Rabbit Genetic Resources Can Provide Several Animal Models to Explain at the Genetic Level the Diversity of Morphological and Physiological Relevant Traits
Abstract
1. Introduction
2. The Domestication Process of the Rabbit and the Origin of the Genetic Diversity in this Species
3. Molecular Characterization of Coat Color Affecting Genes in Rabbits
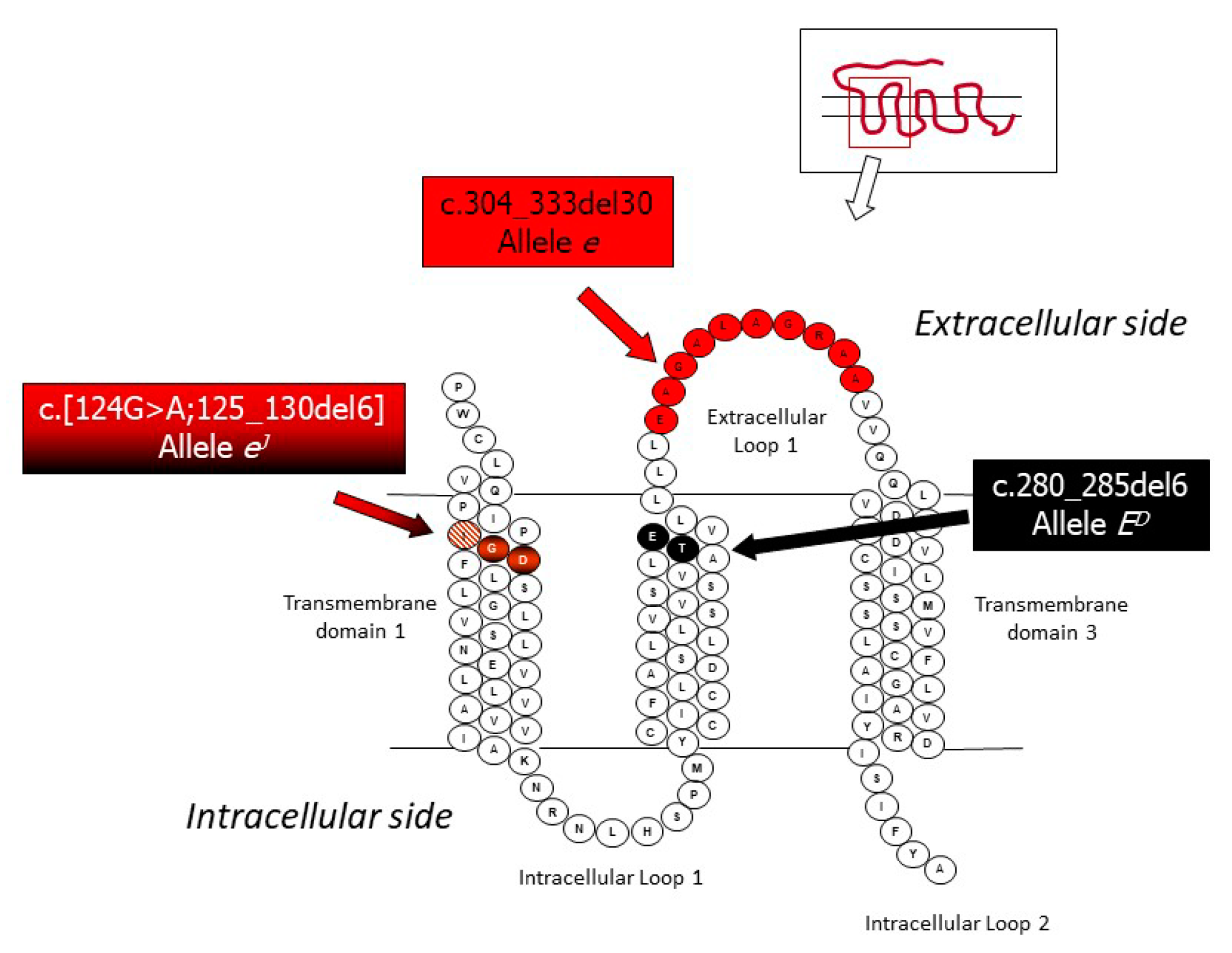
3.1. Mutations in the Melanocortin 1 Receptor (MC1R) Gene and the Extension Locus
3.2. Mutations in the Agouti Signaling Protein (ASIP) Gene and the Agouti Locus
3.3. Mutations in the Tyrosinase (TYR) Gene and the Albino Locus
3.4. Mutations in the Melanophilin (MLPH) Gene and the Dilute Locus
3.5. A Mutation in the Tyrosinase-Related Protein 1 (TYRP1) Gene and the Brown Locus
3.6. The KIT Gene is Responsible for the English Spotting Locus and the Megacolon Defect in the Checkered Giant Breed
4. Molecular Characterization of Genes Affecting Hair and Coat Structure as Models for Hair Growth and Development
4.1. A Mutation in the Lipase Member H (LIPH) Gene Causes the Rex Locus R1
4.2. A Marker in the Fibroblast Growth Factor 5 (FGF5) Gene is Associated to the Angora Locus
5. Molecular Characterization of Other Loci Determining Breed and Strain Specific or Un-Specific Phenotypes
5.1. A Structural Mutation in the High-Mobility Group AT-hook 2 (HMGA2) Gene Determines a Dwarf Locus
5.2. A Mutation in the RAR Related Orphan Receptor B (RORB) Gene and the Acrobat Locus
5.3. Molecular Characterization of a Biochemical Defect: The Yellow Fat Locus
| Locus | Gene Name | Gene Symbol | Alleles 1 | Sequences/Mutations 2 | Defects/Models 5 | References 6 |
|---|---|---|---|---|---|---|
| Extension | Melanocortin 1 receptor | MC1R | E+ | Several wild type alleles 3 | - | [41] |
| ED (dominant black) | 6 bp-in-frame deletion: c.280_285del6 | - | [41] | |||
| ES (steel) | Probably due to the same mutation of ED | - | [41] | |||
| eJ (Japanese brindling) | 6 bp-in frame deletion flanked by a G > A transition in 5’: c.[124G > A;125_130del6] | Potential model for gene expression regulation | [42] | |||
| e (red, non-extension of black) | 30 bp-in frame deletion: c.304_333del30 | - | [41] | |||
| Agouti | Agouti signaling protein | ASIP | A (light-bellied agouti; wild type) | Several wild type alleles 3,4 | - | [60] |
| at (black and tan) | p.L55M, p.K77R and p.L89P/11 kb deletion spanning the promoter and first exon | Potential model for gene expression regulation | [60,61] | |||
| a (recessive black non-agouti) | c.5_6insA | - | [60] | |||
| Albino | Tyrosinase | TYR | C (normal melanin production) | Several wild type alleles 3,4 | - | [63,70] |
| cchd (dark chinchilla) | p.E294G and p.T358I | - | [63,70] | |||
| cchm (medium chinchilla) | Not confirmed by molecular studies | - | - | |||
| cchl (light chinchilla) | Not confirmed by molecular studies | - | - | |||
| ch (Himalayan) | p.E294G (several haplotypes with this mutation) | - | [63,70] | |||
| c (Albino, lack of pigments) | p.T373K (several haplotypes with this mutation) | Potential model for oculocutaneous albinism type IA (OCA1A) | [63,70] | |||
| Dilute | Melanophilin | MLPH | D (wild type, intense black and red) | Several wild type alleles 3,4 | - | [74] |
| d (dilution of black to blue and red to yellow) | c.585delG (g.549853delG)—Two exon skipping mutation: c.111-5C > A | Potential model for Griscelli syndrome type 3 | [74,75,76] | |||
| Brown | Tyrosinase-related protein 1 | TYRP1 | B (wild type, production of black and brown eumelanin) | Several wild type alleles 3 | - | [80] |
| b (production of brown eumelanin) | p.W190ter (g.41360196G > A) | - | [80] | |||
| English spotting | v-kit Hardy-Zuckerman 4 feline sarcoma viral oncogene homolog | KIT | en (wild type, non-spotted coat colour, recessive) | Several wild type alleles 3,4 | - | [87] |
| En (English spotted, spotted patterns, partially dominant, megacolon) | g.93948587T > C (in complete linkage disequilibrium with the En allele) | Potential model for non-aganglionic megacolon | [87] | |||
| Rex 1 | Lipase member H | LIPH | R1 (wild type, normal presence of guard and awn hairs) | Several wild type alleles | - | [98] |
| r1 (absence of guard and awn hairs, fur with down hairs | c.1362delA | Potential model for hypothricosis and related defects | [98] | |||
| Angora | Fibroblast growth factor 5 | FGF5 | L (wild type, normal length of guard hairs) | Several wild type alleles (haplotypes) 3,4 | - | [110] |
| l (long hairs) | A missense mutation in exon 3 in linkage disequilibrium | Potential mode for hair growth regulation | [110] | |||
| Dwarf | High-mobility group AT-hook 2 | HMGA2 | Dw (normal size) | - | - | [122] |
| dw (proportionated dwarf-reduced size) | 12.1 kb deletion (including promoter and the first three exons) | Potential model to study growth and body size | [122] | |||
| Acrobat | RAR related orphan receptor B | RORB | Ak (normal locomotion) | - | - | [127] |
| ak or Sam (walks on forelegs) | a splice-site mutation in an evolutionary conserved nucleotide position | Potential model for neuronal differentiation and locomotion behavior | [127] | |||
| Yellow fat | Beta-carotene oxygenase 2 | BCO2 | Y (wild type, normal color of fat) | Wild type sequence | - | [134] |
| y (yellow fat) | AAT-deletion at codon 248 | Potential model for carotenoid catabolism | [134] |
| Breeds 1 | Coat Colour of the Animals 2 | MC1R 3 | ASIP 4 | TYR 5 | MLPH 6 | TYRP1 7 | KIT 8 |
|---|---|---|---|---|---|---|---|
| Alaska | self black | wt/wt | a/a | wt/wt | wt/wt | wt/wt | - |
| Belgian Hare | reddish laced with black | wt/wt | (wt/wt: haplotype p77R + p.89P) | wt/wt | wt/wt | wt/wt | - |
| Blanc de Hotot | white with black markings | wt/wt | a/a | wt/wt | wt/wt | wt/wt | - |
| Burgundy Fawn | fawn | e/e | wt/wt | wt/wt | wt/wt | wt/wt | - |
| Californian | white with black markings | ED/ED | wt/wt, wt/a, a/a | ch/ch | wt/wt, wt/d | wt/wt | T/T, T/C, C/C |
| Californian | white with blue markings[d/d] | ED/ED | wt/wt, wt/a, a/a | ch/ch | d/d | wt/wt | T/T, T/C, C/C |
| Champagne d’Argent | silver as surface colour and black as under-colour | wt/wt | a/a | wt/wt | wt/wt | wt/w | C/C |
| Checkered Giant | white with black spots | ED/ED, ED/eJ | wt/a, a/a | wt/wt | wt/wt, wt/d | wt/wt | T/C |
| Checkered Giant | white with blue spots | ED/ED | wt/a, a/a | wt/wt | d/d | wt/wt | T/C |
| Dutch | with black markings | wt/wt, wt/ED, ED/ED | a/a | wt/wt | wt/wt | wt/wt | T/T, T/C, C/C |
| Dutch | tricolor | eJ/eJ | a/a | wt/wt | wt/wt | - | - |
| English Lop | shaded yellow/brown | e/e | - | - | - | - | - |
| English Spot | white with black markings | wt/wt | a/a | wt/wt | wt/wt | wt/wt | C/C |
| English Spot | white with blue markings | wt/wt | a/a | wt/wt | d/d | wt/wt | C/C |
| Fairy Marburg | grey-light blue | wt/wt | a/a | - | d/d | - | - |
| Fairy Pearly | pearling grey | wt/wt | wt/a, a/a | - | d/d | - | - |
| Fox | dark blue | wt/wt | - | - | d/d | - | - |
| Giant Chinchilla | chinchilla | wt/wt | wt/wt | cch/cch | wt/wt | wt/wt | T/C, C/C |
| Giant Grey | wild-grey | wt/wt, wt/eJ | wt/wt, wt/a | wt/wt | wt/wt | wt/wt | T/T, T/C- |
| Giant White | white albino | ED/ED | wt/wt | c/c | wt/wt | wt/wt | T/T |
| Gold Saxony | red | e/e | - | - | - | wt/wt | - |
| Havana | brown | wt/wt | a/a | wt/wt | wt/wt | b/b | - |
| Japanese | Japanese brindling | eJ/eJ | - | - | - | wt/wt | C/C |
| Leprino di Viterbo | wild-grey | wt/wt | - | wt/wt | - | wt/wt | - |
| Lop | wild-grey | wt/wt | wt/wt, wt/a, a/a | wt/wt | wt/wt | wt/wt | - |
| New Zealand Red | solid red | e/e | wt/wt | wt/wt | - | wt/wt | - |
| New Zealand White | white-albino | ED/ED | wt/wt, wt/a, a/a | c/c | wt/wt | wt/wt | C/C |
| Rhinelander | white with black and yellow markings | eJ/eJ | wt/wt, wt/a, a/a | wt/wt | wt/wt | wt/wt | T/C |
| Russian | white with black markings | wt/wt | a/a | cch/cch | wt/wt | - | - |
| Silver | black with silvering | wt/wt | a/a | wt/wt | wt/wt | wt/wt | - |
| Tan | black fire | wt/wt | at/at | - | wt/wt | - | - |
| Thuringian | shaded yellow/brown | e/e | a/a | wt/wt | wt/wt | wt/wt | - |
| Vienna Blue | dark blue | wt/wt | a/a | wt/wt | d/d | wt/wt | - |
| Vienna White | white-blue eyes | wt/wt | wt/a, a/a | wt/wt | wt/wt | wt/wt | - |
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Fontanesi, L. The rabbit in the genomics era: Applications and perspectives in rabbit biology and breeding. In Proceedings of the 11th World Rabbit Congress, Qingdao, China, 15–18 June 2016. [Google Scholar]
- Shiomi, M. Rabbit as Model for the Study of Human Diseases. In Rabbit Biotechnology: Rabbit Genomics, Transgenesis, Cloning and Models; Houdebine, L.-M., Fan, J., Eds.; Springer: Dordrecht, Germany, 2009; pp. 49–63. [Google Scholar] [CrossRef]
- Esteves, P.J.; Abrantes, J.; Baldauf, H.M.; BenMohamed, L.; Chen, Y.; Christensen, N.; González-Gallego, J.; Giacani, L.; Hu, J.; Kaplan, G.; et al. The wide utility of rabbits as models of human diseases. Exp. Mol. Med. 2018, 50, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mage, R.G.; Esteves, P.J.; Rader, C. Rabbit models of human diseases for diagnostics and therapeutics development. Dev. Comp. Immunol. 2019, 92, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.; Peng, H.; Rader, C. From rabbit antibody repertoires to rabbit monoclonal antibodies. Exp. Mol. Med. 2017, 49, e305. [Google Scholar] [CrossRef] [PubMed]
- Thompson, H.V.; King, C.M. The European Rabbit: The History and Biology of a Successful Colonizer; Oxford University Press: Oxford, UK, 1994; pp. 1–245. [Google Scholar]
- Ennafaa, H.; Monnerot, M.; El Gaaïed, A.; Mounolou, J.C. Rabbit mitochondrial DNA: Preliminary comparison between some domestic and wild animals. Genet. Sel. Evol. 1987, 19, 279–288. [Google Scholar] [CrossRef]
- Biju-Duval, C.; Ennafaa, H.; Dennebouy, N.; Monnerot, M.; Mignotte, F.; Soriguer, R.; El Gaieed, A.; El Hili, A.; Mounolou, J.-C. Mitochondrial DNA evolution in lagomorphs: Origin of systematic heteroplasmy and organization of diversity in European rabbits. J. Mol. Evol. 1991, 33, 92–102. [Google Scholar] [CrossRef]
- Monnerot, M.; Vigne, J.D.; Biju-Duval, C.; Casane, D.; Callou, C.; Hardy, C.; Mougel, F.; Soriguer, R.; Dennebouy, N.; Mounolou, J.-C. Rabbit and man: Genetic and historic approach. Genet. Sel. Evol. 1994, 26 (Suppl. 1), 167S–182S. [Google Scholar] [CrossRef]
- van der Loo, W.; Ferrand, N.; Soriguer, R. Estimation of gene diversity at the b locus of the constant region of the immunoglobulin light chain in natural populations of European rabbit (Oryctolagus cuniculus) in Portugal, Andalusia and on the Azorean islands. Genetics 1991, 127, 789–799. [Google Scholar]
- Sharples, C.M.; Fa, J.E.; Bell, D.J. Geographical variation in size in the European rabbit Oryctolagus cuniculus (Lagomorpha: Leporidae) in western Europe and North Africa. Zool. J. Linnean Soc. 1996, 117, 141–158. [Google Scholar] [CrossRef]
- Branco, M.; Ferrand, N.; Monnerot, M. Phylogeography of the European rabbit (Oryctolagus cuniculus) on the Iberian Peninsula inferred from RFLP analysis of the cytochrome b gene. Heredity 2000, 85, 307–317. [Google Scholar] [CrossRef]
- Branco, M.; Monnerot, M.; Ferrand, N.; Templeton, A.R. Postglacial dispersal of the European rabbit (Oryctolagus cuniculus) on the Iberian Peninsula reconstructed from nested clade and mismatch analyses of mitochondrial DNA genetic variation. Evolution 2002, 56, 792–803. [Google Scholar] [CrossRef]
- Ferrand, N. Inferring the evolutionary history of the European rabbit (Oryctolagus cuniculus) from molecular markers. In Lagomorph Biology: Evolution, Ecology, and Conservation; Alves, P.C., Ferrand, N., Hackländer, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 47–63. [Google Scholar] [CrossRef]
- Carneiro, M.; Rubin, C.J.; Di Palma, F.; Albert, F.W.; Alföldi, J.; Barrio, A.M.; Pielberg, G.; Rafati, N.; Sayyab, S.; Turner-Maier, J.; et al. Rabbit genome analysis reveals a polygenic basis for phenotypic change during domestication. Science 2014, 345, 1074–1079. [Google Scholar] [CrossRef] [PubMed]
- Alves, J.M.; Carneiro, M.; Afonso, S.; Lopes, S.; Garreau, H.; Boucher, S.; Allain, D.; Queney, G.; Esteves, P.J.; Bolet, G.; et al. Levels and patterns of genetic diversity and population structure in domestic rabbits. PLoS ONE 2015, 10, e0144687. [Google Scholar] [CrossRef] [PubMed]
- Callou, C. Modifications de l’aire de répartition du lapin (Oryctolagus cuniculus) en France et en Espagne, du Pléistocène à l’époque actuelle. Etat de la question. Anthropozoologica 1995, 21, 95–114. [Google Scholar]
- Callou, C. De la garenne au clapier. Étude archéozoologique du Lapin en Europe occidentale; Mémoires do Muséum National d’Histoire Naturelle: Paris, France, 2003. [Google Scholar]
- Zeder, M.A. Central questions in the domestication of plants and animals. Evol. Anthropol. 2006, 15, 105–117. [Google Scholar] [CrossRef]
- Zeder, M.A. The domestication of animals. J. Anthropol. Res. 2012, 68, 161–190. [Google Scholar] [CrossRef]
- Larson, G.; Burger, J. A population genetics view of animal domestication. Trends Genet. 2013, 29, 197–205. [Google Scholar] [CrossRef]
- Irving-Pease, E.K.; Frantz, L.A.; Sykes, N.; Callou, C.; Larson, G. Rabbits and the specious origins of domestication. Trends Ecol. Evol. 2018, 33, 149–152. [Google Scholar] [CrossRef]
- Hardy, C.; Callou, C.; Vigne, J.-D.; Casane, D.; Dennebouy, N.; Mounolou, J.-C.; Monnerot, M. Rabbit mitochondrial DNA diversity from prehistoric to modern times. J. Mol. Evol. 1995, 40, 227–237. [Google Scholar] [CrossRef]
- Queney, G.; Vachot, A.M.; Brun, J.M.; Dennebouy, N.; Mulsant, P.; Monnerot, M. Different levels of human intervention in domestic rabbits: Effects on genetic diversity. J. Hered. 2002, 93, 205–209. [Google Scholar] [CrossRef][Green Version]
- Carneiro, M.; Afonso, S.; Geraldes, A.; Garreau, H.; Bolet, G.; Boucher, S.; Tircazes, A.; Queney, G.; Nachman, M.W.; Ferrand, N. The genetic structure of domestic rabbits. Mol. Biol. Evol. 2011, 28, 1801–1816. [Google Scholar] [CrossRef]
- Zeuner, F.E. A History of Domesticated Animals; Harper & Row Publishers: Evanston, UK, 1963; pp. 409–415. [Google Scholar]
- Carneiro, M.; Piorno, V.; Rubin, C.J.; Alves, J.M.; Ferrand, N.; Alves, P.C.; Andersson, L. Candidate genes underlying heritable differences in reproductive seasonality between wild and domestic rabbits. Anim. Genet. 2015, 46, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Brusini, I.; Carneiro, M.; Wang, C.; Rubin, C.J.; Ring, H.; Afonso, S.; Blanco-Aguiar, J.A.; Ferrand, N.; Rafati, N.; Villafuerte, R.; et al. Changes in brain architecture are consistent with altered fear processing in domestic rabbits. Proc. Natl. Acad. Sci. USA 2018, 115, 7380–7385. [Google Scholar] [CrossRef] [PubMed]
- Whitman, B.D. Domestic Rabbits & Their Histories: Breeds of the World; Leathers Publishing: Overland Parks, KS, USA, 2004; pp. 1–456. [Google Scholar]
- Robinson, R. Genetic studies of the rabbit. Bibl. Genet. 1958, 17, 229–558. [Google Scholar]
- Searle, A.G. Comparative Genetics of Coat Colour in Mammals; Logos Press: London, UK, 1968; pp. 126–137. [Google Scholar]
- Robbins, L.S.; Nadeau, J.H.; Johnson, K.R.; Kelly, M.A.; Roselli-Rehfuss, L.; Baack, E.; Mountjoy, K.G.; Cone, R.D. Pigmentation phenotypes of variant extension locus alleles result from point mutations that alter MSH receptor function. Cell 1993, 72, 827–834. [Google Scholar] [CrossRef]
- Klungland, H.; Våge, D.I.; Gomez-Raya, L.; Adalsteinsson, S.; Lien, S. The role of melanocyte-stimulating hormone (MSH) receptor in bovine coat color determination. Mamm. Genome 1995, 6, 636–639. [Google Scholar] [CrossRef] [PubMed]
- Kijas, J.M.; Wales, R.; Törnsten, A.; Chardon, P.; Moller, M.; Andersson, L. Melanocortin receptor 1 (MC1R) mutations and coat color in pigs. Genetics 1998, 150, 1177–1185. [Google Scholar]
- Fontanesi, L.; Beretti, F.; Riggio, V.; Dall’Olio, S.; González, E.G.; Finocchiaro, R.; Davoli, R.; Russo, V.; Portolano, B. Missense and nonsense mutations in melanocortin 1 receptor (MC1R) gene of different goat breeds: Association with red and black coat colour phenotypes but with unexpected evidences. BMC Genet. 2009, 10, 47. [Google Scholar] [CrossRef]
- Fontanesi, L.; Beretti, F.; Riggio, V.; Dall’Olio, S.; Calascibetta, D.; Russo, V.; Portolano, B. Sequence characterization of the melanocortin 1 receptor (MC1R) gene in sheep with different coat colours and identification of the putative e allele at the ovine Extension locus. Small Rum. Res. 2010, 91, 200–207. [Google Scholar] [CrossRef]
- Punnett, R.C. Inheritance of coat colour in rabbits. J. Genet. 1912, 2, 221–238. [Google Scholar] [CrossRef]
- Punnett, R.C. On the “Japanese” rabbit. J. Genet. 1924, 14, 230–240. [Google Scholar] [CrossRef]
- Punnett, R.C. On the series of allelomorphs connected with the production of black pigment in rabbit. J. Genet. 1930, 23, 265–274. [Google Scholar] [CrossRef]
- Castle, W.E. Genetics of the Japanese rabbit. J. Genet. 1924, 14, 225–229. [Google Scholar] [CrossRef]
- Fontanesi, L.; Tazzoli, M.; Beretti, F.; Russo, V. Mutations in the melanocortin 1 receptor (MC1R) gene are associated with coat colours in the domestic rabbit (Oryctolagus cuniculus). Anim. Genet. 2006, 37, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Fontanesi, L.; Scotti, E.; Colombo, M.; Beretti, F.; Forestier, L.; Dall’Olio, S.; Deretz, S.; Russo, V.; Allain, D.; Oulmouden, A. A composite six bp in-frame deletion in the melanocortin 1 receptor (MC1R) gene is associated with the Japanese brindling coat colour in rabbits (Oryctolagus cuniculus). BMC Genet. 2010, 11, 59. [Google Scholar] [CrossRef] [PubMed]
- Newton, J.M.; Wilkie, A.L.; He, L.; Jordan, S.A.; Metallinos, D.L.; Holmes, N.G.; Jackson, I.J.; Barsh, G.S. Melanocortin 1 receptor variation in the domestic dog. Mamm. Genome 2000, 11, 24–30. [Google Scholar] [CrossRef]
- Everts, R.E.; Rothuizen, J.; Van Oost, B.A. Identification of a premature stop codon in the melanocyte-stimulating hormone receptor gene (MC1R) in Labrador and Golden retrievers with yellow coat colour. Anim. Genet. 2000, 31, 194–199. [Google Scholar] [CrossRef]
- Våge, D.I.; Klungland, H.; Lu, D.; Cone, R.D. Molecular and pharmacological characterization of dominant black coat color in sheep. Mamm. Genome 1999, 10, 39–43. [Google Scholar] [CrossRef]
- Marklund, L.; Moller, M.; Sandberg, K.; Andersson, L. A missense mutation in the gene for melanocyte-stimulating hormone receptor (MC1R) is associated with the chestnut coat color in horses. Mamm. Genome 1996, 7, 895–899. [Google Scholar] [CrossRef]
- Abitbol, M.; Legrand, R.; Tiret, L. A missense mutation in melanocortin 1 receptor is associated with the red coat colour in donkeys. Anim. Genet. 2014, 45, 878–880. [Google Scholar] [CrossRef]
- Kerje, S.; Lind, J.; Schütz, K.; Jensen, P.; Andersson, L. Melanocortin 1-receptor (MC1R) mutations are associated with plumage colour in chicken. Anim. Genet. 2003, 34, 241–248. [Google Scholar] [CrossRef]
- Gustafson, N.A.; Gandolfi, B.; Lyons, L.A. Not another type of potato: MC1R and the russet coloration of Burmese cats. Anim. Genet. 2017, 48, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Abitbol, M.; Gache, V. Copal, a new MC1R allele in the domestic cat. Anim. Genet. 2019, 50, 553–554. [Google Scholar] [CrossRef] [PubMed]
- Bultman, S.J.; Michaud, E.J.; Woychik, R.P. Molecular characterization of the mouse agouti locus. Cell 1992, 71, 1195–1204. [Google Scholar] [CrossRef]
- Lu, D.; Willard, D.; Patel, I.R.; Kadwell, S.; Overton, L.; Kost, T.; Luther, M.; Chen, W.; Woychik, R.P.; Wilkison, W.O.; et al. Agouti protein is an antagonist of the melanocyte-stimulating-hormone receptor. Nature 1994, 371, 799–802. [Google Scholar] [CrossRef] [PubMed]
- Ollmann, M.M.; Lamoreux, M.L.; Wilson, B.D.; Barsh, G.S. Interaction of Agouti protein with the melanocortin 1 receptor in vitro and in vivo. Genes Dev. 1998, 12, 316–330. [Google Scholar] [CrossRef]
- Kuramoto, T.; Nomato, T.; Sugimura, T.; Ushijima, T. Cloning of the rat agouti gene and identification of the rat nonagouti mutation. Mamm. Genome 2001, 12, 469–471. [Google Scholar] [CrossRef]
- Miltenberger, R.J.; Wakumatsu, K.; Ito, S.; Woychik, R.P.; Russell, L.B.; Michaud, E.J. Molecular and phenotypic analysis of 25 recessive, homozygous-viable alleles at the mouse agouti locus. Genetics 2002, 160, 659–674. [Google Scholar]
- Kerns, J.A.; Newton, J.; Berryere, T.G.; Rubin, E.M.; Cheng, J.F.; Schmutz, S.M.; Barsh, G.S. Characterization of the dog Agouti gene and a nonagouti mutation in German Shepherd dogs. Mamm. Genome 2004, 15, 798–808. [Google Scholar] [CrossRef]
- Norris, B.J.; Whan, V.A. A gene duplication affecting expression of the ovine ASIP gene is responsible for white and black sheep. Genome Res. 2008, 18, 1282–1293. [Google Scholar] [CrossRef]
- Fontanesi, L.; Dall’Olio, S.; Beretti, F.; Portolano, B.; Russo, V. Coat colours in the Massese sheep breed are associated with mutations in the agouti signalling protein (ASIP) and melanocortin 1 receptor (MC1R) genes. Animal 2011, 5, 8–17. [Google Scholar] [CrossRef]
- Silvers, W.K. The agouti and extension series of alleles, umbrous, and sable. In The Coat Colors of Mice: A Model for Mammalian Gene Action and Interaction; Silver, W.K., Ed.; Springer: New York, NY, USA, 1979; pp. 6–44. [Google Scholar]
- Fontanesi, L.; Forestier, L.; Allain, D.; Scotti, E.; Beretti, F.; Deretz-Picoulet, S.; Pecchioli, E.; Vernesi, C.; Robinson, T.J.; Malaney, J.L.; et al. Characterization of the rabbit agouti signaling protein (ASIP) gene: Transcripts and phylogenetic analyses and identification of the causative mutation of the nonagouti black coat colour. Genomics 2010, 95, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Letko, A.; Ammann, B.; Jagannathan, V.; Henkel, J.; Leuthard, F.; Schelling, C.; Carneiro, M.; Drögemüller, C.; Leeb, T. A deletion spanning the promoter and first exon of the hair cycle-specific ASIP transcript isoform in black and tan rabbits. Anim. Genet. 2020, 51, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Castle, W.E. The Genetics of Domestic Rabbit; Cambridge Harvard University Press: London, UK, 1930; pp. 1–31. [Google Scholar]
- Aigner, B.; Besenfelder, U.; Müller, M.; Brem, G. Tyrosinase gene variants in different rabbit strains. Mamm. Genome 2000, 11, 700–702. [Google Scholar] [CrossRef] [PubMed]
- Beermann, F.; Orlow, S.J.; Lamoreux, M.L. The Tyr (albino) locus of the laboratory mouse. Mamm. Genome 2004, 15, 749–758. [Google Scholar] [CrossRef]
- Grønskov, K.; Ek, J.; Brondum-Nielsen, K. Oculocutaneous albinism. Orphanet J. Rare Dis. 2007, 2, 43. [Google Scholar] [CrossRef]
- Castle, W.E. Heredity of Coat Characters in Guinea Pigs and Rabbits; Carnegie Institution Washington Publisher: Washington, DC, USA, 1905; Volume 23, pp. 1–78. [Google Scholar]
- Castle, W.E. Genetics of the chinchilla rabbit. Science 1921, 53, 387–388. [Google Scholar] [CrossRef]
- Sawin, P.B. Albino Allelomorphs of the Rabbit with Special Reference to Blue-Eyed Chinchilla and Its Variations; Carnegie Institution Washington Publisher: Washington, DC, USA, 1932; Volume 427, pp. 15–50. [Google Scholar]
- Sawin, P.B. Hereditary variation of the chinchilla rabbit in coat and eye color. J. Hered. 1932, 23, 39–46. [Google Scholar] [CrossRef]
- Utzeri, V.J.; Ribani, A.; Schiavo, G.; Fontanesi, L. Describing variability in the tyrosinase (TYR) gene, the albino coat colour locus, in domestic and wild European rabbits. Ital. J. Anim. Sci. 2021, in press. [Google Scholar]
- Mercer, J.A.; Seperack, P.K.; Strobel, M.C.; Copeland, N.G.; Jenkins, N.A. Novel myosin heavy chain encoded by murine dilute coat colour locus. Nature 1991, 349, 709–713. [Google Scholar] [CrossRef]
- Fontanesi, L.; Scotti, E.; Dall’Olio, S.; Oulmouden, A.; Russo, V. Identification and analysis of single nucleotide polymorphisms in the myosin VA (MYO5A) gene and its exclusion as the causative gene of the dilute coat colour locus in rabbit. World Rabbit. Sci. 2012, 20, 35–41. [Google Scholar] [CrossRef]
- Matesic, L.E.; Yip, R.; Reuss, A.E.; Swing, D.A.; O’Sullivan, T.N.; Fletcher, C.F.; Copeland, N.G.; Jenkins, N.A. Mutations in Mlph, encoding a member of the Rab effector family, cause the melanosome transport defects observed in leaden mice. Proc. Natl. Acad. Sci. USA 2001, 98, 10238–10243. [Google Scholar] [CrossRef] [PubMed]
- Fontanesi, L.; Scotti, E.; Allain, D.; Dall’Olio, S. A frameshift mutation in the melanophilin (MLPH) gene causes the dilute coat colour in rabbit (Oryctolagus cuniculus) breeds. Anim. Genet. 2014, 45, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Lehner, S.; Gähle, M.; Dierks, C.; Stelter, R.; Gerber, J.; Brehm, R.; Distl, O. Two-exon skipping within MLPH is associated with coat color dilution in rabbits. PLoS ONE 2013, 8, e84525. [Google Scholar] [CrossRef] [PubMed]
- Demars, J.; Iannuccelli, N.; Utzeri, V.J.; Auvinet, G.; Riquet, J.; Fontanesi, L.; Allain, D. New insights into the melanophilin (MLPH) gene affecting coat color dilution in rabbits. Genes 2018, 9, 430. [Google Scholar] [CrossRef] [PubMed]
- Sanal, O.; Ersoy, F.; Tezcan, I.; Metin, A.; Yel, L.; Menasche, G.; Gurgey, A.; Berkel, I.; de Basile, G.S. Griscelli disease: Genotype-phenotype correlation in an array of clinical heterogeneity. J. Clin. Immunol. 2002, 22, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Menasche, G.; Ho, C.H.; Sanal, O.; Feldmann, J.; Tezcan, I.; Ersoy, F.; Houdusse, A.; Fischer, A.; de Basile, G.S. Griscelli syndrome restricted to hypopigmentation results from a melanophilin defect (GS3) or a MYO5A F-exon deletion (GS1). J. Clin. Investig. 2003, 112, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Jackson, I.J. A cDNA encoding tyrosinase-related protein maps to the mouse brown locus. Proc. Natl. Acad. Sci. USA 1988, 85, 4391–4396. [Google Scholar] [CrossRef]
- Utzeri, V.J.; Ribani, A.; Fontanesi, L. A premature stop codon in the TYRP1 gene is associated with brown coat colour in the European rabbit (Oryctolagus cuniculus). Anim. Genet. 2014, 45, 600–603. [Google Scholar] [CrossRef]
- Castle, W.E. Studies of heredity in rabbits, rats and mice. Carnegie Inst. Wash. Publ. 1909, 288, 4–28. [Google Scholar]
- Richardson, E.C. Inheritance of white in the “English” rabbit. Heredity 1953, 7, 150. [Google Scholar]
- Nachtsheim, H. Ergebrisse und Probleme der Vergleichenden und Experimentellen Erbpathologie. Jena. Z. für Nat. 1943, 76, 81–108. [Google Scholar]
- Wieberneit, D.; Mahdi, N.; Zacharias, K.; Wegner, W. Zur Problematik der Scheckenzucht bei Kaninchen. 1. Mitteilung: Mast- und Schlachtkörpereigenschaften, Organbefunde. Dtsch. Tierärztliche Wochenschr. 1990, 98, 352–354. [Google Scholar]
- Böderek, D.; Türk, O.; Lovén, E.; Wieberneit, D.; Wegner, W. Pathophysiological and functional aspects of the Megacolon-Syndrome of homozygous Spotted rabbits. J. Vet. Med. A 1995, 42, 549–559. [Google Scholar] [CrossRef]
- Wieberneit, D.; Wegner, W. Albino rabbits can suffer from Megacolon-Syndrome when they are homozygous for the “English-spot” gene (En En). World Rabbit. Sci. 1995, 3, 19–26. [Google Scholar] [CrossRef][Green Version]
- Fontanesi, L.; Vargiolu, M.; Scotti, E.; Latorre, R.; Pellegrini, M.S.F.; Mazzoni, M.; Asti, M.; Chiocchetti, R.; Romeo, G.; Clavenzani, P.; et al. The KIT gene is associated with the English spotting coat color locus and congenital megacolon in Checkered Giant rabbits (Oryctolagus cuniculus). PLoS ONE 2014, 9, e93750. [Google Scholar] [CrossRef] [PubMed]
- Gerlitz, S.; Wessel, G.; Wieberneit, D.; Wegner, W. Zur Problematik der Scheckenzucht bei Kaninchen. 3. Mitteilung: Variabilität des Pigmentierungsgrades, ganglionäre Darmwandversorgung, Beziehung zur Pathogenese—tierzüchterische und tierschützerische Aspekte. Dtsch. Tierärztliche Wochenschr. 1993, 100, 237–239. [Google Scholar]
- Fontanesi, L.; Vargiolu, M.; Scotti, E.; Mazzoni, M.; Clavenzani, P.; De Giorgio, R.; Romeo, G.; Russo, V. Endothelin receptor B (EDNRB) is not the causative gene of the English spotting locus in the domestic rabbit (Oryctolagus cuniculus). Anim. Genet. 2010, 41, 669–670. [Google Scholar] [CrossRef]
- Besmer, P.; Manova, K.; Duttlinger, R.; Huang, E.J.; Packer, A.; Gyssler, C.; Bachvarova, R.F. The kit-ligand (steel factor) and its receptor c-kit/W: Pleiotropic roles in gametogenesis and melanogenesis. Development 1993, 1, 125–137. [Google Scholar]
- Thomas, A.J.; Erickson, C.A. The making of a melanocyte: The specification of melanoblasts from the neural crest. Pigment. Cell Mel. Res. 2008, 21, 598–610. [Google Scholar] [CrossRef]
- Farrugia, G. Interstitial cells of Cajal in health and disease. Neurogastroenterol. Motil. 2008, 20, 54–63. [Google Scholar] [CrossRef]
- Alonso, L.; Fuchs, E. The hair cycle. J. Cell Sci. 2006, 119, 391–393. [Google Scholar] [CrossRef] [PubMed]
- Lienhart, R. Apropos d’une recente mutation chez le lapin domestique, le lapin Castorrex. C. R. Seances Soc. Biol. Ses. Fil. 1927, 97, 386–388. [Google Scholar]
- Létard, E. Le lapin Castorrex, L’histoire d’une mutation. Rev. de Méd. Vét. de Toulouse 1928, 80, 136–143. [Google Scholar]
- Castle, W.E.; Nachtsheim, H. Linkage interrelations of three genes for rex (short) coat in the rabbit. Proc. Natl. Acad. Sci. USA 1933, 19, 1006–1011. [Google Scholar] [CrossRef]
- Fox, R.R. Taxonomy and genetics. In The Biology of the Laboratory Rabbit, 2nd ed.; Manning, P.J., Ringler, D.H., Newcomer, C.E., Eds.; Academic Press: San Diego, CA, USA, 1994; pp. 1–26. [Google Scholar]
- Diribarne, M.; Mata, X.; Chantry-Darmon, C.; Vaiman, A.; Auvinet, G.; Bouet, S.; Deretz, S.; Cribiu, E.P.; de Rochambeau, H.; Allain, D.; et al. A deletion in exon 9 of the LIPH gene is responsible for the rex hair coat phenotype in rabbits (Oryctolagus cuniculus). PLoS ONE 2011, 6, e19281. [Google Scholar] [CrossRef]
- Wen, X.Y.; Bryce, D.M.; Breitman, M.L. Characterization of lpd (lipid defect): A novel mutation on mouse chromosome 16 associated with a defect in triglyceride metabolism. Hum. Mol. Genet. 1998, 7, 743–750. [Google Scholar] [CrossRef][Green Version]
- Kazantseva, A.; Goltsov, A.; Zinchenko, R.; Grigorenko, A.P.; Abrukova, A.V.; Moliaka, Y.K.; Kirillov, A.G.; Guo, Z.; Lyle, S.; Ginter, E.K.; et al. Human hair growth deficiency is linked to a genetic defect in the phospholipase gene LIPH. Science 2006, 314, 982–985. [Google Scholar] [CrossRef]
- Shimomura, Y.; Wajid, M.; Petukhova, L.; Shapiro, L.; Christiano, A.M. Mutations in the lipase H gene underlie autosomal recessive woolly hair/hypotrichosis. J. Investig. Dermatol. 2009, 129, 622–628. [Google Scholar] [CrossRef]
- Kinoshita-Ise, M.; Kubo, A.; Sasaki, T.; Umegaki-Arao, N.; Amagai, M.; Ohyama, M. Identification of factors contributing to phenotypic divergence via quantitative image analyses of autosomal recessive woolly hair/hypotrichosis with homozygous c.736T>A LIPH mutation. Br. J. Dermatol. 2017, 176, 138–144. [Google Scholar] [CrossRef]
- Takeichi, T.; Tanahashi, K.; Taki, T.; Kono, M.; Sugiura, K.; Akiyama, M. Mutational analysis of 29 patients with autosomal-recessive woolly hair and hypotrichosis: LIPH mutations are extremely predominant in autosomal-recessive woolly hair and hypotrichosis in Japan. Br. J. Dermatol. 2017, 177, 290–292. [Google Scholar] [CrossRef]
- Diribarne, M.; Mata, X.; Rivière, J.; Bouet, S.; Vaiman, A.; Chapuis, J.; Reine, F.; Fleurot, R.; Auvinet, G.; Deretz, S.; et al. LIPH expression in skin and hair follicles of normal coat and Rex rabbits. PLoS ONE 2012, 7, e30073. [Google Scholar] [CrossRef] [PubMed]
- Castle, W.E. The heredity of “angora” coat in mammals. Science 1903, 18, 760–761. [Google Scholar] [CrossRef] [PubMed]
- Wucherer, E. Überden character des Angorahaares. Z. J. Tierz. Zuchtungbiol. 1925, 4, 119–143. [Google Scholar]
- Hardy, T.M.P.; Markley, M.H. Microscopic study of coat variation in white New Zealand and Angora rabbits. J. Hered. 1944, 35, 183–192. [Google Scholar] [CrossRef]
- Crary, D.D.; Sawin, P.B. Some factors influencing the growth potential of the skin in the domestic rabbit. J. Exp. Zool. 1953, 124, 31–62. [Google Scholar] [CrossRef]
- Hébert, J.M.; Rosenquist, T.; Götz, J.; Martin, G.R. FGF5 as a regulator of the hair growth cycle: Evidence from targeted and spontaneous mutations. Cell 1995, 78, 1017–1025. [Google Scholar] [CrossRef]
- Mulsant, P.; Rochambeau, H.D.; Thébault, R.G. A note on linkage between the angora and fgf5 genes in rabbits. World Rabbit. Sci. 2004, 12, 1–6. [Google Scholar] [CrossRef]
- Chantry-Darmon, C.; Urien, C.; de Rochambeau, H.; Allain, D.; Pena, B.; Hayes, H.; Grohs, C.; Cribiu, E.P.; Deretz-Picoulet, S.; Larzul, C.; et al. A first-generation microsatellite-based integrated genetic and cytogenetic map for the European rabbit (Oryctolagus cuniculus) and localization of angora and albino. Anim. Genet. 2006, 37, 335–341. [Google Scholar] [CrossRef]
- Zhao, B.; Chen, Y.; Hao, Y.; Yang, N.; Wang, M.; Mei, M.; Wang, J.; Qiu, X.; Wu, X. Transcriptomic analysis reveals differentially expressed genes associated with wool length in rabbit. Anim. Genet. 2018, 49, 428–437. [Google Scholar] [CrossRef]
- Ding, H.; Zhao, H.; Cheng, G.; Yang, Y.; Wang, X.; Zhao, X.; Qi, Y.; Huang, D. Analyses of histological and transcriptome differences in the skin of short-hair and long-hair rabbits. BMC Genomics 2019, 20, 140. [Google Scholar] [CrossRef]
- Higgins, C.A.; Petukhova, L.; Harel, S.; Ho, Y.Y.; Drill, E.; Shapiro, L.; Wajid, M.; Christiano, A.M. FGF5 is a crucial regulator of hair length in humans. Proc. Natl. Acad. Sci. USA 2014, 111, 10648–10653. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, H.; Pan, H.; Wang, X.; Zhang, Y.; Yao, B.; Li, N.; Lai, L.; Li, Z. CRISPR/Cas9-mediated disruption of Fibroblast Growth Factor 5 in rabbits results in a systemic long hair phenotype by prolonging anagen. Genes 2020, 11, 297. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Cheng, G.; Leng, J.; Yang, Y.; Zhao, X.; Wang, X.; Qi, Y.; Huang, D.; Zhao, H. Analysis of histological and microRNA profiles changes in rabbit skin development. Sci. Rep. 2020, 10, 454. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Chen, Y.; Hu, S.; Yang, N.; Wang, M.; Liu, M.; Li, J.; Xiao, Y.; Wu, X. Systematic analysis of non-coding RNAs involved in the Angora rabbit (Oryctolagus cuniculus) hair follicle cycle by RNA sequencing. Front. Genet. 2019, 10, 407. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Bao, Z.; Zhao, B.; Zhou, T.; Li, J.; Liu, M.; Hu, S.; Yang, N.; Chen, Y.; Wu, X. Characterization and functional analysis of Krtap11-1 during hair follicle development in Angora rabbits (Oryctolagus cuniculus). Genes Genomics 2020, 42, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Chen, Y.; Hu, S.; Yang, N.; Liu, M.; Li, J.; Bao, Z.; Wu, X. Characterization of HTATIP2 and its role during hair follicle cycles in Angora rabbit. Genome 2020, 63, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Greene, H.S.; Hu, C.K.; Brown, W.H. A lethal dwarf mutation in the rabbit with stigmata of endocrine abnormality. Science 1934, 79, 487–488. [Google Scholar] [CrossRef]
- Greene, H.S. A dwarf mutation in the rabbit: The constitutional influence on homozygous and heterozygous individuals. J. Exp. Med. 1940, 71, 839–856. [Google Scholar] [CrossRef]
- Carneiro, M.; Hu, D.; Archer, J.; Feng, C.; Afonso, S.; Chen, C.; Blanco-Aguiar, J.A.; Garreau, H.; Boucher, S.; Ferreira, P.G.; et al. Dwarfism and altered craniofacial development in rabbits is caused by a 12.1 kb deletion at the HMGA2 locus. Genetics 2017, 205, 955–965. [Google Scholar] [CrossRef]
- Létard, E. Une mutation nouvelle chez le lapin. Bull. Acad. Vét. Fr. 1935, 8, 608–610. [Google Scholar]
- Létard, E. Troubles de la locomotion et de la vision chez le lapin, liaison hereditaire. Bull. Acad. Vét. Fr. 1943, 16, 184–192. [Google Scholar]
- Boucher, S. Le lapin Sauteur d’Alfort: Un symbole au service de la pathologie cunicole. La Sem. Vet. 1994, 746, 17–18. [Google Scholar]
- Audigier, I.; Renous, S. Les allures du lapin normal peuvent-elles expliquer la marche acrobatique du lapin sauteur d’Alfort? Mammalia 2002, 66, 563–578. [Google Scholar] [CrossRef]
- Carneiro, M.; Vieillard, J.; Andrade, P.; Boucher, S.; Afonso, S.; Blanco-Aguiar, J.A.; Santos, N.; Branco, J.; Esteves, P.J.; Ferrand, N.; et al. Retinoid-related orphan nuclear receptor RORB is required for saltatorial locomotion in rabbits. Curr. Biol. 2019. [Google Scholar] [CrossRef]
- Pease, M. Yellow fat in rabbits, a linked character? Z. für Indukt. Abstamm. und Vererb. 1928, 2, 1153–1156. [Google Scholar]
- Wilson, W.K.; Dudley, F.J. Fat colour and fur colour in different varieties of rabbit. J. Genet. 1946, 47, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Berry, S.D.; Davis, S.R.; Beattie, E.M.; Thomas, N.L.; Burrett, A.K.; Ward, H.E.; Stanfield, A.M.; Biswas, M.; Ankersmit-Udy, A.E.; Oxley, P.E.; et al. Mutation in bovine beta-carotene oxygenase 2 affects milk color. Genetics 2009, 182, 923–926. [Google Scholar] [CrossRef]
- Tian, R.; Pitchford, W.S.; Morris, C.A.; Cullen, N.G.; Bottema, C.D. Genetic variation in the beta, beta-carotene-9′, 10′-dioxygenase gene and association with fat colour in bovine adipose tissue and milk. Anim. Genet. 2010, 41, 253–259. [Google Scholar] [CrossRef]
- Våge, D.I.; Boman, I.A. A nonsense mutation in the beta-carotene oxygenase 2 (BCO2) gene is tightly associated with accumulation of carotenoids in adipose tissue in sheep (Ovis aries). BMC Genet. 2010, 11, 10. [Google Scholar] [CrossRef]
- Fallahshahroudi, A.; Sorato, E.; Altimiras, J.; Jensen, P. The domestic BCO2 allele buffers low-carotenoid diets in chickens: Possible fitness increase through species hybridization. Genetics 2019, 212, 1445–1452. [Google Scholar] [CrossRef]
- Strychalski, J.; Brym, P.; Czarnik, U.; Gugołek, A. A novel AAT-deletion mutation in the coding sequence of the BCO2 gene in yellow-fat rabbits. J. Appl. Genet. 2015, 56, 535–537. [Google Scholar] [CrossRef] [PubMed]
- Strychalski, J.; Gugołek, A.; Antoszkiewicz, Z.; Kowalska, D.; Konstantynowicz, M. Biologically active compounds in selected tissues of white-fat and yellow-fat rabbits and their production performance parameters. Livest. Sci. 2016, 183, 92–97. [Google Scholar] [CrossRef]
- Strychalski, J.; Gugołek, A.; Brym, P.; Antoszkiewicz, Z. Effect of the β-carotene oxygenase 2 genotype on the content of carotenoids, retinol and α-tocopherol in the liver, fat and milk of rabbit does, reproduction parameters and kitten growth. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1585–1593. [Google Scholar] [CrossRef] [PubMed]
- Strychalski, J.; Gugołek, A.; Brym, P.; Antoszkiewicz, Z.; Chwastowska-Siwiecka, I. Polymorphism of the BCO2 gene and the content of carotenoids, retinol, and α-tocopherol in the liver and fat of rabbits. Rev. Bras. Zootec. 2019, 48, e20180243. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fontanesi, L. Rabbit Genetic Resources Can Provide Several Animal Models to Explain at the Genetic Level the Diversity of Morphological and Physiological Relevant Traits. Appl. Sci. 2021, 11, 373. https://doi.org/10.3390/app11010373
Fontanesi L. Rabbit Genetic Resources Can Provide Several Animal Models to Explain at the Genetic Level the Diversity of Morphological and Physiological Relevant Traits. Applied Sciences. 2021; 11(1):373. https://doi.org/10.3390/app11010373
Chicago/Turabian StyleFontanesi, Luca. 2021. "Rabbit Genetic Resources Can Provide Several Animal Models to Explain at the Genetic Level the Diversity of Morphological and Physiological Relevant Traits" Applied Sciences 11, no. 1: 373. https://doi.org/10.3390/app11010373
APA StyleFontanesi, L. (2021). Rabbit Genetic Resources Can Provide Several Animal Models to Explain at the Genetic Level the Diversity of Morphological and Physiological Relevant Traits. Applied Sciences, 11(1), 373. https://doi.org/10.3390/app11010373





