Abstract
This study analyzed the influence of different ammonia stripping parameters on ammonia removal efficiency and mass transfer rate. Ammonia stripping was performed on two devices, a column and a packed tower, with artificial ammonium hydroxide wastewater. First, ammonia concentration and pH were varied in a column without liquid circulation. At the same pH, the removal efficiency and mass transfer rate were constant, irrespective of initial ammonia concentration. When pH was increased, the ammonia fraction also increased, resulting in higher removal efficiency and mass transfer rate. Second, the effects of stripping were assessed using a packed tower with fluid circulation. The ammonium hydroxide concentration did not affect the removal efficiency or mass transfer rate. Furthermore, at apparatus liquid-gas ratios of 26.8–107.2 L/m3, a lower liquid-gas ratio led to increased ammonia removal efficiency and mass transfer rate. Conversely, the lower the liquid-gas ratio, the greater the air consumption. In conclusion, considering the removal rate and volume of air supply, the range of optimal liquid-gas ratio was determined as 26.8–53.6 L/m3. In particular, the 26.8 L/m3 condition achieved the best ammonia removal rate of 63.0% through only 6 h of stripping at 70 °C and pH 8.5.
1. Introduction
With industrial development and population growth, there has been a rapid increase in the amount of wastewater containing ammonia from various sources, including sewage sludge, cattle excrement, food waste, and biomass []. Ammonia containing wastewater is also generated in the energizing process of waste resources [,]. Ammonia in wastewater should be removed because it causes eutrophication and ecotoxicity when it leaks into ecosystems [,].
There are various methods for removing ammoniacal nitrogen from solution, including physical, electronic, and biological methods. Ammonia stripping is a particularly efficient method for ammonia recovery from high concentration wastewater [,], In ammonia stripping system, ammoniacal nitrogen is removed as a gas by supplying gas such as air or steam [,,,]. The reactor is usually a fixed-bed column or packed bed tower [,,,]. In tower-type reactors, ammonia mass transfer is achieved by gas-liquid contact within the packing material []. Therefore, the liquid-gas ratio should be carefully considered to optimize the operating conditions of packed towers.
In this regard, substantial research and development have focused on the operating conditions and other factors affecting stripping towers for different types of wastewater [,,,,,]. For example, Ferraz et al. [] performed ammonia stripping from landfill leachate using a packed tower at room temperature and pH 11 and achieved a removal efficiency of 98% after 24 h of stripping at a liquid-gas ratio (L/G) of 6.7–20 L/m3. Guštin et al. [] used a continuous flow packed tower to strip ammoniacal nitrogen from anaerobic digestate at a liquid-gas ratio of 2.5 L/m3, achieving a removal efficiency of 55% at 50 °C and pH 10. Liu et al. [] evaluated the ammonia removal efficiency and rate for the urine with temperature, pH, concentration, and liquid-gas ratio. Zhu et al. [] and Li et al. [] also analyzed ammonia removal rate by temperature, pH and air supply condition.
Conventional ammonia stripping processes are typically performed at a temperature between room temperature and 50 °C and in a pH range of 10–12 [,,,,,]. These process require a large amount of reagents to adjust the pH [,]. In the ammonia stripping process, a basic reagent is used to increase the pH. After ammonia removal, the pH must be adjusted to neutral for wastewater treatment and discharge. One method for reducing the use of these reagents is high-temperature stripping; at a high temperature, the same removal efficiency can be achieved with a smaller quantity of reagent []. Although there is a problem that a heat is required for high-temperature stripping, the heat energy consumption can be minimized by using waste heat. For example, the hydrothermal thermal carbonization (HTC) reaction is carried out at 180–260 °C [,], and it is possible to raise the temperature of the HTC wastewater up to 70–80 °C by using HTC heat. In addition, the high-temperature process can be operated more economically by reducing the air supply [].
The purpose of this study is to design a high-temperature stripping tower and derive the optimal operating factors that will reduce the use of reagents and air supply while maintaining the ammonia fraction at an operating temperature of 70 °C. To this end, the ammonia removal efficiency and mass transfer coefficient are assessed at different temperatures, pH, ammonium hydroxide concentration, and airflow volume. Specifically, by deriving the optimal liquid-gas ratio and air supply volume for efficient liquid-to-gas contact in the packing layer, this study provides useful data on the key factors in the design of high-temperature ammonia stripping devices.
2. Theoretical Considerations
2.1. Ammonia–Water System
In aqueous solution, total ammonia exists as both free ammonia () and ammonium ions (), as shown in Equation (1), and the water is dissociated into ions, as shown in Equation (2) [].
The total ammonia concentration in the solution is expressed by Equation (3).
where , , and mean the molar concentration (mol/L) of total ammonia, free ammonia, and ammonium ion, respectively.
The ionization constants for water () and ammonia () are known in Equations (4) and (5), respectively.
where and are the molar concentration (mol/L) of hydronium ion and hydroxide ion, respectively.
In addition, the ionization constants are expressed as a function of absolute temperature (K), as shown in Equations (6) and (7) [,,,].
2.2. Ammonia Stripping
In aqueous solution, the free ammonia can be stripped through transfer from liquid to gas. Thus, the concentration ratio of the free ammonia to total ammonia is a major factor in stripping and is defined as the ammonia fraction () in Equation (8) [].
The ammonia fraction is also expressed by Equation (9) from Equations (4) and (5).
Since the ionization constants is described as a function of absolute temperature in Equation (6), the ammonia fraction is dependent on both temperature and pH.
The percentage of ammonia removal () is calculated as the amount of ammonia removed compared to the initial ammonia and is expressed as Equation (10):
2.3. Mass Transfer of Ammonia
In the process of ammonia stripping, the mass transfer rate of ammonia () from liquid to gas phase is given by Equation (11) [,,].
where is time and is the total volume of liquid.
In a batch reactor, the mass transfer rate for liquid ammonia to gas is defined by Matter-Mueller, as shown in Equation (12) [,]:
where is the gas flow rate and is the dimensionless Henry’s law constant. is the mass transfer coefficient of liquid ammonia. The interfacial area per unit volume between liquid and gas is indicated by .
Combining Equations (8), (10) and (12) and integrating the total ammonia concentration over time gives Equation (13):
The slope for the logarithm of ammonia concentration ratio over time is then given by Equation (14):
From Equations (13) and (14), the overall mass transfer coefficient for liquid ammonia to gas is calculated as follows:
The main variables in ammonia stripping are the ammonia fraction, the rate of change in the ammonia concentration, the volume of gas supplied, and the overall mass transfer coefficient according to the type of reactor as shown in Equations (12) and (14). Hence, in this study, we analyzed the effect of the major stripping factors—ammonia concentration, fraction and gas flow rate—on the ammonia removal efficiency and mass transfer coefficient.
3. Materials and Methods
3.1. Preparation
In this study, air stripping experiments were conducted and stripping factors were evaluated for high ammonia concentration wastewater such as HTC or anaerobic liquids [,,,,,,]. These wastewaters contain ammonia up to 4000 mg/L [,,]. To simulate artificial wastewater, 1000–3700 mg/L ammonia solution was prepared by diluting a 28–30% ammonia solution (SAMCHUN) with tap water. To evaluate the physical degassing properties of ammonia, the effects on trace amounts of nitrate, nitrite were excluded.
3.2. Stripping Column
A column-type ammonia stripping device was configured as shown in Figure 1. The capacity of the column was 2 L, and a disk-type air diffuser was installed at the bottom, through which external air could be supplied. The air required for stripping was controlled at a pressure of ≤30 Pa using a blower and regulator, and a flow meter was used to control the volume. To control the pH of the solution, NaOH solution was supplied using an auto titration system (905 Titrando, Metrohm AG, Switzerland). Stripping was performed on 1 L of artificial wastewater, and the air flow rate was set to 900 L/h. The stripping factors were assessed while varying the ammonia concentration and the pH of the wastewater, under the conditions shown in Table 1.
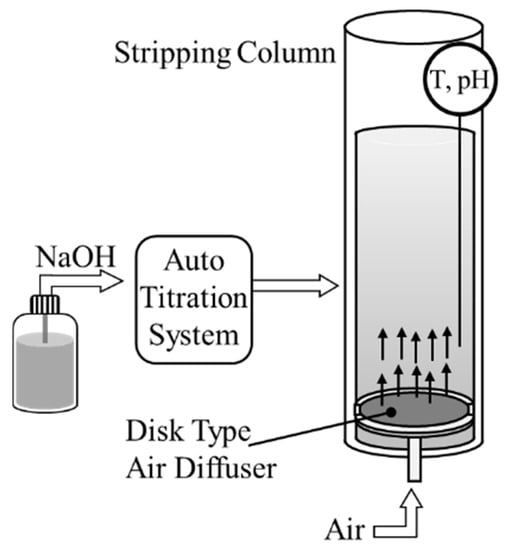
Figure 1.
Schematic view of the ammonia stripping column.

Table 1.
Experimental conditions of NH3 stripping using a stripping column.
3.3. Packed Tower
Figure 2 shows the packed tower system constructed to determine the effect of the liquid-gas contact ratio on the stripping factors. The tower was designed with a diameter of 0.2 m and a height of 1.5 m to enable observation of the physical characteristics of NH3 air stripping at the bench scale. The precise specifications are shown in Table 2. The internal packing material consisted of 18 mm PP pall rings, and the height of the packing was 0.48 m. The maximum capacity of the tank was 147 L; the effective capacity for the stripping experiment was set to 100 L. The stripping temperature was set to 70 °C, and a heater in the tank and a heat gun were used to control the stripping temperature. Air was supplied using the heat gun into the bottom of the tower and was emitted from the top of the tower. The flow rate of air was controlled using a valve and measured through a flow meter. The liquid was supplied into the top of the tower from the tank using a pump; after spraying through a nozzle, the liquid passes through the packing layer, then cycled back into the tank. The cycling rate of the liquid and the flow rate of the gas are specified along with the other operating conditions in Table 3. A temperature-adjusted pH sensor was installed inside the tank to monitor the pH and temperature, and the NaOH solution was supplied into the tank using a pump to control the pH. Ammonia stripping using the packed tower was performed at 70 °C and pH 8.5 (NH3 fraction 76.1%). To ascertain the optimal operating conditions for the tower, the liquid flow rate was fixed at 117.0 L/h, and the stripping efficiency was assessed according to the liquid-gas ratio by varying the gas flow rate.
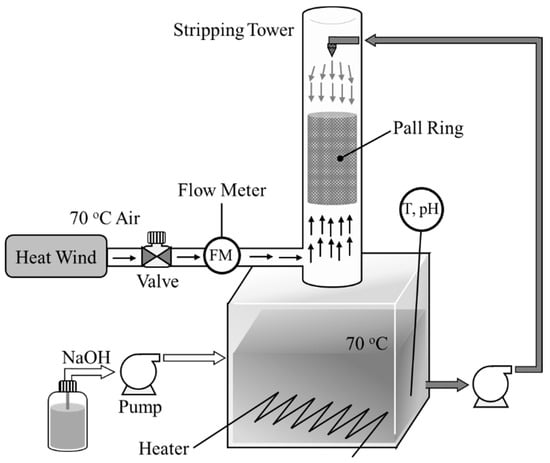
Figure 2.
Schematic view of the ammonia stripping packed tower system.

Table 2.
Design parameters of the stripping tower.

Table 3.
Experimental conditions of NH3 stripping using a packed tower.
3.4. Analysis
The ammonia concentration in the aqueous solution was analyzed to assess the stripping efficiency. Solution samples of 10 mL were taken from stripping column and packed tower tank at regular time intervals. All samples were treated with sulfonic acid to pH 2.5, and the concentrations of ammonium ions were quantified using ion chromatography (930 Compact IC Flex, Metrohm AG, Herisau, Switzerland) equipped with a cation separation column (Metrosep C 4 150/4.0, Metrohm AG, Herisau, Switzerland).
4. Results
4.1. Air Stripping without Liquid Circulation
Ammonia stripping was performed using the stripping column in Figure 1. In the column reactor, the contact surface between liquid and gas is the interface of the air bubble []. The mass transfer interfacial area is related to the size and number of air bubbles. Therefore, it is possible to compare the mass transfer coefficient in the constant conditions: total liquid volume; air flow rate; temperature []. As an experimental condition, the airflow rate was constant of 900 L/h on 1 L of artificial wastewater. The stripping efficiency and mass transfer rate of ammonia were analyzed according to initial concentration and pH. Ammonia removal efficiency and mass transfer coefficient were calculated by Equations (10), (15), and (16).
Figure 3 shows the results of stripping at different ammonium hydroxide concentrations at 20 °C and pH 9.4. Figure 3a shows the ammonia removal rate according to the concentration. The removal rate increased with increasing time. After 3 h, similar results of 38.4%, 38.5%, and 38.3% were observed for concentrations of 1500, 2200, and 2900 mg/L, respectively.
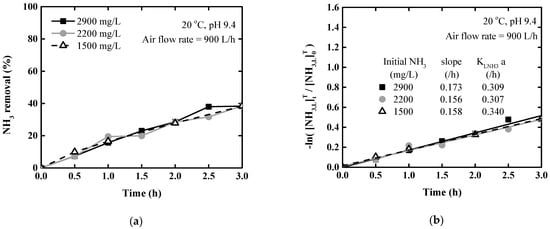
Figure 3.
Ammonia stripping performance according to initial concentration at 20 °C and pH 9.4: (a) Ammonia removal rate; (b) logarithm of the ammonia concentration ratio.
Figure 3b shows the natural logarithm of the relative change in total ammonia concentration over time. A linear regression analysis was conducted against time; the mean slope was 0.16 ± 20.011. The R2 values were 0.980, 0.973, and 0.996 for each respective concentration, indicating that the results were reliable. The overall volumetric mass transfer coefficients on the liquid phase () were calculated from the slope of Figure 3a and Equation (16). The mean was 0.319 h−1, which remained constant regardless of the initial ammonia concentration in the solution. Therefore, the ammonium hydroxide concentration had little effect on the removal efficiency or rate.
The change of pH in the ammonia solution causes a change of ammonia fraction, which means that the higher pH, the more ammonia can be removed. pH is an important parameter affecting the stripping efficiency and mass transfer coefficient.
Figure 4 represents the results of ammonia stripping according to pH at 2900 mg/L ammonia concentration and 20 °C. The ammonia removal rate was shown as a linear increase over time; after 2.5 h of stripping, the removal rate increased from 13.9% to 72.6% when pH was increased from 8.9 to 10.8. To analyze the influence of pH on ammonia removal, the ratio of removal rate to ammonia fraction ( was compared. was approximately constant for pH 9.4–10.8; The ratios were 72.0%, 71.2%, and 75.2% at pH 9.4, 10.2 and 10.8, respectively. From these results, it was confirmed that the ammonia removal rate was linearly affected by the ammonia fraction according to the pH change.
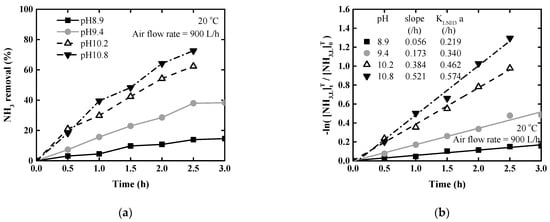
Figure 4.
Ammonia stripping performance according to pH at 20 °C: (a) Ammonia removal rate; (b) logarithm of ammonia concentration ratio.
Figure 4b shows the natural logarithm of the relative change in total ammonia concentration against time; the slopes for each pH value were obtained by linear regression (R2 = 0.997, 0.980, 0.993, 0.989). As a result, the slope and mass transfer coefficient increased proportionally with increasing pH.
The amount of NaOH which was used to adjust the pH was determined. NaOH consumption was 0.74, 1.44, 1.84, and 1.92 g of 100% NaOH/L liquid for pH 8.9, 9.4, 10.2, and 10.8, respectively, to maintain pH at 20 °C. When pH increased, the ammonia removal rate and mass transfer rate were increased, but the amount of chemical required also increased.
4.2. Packed Tower Air Stripping with Liquid Circulation
To analyze the effect of liquid-to-gas contact efficiency on the stripping tower operating factors, the stripping performance was assessed using the packed tower in Figure 2. In the packed tower, the mass transfer interfacial area is a water film on the surface of packed materials. In this study, tower design, packed layer height, and liquid flow rate were constant as shown in Table 2 and Table 3. In consequence, a relative comparison of mass transfer coefficients is possible using overall volumetric mass transfer coefficients.
Stripping was performed on 100 L of artificial wastewater at ammonia concentrations of 1100 and 3700 mg/L with the packed tower. The liquid flow rate was 117.0 L/h and the gas flow rate was 26,200 L/h. The stripping results for ammonia concentration in the packed tower were shown in Figure 5.
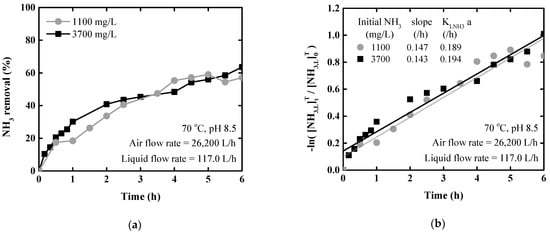
Figure 5.
Ammonia stripping performance according to ammonia concentration for the packed tower at 70 °C and pH 8.5: (a) Ammonia removal rate; (b) logarithm of ammonia concentration ratio.
Figure 5a shows the ammonia removal rate over time. After 1 h, the removal rate reached 30.1% and 18.4%, in 1100 and 3700 mg/L solutions, respectively. The reason for the difference in ammonia removal rate is that pH of the 1100 mg/L solution decrease to 8. Thereafter, NaOH was used to maintain the pH at approximately 8.5, and the ammonia removal rate showed similar trends over total stripping time.
Figure 5b shows the natural logarithm of the change in the ammonia concentration ratio. As a result of linear regression analysis against time, the R2 values were relatively low (0.923–0.957), but reliable results were obtained. The slopes were similar between the two solution concentrations and the mass transfer coefficients of liquid ammonia were also similar. At 70 °C, pH 8.5, and 4.5 L/m3 L/G ratio, the mean mass transfer coefficient was 0.192 ± 0.003 h−1. Stripping using the packed tower exhibited similar patterns regardless of the solution concentration as shown in Figure 3. Finally, the ammonia concentration of solution had little effect on the removal rate or mass transfer rate in both the stripping column and packed tower.
To identify the optimal liquid-to-gas contact range for the tower, the stripping performance was compared at different gas flow rates in the range of 4.5–107.2 L/m3. Figure 6a shows the removal rate for 1100 mg/L ammonium hydroxide solution at different L/G ratios. The ammonia removal rate tended to increase as the L/G ratio decreased. A decrease in the L/G ratio corresponds to an increase in the volume of air supplied per unit effective liquid capacity, which improves the ammonia removal efficiency [,]. However, when the L/G ratio was excessively reduced (4.5 L/m3 level here), airflow rate was too much, resulting in a reduction in ammonia removal efficiency. In Figure 6a, the removal rates after 6 h were 57.1%, 63.0%, and 57.1% for L/G of 4.5, 26.8, 53.6 L/m3, respectively. The most effective removal performance was shown at L/G 26.8 L/m3.
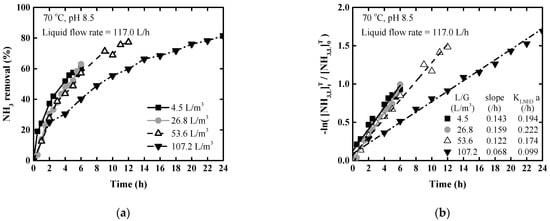
Figure 6.
Ammonia stripping performance according to the liquid-gas ratio at 70 °C and pH 8.5: (a) Ammonia removal rate; (b) logarithm of ammonia concentration ratio.
Figure 6b shows the natural logarithms of the relative concentration according to L/G ratios. Figure 6b indicates that the slope and mass transfer coefficients increased with decreasing L/G ratio, except for 4.5 L/m3. The reason the mass transfer coefficient increases with reducing L/G is that contact efficiency improves at the liquid-to-gas interface due to increased airflow rate. Base on the L/G ratio of 107.2 L/m3, when it decreases by 1/2 or 1/4, the gas flow rate increases by two or four times, and the mass transfer coefficient increased 1.4 or 1.6 times, respectively. However, when L/G reduced to 4.5 L/m3, the gas flow rate increases significantly by 24 times, whereas the mass transfer coefficient increases by 1.5 times. As a result, it was derived that the mass transfer coefficient increased according to L/G decrease but decreases below a certain level. The gas flow rate and the mass transfer coefficient are not necessarily proportional, and the optimal point should be derived.
The amount of NaOH consumed was 1.09 g of 100% NaOH/L liquid to maintain pH 8.5 at L/G 107.2 L/m3 for 24 h. When compared to the results by pH at 20 °C, the ammonia removal efficiency is excellent despite less air supply and NaOH consumption for the unit liquid.
Figure 7 shows the total air supplied against the ammonia removal rate at different L/G ratios. Here, the total air supplied refers to the total cumulative air for 1 L of artificial wastewater. To compare the total air supplied according to L/G ratio, it was revealed that the lower L/G ratio, the greater the total air consumed at the same removal rate. The total volume of air required was 240 L/L liquid at 53.6 L/m3 and 212 L/L water at 107.2 L/m3 to achieve 76.1%, where the ammonia removal rate was equal to the ammonia fraction ( = 1). At L/G ratios of 4.5–26.8 L/m3, it is predicted that a greater volume of air will be required more than 1600 and 300 L/L liquid, respectively.
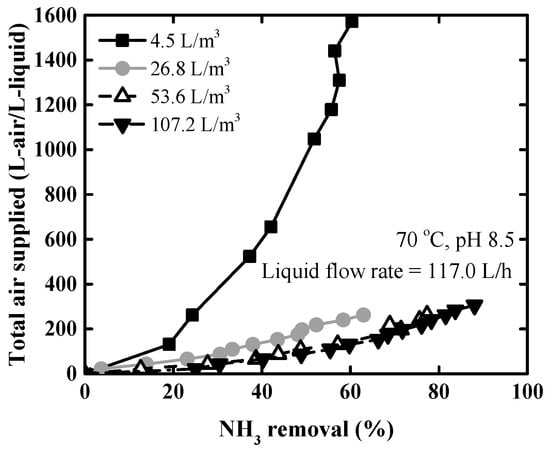
Figure 7.
Ammonia removal rate according to the volume of air supplied at 70 °C, pH 8.5, and different L/G ratios.
From Figure 6 and Figure 7, it was found that as L/G ratio increased, the mass transfer rate improved, and the more air supply was required to ensure the same ammonia removal rate. To increase the air supply, the size and power costs of the blower need to be increased. Therefore, it is necessary to determine the optimal L/G ratio considering removal efficiency, air supply, and time. In this study, the most efficient L/G condition was 26.8 L/m3, and 53.6 L/m3 also seemed to be appropriate when trying to lower the total air supply. In consequence, a liquid-gas ratio of 26.8–53.6 L/m3 was selected as an optimal condition for packed tower operating at 70 °C, pH 8.5.
4.3. The Comparison of Results with Literature
Table 4 shows the comparison of results for ammonia stripping system with the literature. First, the stripping column results according to pH were compared. In this study, the ammonia removal efficiency increased in proportion to ammonia fraction as the pH increased. Zhu et al. [] reported that an increase in pH increase leads to improve ammonia stripping rate and mass transfer rate. When ammonia fraction and air supply are similar, the removal rate and mass transfer coefficients are also similar to those derived from literature [].

Table 4.
The comparison of removal rate and overall volumetric mass transfer coefficient based on the liquid phase of ammonia.
Secondly, packed tower results were compared. It is difficult to compare the mass transfer coefficient arithmetically because the interfacial area for the packed tower varies depending on the design of the tower and the packed material. However, it is possible to compare the approximate mass transfer rates by considering the ammonia fraction, air supply, and liquid ratio.
Liu et al. [] derived the mass transfer coefficient according to air flow rate. Although there is no mention of the L/G ratio, it can be interpreted that the lower L/G, the higher , since the L/G decreases as the air flow rate increases. When compared with this study, it was confirmed that was almost same under similar air supply conditions. It is considered that the calculated values are within a reasonable range when compared with the reports by Liu et al. [] and Ferraz et al. []. Li et al. [] reported the highest value of values under very low L/G ratio when compared to other literatures.
In addition, ammonia removal rate was also compared for the jet loop reactor and the water-sparged aerocyclone. In these two reactors, as in the column and packed tower, the mass transfer coefficient increased as the liquid to gas ratio decreased.
5. Conclusions
Stripping experiments were performed using an air stripping column and a packed tower on an ammonium hydroxide solution to calculate the major operating factors for each device. For ammonia stripping with a column, an increase in pH resulted in higher recovery efficiency and a larger mass transfer coefficient, and a proportional relationship was observed between ammonia removal efficiency and ammonia fraction. However, ammonia concentration did not affect ammonia removal rate or the mass transfer coefficient. For the packed tower, the operating factors were analyzed at 70 °C, pH 8.5. As the liquid-gas ratio decreased in the range of 26.8–107.2 L/m3, the mass transfer rate and air consumption increased. The 26.8 L/m3 condition achieved the largest mass transfer coefficient and 63.0% of ammonia removal rate by only 6 h operation with 262 L-air/L-liquid. And at L/G of 53.6 L/m3, ammonia removal was 77.3% for 12 h with same air consumption. Therefore, the optimal liquid-gas ratio was chosen as 26.8–53.6 L/m3, by considering the optimal mass transfer coefficient and air supply. In addition, through high-temperature stripping, excellent ammonia removal rate was achieved with low NaOH consumption. Finally, overall operating conditions such as temperature, pH, and air consumption were optimized to ensure both performance and economics for ammonia stripping devices design.
Author Contributions
Conceptualization, E.J.K.; writing—original draft preparation, E.J.K.; funding acquisition, H.K.; project administration, H.K.; writing—review and editing, E.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Korea Agency for Infrastructure Technology Advancement (KAIA) grant funded by the Ministry of Land, Infrastructure and Transport (Grant 21UGCP-B157945-02).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available in a publicly accessible repository.
Acknowledgments
This subject was supported by the Korea Agency for Infrastructure Technology Advancement (KAIA) grant funded by the Ministry of Land, Infrastructure and Transport (Grant 21UGCP-B157945-02).
Conflicts of Interest
There are no conflict of interest to declare.
References
- Bousek, J.; Scroccaro, D.; Sima, J.; Weissenbacher, N.; Fuchs, W. Influence of the gas composition on the efficiency of ammonia stripping. Bioresour. Technol. 2016, 203, 259–266. [Google Scholar] [CrossRef]
- Wang, W.; Ding, Y.; Wang, Y.; Song, X.; Ambrose, R.F.; Ullman, J.L.; Winfrey, B.K.; Wang, J.; Gong, J. Treatment of rich ammonia nitrogen wastewater with polyvinyl alcohol immobilized nitrifier biofortified constructed wetlands. Ecol. Eng. 2016, 94, 7–11. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, Q. Research on the transformation of nitrogen during hydrothermal carbonization of sludge. MATEC Web Conf. 2018, 175, 1–3. [Google Scholar] [CrossRef][Green Version]
- Panequea, M.; Rosa, D.L.J.M.; Kern, J.; Reza, M.T.; Knicker, H. Hydrothermal carbonization and pyrolysis of sewage sludges: What happen to carbon and nitrogen? J. Anal. Appl. Pyrolysis 2017, 128, 314–323. [Google Scholar] [CrossRef]
- Vecino, X.; Reig, M.; Bhushan, B.; Gibert, O.; Valderrama, C.; Cortina, J.L. Liquid fertilizer production by ammonia recovery from treated ammonia-rich regenerated streams using liquid-liquid membrane contactors. Chem. Eng. J. 2019, 360, 890–899. [Google Scholar] [CrossRef]
- Bonmatí, A.; Flotats, X. Air stripping of ammonia from pig slurry: Characterisation and feasibility as a pre- or post-treatment to mesophilic anaerobic digestion. Waste Manag. 2003, 23, 261–272. [Google Scholar] [CrossRef]
- Vaddella, V.K.; Ndegwa, P.M.; Ullman, J.L.; Jiang, A. Mass transfer coefficients of ammonia for liquid dairy manure. Atmos. Environ. 2013, 66, 107–113. [Google Scholar] [CrossRef]
- Jia, D.; Lua, W.; Zhang, Y. Research on mechanism of air stripping enabled ammonia removal from Industrial wastewater and Its application. Chem. Eng. Trans. 2017, 62, 115–120. [Google Scholar] [CrossRef]
- Zeng, L.; Mangan, C.; Li, X. Ammonia recovery from anaerobically digested cattle manure by steam stripping. Water Sci. Technol. 2006, 54, 137–145. [Google Scholar] [CrossRef]
- Kinidi, L.; Tan, I.A.W.; Wahab, N.B.A.; Tamrin, K.F.B.; Hipolito, C.N.; Salleh, S.F. Recent Development in ammonia stripping process for industrial wastewater treatment. Int. J. Chem. Eng. 2018, 1–14. [Google Scholar] [CrossRef]
- Viotti, P.; Gavasci, R. Scaling of ammonia stripping towers in the treatment of groundwater polluted by municipal solid waste landfill leachate: Study of the causes of scaling and its effects on stripping performance. Rev. Ambiente Agua 2015, 10, 241–252. [Google Scholar] [CrossRef]
- Ferraz, F.M.; Povinelli, J.; Vieira, E.M. Ammonia removal from landfill leachate by air stripping and absorption. Environ. Technol. 2013, 34, 2317–2326. [Google Scholar] [CrossRef]
- Guštin, S.; Marinšek-Logar, R. Effect of pH, temperature and air flow rate on the continuous ammonia stripping of the anaerobic digestion effluent. Process Saf. Environ. Prot. 2011, 89, 61–65. [Google Scholar] [CrossRef]
- Liu, B.; Giannis, A.; Zhang, J.; Chang, V.W.C.; Wang, J.Y. Air stripping process for ammonia recovery from source-separated urine: Modeling and optimization. J. Chem. Technol. Biotechnol. 2015, 90, 2208–2217. [Google Scholar] [CrossRef]
- Zhu, L.; Dong, D.M.; Hua, X.Y.; Xu, Y.; Guo, Z.Y. Ammonia nitrogen removal and recovery from acetylene purification wastewater by air stripping. Water Sci. Technol. 2017, 75, 2538–2542. [Google Scholar] [CrossRef]
- Li, L.; Wang, H.W.; Lu, J.H. Nitrogen removal using air stripping tower in urban wastewater treatment plant. China Water Wastewater 2006, 22, 92–95. [Google Scholar]
- Jiang, A.; Zhang, T.; Zhao, Q.B.; Li, X.; Chen, S.; Frear, C.S. Evaluation of an integrated ammonia stripping, recovery, and biogas scrubbing system for use with anaerobically digested dairy manure. Biosyst. Eng. 2014, 119, 117–126. [Google Scholar] [CrossRef]
- Escala, M.; Zumbuhl, T.; Koller, C.H.; Junge, R.; Krebs, R. Hydrothermal Carbonization as an Energy-Efficient Alternative to Established Drying Technologies for Sewage Sludge: A Feasibility Study on a Laboratory Scale. Energy Fuels 2013, 27, 454–460. [Google Scholar] [CrossRef]
- Wang, T.F.; Zhai, Y.; Zhu, Y.; Peng, C.; Xu, B.; Wang, T.; Li, C.; Zeng, G. Influence of temperature on nitrogen fate during hydrothermal carbonization of food waste. Bioresour. Technol. 2018, 247, 182–189. [Google Scholar] [CrossRef]
- Fritz, U.; Matthias, B. Ullmann’s Encyclopedia of Industrial Chemistry, 7th ed.; Willey VCH: Weinheim, Germany, 2011. [Google Scholar]
- Matter-Müller, C.; Gujer, W.; Giger, W. Transfer of volatile substances from water to the atmosphere. Water Res. 1981, 15, 1271–1279. [Google Scholar] [CrossRef]
- Quan, X.; Wang, F.; Zhao, Q.; Zhao, T.; Xiang, J. Air stripping of ammonia in a water-sparged aerocyclone reactor. J. Hazard. Mater. 2009, 170, 983–988. [Google Scholar] [CrossRef]
- Agnieszka, U.; Małgorzata, K.K.; Mateusz, W.; Przemysław, S.; Marcin, B.; Halina, P.K.; Monika, S.T.; Krystian, K.; Lukasz, N. Treatment of liquid by-products of hydrothermal carbonization (HTC) of agricultural digestate using membrane separation. Energies 2020, 13, 262. [Google Scholar] [CrossRef]
- Degermenci, N.; Ata, O.N.; Yildız, E. Ammonia removal by air stripping in a semi-batch jet loop reactor. J. Ind. Eng. Chem. 2012, 18, 399–404. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).