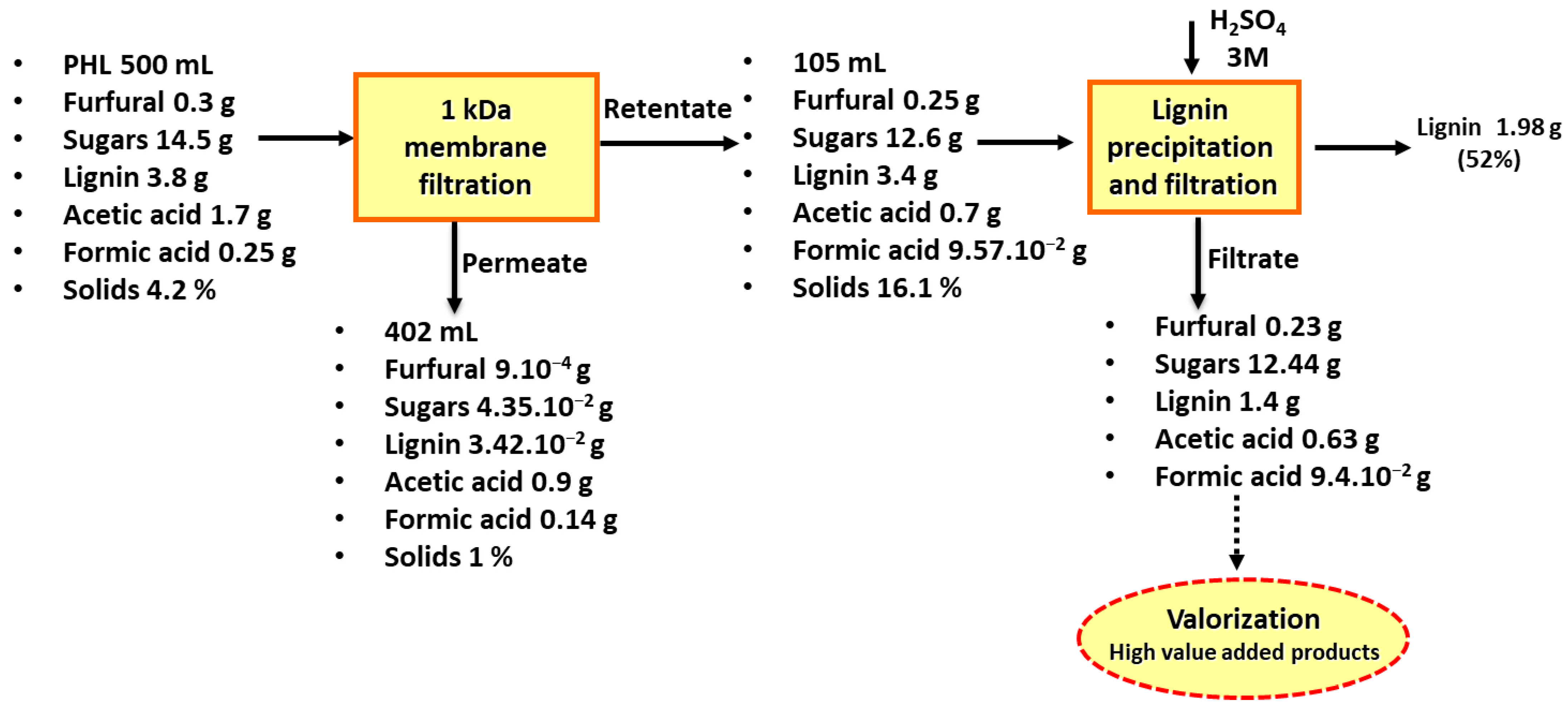
2.2. Pre-Hydrolysate Concentration
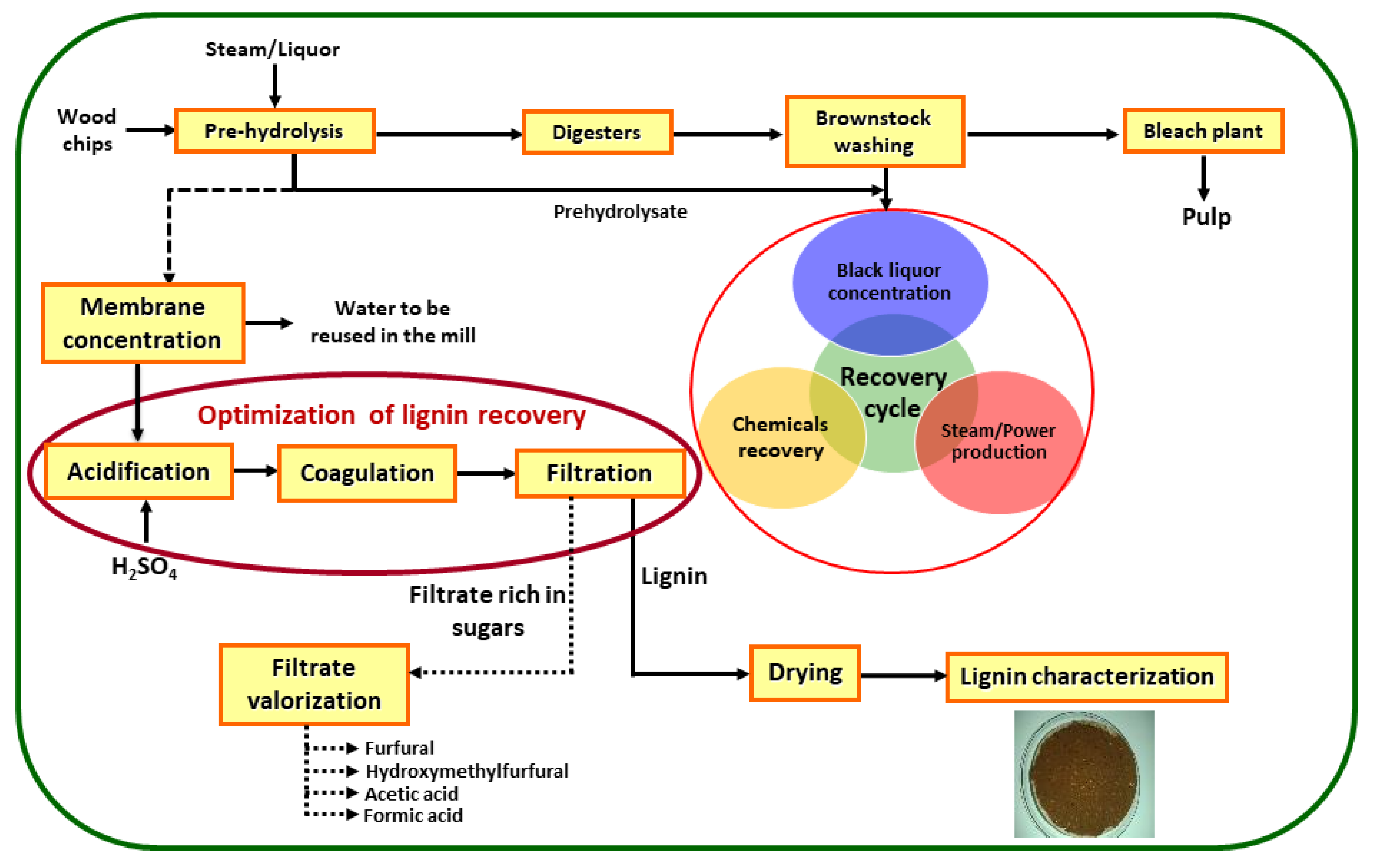
2.3. Lignin Recovery Optimization
2.4. Chemical Analyses
2.4.1. Insoluble Lignin
2.4.2. Soluble Lignin
2.4.3. Monomeric Sugar Content
2.4.4. Oligomeric Sugar Content
2.4.5. Sodium and Sulfur
2.4.6. Molecular Weight
2.4.7. Fourier-Transform Infrared Spectroscopy (FTIR) Analysis
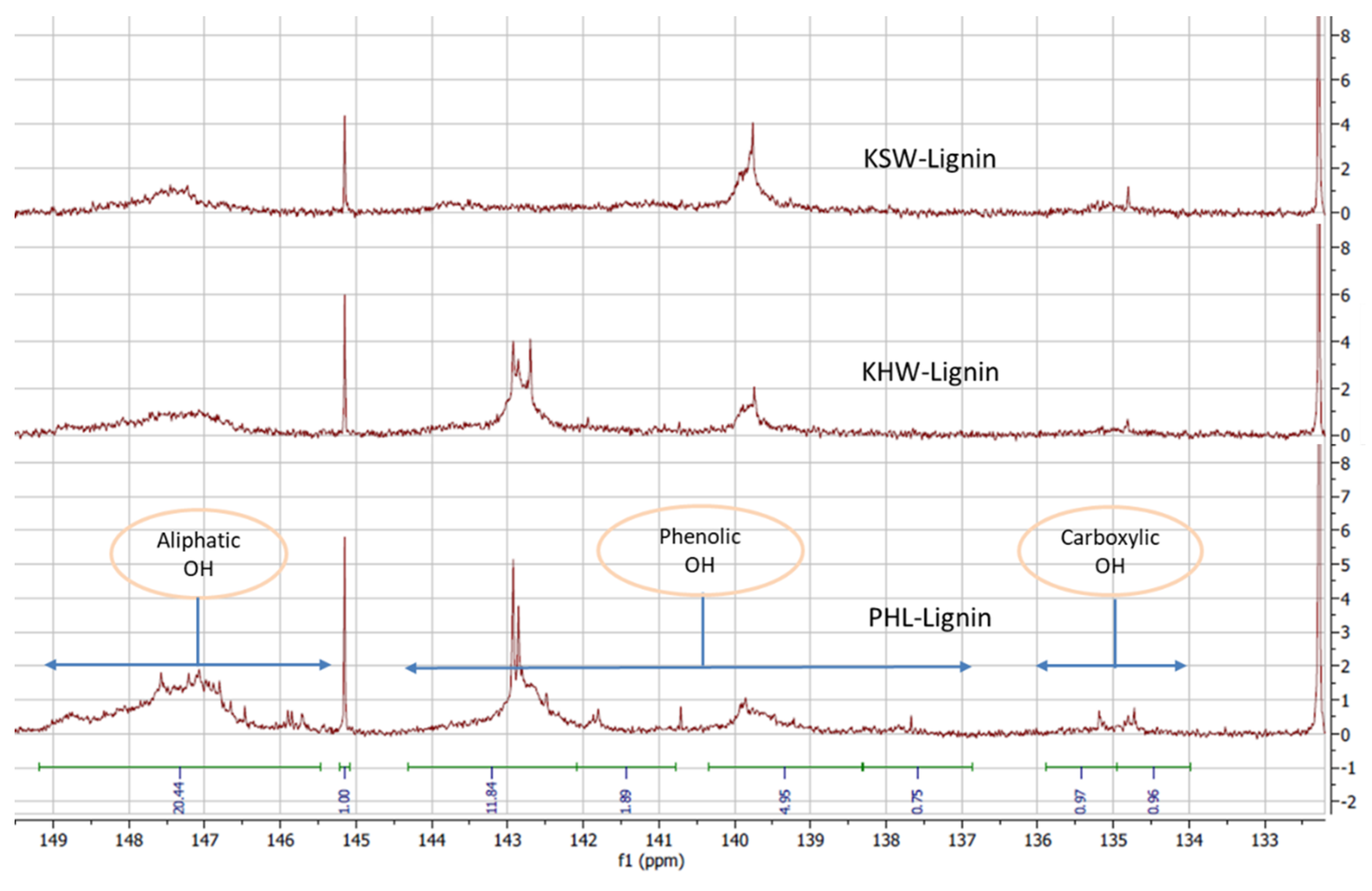
2.4.8. Functional Groups
2.4.9. Calculations
- -
- mr and mi are solute mass in the retentate and the pre-hydrolysate, respectively;
- -
- Cr and Ci are solute concentrations in the retentate and the pre-hydrolysate, respectively; and
- -
- Vr and Vi are retentate and pre-hydrolysate volume, respectively.
2.4.10. Statistics
3. Results
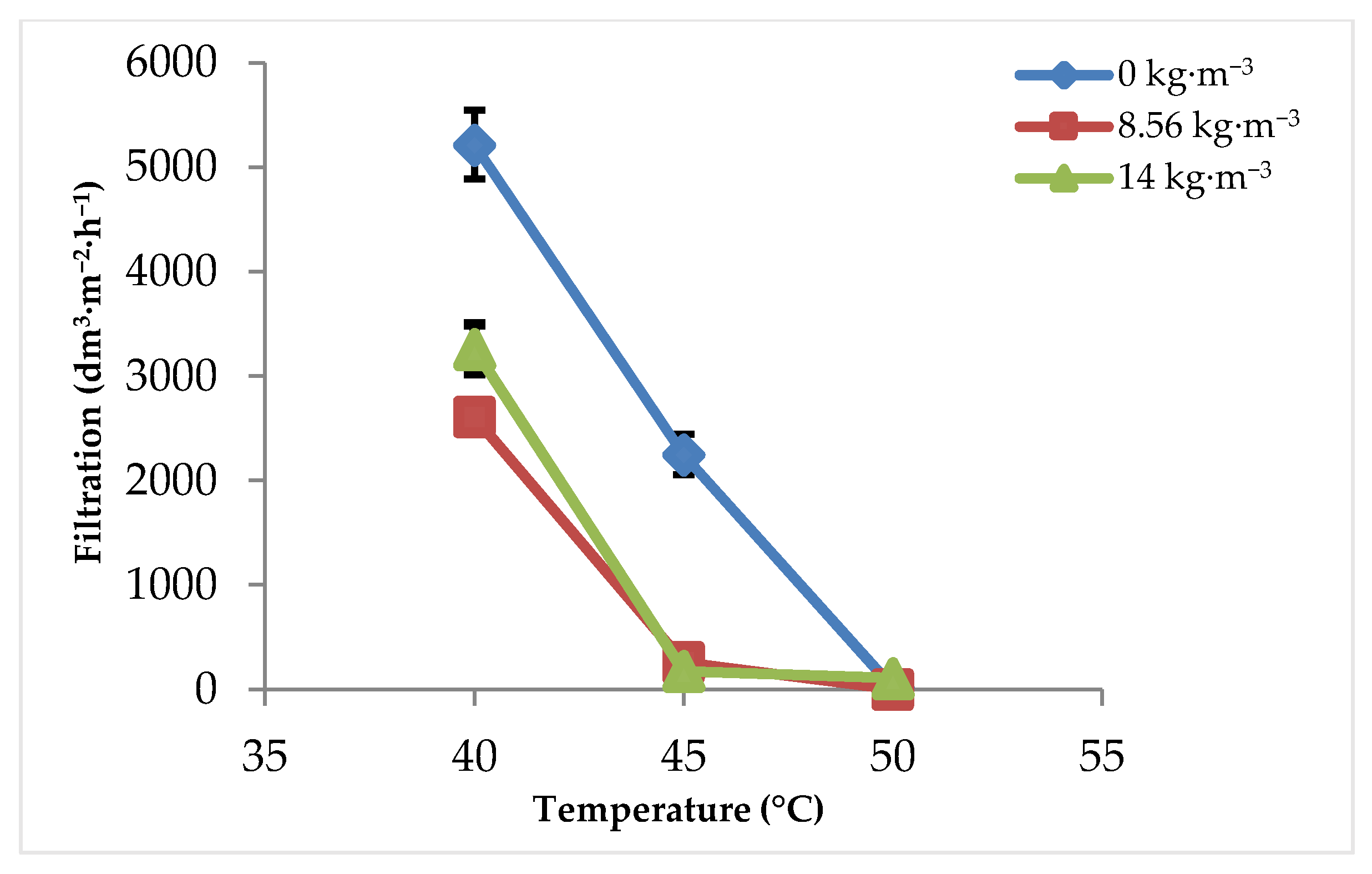
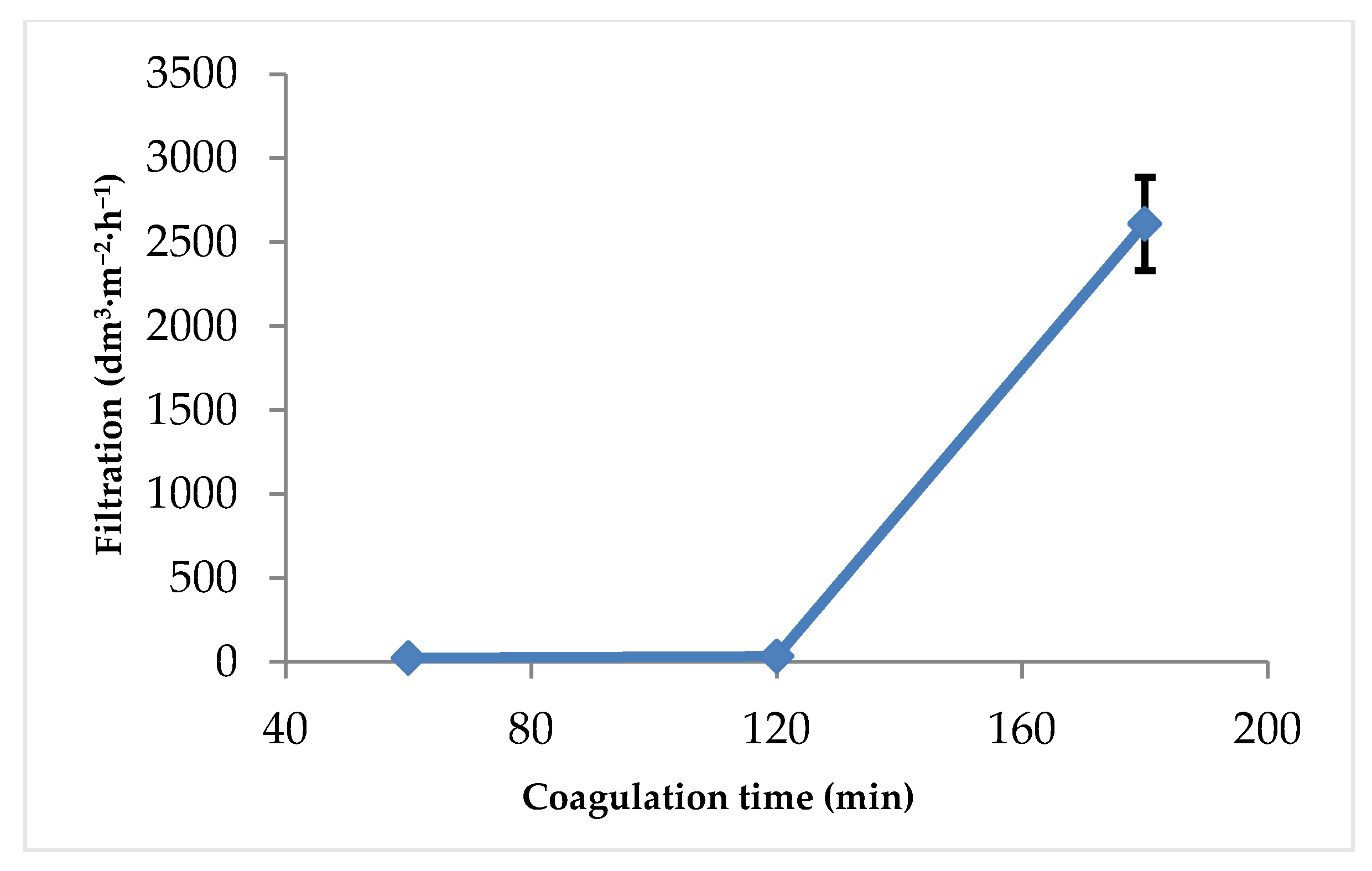
3.1. Filtration Rate
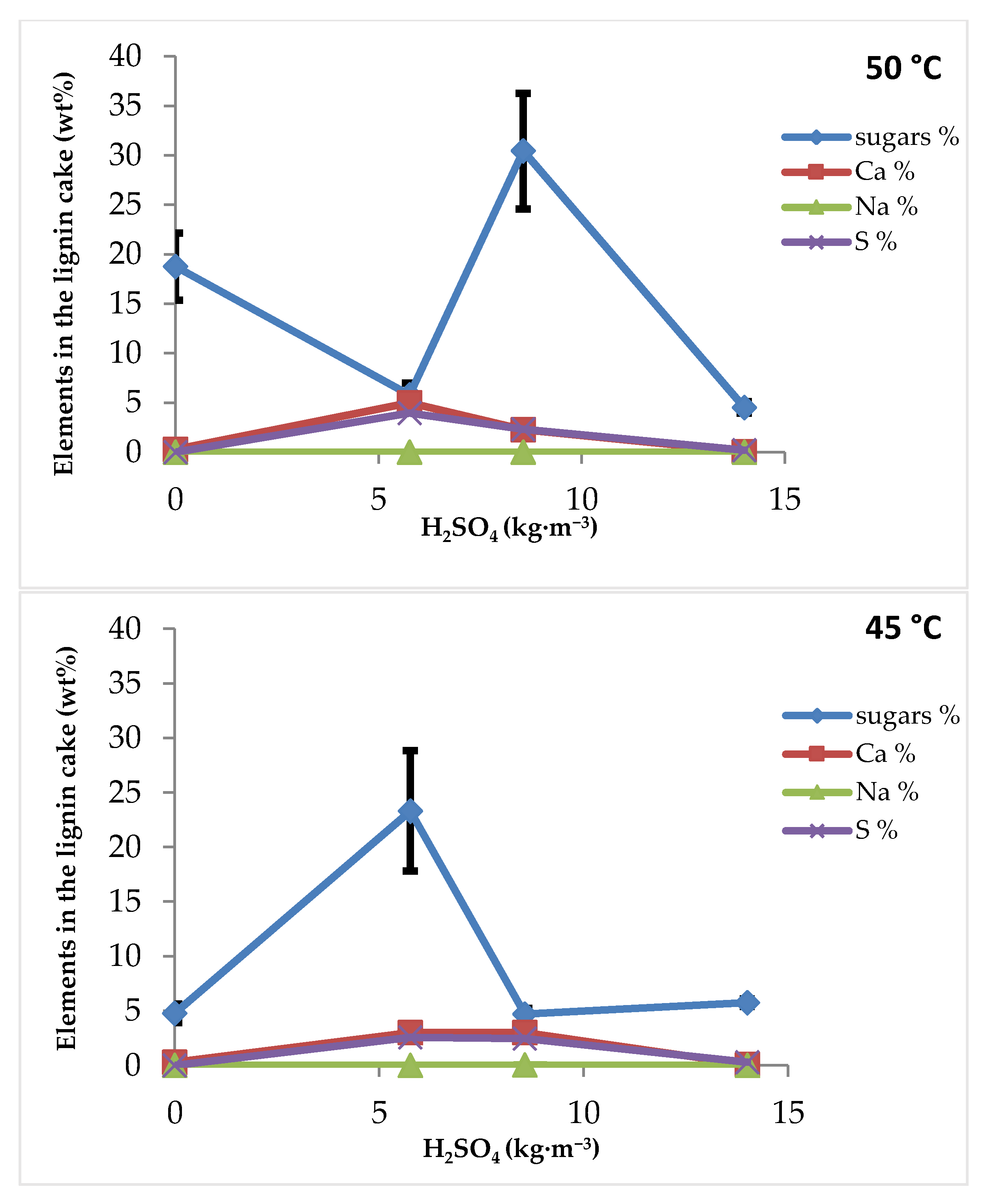
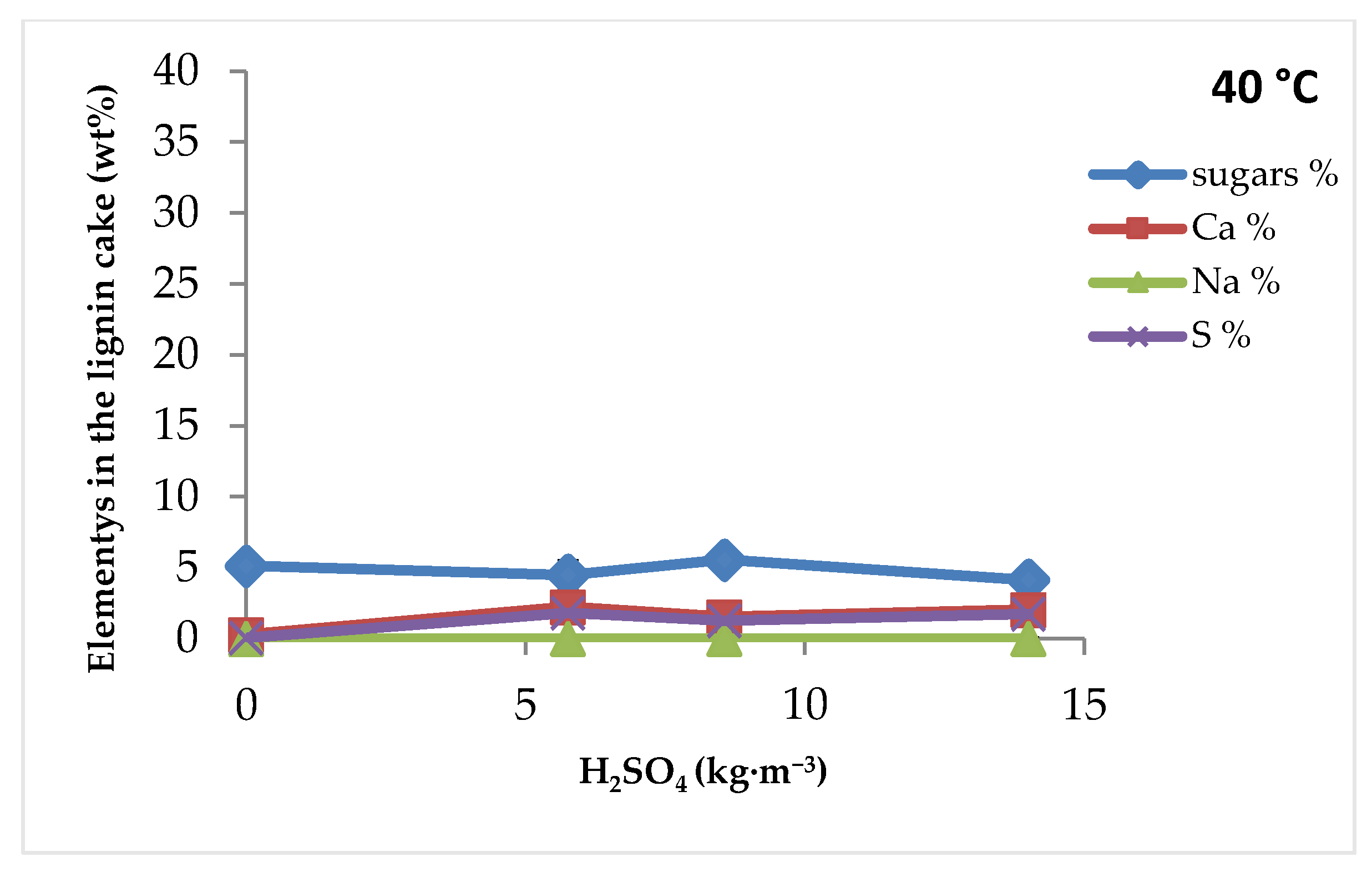
3.1.1. Effect of Sulfuric Acid Concentration and Filtration Temperature
3.1.2. Effect of Coagulation Time
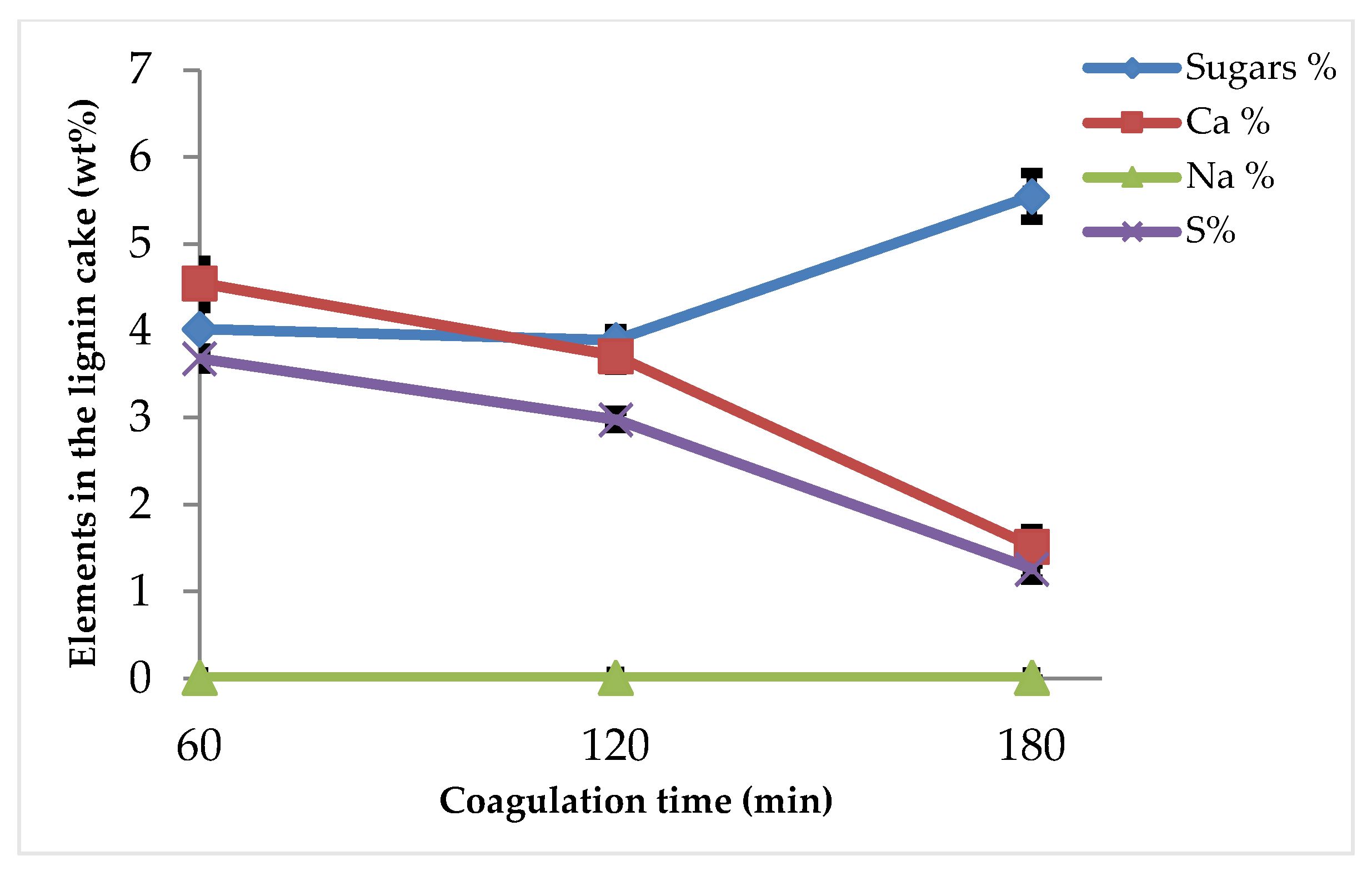
3.2. Purity of Lignin
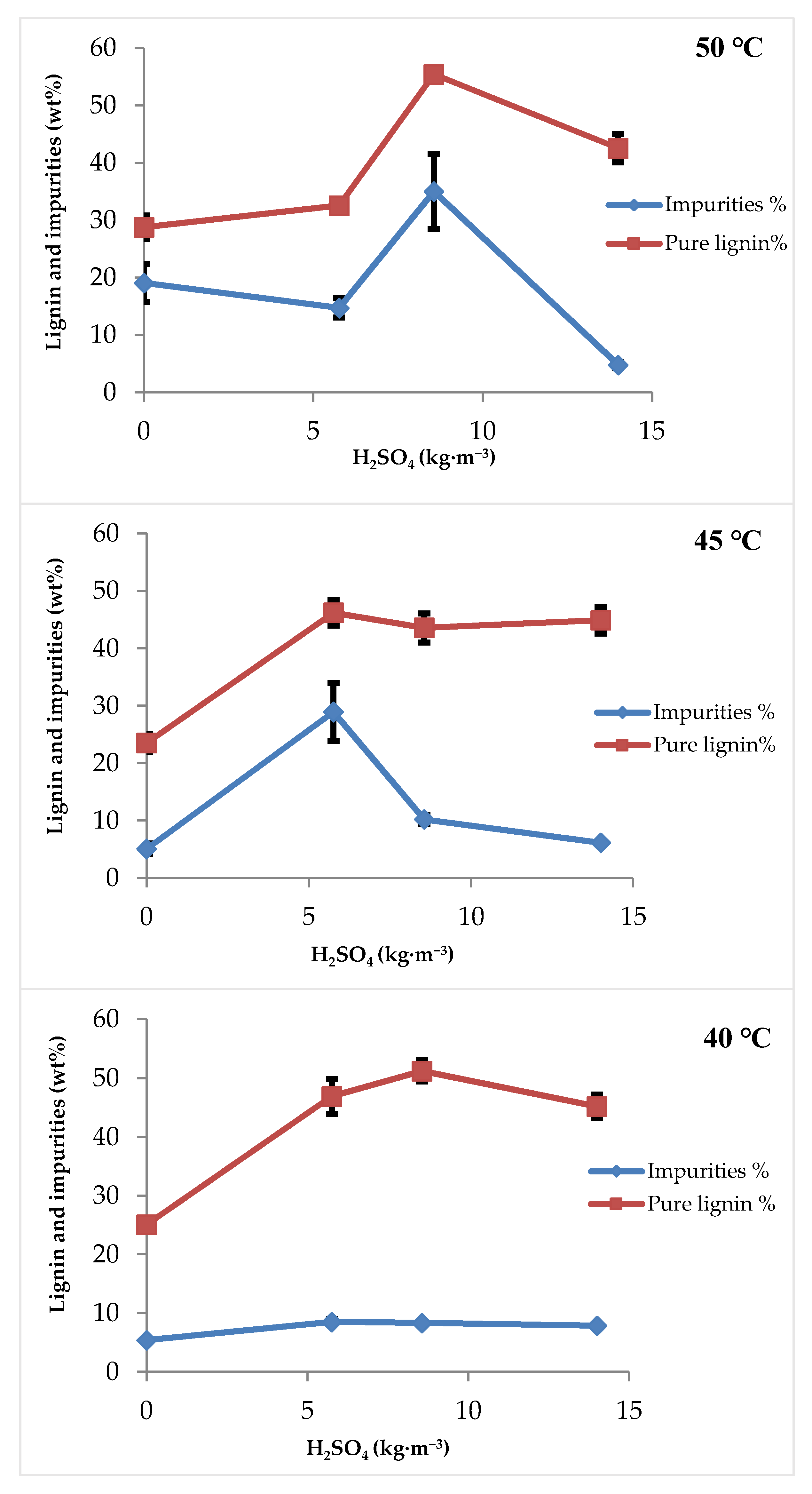
3.2.1. Effect of Coagulation Time
3.2.2. Effect of Sulfuric Acid Concentration and Filtration Temperature
3.3. Lignin Quantity
3.3.1. Effect of Coagulation Time
3.3.2. Effect of Sulfuric Acid Concentration and Filtration Temperature
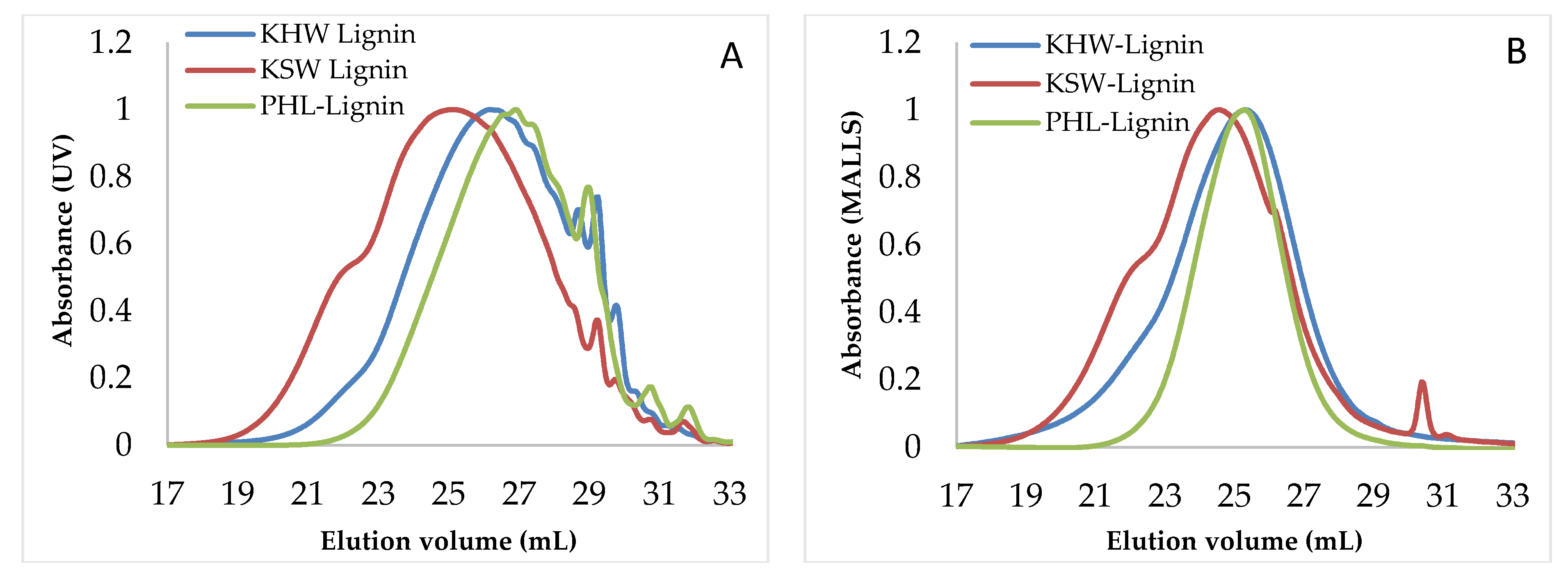
3.4. Molecular Weight and Molecular Weight Distribution
3.4.1. Effect of Filtration Temperature
3.4.2. Effect of Coagulation Time
3.4.3. Effect of Acid Concentration
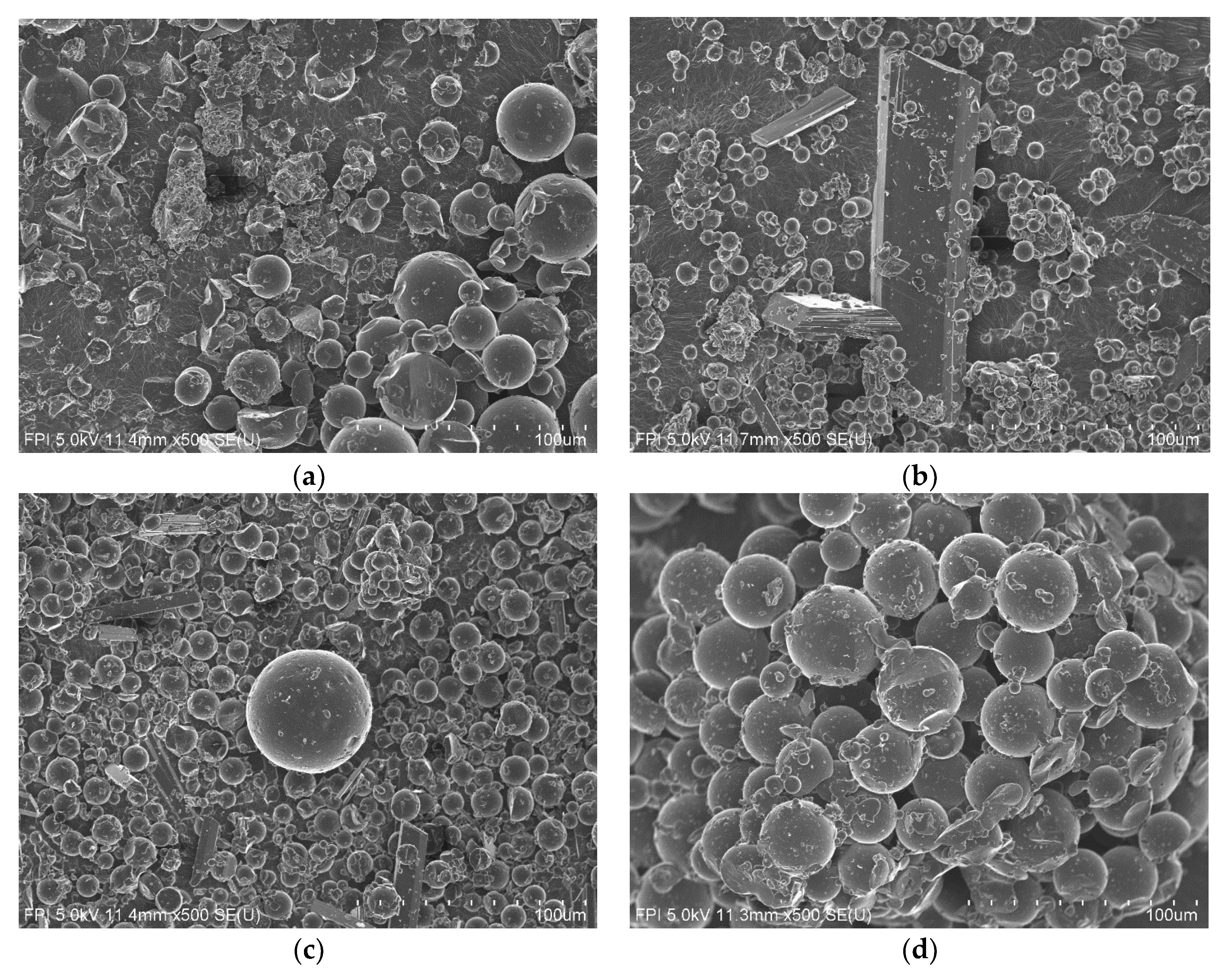
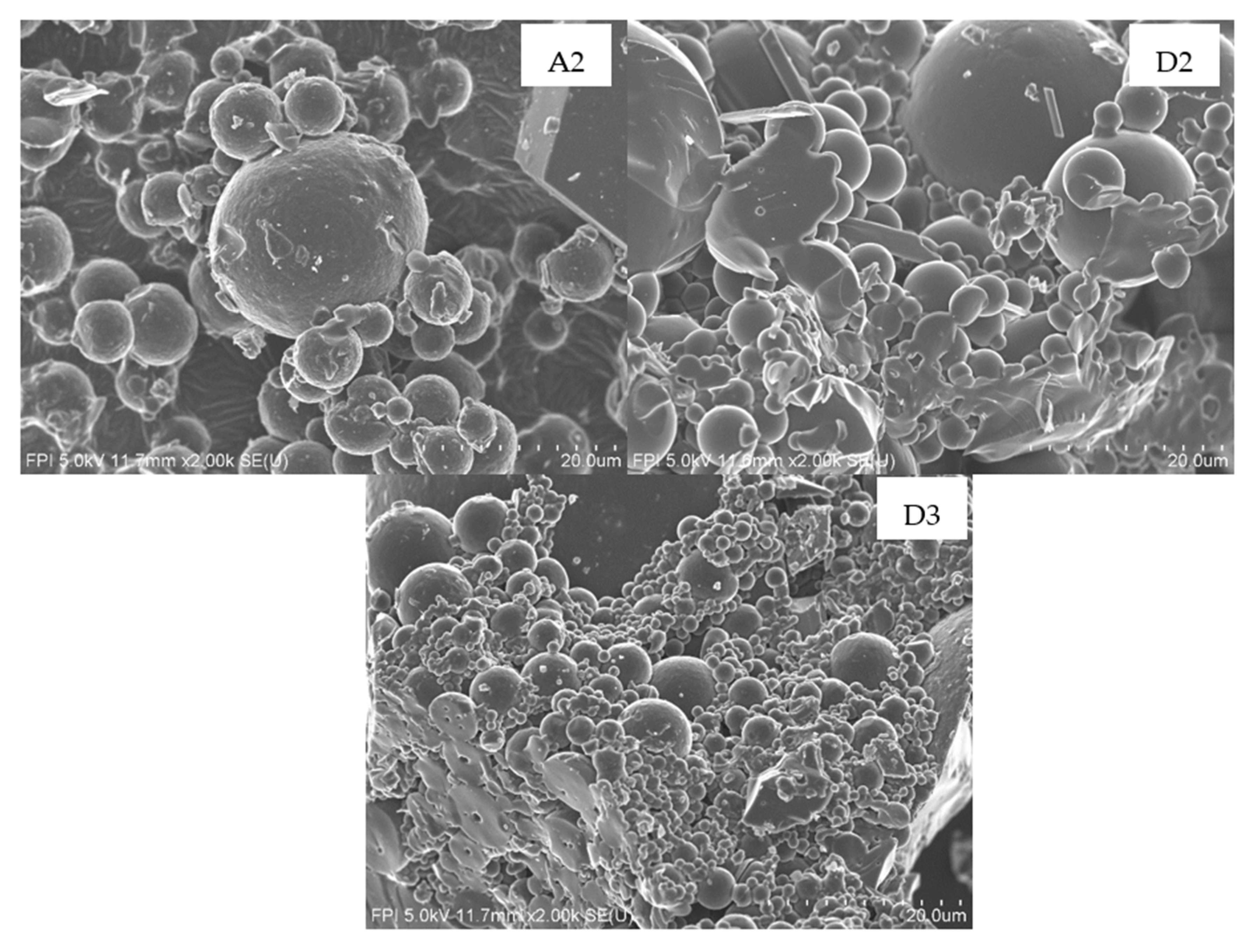
3.5. Particles Size
3.5.1. Effect of Acid Concentration
3.5.2. Effect of Coagulation Time
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Macdonald, C. Dissolving pulp draws renewed interest. Pulp Pap. Can. 2011, 112, 19–26. [Google Scholar]
- Huang. S. Reductive Depolymerization of Kraft Lignin for Chemicals and Fuels Using Formic Acid as an In-Situ Hydrogen Source. Master’s Thesis, University of Western Ontario, London, ON, Canada, 2014; p. 2.
- Tran, H.N.; Elliott, M.; Barham, D.; Reeve, D. Recovery boiler operation and plugging in Canada. Pulp Pap. Can. 1986, 87, 58–64. [Google Scholar]
- Kouisni, L.; Paleologou, M. Method for Separating Lignin from Black Liquor. U.S. Patent No. 8,940,130. U.S. Patent and Trademark Office: Washington, DC, USA, 9 April 2015. [Google Scholar]
- Kouisni, L.; Holt-Hindle, P.; Maki, K.; Paleologou, M. The Lignoforce system: A new process for the production of high quality lignin from black liquor. J. Sci. Tech. Forest Prod. Proc. 2012, 2, 6–10. [Google Scholar]
- Ohman, F.; Theliander, H.; Tomani, P.; Axegard, P. Method for Separating Lignin from Black Liquor. International Patent Application, WO2006/031175 A1, 23 March 2006. [Google Scholar]
- Mazar, A.; Jemaa, N.; Al Dajani, W.W.; Marinova, M.; Perrier, M. Furfural production from a pre-hydrolysate generated using aspen and maple chips. Biomass Bioenergy 2017, 104, 8–16. [Google Scholar] [CrossRef]
- Liu, H.; Hu, H.; Jahan, M.S.; Ni, Y. Improvement of Furfural Production from Concentrated PreHydrolysis Liquor (PHL) of a Kraft-Based Hardwood Dissolving Pulp Production Process. J. Wood Chem. Technol. 2015, 35, 260–269. [Google Scholar] [CrossRef]
- Liu, H.; Hu, H.; Jahan, M.S.; Ni, Y. Furfural formation from the pre-hydrolysis liquor of a hardwood kraft-based dissolving pulp production process. Bioresour. Technol. 2013, 131, 315–320. [Google Scholar] [CrossRef]
- Baktash, M.M.; Ahsan, L.; Ni, Y. Production of furfural from an industrial pre-hydrolysis liquor. Sep. Purif. Technol. 2015, 149, 407–412. [Google Scholar] [CrossRef]
- Mazar, A.; Jemaa, N.; Al Dajani, W.W.; Marinova, M.; Perrier, M. Comparative study: Furfural production from two types of pre-hydrolysates produced using aspen and maple chips. Biomass Bioenergy 2018, 111, 103–113. [Google Scholar] [CrossRef]
- Jahan, M.S.; Liu, Z.; Wang, H.; Saeed, A.; Ni, Y. Isolation and characterization of lignin from prehydrolysis liquor of kraft-based dissolving pulp production. Cellul. Chem. Technol. 2012, 46, 261–267. [Google Scholar]
- Shi, H.; Fatehi, P.; Xiao, H.; Ni, Y. A combined acidification/PEO flocculation process to improve the lignin removal from the pre-hydrolysis liquor of kraft-based dissolving pulp production process. Bioresour. Technol. 2011, 102, 5177–5182. [Google Scholar] [CrossRef]
- Yasarla, L.R.; Ramarao, B.V. Dynamics of Flocculation of Lignocellulosic Hydrolyzates by Polymers. Ind. Eng. Chem. Res. 2012, 51, 6847–6861. [Google Scholar] [CrossRef]
- Shi, H.; Fatehi, P.; Xiao, H.; Ni, Y. A process for isolating lignin of pre-hydrolysis liquor of kraft pulping process based on surfactant and calcium oxide treatments. Biochem. Eng. J. 2012, 68, 19–24. [Google Scholar] [CrossRef]
- Wang, Q.; Jahan, M.S.; Liu, S.; Miao, Q.; Ni, Y. Lignin removal enhancement from prehydrolysis liquor of kraft-based dissolving pulp production by laccase-induced polymerization. Bioresour. Technol. 2014, 164, 380–385. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, S.; Yang, G.; Chen, J. Modeling laccase-induced lignin removal in prehydrolysis liquor from kraft-based dissolving pulp production. Bioresour. Technol. 2015, 175, 638–641. [Google Scholar] [CrossRef] [PubMed]
- Mazar, A.; Jemaa, N.; Al Dajani, W.W.; Marinova, M.; Perrier, M. Influence of membrane filtration on extraction and characteristics of lignin from a kraft dissolving pulp mill pre-hydrolysate. Ind. Crop. Prod. 2018, 124, 726–734. [Google Scholar] [CrossRef]
- Ajao, O.; Le Hir, M.; Rahni, M.; Marinova, M.; Chadjaa, H.; Savadogo, O. Concentration and Detoxification of Kraft Prehydrolysate by Combining Nanofiltration with Flocculation. Ind. Eng. Chem. Res. 2015, 54, 1113–1122. [Google Scholar] [CrossRef]
- Tavares, L.B.; Boas, C.V.; Schleder, G.R.; Nacas, A.M.; Rosa, D.S.; Santos, D.J. Bio-based polyurethane prepared from Kraft lignin and modified castor oil. Express Polym. Lett. 2016, 10, 927–940. [Google Scholar] [CrossRef]
- Gómez-Fernández, S.; Ugarte, L.; Calvo-Correas, T.; Peña-Rodríguez, C.; Corcuera, M.A.; Eceiza, A. Properties of flexible polyurethane foams containing isocyanate functionalized kraft lignin. Ind. Crop. Prod. 2017, 100, 51–64. [Google Scholar] [CrossRef]
- Solt, P.; Rößiger, B.; Konnerth, J.; Van Herwijnen, H.W. Lignin phenol formaldehyde resoles using base-catalysed de-polymerized Kraft lignin. Polymers 2018, 10, 1162. [Google Scholar] [CrossRef]
- Brazil, T.R.; Gonçalves, M.; Junior, M.S.; Rezende, M.C. A statistical approach to optimize the activated carbon pro-duction from Kraft lignin based on conventional and microwave processes. Micropor. Mesopor. Mat. 2020, 308, 110485. [Google Scholar] [CrossRef]
- Lourençon, T.V.; De Lima, G.G.; Ribeiro, C.S.; Hansel, F.A.; Maciel, G.M.; Da Silva, K.; Winnischofer, S.M.; De Muniz, G.I.; Magalhães, W.L. Antioxidant, antibacterial and antitumoural activities of kraft lignin from hardwood fractionated by acid precipitation. Int. J. Biol. Macromol. 2021, 166, 1535–1542. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Fatehi, P.; Xiao, H.; Ni, Y. Optimizing the poly ethylene oxide flocculation process for isolating lignin of prehy-drolysis liquor of a kraft-based dissolving pulp production process. Ind. Eng. Chem. Res. 2012, 51, 5330–5335. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Z.; Fu, Y.; Li, Z.; Qin, M. Specific lignin precipitation for oligosaccharides recovery from hot water wood extract. Bioresour. Technol. 2014, 152, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Norgren, M.; Edlund, H.; Wågberg, L.; Lindström, B.; Annergren, G. Aggregation of kraft lignin derivatives under con-ditions relevant to the process, part I: Phase behaviour. Colloids Surf. A 2001, 194, 85–96. [Google Scholar] [CrossRef]
- Öhman, F.; Theliander, H. Filtration properties of lignin precipitated from black liquor. TAPPI J. 2007, 6, 3–9. [Google Scholar]
- Faix, O. Classification of Lignins from Different Botanical Origins by FT-IR Spectroscopy. Holzforschung 1991, 45, 21–28. [Google Scholar] [CrossRef]
- Gomez-Serrano, V.; Pastor-Villegas, J.; Perez-Florindo, A.; Duran-Valle, C.; Valenzuela-Calahorro, C. FT-IR study of rockrose and of char and activated carbon. J. Anal. Appl. Pyrolysis 1996, 36, 71–80. [Google Scholar] [CrossRef]
- Li, B.; Zhou, M.; Huo, W.; Cai, D.; Qin, P.; Cao, H.; Tan, T. Fractionation and oxypropylation of corn-stover lignin for the production of biobased rigid polyurethane foam. Ind. Crop. Prod. 2020, 143, 111887. [Google Scholar] [CrossRef]
- Toledano, A.; García, A.; Mondragon, I.; Labidi, J. Lignin separation and fractionation by ultrafiltration. Sep. Purif. Technol. 2010, 71, 38–43. [Google Scholar] [CrossRef]
- Yuan, T.-Q.; He, J.; Xu, F.; Sun, R. Fractionation and physico-chemical analysis of degraded lignins from the black liquor of Eucalyptus pellita KP-AQ pulping. Polym. Degrad. Stab. 2009, 94, 1142–1150. [Google Scholar] [CrossRef]
- Sun, R.; Tomkinson, J. Fractional separation and physico-chemical analysis of lignins from the black liquor of oil palm trunk fibre pulping. Sep. Purif. Technol. 2001, 24, 529–539. [Google Scholar] [CrossRef]
- Saito, T.; Perkins, J.H.; Vautard, F.; Meyer, H.M.; Messman, J.M.; Tolnai, B.; Naskar, A.K. Methanol Fractionation of Softwood Kraft Lignin: Impact on the Lignin Properties. ChemSusChem 2014, 7, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Brodin, I.; Sjöholm, E.; Gellerstedt, G. Kraft lignin as feedstock for chemical products: The effects of membrane filtration. Holzforschung 2009, 63, 290–297. [Google Scholar] [CrossRef]
- Mörck, R.; Reimann, A.; Kringstad, K.P. Fractionation of kraft lignin by successive extraction with organic solvents: Functional groups, 13C NMR spectra and molecular weight distributions. Holzforschung 1986, 40, 51–60. [Google Scholar]
- Thring, R.; Vanderlaan, M.; Griffin, S. Fractionation of Alcell® Lignin by Sequential Solvent Extraction. J. Wood Chem. Technol. 1996, 16, 139–154. [Google Scholar] [CrossRef]
- Yoshida, H.; Mörck, R.; Kringstad, K.P.; Hatakeyama, H. Fractionation of kraft lignin by successive extraction with or-ganic solvents. II. Thermal properties of kraft lignin fractions. Holzforschung 1987, 41, 171–176. [Google Scholar] [CrossRef]
- Wallberg, O.; Jönsson, A.S.; Wimmerstedt, R. Fractionation and concentration of kraft black liquor lignin with ultrafil-tration. Desalination 2003, 154, 187–199. [Google Scholar] [CrossRef]
- Jönsson, A.-S.; Nordin, A.-K.; Wallberg, O. Concentration and purification of lignin in hardwood kraft pulping liquor by ultrafiltration and nanofiltration. Chem. Eng. Res. Des. 2008, 86, 1271–1280. [Google Scholar] [CrossRef]
- Norgren, M.; Lindström, B. Physico-chemical characterization of a fractionated kraft lignin. Holzforschung 2000, 54, 528–534. [Google Scholar] [CrossRef]
- Helander, M. The Use of Membrane Filtration to Improve the Properties of Extracted Wood Componets. Ph.D. Thesis, KTH Royal Institute of Technology, Stockholm, Sweden, 2014. [Google Scholar]
- Beauchet, R.; Monteil-Rivera, F.; Lavoie, J.-M. Conversion of lignin to aromatic-based chemicals (L-chems) and biofuels (L-fuels). Bioresour. Technol. 2012, 121, 328–334. [Google Scholar] [CrossRef]
- Diop, A. Extraction, Dépolymérisation et Valorisation de la Lignine Kraft de la Liqueur Noire. Ph.D. Thesis, Sciences et Génie des Matériaux Lignocellulosiques, Université de Québec à Trois Rivières, Trois-Rivières, QC, Canada, 2014. [Google Scholar]
- Madhav, P.P. Lignin Depolymerisation and Conversion: A Review of Thermochemical Methods. Chem. Eng. Technol. 2011, 34, 29–41. [Google Scholar]
- Khitrin, K.S.; Fuks, S.L.; Khitrin, S.V.; Kazienkov, S.A.; Meteleva, D.S. Lignin utilization options and methods. Russ. J. Gen. Chem. 2012, 82, 977–984. [Google Scholar] [CrossRef]
- Ahmad, Z.; Mahmood, N.; Yuan, Z.; Paleologou, M.; Xu, C.C. Effects of Process Parameters on Hydrolytic Treatment of Black Liquor for the Production of Low-Molecular-Weight Depolymerized Kraft Lignin. Molecules 2018, 23, 2464. [Google Scholar] [CrossRef]
- Yuan, Z.; Tymchyshyn, M.; Xu, C. (Charles) Reductive Depolymerization of Kraft and Organosolv Lignin in Supercritical Acetone for Chemicals and Materials. ChemCatChem 2016, 8, 1968–1976. [Google Scholar] [CrossRef]
- Jemaa, N.; Mazar, A.; AL DAJANI, W.W.; Paleologou, M. Lignin Recovery and Furfural Production from Prehydrolysate Streams. U.S. Patent Application No.16/088,980, October 15, 2020. [Google Scholar]
- Mazar, A.; Ajao, O.; Benali, M.; Jemaa, N.; Al-Dajani, W.W.; Paleologou, M. Integrated Multiproduct Biorefinery for Furfural Production with Acetic Acid and Lignin Recovery: Design, Scale-Up Evaluation, and Technoeconomic Analysis. ACS Sustain. Chem. Eng. 2020, 8, 17345–17358. [Google Scholar] [CrossRef]


| Species | Value |
|---|---|
| Phenolics, kg·m−3 | 2.1 |
| Furfural, kg·m−3 | 0.6 |
| Hydroxymethylfurfural (HMF), kg·m−3 | 0.1 |
| Solid, % (w/w) | 4.3 |
| Total lignin, kg·m−3 | 7.6 |
| Acetic acid, kg·m−3 | 3.4 |
| Formic acid, kg·m−3 | 0.5 |
| Total sugars, kg·m−3 | 29 |
| Total monomeric sugars, kg·m−3 | 5 |
| Total oligomeric sugars, kg·m−3 | 24 |
| pH | 3.5 |
| Lignin Precipitation Parameters | MALLS | ||
|---|---|---|---|
| MW | Mn | MW/Mn | |
| A1 (40 °C; 14 kg·m−3, 180 min) | 2160 | 1860 | 1.16 |
| A2 (40 °C; 8.5 kg·m−3, 180 min) | 1270 | 1040 | 1.22 |
| A3 (40 °C; 5.7 kg·m−3, 180 min) | 1540 | 1310 | 1.18 |
| A4 (40 °C; 0 kg·m−3, 180 min) | 4285 | 3725 | 1.15 |
| B1 (45 °C; 14 kg·m−3, 180 min) | 3280 | 2555 | 1.28 |
| B2 (45 °C; 8.5 kg·m−3, 180 min) | 2865 | 2115 | 1.35 |
| B3 (45 °C; 5.7 kg·m−3, 180 min) | 2610 | 1445 | 1.81 |
| B4 (45 °C; 0 kg·m−3, 180 min) | 4160 | 3740 | 1.11 |
| C1 (50 °C; 14 kg·m−3, 180 min) | 4015 | 3090 | 1.30 |
| C2 (50 °C; 8.5 kg·m−3, 180 min) | 3550 | 2350 | 1.51 |
| C3 (50 °C; 5.7 kg·m−3, 180 min) | 2890 | 1980 | 1.46 |
| C4 (50 °C; 0 kg·m−3, 180 min) | 2610 | 2140 | 1.22 |
| D2 (40 °C; 8.5 kg·m−3, 120 min) | 1890 | 1095 | 1.73 |
| D3 (40 °C; 8.5 kg·m−3, 60 min) | 2320 | 1530 | 1.52 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazar, A.; Jemaa, N.; Wafa Al Dajani, W.; Marinova, M.; Perrier, M. Optimization of Lignin Recovery from the Pre-Hydrolysate of Kraft-Based Dissolving Pulp Production Processes. Appl. Sci. 2021, 11, 454. https://doi.org/10.3390/app11010454
Mazar A, Jemaa N, Wafa Al Dajani W, Marinova M, Perrier M. Optimization of Lignin Recovery from the Pre-Hydrolysate of Kraft-Based Dissolving Pulp Production Processes. Applied Sciences. 2021; 11(1):454. https://doi.org/10.3390/app11010454
Chicago/Turabian StyleMazar, Adil, Naceur Jemaa, Waleed Wafa Al Dajani, Mariya Marinova, and Michel Perrier. 2021. "Optimization of Lignin Recovery from the Pre-Hydrolysate of Kraft-Based Dissolving Pulp Production Processes" Applied Sciences 11, no. 1: 454. https://doi.org/10.3390/app11010454
APA StyleMazar, A., Jemaa, N., Wafa Al Dajani, W., Marinova, M., & Perrier, M. (2021). Optimization of Lignin Recovery from the Pre-Hydrolysate of Kraft-Based Dissolving Pulp Production Processes. Applied Sciences, 11(1), 454. https://doi.org/10.3390/app11010454







