Abstract
The rabbit (Oryctolagus cuniculus) is a unique multipurpose domestic species that has relevant economic impacts in several contexts. This review is focused on rabbit genetic resources that have been mainly bred for the fixation of differentiating features (e.g., exterior traits) that have been already genetically characterized. Several naturally occurring rabbit mutants could be useful as animal models for the investigation of the biological mechanisms determining their characterizing aspects, with translational potentials. A historical overview of the origin of the domesticated rabbit populations and of their genetic differentiation into many breeds is summarized. Then, a detailed analysis of the genetic features that characterize the different breeds is reported, starting from coat color and coat structure affecting genes (MC1R, ASIP, TYR, MLPH, TYRP1, KIT, LIPH, and FGF5), determining major loci described by classical genetic studies. Mutations in these genes have implications in pigmentation features, hair growth, and related defects. Other gene mutations affecting body size or shapes (HMGA2) and other physiological disfunctions (RORB and BCO2) are also described Additional studies are needed to complete the genetic characterization of some of these loci and to investigate the large genetic variability available in fancy breeds and commercial meat and fur lines.
1. Introduction
The European rabbit (Oryctolagus cuniculus), known simply as rabbit, is a unique multipurpose domestic species, that has relevant economic impacts in several contexts and that, with the wild or feral counterpart, plays important roles in ecological niches [1]. The rabbit is a livestock raised for meat and fur productions. These uses can be considered the original human-oriented purposes of this species. Many commercial rabbit lines and populations have been selected for these purposes, and selection and breeding programs are currently under way in different countries to strengthen and improve production performances. The rabbit is also an important animal model for biomedical investigations and applications [2,3,4]. For these purposes, it is used to explore basic aspects of biological questions, and several advancements in immunology, neural, muscle, and eye physiology and anatomy as well as in many other branches of biology have been achieved by investigating this species. The rabbit is also a biotech tool used for the production of pharmacological active and biotechnological relevant molecules. For example, most of the commercially available polyclonal antibodies are produced in this animal [5]. The rabbit is a wild animal resource in its native region, i.e., the Iberian peninsula and part of the South of France, and in many other regions in the world in which it has been subsequently introduced starting from wild or domesticated (feral) stocks [6]. Consequently, the rabbit is also considered a pest as, in many non-native regions, its impressive population growth cannot be controlled by predators or other natural enemies, creating many ecological disruptions and damages. Last but not least, the rabbit is also a fancy species with a broad phenotypic diversity that defines many different lines or breeds, which are genetically differentiated by selective breeding processes that, in most cases, have fixed naturally and randomly occurring mutations. In addition, a large number of lines or strains has been developed for other aims, starting from natural mutations.
This review is focused on rabbit genetic resources that have been mainly bred for the fixation of differentiating features (e.g., exterior traits) that have been already genetically characterized (at least in part), and that could be useful as animal models for the exploitation of the biological mechanisms determining their characterizing aspects with translational potentials. First, a historical overview of the origin of the domesticated rabbit populations and of their genetic differentiation into many breeds is summarized here. Then, a detailed analysis of the genetic features that characterize the different breeds is reported starting from coat color and coat structure affecting genes, which have implications in pigmentation features, hair growth, and related defects. The review also includes an analysis of other gene mutations affecting body size or shapes and other physiological disfunctions.
2. The Domestication Process of the Rabbit and the Origin of the Genetic Diversity in this Species
Using morphometric analyses and molecular markers (starting from biochemical markers and then by analyzing mitochondrial DNA and nuclear genome differences), two subspecies of wild European rabbits have been described: O. c. algirus (originally spread in the southwest of the Iberian Peninsula) and O. c. cuniculus (originally distributed in the northeastern regions of the Iberian Peninsula and South of France [7,8,9,10,11,12,13]). At the molecular level, these two subspecies were first reported to carry two separated mitochondrial DNA (mtDNA) lineages (clades A and B) that have about 4.5% nucleotide differences [8,9,12,13]. Differences between these two subspecies were subsequently also clearly evidenced at the nuclear DNA level (e.g., [10,14,15]. The phylogeographical pattern of these wild European rabbit populations suggests that the two groups remained isolated for a certain time and evolved independently. Climatic-generated barriers were probably determined by the glacial dynamics in the Iberian Peninsula that occurred during the Quaternary ice-ages. When glacial barriers later disappeared the two groups expanded their previously confined distribution areal, creating a sort of transversal line of diluted overlapping and gradual separation that goes from the North-East to the South-West of this peninsula [14]. As a consequence of the related population genetic histories, the subspecies of the mtDNA clade A maintained more genetic diversity than the subspecies of the other clade [12,13]. A reduced level of genetic diversity observed in the South of France populations is the result of bottlenecks due to the origin of these populations from the contiguous Iberian O. c. cuniculus populations [9,12,13,15,16]. In historical time, wild populations of European rabbit expanded in the North of France and North of Europe by means of human translocation activities that mainly started during the Middle Age and continued over the subsequent centuries [17,18]. The domestication process relied on the genetic stock constituted by the subspecies O. c. cuniculus that first colonized France and then other regions of the North of Europe. Animal domestication can be usually described with a gradual morphological, biological, and behavioral modification of the animals in one or more populations, and a few domestication pathways have been proposed [19]. The domestication pathway that could better describe what probably occurred in the rabbit is the directed pathway that, in the case of this species, cannot be simply derived by few events. This model does not involve any preliminary steps of habituation to the humans or preliminary management of the animals and directly begins with the capture of wild forms with the aim to control their reproduction and manage their breeding [20,21,22]. It is however not completely clear if preliminary habituation to anthropogenic environments and human derived management conditions of the wild genetic pool could have, at least in part, contributed to shape and lead the domestication process of the rabbit.
Domestic rabbits carry a subset of the B mitotypes (B1 is the most frequent) also identified in the wild rabbit populations of the North-East Iberia and South-West of France, demonstrating that the domestication of this species was based on the wild populations that were present in France [9,23,24]. The domestication processes produced another reduction of genetic diversity that, however, was stronger than that observed during the transition from the Iberian to the French wild rabbits [15,16,25]. This reduction is in agreement with a small effective population size of the early domesticated genetic pool, that is consistent with the fact that the relevant domestication events that involved wild rabbits occurred mainly in a short time window and in a limited geographic area (Middle Age and close to French castles and monasteries [18,26]) and that subsequent crossbreeding with wild rabbits was not frequent [16].
Carneiro et al. [15], using whole genome resequencing data from wild rabbits of the two subspecies and from domestic rabbits of different breeds, have reported that very few loci have gone to complete fixation in domestic populations in comparison with the variability present in the wild ancestral populations. Moreover, the directional selection determined by the domestication process slightly modified allele frequencies at many loci in the domesticated populations, suggesting that the domestication acted softly on standing genetic variation in many regulatory regions of the rabbit genome [15]. An over-representation of the shifted alleles in regulatory regions of genes affecting brain and neuronal development indicates that the behavior of the domesticated animals changed allowing the animals to tolerate the human environment and to adapt to the production system in which they were used [15,27]. As consequence of the domestication, brain architecture was probably modified: amygdala volume decreased, with a possible reduction of fear, and the medial prefrontal cortex volume increased, which might give an increased response to negative effects [28].
The domestication process was followed or was, in some way, part of the process that led to the constitution of the breeds, with an additional reduction of genetic diversity [16]. Bottleneck and founder effects, that usually occur in breed formation, might have generated this reduction that culminates in a small effective population size of the resulted breeds, which can be defined as phenotypically homogeneous populations. The current rabbit breeds exhibit a clear and detectable genetic differentiation mainly derived by differences in allele frequencies and fixation of unique genetically determined exterior features [1,16]. Most modern rabbit breeds and recently constituted lines or strains have been derived by cross-breeding between pre-existing morphs or varieties. Moreover, historical and genetic evidences indicate that introgression has been frequently used to introduce desirable coat color variants into other varieties [1,16,29].
The most important exterior traits that differentiate many rabbit breeds are determined by several coat colors and color patterns that have been selected by fancy breeders and then fixed in the breed populations. The molecular characterization of the most relevant coat color loci can provide useful information to understand some mechanisms of the biology of pigmentation in mammals. Other phenotypic differences are related to the type of fur, the body size, and length and shape of the ears and face and some biochemical features. For a few of these traits, the mutated genes have been identified and the causative variants have been reported.
3. Molecular Characterization of Coat Color Affecting Genes in Rabbits
Rabbits with different color morphs were depicted in old books or paintings. For example, a few illustrations in the Livre de Chasse of Gaston Phoebus (Gaston III, Count of Foix; written between 1387 and 1389) and the paintings of Ridolfo Ghirlandaio (Portrait of a Lady with a Rabbit, ca. 1508) and Titian (Madonna with Rabbit, c. 1530) indicated that several color varieties could be quite common in the rabbits at that time. It is however not clear if these morphs could be the same that are observed nowadays or if more recent occurring variants should be considered the mutants that have shaped the current color diversity available in this species. Most coat color morphs and varieties in rabbits however emerged and were selected over the last couple of centuries and constituted the basic elements for the formation of distinctive populations that are identified as breeds [29]. Many modern breeds are named with words that directly indicate a coat color or color pattern. Coat color loci in rabbits were first described using classical genetic approaches, and comparative analyses across species established homologous loci [30,31]. In a few cases, homology of loci identified in rabbits with those established in other species was not confirmed with molecular investigations. Table 1 reports the list of the coat color loci that have been analyzed at molecular level and for which causative mutations or associated DNA markers have been identified. Some of them can be considered interesting examples of the effects of mutations (and their interactions) in determining phenotypic traits and could be used to better understand the fine molecular mechanisms involved. A few also have pleotropic effects on other biological aspects, apart from pigmentation, and for this reason they have been investigated with the aim to establish new biomedical models. Table 2 summarizes the genetic information available for coat color gene mutations in the most common breeds. Other coat color loci (Dutch, Viennese white, Silver, Red eye, Wide band) have been described by classical genetic studies [30,31] but have not been characterized at the molecular level yet and are not discussed in this review.
3.1. Mutations in the Melanocortin 1 Receptor (MC1R) Gene and the Extension Locus
In many species, mutations in the single-exon melanocortin 1 receptor (MC1R) gene determine the multi-allelic series at the Extension (E) locus (e.g., [32,33,34,35,36]). MC1R encodes for a seven transmembrane G-protein coupled receptor that is localized within the membrane of the melanocytes. The melanocytes are specialized cells that produce melanin of different types that are the final molecular products of the biological pathway that lead to the pigmentation. This locus is epistatic over the Agouti locus. Therefore, the types of produced melanin are directly related to the allelic structure at the Extension and Agouti loci.
Classical genetic studies carried out in rabbits have suggested that the Extension series in this species is determined by five alleles [30,31,37,38,39,40]. Allele ED determines the dominant black coat color and is caused by one in-frame deletion of 6 bp (c.280_285del6 or D6 allele), which eliminates two amino acids in the second transmembrane domain [41]. The black color of the spots of the Checkered Giant breed is due to this allele. Allele ES (steel) is considered a weaker version of ED, even if it could be probably determined by the same mutation causing ED, as another MC1R variant associated to this allele has not been described so far [41]. The steel coat color effect associated to this allele could be due to the involvement of modifier genes or heterozygous combinations at the Extension locus. Allele E or E+ is the wild type allele that determines the normal grey or the normal extension of black. Two wild type haplotypes have been described at the Extension locus that can determine the same phenotypic effect [41]. Another in-frame deletion of 6 bp flanked by a G > A transition in 5’ (c.[124G > A;125_130del6]) is the causative composite mutation of the eJ allele, which produces the Japanese brindling coat color phenotype [42]. This allele determines a mosaic distribution of black and yellow pigmentation and could be an interesting model of alternative fixation of the production of eumelanin and pheomelanin in specifically devoted melanocytes. Epigenetic regulations might be involved in determining this unique coat color phenotype [42]. Two breeds have the characteristic coat color determined by the homozygous state of the eJ allele: Japanese and Rhinelander (knows also as Tricolor) breeds. Allele eJ is recessive over all other alleles described above but is dominant or partially dominant over allele e, which determines the non-extension of black and the yellow/red (with white belly) coat color [39,42]. Allele e is caused by a 30 nucleotides in-frame deletion (c.304_333del30; ∆30 allele), which eliminates 10 amino acids of the first extracellular loop of the transmembrane MC1R protein [41]. The modified protein is not functional; thus, the melanocytes of the homozygous e/e rabbits can only produce pheomelanin. Allele e is fixed in all rabbit breeds that have a yellow/red characterizing coat color (e.g., Burgundy Fawn, Gold Saxony, New Zealand Red, and Thuringian [41]).
The allele series at this locus in rabbit is probably unique if compared to those of all other domestic animals. In rabbit, all mutant alleles (dominant and recessive, if related to the wild type forms) are caused by in-frame deletions that eliminate a few amino acids in the translated protein (Figure 1). In dog, cattle, sheep, goat, pig, horse, donkey, chicken, and in several other species that have been investigated at the MC1R gene, variants affecting the function of this gene are due to missense mutations, frameshift mutations, non-sense mutations, or structural mutations that alter the function of the protein or completely disrupt it (e.g., [32,33,34,35,36,43,44,45,46,47,48]). Thus far, only in cats, in-frame deletions determining pheomelanic recessive alleles have been reported [49,50] but in this species, no dominant in-frame deletion has been described. The peculiarity of the natural occurring alleles in rabbits can give the opportunity to study the function and effect of micro-eliminations of residues from MC1R primary structure, particularly for the dominant allele, that could be also useful to understand the role of the domains and amino acid residues in this protein.
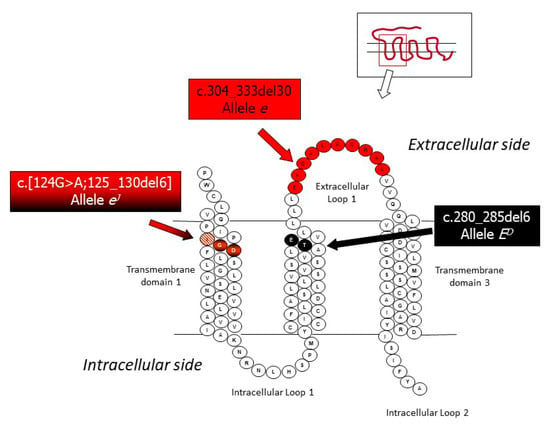
Figure 1.
A detail of the 2D structure of the rabbit MC1R protein with indicated the amino acids that are eliminated by the three in-frame deletions that cause the ED (and/or ES; see the text), eJ, and e alleles at the Extension locus (modified from [42]).
3.2. Mutations in the Agouti Signaling Protein (ASIP) Gene and the Agouti Locus
The agouti signaling protein (ASIP) gene, structurally constituted by three coding exons, encodes for a small protein that regulates the eumelanin to pheomelanin synthesis switch in the melanocytes [51]. The wild type agouti coat color that is characterized by banded-hairs is obtained by the alternative and mutually exclusive binding of MC1R by the α-melanocyte-stimulating hormone or by ASIP [52,53]. Mutations in the ASIP gene or in the regulatory motifs controlling ASIP expression that disrupt the protein structure or reduce the expression level and protein synthesis are usually recessive and impair the ASIP regulation activity on MC1R. This altered interaction between the two proteins, in turn, determines the production of eumelanin and the absence of pheomelanin, resulting in a non-agouti black coat color (e.g., [51,54,55,56,57,58]).
Classical genetic studies have described three alleles at the rabbit Agouti locus [30,31]. The wild type allele (A or Aw, as it resembles the murine Aw light-bellied agouti allele [59]) produces a greyish-brown or grey dorsal fur with a lighter (almost white) underside with a particular distribution of pheomelanin and eumelanin in the different types of rabbit hairs [30,31]. The wild type allele is dominant over the other two alleles described in rabbits at this locus: at (black and tan pattern) and a (non-agouti or self).
The rabbit at allele is characterized by a unique haplotype in the coding region determined by three missense mutations (p.L55M, pK77R and p.L89P), one of which (p.L55M; located in a conserved position of the basic amino-terminal domain of the protein) was reported only in Tan rabbits that are homozygous for the at allele [60]. The at haplotype extends for a large chromosome region spanning the ASIP gene and includes the putative causative mutation determined by a 11 kb deletion encompassing the hair cycle-specific ASIP promoter [61]. Rabbits homozygous for the at allele have non-agouti (not banded) hairs on the dorsal surface of the body, whereas the belly and other regions of the body (i.e., eye-circles, line of jowl, inside the ears, undertail, and feet pads) have whitish color [30,31]. The at allele is dominant over the a allele.
Homozygous rabbits for the a allele are self-colored (i.e., they are completely black). The a allele is determined by a frameshift mutation (c.5_6insA) caused by one nucleotide insertion in the coding region of exon 2 [60]. The resulting shift of the reading frame determines the production of a truncated and non-functional protein of only 21 amino acids. The homozygous c.5_6insA mutated genotype has been observed in all rabbit breeds with black coat color or variation of black (e.g., blue or silver) that also carried a wild type MC1R allele [41,60]: Alaska, Blanc de Hotot, Champagne d’Argent, English Spot, Havana, Mini Silver, Russian, Silver, and Vienna Blue. Other breeds (Californian, Checkered Giant, Checkered Small, and Dutch) that have black coat color patterns but that also carry the dominant allele at the Extension locus [41] are fixed for or have high frequency of the ASIP c.5_6insA mutated allele. The non-agouti allele has been also identified in other breeds (New Zealand White, Thuringian, and White Vienna) for which mutations in other loci that have epistatic effects over the Agouti locus (Albino, Extension, and Viennese White, respectively) determine their coat color [30,31,62,63]. The non-agouti allele has been also identified in Giant Grey rabbits but in heterozygous condition with the wild type allele [60]. Genotyping data at this gene available for a large number of breeds is reported in Table 2.
3.3. Mutations in the Tyrosinase (TYR) Gene and the Albino Locus
Tyrosinase (TYR) is the rate-limiting enzyme needed for the synthesis of melanin in the melanosomes. Mutations in this gene that alter or impair the function of the encoded enzyme determine a few forms of albinism in many species [64,65]. Six alleles have been reported at the rabbit Albino locus by classical genetic studies [30,31,66,67,68,69]. These classical works indicated that a wild type allele (allele C) is needed for the production of both eumelanin and pheomelanin. Subsequent analyses of the rabbit TYR gene indicated the presence of more than one wild type allelic form that might encode isoforms with biochemically similar activities [70]. All other mutated alleles that are recessive to C lead to a progressive decline in quantity of pigments produced. Three alleles that can give three variants of chinchilla coat color have been reported by classical genetic studies [30,31]: the dark chinchilla allele (cchd), dominant over the other alleles below it, causes a reduction of phaeomelanin, whereas eumelanin remains unaffected; the medium chinchilla allele (cchm), leads to a slight reduction of the production of eumelanin and a complete reduction of the production of pheomelanin; the light chinchilla allele (cchl) has an effect similar to the medium chinchilla allele but with a more pronounced reduction in eumelanin production. However, molecular characterization of the rabbit TYR gene has identified only one mutated haplotype associated to the chinchilla phenotype and determined by two missense mutations (p.E294G and p.T358I [63,70]). The Himalayan allele (ch), which has a temperature-dependent action and can lead to the production of eumelanin at the extremities of the body where the temperature is lower than in the rest of the body [30,31], is determined by only the p.E294G missense mutation [63,70]. Californian rabbits are homozygous for this mutation. The last allele of this series (the albino allele or c), which is recessive to all other alleles when it is in homozygous condition, determines the complete absence of all eumelanin and pheomelanin pigments. This allele is caused by a missense mutation that determines the p.T373K amino acid substitution in a conserved position of the CuB binding site of the TYR enzyme [63,70]. A list of breeds carrying the described alleles is reported in Table 2.
3.4. Mutations in the Melanophilin (MLPH) Gene and the Dilute Locus
An altered distribution of eumelanin and pheomelanin in the hairs is determined by the Dilute locus that produces a coat color dilution. The murine Dilute locus is caused by mutations in the in the myosin Va (Myo5a) gene, which encodes for an actin-binding protein that is involved in the process of melanosome transport [71]. Considering the potential homology between the mouse and rabbit Dilute loci, the rabbit MYO5A gene was first investigated to evaluate if its mutations could be responsible for the coat color dilution observed in a few rabbit breeds, such as Vienna Blue. Fontanesi et al. [72] excluded that the rabbit MYO5A gene was involved in determining the Dilute locus in this lagomorph species. Then, the rabbit melanophilin (MLPH) gene that in mice is responsible for a similar coat color phenotype determined by the Leaden locus [73] was investigated. A family-based segregation analysis that followed the transmission of the mutated allele d revealed that the rabbit MLPH gene determines the Dilute locus in Oryctolagus cuniculus [74]. Sequence and gene expression analyses identified two candidate mutations for the d allele [74,75]: the c.111-5C > A mutation, located within intron 2 in an acceptor site for splicing, leading to the skipping of exons 3 and 4 from the normal rabbit MLPH transcript; and a 1-bp deletion in exon 6 (c.585delG, indicated also g.549853delG, if referred to its position on the scaffold GL018840 of the oryCun2.0 genome version) that leads to a frameshift and an altered amino acid sequence, with a premature stop codon in a downstream exon. Subsequent segregation and gene expression investigations strongly supported the causative role of the frameshift mutation (c.585delG) and tended to exclude the intron 2 mutation as being the responsible variant of the d allele in most coat color diluted breeds [76]. All self blue breeds and lines (Vienna Blue, Castor Rex, and Chinchilla diluted) and spotted blue rabbits of different breeds (Californian, Checkered Giant, and English Spotted rabbits) and light grey or cream rabbits (Fairy Marburg and Fairy Pearly rabbits) were homozygous for the c.585delG frameshift mutation [74,76]. This recessive allele was also identified in a few other breeds in which it was masked by the wild type D allele [74]. A complete list of breeds in which the c.585delG mutation was identified is reported in Table 2.
These results, which confirmed that the rabbit MLPH gene is involved in this coat color phenotype, make it possible to consider the rabbit as a natural animal model for the human Griscelli syndrome type 3 where mutations in the same gene produce coat color defects in humans since infancy, with unusually hypopigmented skin and light silvery-gray hair [77,78].
3.5. A Mutation in the Tyrosinase-Related Protein 1 (TYRP1) Gene and the Brown Locus
The Brown locus in rabbits has been first described by family based studies in the last century that suggested the presence of a wild type B allele that can produce dense eumelanin throughout the coat and a recessive b allele that is unable to produce black pigmentation, with the result that only brown pigments are obtained [30,31,62]. Using a candidate gene approach derived by the fact that in mice mutations in the tyrosinase-related protein 1 (Tyrp1) gene are responsible for the same locus [79], the rabbit TYRP1 gene was sequenced in brown rabbits as well as in rabbits with several other coat colors not affected by the Brown locus [80]. A non-sense mutation in exon 2 (g.41360196G > A), leading to a premature stop codon at position 190 of the deduced protein sequence (p.W190ter), was identified only in Havana rabbits that have a characteristic brown coat color. Therefore, this disrupting mutation was considered the only candidate mutation determining the recessive b allele at this locus in rabbit [80].
3.6. The KIT Gene is Responsible for the English Spotting Locus and the Megacolon Defect in the Checkered Giant Breed
A dominant allele (En) is considered the determining genetic factor for the spotted phenotype, very variable in extent, attributed to the English spotting locus. The recessive wild-type allele (en) determines a self-colored phenotype [81]. Rabbits with the heterozygous genotype En/en have far larger patches of colored fur than rabbits with the homozygous En/En genotype [30]. Heterozygous En/en rabbits have the characteristic spotted patterns described in the English spot (or spotted), Butterfly, and Checkered Giant breeds [67]. This is a unique allelic combination that is needed to produce breed standards desired by fancy breeders. The patches of the spotted phenotypes can be of different colors according to the allelic combination at other loci. Other genes are probably involved in determining the degree of extension of the colored patterns versus the white areas [82]. Dominant homozygous En/En rabbits are affected by an underlying megacolon defect and are usually subvital compared to the animals with the other two genotypes, i.e., En/en and en/en [30,82,83,84,85,86,87]. The defect has incomplete penetrance, it is influenced by environmental factors (age, diet, stressors) and is recessive, as En/en rabbits are not affected [87]. Etiopathogenetic analyses of rabbits having this megacolon pointed out that abnormalities of the enteric nervous system throughout the colon might be involved in this defect [85,86,88].
Several genes that are implicated both in coat color spotted patterns and in similar disfunctions of the digestive tract have been described in rodents and other mammals. Two of these genes were also investigated in association studies with the coat color and megacolon phenotypes in rabbits. The endothelin receptor B (EDNRB) gene was first excluded to be involved in these traits in Checkered Giant rabbits [89]. Subsequently, the analysis of KIT gene markers in Checkered Giant families indicated that a synonymous polymorphism in exon 5 of this gene is associated with both traits [87]. The KIT gene encodes the mast/stem cell growth factor receptor that is mainly involved in the differentiation of melanoblasts and in regulating their migration from the neural crest along the dorsolateral pathway to reach the final destinations in the skin [90,91]. KIT gene expression in the gut musculature is prominent only in interstitial cells of Cajal (ICC) that are essential in gut motility [92]. KIT gene expression in cecum and colon specimens of En/En rabbits resulted very low compared to the expression in the same tissues observed in en/en rabbits. Reduced and altered c-kit immunolabelled ICC, together with neuronal and ICC abnormalities in cecum and colon tissues, were identified in En/En rabbits [87]. Therefore, in addition to the effect of the English spotting locus on a coat color spotted phenotype evidenced in Checkered Giant rabbits, the neuro-ICC changes in rabbits with En/En genotype are reminiscent of the human non-aganglionic megacolon [87]. Therefore, En/En rabbits might be considered as new animal models for this type of defect in humans [87]. The complete sequence characterization of the rabbit KIT gene and of the upstream and downstream regions is needed to identify the causative mutation(s) of the coat color pattern and of the megacolon defect described in this model. Other coat color patterns might have additional variants affecting the KIT gene.
4. Molecular Characterization of Genes Affecting Hair and Coat Structure as Models for Hair Growth and Development
The fur of the rabbit includes three types of hairs. The guard hairs are the longest and thickest hairs (about 3–4 cm of length, with a diameter of 50–60 µm), which together with the awn hairs (about 3–3.5 cm of length, with a diameter of about 25–30 µm) constitute the outer coat and mainly play the function of physical barrier and protection. The down hairs are the shortest and thinnest hairs (2.5–3 cm; 15 µm) that are usually the most abundant types of hairs of a rabbit fur (90–95% of all hairs). Down hairs constitute the inner coat that represents the main external thermal barrier of the rabbits. The hair cycle is divided into three phases [93]: (i) anagen, which is the growth phase when follicles produce an entire hair shaft from tip to root; (ii) catagen, which constitutes the regression or involution phase when hairs cease to elongate; and (iii) telogen, which is the quiescence phase when follicles lie dormant in a resting phase. The hair cycle is a model to study the regulation of stem cell activation and quiescence, cell differentiation, and apoptosis [93].
A few loci described in rabbit affect hair growth and structure. Among these loci, Rex and Angora (Table 1) are exploited industrially for the production of fibers and furs and have been characterized or partially analyzed at the molecular level. Other loci (Satin, Waved or Curly, Wuzzy and Furless or Naked) have been described by classical genetic studies, but they have not been investigated at the molecular level yet [30].
4.1. A Mutation in the Lipase Member H (LIPH) Gene Causes the Rex Locus R1
Rex rabbits show an interesting breed-specific phenotype mainly determined by the absence (or almost complete absence) of guard and awn hairs that gives a fur constituted by short and soft hair coat. Early classical genetic studies analyzed the segregation of three independent natural Rex mutants that occurred in three independent rabbit populations and established a similar genetic determinism, each due to an autosomal recessive allele [30]. Due to uncertainty in establishing homology across populations, the presence of three independent Rex loci (R1, R2, and R3, having the recessive alleles indicated as r1, r2, and r3, respectively) was suggested [94,95,96,97]. The mutation that occurred in the French rabbit strain (r1) was first assigned to rabbit chromosome 14 in an experimental design based on microsatellite analysis of rabbit families established to follow the segregation of the derived Rex phenotypic variant [98]. A refined analysis, using the same approach and applying a comparative mapping, identified that a single nucleotide deletion in exon 9 of the lipase member H (LIPH) gene was the causative mutation of this Rex locus [98]. This frameshift mutation (indicated with c.1362delA) introduces a downstream premature stop codon that alters the C-terminal region of the protein. LIPH encodes a membrane-bound member of the mammalian triglyceride lipase family that catalyzes the production of 2-acyl lysophosphatidic acid (LPA). This molecule is a lipid mediator with several biological properties on smooth muscle contraction, platelet aggregation, and stimulation of cell proliferation and motility. Some mutations affecting the structure and function of this gene in mice and humans have been shown to affect hair structure and growth among other defects (e.g., [99,100,101,102,103]). Transcriptional analysis of this gene in Rex and normal rabbit skins in both fetal and adult stages indicated that the mutated phenotype is associated to a three-time reduced expression level of LIPH in rabbits homozygous for the c.1362delA mutation [104].
4.2. A Marker in the Fibroblast Growth Factor 5 (FGF5) Gene is Associated to the Angora Locus
The Angora locus is due to an autosomal recessive allele (indicated with “l”) that determines an abnormal long hair growth with no other modifications on fiber structure or coat composition [105,106,107,108]. Hair growth lasts about 13 weeks in Angora rabbits, whereas it lasts only six weeks in the rabbits that are not homozygous for the mutated l allele. In mice, mutations disrupting the fibroblast growth factor 5 (Fgf5) gene produce an angora-like phenotype [109]. Using a candidate gene approach based on the evidences reported in mice, Mulsant et al. [110] investigated the rabbit FGF5 gene and identified a missense mutation in exon 3 that was in strong (even if not complete) linkage disequilibrium with the Angora allele. This gene, assigned to rabbit chromosome 15, was included in the chromosome region in which the Angora locus was located based on segregation analysis followed by microsatellite markers in rabbit families, further supporting the candidacy of FGF5 in this phenotype [111]. Transcriptome analyses based on RNA-seq of skin of long-hair Angora and short-hair Rex rabbits have shown that FGF5 gene expression was upregulated in long-haired rabbits [112,113]. This high FGF5 expression level in Angora rabbits is quite surprising considering that FGF5 has a role in inhibiting hair growth and inducing catagen through a regression of hair follicles [114]. This inhibiting role has been also demonstrated in rabbits when CRISPR/Cas9 mediated disruption of the FGF5 gene produced a long hair phenotype by prolonging anagen [115]. It could be possible that alternatively spliced FGF5 transcripts or other regulatory events might be involved in suppressing FGF5 activities in Angora rabbit skin [113,116]. Other gene expression studies in the skin of Angora rabbits have been carried out to clarify the regulating mechanisms determining anagen length in this model and the changes in gene expression over the hair cycle [117,118,119], but the causative mutation(s) of the Angora phenotype in rabbits has (have) not been identified yet.
5. Molecular Characterization of Other Loci Determining Breed and Strain Specific or Un-Specific Phenotypes
Other phenotypic traits that characterize different rabbit breeds or strains and that have been investigated at the molecular level are related to the body size and type of locomotion. Two of them specifically identify pet rabbits (dwarfism) or a unique strain with a peculiar locomotion behavior (Table 1). On the other hand, among the altered phenotypes, a few have (or had in the past) a quite broad diffusion in different breeds. This is the case of the yellow fat trait caused by the Yellow fat locus that has been characterized at the molecular level (Table 1). A more detailed description of the mutants and related causative variants or associated DNA markers is reported below.
Several putative loci affecting morphological traits (e.g., ear length, vertebral number) or causing gross abnormalities of the eyes, nervous system, and skeleton as well as of several other tissues and systems have been described in some other lines or strains [30] but have not been analyzed at the molecular level yet.
5.1. A Structural Mutation in the High-Mobility Group AT-hook 2 (HMGA2) Gene Determines a Dwarf Locus
Several dwarf breeds and strains, mainly used as pets, have been developed in rabbits. A few loci determining dwarfism have been described in this species in the pre-genomic era [30]. It is however not completely clear if they can be considered distinctive loci, or if they are determined by different alleles at the same locus and if modifying genes could be involved in further reducing body size.
The first form of dwarfism was described in a progeny of a Polish buck raised in US [120,121]. The condition was attributed to an incompletely recessive allele that was designated as dw. Homozygous dw/dw rabbits, called peanuts, are usually born inviable with about one third of the weight of the normal litter mates, with swollen head and other malformations. The heterozygous Dw/dw rabbits are viable and in adulthood can reach about two thirds of the size of homozygous Dw/Dw. Heterozygous Dw/dw rabbits also have a short snout determined by a modified craniofacial development, small ears, and a large disproportionate head in comparison to the compact and rounded body [30]. Carneiro et al. [122] sequenced the whole genome of rabbits with the three genotypes and identified the causal mutation of the dw allele that is determined by a 12.1 kb deletion affecting the high mobility AT-hook 2 (HMGA2) gene. HMGA2 encodes for an architectural factor that is a component of the enhancesome, which plays regulator roles during embryo development. This large deletion eliminates the promoter and the first three exons of this gene, resulting in its inactivation by suppressing the transcriptional regulation [122]. As a cascade effect, insulin-like growth factor 2 mRNA-binding protein 2 (IGF2BP2) expression is inhibited together with altered expression of several other genes that might help to disentangle the altered processes and related pathways leading to reduced size of the animals [122]. Based on these results, HMGA2 has been proposed as a major regulator of body size in mammals [122].
5.2. A Mutation in the RAR Related Orphan Receptor B (RORB) Gene and the Acrobat Locus
A peculiar behavior affecting the gait of the rabbits has been described by Létard [123,124]. The rabbits of the strain that was then developed (indicated with the name of sauteur d’Alfort or Alfort jumping rabbits) carry a recessive allele with symbol ak or Sam [97,123,124,125,126]. The homozygous ak/ak rabbits usually show an altered locomotion behavior based on a bipedal gait that makes use of the front legs [123,124,125,126]. This atypical gait is derived by a neurological alteration caused by a splice-site mutation in an evolutionary conserved nucleotide position of the RAR related orphan receptor B (RORB) gene, which produces aberrant transcripts [127]. This natural rabbit mutant has been important to illuminate the key role of RORB in neuronal differentiation of the rabbit spinal cord and the derived role in determining the normal locomotion behavior [127].
5.3. Molecular Characterization of a Biochemical Defect: The Yellow Fat Locus
A Mendelian recessive allele at the Yellow fat locus determines the excessive accumulation of xanthophylls in the fat, because these molecules cannot be degraded due to the absence of a key enzyme of this process. This biochemical defect has been reported in many rabbit breeds [128,129], but its occurrence is becoming rarer nowadays due to the negative selection against meat rabbits that have yellow fat driven by a reduced acceptance of this carcass appearance by the consumers in most countries.
To identify the causative mutation in rabbits, a candidate gene approach was used, considering that similar defects have been already characterized in other species. For example, mutations in the beta-carotene oxygenase 2 (BCO2) gene have been already associated with yellow fat in cattle, sheep, and chicken [130,131,132,133]. Then, Strychalski et al. [134] investigated the BCO2 gene in normal rabbits and in rabbits with the Yellow fat phenotype. An in-frame deletion of three nucleotides in the coding sequence of this gene gene was reported to be the causative mutation of the Yellow fat recessive allele [134]. Homozygous rabbits for this mutation have an increased accumulation of xanthophylls and beta-carotene in the liver, muscle, adipose tissue, and milk without any negative effects on growth and maternal performances compared to heterozygous or homozygous rabbits for the wild type allele [135,136,137].

Table 1.
Rabbit loci that have been characterized or partially described at the molecular level, corresponding mutations, and potential biomedical models of the related mutants.
Table 1.
Rabbit loci that have been characterized or partially described at the molecular level, corresponding mutations, and potential biomedical models of the related mutants.
| Locus | Gene Name | Gene Symbol | Alleles 1 | Sequences/Mutations 2 | Defects/Models 5 | References 6 |
|---|---|---|---|---|---|---|
| Extension | Melanocortin 1 receptor | MC1R | E+ | Several wild type alleles 3 | - | [41] |
| ED (dominant black) | 6 bp-in-frame deletion: c.280_285del6 | - | [41] | |||
| ES (steel) | Probably due to the same mutation of ED | - | [41] | |||
| eJ (Japanese brindling) | 6 bp-in frame deletion flanked by a G > A transition in 5’: c.[124G > A;125_130del6] | Potential model for gene expression regulation | [42] | |||
| e (red, non-extension of black) | 30 bp-in frame deletion: c.304_333del30 | - | [41] | |||
| Agouti | Agouti signaling protein | ASIP | A (light-bellied agouti; wild type) | Several wild type alleles 3,4 | - | [60] |
| at (black and tan) | p.L55M, p.K77R and p.L89P/11 kb deletion spanning the promoter and first exon | Potential model for gene expression regulation | [60,61] | |||
| a (recessive black non-agouti) | c.5_6insA | - | [60] | |||
| Albino | Tyrosinase | TYR | C (normal melanin production) | Several wild type alleles 3,4 | - | [63,70] |
| cchd (dark chinchilla) | p.E294G and p.T358I | - | [63,70] | |||
| cchm (medium chinchilla) | Not confirmed by molecular studies | - | - | |||
| cchl (light chinchilla) | Not confirmed by molecular studies | - | - | |||
| ch (Himalayan) | p.E294G (several haplotypes with this mutation) | - | [63,70] | |||
| c (Albino, lack of pigments) | p.T373K (several haplotypes with this mutation) | Potential model for oculocutaneous albinism type IA (OCA1A) | [63,70] | |||
| Dilute | Melanophilin | MLPH | D (wild type, intense black and red) | Several wild type alleles 3,4 | - | [74] |
| d (dilution of black to blue and red to yellow) | c.585delG (g.549853delG)—Two exon skipping mutation: c.111-5C > A | Potential model for Griscelli syndrome type 3 | [74,75,76] | |||
| Brown | Tyrosinase-related protein 1 | TYRP1 | B (wild type, production of black and brown eumelanin) | Several wild type alleles 3 | - | [80] |
| b (production of brown eumelanin) | p.W190ter (g.41360196G > A) | - | [80] | |||
| English spotting | v-kit Hardy-Zuckerman 4 feline sarcoma viral oncogene homolog | KIT | en (wild type, non-spotted coat colour, recessive) | Several wild type alleles 3,4 | - | [87] |
| En (English spotted, spotted patterns, partially dominant, megacolon) | g.93948587T > C (in complete linkage disequilibrium with the En allele) | Potential model for non-aganglionic megacolon | [87] | |||
| Rex 1 | Lipase member H | LIPH | R1 (wild type, normal presence of guard and awn hairs) | Several wild type alleles | - | [98] |
| r1 (absence of guard and awn hairs, fur with down hairs | c.1362delA | Potential model for hypothricosis and related defects | [98] | |||
| Angora | Fibroblast growth factor 5 | FGF5 | L (wild type, normal length of guard hairs) | Several wild type alleles (haplotypes) 3,4 | - | [110] |
| l (long hairs) | A missense mutation in exon 3 in linkage disequilibrium | Potential mode for hair growth regulation | [110] | |||
| Dwarf | High-mobility group AT-hook 2 | HMGA2 | Dw (normal size) | - | - | [122] |
| dw (proportionated dwarf-reduced size) | 12.1 kb deletion (including promoter and the first three exons) | Potential model to study growth and body size | [122] | |||
| Acrobat | RAR related orphan receptor B | RORB | Ak (normal locomotion) | - | - | [127] |
| ak or Sam (walks on forelegs) | a splice-site mutation in an evolutionary conserved nucleotide position | Potential model for neuronal differentiation and locomotion behavior | [127] | |||
| Yellow fat | Beta-carotene oxygenase 2 | BCO2 | Y (wild type, normal color of fat) | Wild type sequence | - | [134] |
| y (yellow fat) | AAT-deletion at codon 248 | Potential model for carotenoid catabolism | [134] |
1 Alleles described by classical genetic studies and the reported effect [30,31]. 2 Sequences and mutations that can describe the different alleles. Some alleles described by classical genetic studies have not been confirmed by molecular studies so far. 3 The sequences of the wild type forms of the gene reported synonymous mutations in the coding region and/or polymorphisms in the non-coding regions. 4 The sequences of the wild type forms of the gene reported missense mutations. 5 List of potential models for different biological functions. All loci affecting coat color and color patterns can be useful to explain some basic mechanisms of pigmentation and, for brevity, their role has not been reported in this column. 6 Only references that described the molecular characterization of the loci have been reported.

Table 2.
Genotypes reported at six coat color genes (described in Table 1) in different rabbit breeds. Genotyping information reported for the listed breeds and coat color morphs has been compiled from [41,42,60,63,70,74,76,80,87]. Alleles (and genotypes) have been named following the classical nomenclature for all genes except for the KIT gene, where molecular information derived by the g.93948587T > C SNP was reported [87].
Table 2.
Genotypes reported at six coat color genes (described in Table 1) in different rabbit breeds. Genotyping information reported for the listed breeds and coat color morphs has been compiled from [41,42,60,63,70,74,76,80,87]. Alleles (and genotypes) have been named following the classical nomenclature for all genes except for the KIT gene, where molecular information derived by the g.93948587T > C SNP was reported [87].
| Breeds 1 | Coat Colour of the Animals 2 | MC1R 3 | ASIP 4 | TYR 5 | MLPH 6 | TYRP1 7 | KIT 8 |
|---|---|---|---|---|---|---|---|
| Alaska | self black | wt/wt | a/a | wt/wt | wt/wt | wt/wt | - |
| Belgian Hare | reddish laced with black | wt/wt | (wt/wt: haplotype p77R + p.89P) | wt/wt | wt/wt | wt/wt | - |
| Blanc de Hotot | white with black markings | wt/wt | a/a | wt/wt | wt/wt | wt/wt | - |
| Burgundy Fawn | fawn | e/e | wt/wt | wt/wt | wt/wt | wt/wt | - |
| Californian | white with black markings | ED/ED | wt/wt, wt/a, a/a | ch/ch | wt/wt, wt/d | wt/wt | T/T, T/C, C/C |
| Californian | white with blue markings[d/d] | ED/ED | wt/wt, wt/a, a/a | ch/ch | d/d | wt/wt | T/T, T/C, C/C |
| Champagne d’Argent | silver as surface colour and black as under-colour | wt/wt | a/a | wt/wt | wt/wt | wt/w | C/C |
| Checkered Giant | white with black spots | ED/ED, ED/eJ | wt/a, a/a | wt/wt | wt/wt, wt/d | wt/wt | T/C |
| Checkered Giant | white with blue spots | ED/ED | wt/a, a/a | wt/wt | d/d | wt/wt | T/C |
| Dutch | with black markings | wt/wt, wt/ED, ED/ED | a/a | wt/wt | wt/wt | wt/wt | T/T, T/C, C/C |
| Dutch | tricolor | eJ/eJ | a/a | wt/wt | wt/wt | - | - |
| English Lop | shaded yellow/brown | e/e | - | - | - | - | - |
| English Spot | white with black markings | wt/wt | a/a | wt/wt | wt/wt | wt/wt | C/C |
| English Spot | white with blue markings | wt/wt | a/a | wt/wt | d/d | wt/wt | C/C |
| Fairy Marburg | grey-light blue | wt/wt | a/a | - | d/d | - | - |
| Fairy Pearly | pearling grey | wt/wt | wt/a, a/a | - | d/d | - | - |
| Fox | dark blue | wt/wt | - | - | d/d | - | - |
| Giant Chinchilla | chinchilla | wt/wt | wt/wt | cch/cch | wt/wt | wt/wt | T/C, C/C |
| Giant Grey | wild-grey | wt/wt, wt/eJ | wt/wt, wt/a | wt/wt | wt/wt | wt/wt | T/T, T/C- |
| Giant White | white albino | ED/ED | wt/wt | c/c | wt/wt | wt/wt | T/T |
| Gold Saxony | red | e/e | - | - | - | wt/wt | - |
| Havana | brown | wt/wt | a/a | wt/wt | wt/wt | b/b | - |
| Japanese | Japanese brindling | eJ/eJ | - | - | - | wt/wt | C/C |
| Leprino di Viterbo | wild-grey | wt/wt | - | wt/wt | - | wt/wt | - |
| Lop | wild-grey | wt/wt | wt/wt, wt/a, a/a | wt/wt | wt/wt | wt/wt | - |
| New Zealand Red | solid red | e/e | wt/wt | wt/wt | - | wt/wt | - |
| New Zealand White | white-albino | ED/ED | wt/wt, wt/a, a/a | c/c | wt/wt | wt/wt | C/C |
| Rhinelander | white with black and yellow markings | eJ/eJ | wt/wt, wt/a, a/a | wt/wt | wt/wt | wt/wt | T/C |
| Russian | white with black markings | wt/wt | a/a | cch/cch | wt/wt | - | - |
| Silver | black with silvering | wt/wt | a/a | wt/wt | wt/wt | wt/wt | - |
| Tan | black fire | wt/wt | at/at | - | wt/wt | - | - |
| Thuringian | shaded yellow/brown | e/e | a/a | wt/wt | wt/wt | wt/wt | - |
| Vienna Blue | dark blue | wt/wt | a/a | wt/wt | d/d | wt/wt | - |
| Vienna White | white-blue eyes | wt/wt | wt/a, a/a | wt/wt | wt/wt | wt/wt | - |
1 Only breeds for which at least two loci have been genotyped are listed here. For a few breeds, more entries were included when the genotyped animals had different coat colors. Some breeds could have different names in different countries. For some breeds, genotyping information has been obtained on a limited number of animals. When no genotyping information was available, the symbol “-” was included. 2 Description of the coat color and color patterns of the genotyped animals. 3 Genotyping information at the melanocortin 1 receptor (MC1R) gene (Extension or E locus): wt = wild type allele(s); ED allele (and/or ES allele) = Δ6 or c.280_285del6; eJ allele = Δ6J or c.[124G>A;125_130del6]; e allele = Δ30 or c.304_333del30 [41,42]. 4 Genotyping information at the agouti signaling protein (ASIP) gene (Agouti or A locus): wt = wild type allele(s); the wild type allele identified in the Belgian Hare breed has been reported using the haplotype information of the two missense mutations (p.K77R and p.L89P); at (black and tan allele) characterized by a large haplotype that includes three missense mutations (p.L55M, p.K77R and p.L89P) and a 11 kb deletion spanning the promoter and first exon; a (non-agouti black allele) determined by the c.5_6insA insertion [60,61]. 5 Genotyping information at the tyrosinase (TYR) gene (Albino or C locus): wt = wild type allele(s); cch (chinchilla allele) = haplotype at the two missense mutations p.294G and p.358I (p.G294 + p.I358); ch (Himalayan allele) = allele p.G294 at the p.E294G missense mutation; c (albino allele) = allele p.K373 at the p.T373K missense mutation [63,70]. 6 Genotyping information at the melanophilin (MLPH) gene (Dilute or D locus): wt = wild type allele(s); d (dilute allele) = c.585delG (deletion of one G). 7 Genotyping information at the tyrosinase-related protein 1 (TYRP1) gene (Brown or B locus): wt = wild type allele(s); b (brown allele) = g.41360196A determining a premature stop codon [80]. 8 Genotyping information at the v-kit Hardy-Zuckerman 4 feline sarcoma viral oncogene homolog (KIT) gene for the g.93948587T > C single nucleotide polymorphism associated with the English spotted locus [87].
6. Conclusions
Rabbit genetic resources that have been developed by fancy breeders (mainly to support the request of pets) and by commercial breeders (for meat and fur production) through selection and, in many cases, fixation of monogenic or polygenic traits constitute an important reservoir of genetic variability that can be exploited for different purposes. Monogenic or oligogenic traits described in this review can provide interesting models to understand basic biological processes and physiological mechanisms that could have relevant roles to complement studies in rodents and humans. Genomic tools available in rabbits will provide additional information on genes affecting complex traits through genome-wide association studies. Gene editing approaches will find many applications in rabbit to confirm the effect of natural occurred mutations.
Funding
This study was supported by University of Bologna RFO 2019-2020 funds.
Acknowledgments
This study is associated with the activities of the RGB-Net COST Action TD1101.
Conflicts of Interest
The author declares no conflict of interest. The funder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Fontanesi, L. The rabbit in the genomics era: Applications and perspectives in rabbit biology and breeding. In Proceedings of the 11th World Rabbit Congress, Qingdao, China, 15–18 June 2016. [Google Scholar]
- Shiomi, M. Rabbit as Model for the Study of Human Diseases. In Rabbit Biotechnology: Rabbit Genomics, Transgenesis, Cloning and Models; Houdebine, L.-M., Fan, J., Eds.; Springer: Dordrecht, Germany, 2009; pp. 49–63. [Google Scholar] [CrossRef]
- Esteves, P.J.; Abrantes, J.; Baldauf, H.M.; BenMohamed, L.; Chen, Y.; Christensen, N.; González-Gallego, J.; Giacani, L.; Hu, J.; Kaplan, G.; et al. The wide utility of rabbits as models of human diseases. Exp. Mol. Med. 2018, 50, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mage, R.G.; Esteves, P.J.; Rader, C. Rabbit models of human diseases for diagnostics and therapeutics development. Dev. Comp. Immunol. 2019, 92, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.; Peng, H.; Rader, C. From rabbit antibody repertoires to rabbit monoclonal antibodies. Exp. Mol. Med. 2017, 49, e305. [Google Scholar] [CrossRef] [PubMed]
- Thompson, H.V.; King, C.M. The European Rabbit: The History and Biology of a Successful Colonizer; Oxford University Press: Oxford, UK, 1994; pp. 1–245. [Google Scholar]
- Ennafaa, H.; Monnerot, M.; El Gaaïed, A.; Mounolou, J.C. Rabbit mitochondrial DNA: Preliminary comparison between some domestic and wild animals. Genet. Sel. Evol. 1987, 19, 279–288. [Google Scholar] [CrossRef]
- Biju-Duval, C.; Ennafaa, H.; Dennebouy, N.; Monnerot, M.; Mignotte, F.; Soriguer, R.; El Gaieed, A.; El Hili, A.; Mounolou, J.-C. Mitochondrial DNA evolution in lagomorphs: Origin of systematic heteroplasmy and organization of diversity in European rabbits. J. Mol. Evol. 1991, 33, 92–102. [Google Scholar] [CrossRef]
- Monnerot, M.; Vigne, J.D.; Biju-Duval, C.; Casane, D.; Callou, C.; Hardy, C.; Mougel, F.; Soriguer, R.; Dennebouy, N.; Mounolou, J.-C. Rabbit and man: Genetic and historic approach. Genet. Sel. Evol. 1994, 26 (Suppl. 1), 167S–182S. [Google Scholar] [CrossRef]
- van der Loo, W.; Ferrand, N.; Soriguer, R. Estimation of gene diversity at the b locus of the constant region of the immunoglobulin light chain in natural populations of European rabbit (Oryctolagus cuniculus) in Portugal, Andalusia and on the Azorean islands. Genetics 1991, 127, 789–799. [Google Scholar]
- Sharples, C.M.; Fa, J.E.; Bell, D.J. Geographical variation in size in the European rabbit Oryctolagus cuniculus (Lagomorpha: Leporidae) in western Europe and North Africa. Zool. J. Linnean Soc. 1996, 117, 141–158. [Google Scholar] [CrossRef]
- Branco, M.; Ferrand, N.; Monnerot, M. Phylogeography of the European rabbit (Oryctolagus cuniculus) on the Iberian Peninsula inferred from RFLP analysis of the cytochrome b gene. Heredity 2000, 85, 307–317. [Google Scholar] [CrossRef]
- Branco, M.; Monnerot, M.; Ferrand, N.; Templeton, A.R. Postglacial dispersal of the European rabbit (Oryctolagus cuniculus) on the Iberian Peninsula reconstructed from nested clade and mismatch analyses of mitochondrial DNA genetic variation. Evolution 2002, 56, 792–803. [Google Scholar] [CrossRef]
- Ferrand, N. Inferring the evolutionary history of the European rabbit (Oryctolagus cuniculus) from molecular markers. In Lagomorph Biology: Evolution, Ecology, and Conservation; Alves, P.C., Ferrand, N., Hackländer, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 47–63. [Google Scholar] [CrossRef]
- Carneiro, M.; Rubin, C.J.; Di Palma, F.; Albert, F.W.; Alföldi, J.; Barrio, A.M.; Pielberg, G.; Rafati, N.; Sayyab, S.; Turner-Maier, J.; et al. Rabbit genome analysis reveals a polygenic basis for phenotypic change during domestication. Science 2014, 345, 1074–1079. [Google Scholar] [CrossRef] [PubMed]
- Alves, J.M.; Carneiro, M.; Afonso, S.; Lopes, S.; Garreau, H.; Boucher, S.; Allain, D.; Queney, G.; Esteves, P.J.; Bolet, G.; et al. Levels and patterns of genetic diversity and population structure in domestic rabbits. PLoS ONE 2015, 10, e0144687. [Google Scholar] [CrossRef] [PubMed]
- Callou, C. Modifications de l’aire de répartition du lapin (Oryctolagus cuniculus) en France et en Espagne, du Pléistocène à l’époque actuelle. Etat de la question. Anthropozoologica 1995, 21, 95–114. [Google Scholar]
- Callou, C. De la garenne au clapier. Étude archéozoologique du Lapin en Europe occidentale; Mémoires do Muséum National d’Histoire Naturelle: Paris, France, 2003. [Google Scholar]
- Zeder, M.A. Central questions in the domestication of plants and animals. Evol. Anthropol. 2006, 15, 105–117. [Google Scholar] [CrossRef]
- Zeder, M.A. The domestication of animals. J. Anthropol. Res. 2012, 68, 161–190. [Google Scholar] [CrossRef]
- Larson, G.; Burger, J. A population genetics view of animal domestication. Trends Genet. 2013, 29, 197–205. [Google Scholar] [CrossRef]
- Irving-Pease, E.K.; Frantz, L.A.; Sykes, N.; Callou, C.; Larson, G. Rabbits and the specious origins of domestication. Trends Ecol. Evol. 2018, 33, 149–152. [Google Scholar] [CrossRef]
- Hardy, C.; Callou, C.; Vigne, J.-D.; Casane, D.; Dennebouy, N.; Mounolou, J.-C.; Monnerot, M. Rabbit mitochondrial DNA diversity from prehistoric to modern times. J. Mol. Evol. 1995, 40, 227–237. [Google Scholar] [CrossRef]
- Queney, G.; Vachot, A.M.; Brun, J.M.; Dennebouy, N.; Mulsant, P.; Monnerot, M. Different levels of human intervention in domestic rabbits: Effects on genetic diversity. J. Hered. 2002, 93, 205–209. [Google Scholar] [CrossRef][Green Version]
- Carneiro, M.; Afonso, S.; Geraldes, A.; Garreau, H.; Bolet, G.; Boucher, S.; Tircazes, A.; Queney, G.; Nachman, M.W.; Ferrand, N. The genetic structure of domestic rabbits. Mol. Biol. Evol. 2011, 28, 1801–1816. [Google Scholar] [CrossRef]
- Zeuner, F.E. A History of Domesticated Animals; Harper & Row Publishers: Evanston, UK, 1963; pp. 409–415. [Google Scholar]
- Carneiro, M.; Piorno, V.; Rubin, C.J.; Alves, J.M.; Ferrand, N.; Alves, P.C.; Andersson, L. Candidate genes underlying heritable differences in reproductive seasonality between wild and domestic rabbits. Anim. Genet. 2015, 46, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Brusini, I.; Carneiro, M.; Wang, C.; Rubin, C.J.; Ring, H.; Afonso, S.; Blanco-Aguiar, J.A.; Ferrand, N.; Rafati, N.; Villafuerte, R.; et al. Changes in brain architecture are consistent with altered fear processing in domestic rabbits. Proc. Natl. Acad. Sci. USA 2018, 115, 7380–7385. [Google Scholar] [CrossRef] [PubMed]
- Whitman, B.D. Domestic Rabbits & Their Histories: Breeds of the World; Leathers Publishing: Overland Parks, KS, USA, 2004; pp. 1–456. [Google Scholar]
- Robinson, R. Genetic studies of the rabbit. Bibl. Genet. 1958, 17, 229–558. [Google Scholar]
- Searle, A.G. Comparative Genetics of Coat Colour in Mammals; Logos Press: London, UK, 1968; pp. 126–137. [Google Scholar]
- Robbins, L.S.; Nadeau, J.H.; Johnson, K.R.; Kelly, M.A.; Roselli-Rehfuss, L.; Baack, E.; Mountjoy, K.G.; Cone, R.D. Pigmentation phenotypes of variant extension locus alleles result from point mutations that alter MSH receptor function. Cell 1993, 72, 827–834. [Google Scholar] [CrossRef]
- Klungland, H.; Våge, D.I.; Gomez-Raya, L.; Adalsteinsson, S.; Lien, S. The role of melanocyte-stimulating hormone (MSH) receptor in bovine coat color determination. Mamm. Genome 1995, 6, 636–639. [Google Scholar] [CrossRef] [PubMed]
- Kijas, J.M.; Wales, R.; Törnsten, A.; Chardon, P.; Moller, M.; Andersson, L. Melanocortin receptor 1 (MC1R) mutations and coat color in pigs. Genetics 1998, 150, 1177–1185. [Google Scholar]
- Fontanesi, L.; Beretti, F.; Riggio, V.; Dall’Olio, S.; González, E.G.; Finocchiaro, R.; Davoli, R.; Russo, V.; Portolano, B. Missense and nonsense mutations in melanocortin 1 receptor (MC1R) gene of different goat breeds: Association with red and black coat colour phenotypes but with unexpected evidences. BMC Genet. 2009, 10, 47. [Google Scholar] [CrossRef]
- Fontanesi, L.; Beretti, F.; Riggio, V.; Dall’Olio, S.; Calascibetta, D.; Russo, V.; Portolano, B. Sequence characterization of the melanocortin 1 receptor (MC1R) gene in sheep with different coat colours and identification of the putative e allele at the ovine Extension locus. Small Rum. Res. 2010, 91, 200–207. [Google Scholar] [CrossRef]
- Punnett, R.C. Inheritance of coat colour in rabbits. J. Genet. 1912, 2, 221–238. [Google Scholar] [CrossRef]
- Punnett, R.C. On the “Japanese” rabbit. J. Genet. 1924, 14, 230–240. [Google Scholar] [CrossRef]
- Punnett, R.C. On the series of allelomorphs connected with the production of black pigment in rabbit. J. Genet. 1930, 23, 265–274. [Google Scholar] [CrossRef]
- Castle, W.E. Genetics of the Japanese rabbit. J. Genet. 1924, 14, 225–229. [Google Scholar] [CrossRef]
- Fontanesi, L.; Tazzoli, M.; Beretti, F.; Russo, V. Mutations in the melanocortin 1 receptor (MC1R) gene are associated with coat colours in the domestic rabbit (Oryctolagus cuniculus). Anim. Genet. 2006, 37, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Fontanesi, L.; Scotti, E.; Colombo, M.; Beretti, F.; Forestier, L.; Dall’Olio, S.; Deretz, S.; Russo, V.; Allain, D.; Oulmouden, A. A composite six bp in-frame deletion in the melanocortin 1 receptor (MC1R) gene is associated with the Japanese brindling coat colour in rabbits (Oryctolagus cuniculus). BMC Genet. 2010, 11, 59. [Google Scholar] [CrossRef] [PubMed]
- Newton, J.M.; Wilkie, A.L.; He, L.; Jordan, S.A.; Metallinos, D.L.; Holmes, N.G.; Jackson, I.J.; Barsh, G.S. Melanocortin 1 receptor variation in the domestic dog. Mamm. Genome 2000, 11, 24–30. [Google Scholar] [CrossRef]
- Everts, R.E.; Rothuizen, J.; Van Oost, B.A. Identification of a premature stop codon in the melanocyte-stimulating hormone receptor gene (MC1R) in Labrador and Golden retrievers with yellow coat colour. Anim. Genet. 2000, 31, 194–199. [Google Scholar] [CrossRef]
- Våge, D.I.; Klungland, H.; Lu, D.; Cone, R.D. Molecular and pharmacological characterization of dominant black coat color in sheep. Mamm. Genome 1999, 10, 39–43. [Google Scholar] [CrossRef]
- Marklund, L.; Moller, M.; Sandberg, K.; Andersson, L. A missense mutation in the gene for melanocyte-stimulating hormone receptor (MC1R) is associated with the chestnut coat color in horses. Mamm. Genome 1996, 7, 895–899. [Google Scholar] [CrossRef]
- Abitbol, M.; Legrand, R.; Tiret, L. A missense mutation in melanocortin 1 receptor is associated with the red coat colour in donkeys. Anim. Genet. 2014, 45, 878–880. [Google Scholar] [CrossRef]
- Kerje, S.; Lind, J.; Schütz, K.; Jensen, P.; Andersson, L. Melanocortin 1-receptor (MC1R) mutations are associated with plumage colour in chicken. Anim. Genet. 2003, 34, 241–248. [Google Scholar] [CrossRef]
- Gustafson, N.A.; Gandolfi, B.; Lyons, L.A. Not another type of potato: MC1R and the russet coloration of Burmese cats. Anim. Genet. 2017, 48, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Abitbol, M.; Gache, V. Copal, a new MC1R allele in the domestic cat. Anim. Genet. 2019, 50, 553–554. [Google Scholar] [CrossRef] [PubMed]
- Bultman, S.J.; Michaud, E.J.; Woychik, R.P. Molecular characterization of the mouse agouti locus. Cell 1992, 71, 1195–1204. [Google Scholar] [CrossRef]
- Lu, D.; Willard, D.; Patel, I.R.; Kadwell, S.; Overton, L.; Kost, T.; Luther, M.; Chen, W.; Woychik, R.P.; Wilkison, W.O.; et al. Agouti protein is an antagonist of the melanocyte-stimulating-hormone receptor. Nature 1994, 371, 799–802. [Google Scholar] [CrossRef] [PubMed]
- Ollmann, M.M.; Lamoreux, M.L.; Wilson, B.D.; Barsh, G.S. Interaction of Agouti protein with the melanocortin 1 receptor in vitro and in vivo. Genes Dev. 1998, 12, 316–330. [Google Scholar] [CrossRef]
- Kuramoto, T.; Nomato, T.; Sugimura, T.; Ushijima, T. Cloning of the rat agouti gene and identification of the rat nonagouti mutation. Mamm. Genome 2001, 12, 469–471. [Google Scholar] [CrossRef]
- Miltenberger, R.J.; Wakumatsu, K.; Ito, S.; Woychik, R.P.; Russell, L.B.; Michaud, E.J. Molecular and phenotypic analysis of 25 recessive, homozygous-viable alleles at the mouse agouti locus. Genetics 2002, 160, 659–674. [Google Scholar]
- Kerns, J.A.; Newton, J.; Berryere, T.G.; Rubin, E.M.; Cheng, J.F.; Schmutz, S.M.; Barsh, G.S. Characterization of the dog Agouti gene and a nonagouti mutation in German Shepherd dogs. Mamm. Genome 2004, 15, 798–808. [Google Scholar] [CrossRef]
- Norris, B.J.; Whan, V.A. A gene duplication affecting expression of the ovine ASIP gene is responsible for white and black sheep. Genome Res. 2008, 18, 1282–1293. [Google Scholar] [CrossRef]
- Fontanesi, L.; Dall’Olio, S.; Beretti, F.; Portolano, B.; Russo, V. Coat colours in the Massese sheep breed are associated with mutations in the agouti signalling protein (ASIP) and melanocortin 1 receptor (MC1R) genes. Animal 2011, 5, 8–17. [Google Scholar] [CrossRef]
- Silvers, W.K. The agouti and extension series of alleles, umbrous, and sable. In The Coat Colors of Mice: A Model for Mammalian Gene Action and Interaction; Silver, W.K., Ed.; Springer: New York, NY, USA, 1979; pp. 6–44. [Google Scholar]
- Fontanesi, L.; Forestier, L.; Allain, D.; Scotti, E.; Beretti, F.; Deretz-Picoulet, S.; Pecchioli, E.; Vernesi, C.; Robinson, T.J.; Malaney, J.L.; et al. Characterization of the rabbit agouti signaling protein (ASIP) gene: Transcripts and phylogenetic analyses and identification of the causative mutation of the nonagouti black coat colour. Genomics 2010, 95, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Letko, A.; Ammann, B.; Jagannathan, V.; Henkel, J.; Leuthard, F.; Schelling, C.; Carneiro, M.; Drögemüller, C.; Leeb, T. A deletion spanning the promoter and first exon of the hair cycle-specific ASIP transcript isoform in black and tan rabbits. Anim. Genet. 2020, 51, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Castle, W.E. The Genetics of Domestic Rabbit; Cambridge Harvard University Press: London, UK, 1930; pp. 1–31. [Google Scholar]
- Aigner, B.; Besenfelder, U.; Müller, M.; Brem, G. Tyrosinase gene variants in different rabbit strains. Mamm. Genome 2000, 11, 700–702. [Google Scholar] [CrossRef] [PubMed]
- Beermann, F.; Orlow, S.J.; Lamoreux, M.L. The Tyr (albino) locus of the laboratory mouse. Mamm. Genome 2004, 15, 749–758. [Google Scholar] [CrossRef]
- Grønskov, K.; Ek, J.; Brondum-Nielsen, K. Oculocutaneous albinism. Orphanet J. Rare Dis. 2007, 2, 43. [Google Scholar] [CrossRef]
- Castle, W.E. Heredity of Coat Characters in Guinea Pigs and Rabbits; Carnegie Institution Washington Publisher: Washington, DC, USA, 1905; Volume 23, pp. 1–78. [Google Scholar]
- Castle, W.E. Genetics of the chinchilla rabbit. Science 1921, 53, 387–388. [Google Scholar] [CrossRef]
- Sawin, P.B. Albino Allelomorphs of the Rabbit with Special Reference to Blue-Eyed Chinchilla and Its Variations; Carnegie Institution Washington Publisher: Washington, DC, USA, 1932; Volume 427, pp. 15–50. [Google Scholar]
- Sawin, P.B. Hereditary variation of the chinchilla rabbit in coat and eye color. J. Hered. 1932, 23, 39–46. [Google Scholar] [CrossRef]
- Utzeri, V.J.; Ribani, A.; Schiavo, G.; Fontanesi, L. Describing variability in the tyrosinase (TYR) gene, the albino coat colour locus, in domestic and wild European rabbits. Ital. J. Anim. Sci. 2021, in press. [Google Scholar]
- Mercer, J.A.; Seperack, P.K.; Strobel, M.C.; Copeland, N.G.; Jenkins, N.A. Novel myosin heavy chain encoded by murine dilute coat colour locus. Nature 1991, 349, 709–713. [Google Scholar] [CrossRef]
- Fontanesi, L.; Scotti, E.; Dall’Olio, S.; Oulmouden, A.; Russo, V. Identification and analysis of single nucleotide polymorphisms in the myosin VA (MYO5A) gene and its exclusion as the causative gene of the dilute coat colour locus in rabbit. World Rabbit. Sci. 2012, 20, 35–41. [Google Scholar] [CrossRef]
- Matesic, L.E.; Yip, R.; Reuss, A.E.; Swing, D.A.; O’Sullivan, T.N.; Fletcher, C.F.; Copeland, N.G.; Jenkins, N.A. Mutations in Mlph, encoding a member of the Rab effector family, cause the melanosome transport defects observed in leaden mice. Proc. Natl. Acad. Sci. USA 2001, 98, 10238–10243. [Google Scholar] [CrossRef] [PubMed]
- Fontanesi, L.; Scotti, E.; Allain, D.; Dall’Olio, S. A frameshift mutation in the melanophilin (MLPH) gene causes the dilute coat colour in rabbit (Oryctolagus cuniculus) breeds. Anim. Genet. 2014, 45, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Lehner, S.; Gähle, M.; Dierks, C.; Stelter, R.; Gerber, J.; Brehm, R.; Distl, O. Two-exon skipping within MLPH is associated with coat color dilution in rabbits. PLoS ONE 2013, 8, e84525. [Google Scholar] [CrossRef] [PubMed]
- Demars, J.; Iannuccelli, N.; Utzeri, V.J.; Auvinet, G.; Riquet, J.; Fontanesi, L.; Allain, D. New insights into the melanophilin (MLPH) gene affecting coat color dilution in rabbits. Genes 2018, 9, 430. [Google Scholar] [CrossRef] [PubMed]
- Sanal, O.; Ersoy, F.; Tezcan, I.; Metin, A.; Yel, L.; Menasche, G.; Gurgey, A.; Berkel, I.; de Basile, G.S. Griscelli disease: Genotype-phenotype correlation in an array of clinical heterogeneity. J. Clin. Immunol. 2002, 22, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Menasche, G.; Ho, C.H.; Sanal, O.; Feldmann, J.; Tezcan, I.; Ersoy, F.; Houdusse, A.; Fischer, A.; de Basile, G.S. Griscelli syndrome restricted to hypopigmentation results from a melanophilin defect (GS3) or a MYO5A F-exon deletion (GS1). J. Clin. Investig. 2003, 112, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Jackson, I.J. A cDNA encoding tyrosinase-related protein maps to the mouse brown locus. Proc. Natl. Acad. Sci. USA 1988, 85, 4391–4396. [Google Scholar] [CrossRef]
- Utzeri, V.J.; Ribani, A.; Fontanesi, L. A premature stop codon in the TYRP1 gene is associated with brown coat colour in the European rabbit (Oryctolagus cuniculus). Anim. Genet. 2014, 45, 600–603. [Google Scholar] [CrossRef]
- Castle, W.E. Studies of heredity in rabbits, rats and mice. Carnegie Inst. Wash. Publ. 1909, 288, 4–28. [Google Scholar]
- Richardson, E.C. Inheritance of white in the “English” rabbit. Heredity 1953, 7, 150. [Google Scholar]
- Nachtsheim, H. Ergebrisse und Probleme der Vergleichenden und Experimentellen Erbpathologie. Jena. Z. für Nat. 1943, 76, 81–108. [Google Scholar]
- Wieberneit, D.; Mahdi, N.; Zacharias, K.; Wegner, W. Zur Problematik der Scheckenzucht bei Kaninchen. 1. Mitteilung: Mast- und Schlachtkörpereigenschaften, Organbefunde. Dtsch. Tierärztliche Wochenschr. 1990, 98, 352–354. [Google Scholar]
- Böderek, D.; Türk, O.; Lovén, E.; Wieberneit, D.; Wegner, W. Pathophysiological and functional aspects of the Megacolon-Syndrome of homozygous Spotted rabbits. J. Vet. Med. A 1995, 42, 549–559. [Google Scholar] [CrossRef]
- Wieberneit, D.; Wegner, W. Albino rabbits can suffer from Megacolon-Syndrome when they are homozygous for the “English-spot” gene (En En). World Rabbit. Sci. 1995, 3, 19–26. [Google Scholar] [CrossRef][Green Version]
- Fontanesi, L.; Vargiolu, M.; Scotti, E.; Latorre, R.; Pellegrini, M.S.F.; Mazzoni, M.; Asti, M.; Chiocchetti, R.; Romeo, G.; Clavenzani, P.; et al. The KIT gene is associated with the English spotting coat color locus and congenital megacolon in Checkered Giant rabbits (Oryctolagus cuniculus). PLoS ONE 2014, 9, e93750. [Google Scholar] [CrossRef] [PubMed]
- Gerlitz, S.; Wessel, G.; Wieberneit, D.; Wegner, W. Zur Problematik der Scheckenzucht bei Kaninchen. 3. Mitteilung: Variabilität des Pigmentierungsgrades, ganglionäre Darmwandversorgung, Beziehung zur Pathogenese—tierzüchterische und tierschützerische Aspekte. Dtsch. Tierärztliche Wochenschr. 1993, 100, 237–239. [Google Scholar]
- Fontanesi, L.; Vargiolu, M.; Scotti, E.; Mazzoni, M.; Clavenzani, P.; De Giorgio, R.; Romeo, G.; Russo, V. Endothelin receptor B (EDNRB) is not the causative gene of the English spotting locus in the domestic rabbit (Oryctolagus cuniculus). Anim. Genet. 2010, 41, 669–670. [Google Scholar] [CrossRef]
- Besmer, P.; Manova, K.; Duttlinger, R.; Huang, E.J.; Packer, A.; Gyssler, C.; Bachvarova, R.F. The kit-ligand (steel factor) and its receptor c-kit/W: Pleiotropic roles in gametogenesis and melanogenesis. Development 1993, 1, 125–137. [Google Scholar]
- Thomas, A.J.; Erickson, C.A. The making of a melanocyte: The specification of melanoblasts from the neural crest. Pigment. Cell Mel. Res. 2008, 21, 598–610. [Google Scholar] [CrossRef]
- Farrugia, G. Interstitial cells of Cajal in health and disease. Neurogastroenterol. Motil. 2008, 20, 54–63. [Google Scholar] [CrossRef]
- Alonso, L.; Fuchs, E. The hair cycle. J. Cell Sci. 2006, 119, 391–393. [Google Scholar] [CrossRef] [PubMed]
- Lienhart, R. Apropos d’une recente mutation chez le lapin domestique, le lapin Castorrex. C. R. Seances Soc. Biol. Ses. Fil. 1927, 97, 386–388. [Google Scholar]
- Létard, E. Le lapin Castorrex, L’histoire d’une mutation. Rev. de Méd. Vét. de Toulouse 1928, 80, 136–143. [Google Scholar]
- Castle, W.E.; Nachtsheim, H. Linkage interrelations of three genes for rex (short) coat in the rabbit. Proc. Natl. Acad. Sci. USA 1933, 19, 1006–1011. [Google Scholar] [CrossRef]
- Fox, R.R. Taxonomy and genetics. In The Biology of the Laboratory Rabbit, 2nd ed.; Manning, P.J., Ringler, D.H., Newcomer, C.E., Eds.; Academic Press: San Diego, CA, USA, 1994; pp. 1–26. [Google Scholar]
- Diribarne, M.; Mata, X.; Chantry-Darmon, C.; Vaiman, A.; Auvinet, G.; Bouet, S.; Deretz, S.; Cribiu, E.P.; de Rochambeau, H.; Allain, D.; et al. A deletion in exon 9 of the LIPH gene is responsible for the rex hair coat phenotype in rabbits (Oryctolagus cuniculus). PLoS ONE 2011, 6, e19281. [Google Scholar] [CrossRef]
- Wen, X.Y.; Bryce, D.M.; Breitman, M.L. Characterization of lpd (lipid defect): A novel mutation on mouse chromosome 16 associated with a defect in triglyceride metabolism. Hum. Mol. Genet. 1998, 7, 743–750. [Google Scholar] [CrossRef][Green Version]
- Kazantseva, A.; Goltsov, A.; Zinchenko, R.; Grigorenko, A.P.; Abrukova, A.V.; Moliaka, Y.K.; Kirillov, A.G.; Guo, Z.; Lyle, S.; Ginter, E.K.; et al. Human hair growth deficiency is linked to a genetic defect in the phospholipase gene LIPH. Science 2006, 314, 982–985. [Google Scholar] [CrossRef]
- Shimomura, Y.; Wajid, M.; Petukhova, L.; Shapiro, L.; Christiano, A.M. Mutations in the lipase H gene underlie autosomal recessive woolly hair/hypotrichosis. J. Investig. Dermatol. 2009, 129, 622–628. [Google Scholar] [CrossRef]
- Kinoshita-Ise, M.; Kubo, A.; Sasaki, T.; Umegaki-Arao, N.; Amagai, M.; Ohyama, M. Identification of factors contributing to phenotypic divergence via quantitative image analyses of autosomal recessive woolly hair/hypotrichosis with homozygous c.736T>A LIPH mutation. Br. J. Dermatol. 2017, 176, 138–144. [Google Scholar] [CrossRef]
- Takeichi, T.; Tanahashi, K.; Taki, T.; Kono, M.; Sugiura, K.; Akiyama, M. Mutational analysis of 29 patients with autosomal-recessive woolly hair and hypotrichosis: LIPH mutations are extremely predominant in autosomal-recessive woolly hair and hypotrichosis in Japan. Br. J. Dermatol. 2017, 177, 290–292. [Google Scholar] [CrossRef]
- Diribarne, M.; Mata, X.; Rivière, J.; Bouet, S.; Vaiman, A.; Chapuis, J.; Reine, F.; Fleurot, R.; Auvinet, G.; Deretz, S.; et al. LIPH expression in skin and hair follicles of normal coat and Rex rabbits. PLoS ONE 2012, 7, e30073. [Google Scholar] [CrossRef] [PubMed]
- Castle, W.E. The heredity of “angora” coat in mammals. Science 1903, 18, 760–761. [Google Scholar] [CrossRef] [PubMed]
- Wucherer, E. Überden character des Angorahaares. Z. J. Tierz. Zuchtungbiol. 1925, 4, 119–143. [Google Scholar]
- Hardy, T.M.P.; Markley, M.H. Microscopic study of coat variation in white New Zealand and Angora rabbits. J. Hered. 1944, 35, 183–192. [Google Scholar] [CrossRef]
- Crary, D.D.; Sawin, P.B. Some factors influencing the growth potential of the skin in the domestic rabbit. J. Exp. Zool. 1953, 124, 31–62. [Google Scholar] [CrossRef]
- Hébert, J.M.; Rosenquist, T.; Götz, J.; Martin, G.R. FGF5 as a regulator of the hair growth cycle: Evidence from targeted and spontaneous mutations. Cell 1995, 78, 1017–1025. [Google Scholar] [CrossRef]
- Mulsant, P.; Rochambeau, H.D.; Thébault, R.G. A note on linkage between the angora and fgf5 genes in rabbits. World Rabbit. Sci. 2004, 12, 1–6. [Google Scholar] [CrossRef]
- Chantry-Darmon, C.; Urien, C.; de Rochambeau, H.; Allain, D.; Pena, B.; Hayes, H.; Grohs, C.; Cribiu, E.P.; Deretz-Picoulet, S.; Larzul, C.; et al. A first-generation microsatellite-based integrated genetic and cytogenetic map for the European rabbit (Oryctolagus cuniculus) and localization of angora and albino. Anim. Genet. 2006, 37, 335–341. [Google Scholar] [CrossRef]
- Zhao, B.; Chen, Y.; Hao, Y.; Yang, N.; Wang, M.; Mei, M.; Wang, J.; Qiu, X.; Wu, X. Transcriptomic analysis reveals differentially expressed genes associated with wool length in rabbit. Anim. Genet. 2018, 49, 428–437. [Google Scholar] [CrossRef]
- Ding, H.; Zhao, H.; Cheng, G.; Yang, Y.; Wang, X.; Zhao, X.; Qi, Y.; Huang, D. Analyses of histological and transcriptome differences in the skin of short-hair and long-hair rabbits. BMC Genomics 2019, 20, 140. [Google Scholar] [CrossRef]
- Higgins, C.A.; Petukhova, L.; Harel, S.; Ho, Y.Y.; Drill, E.; Shapiro, L.; Wajid, M.; Christiano, A.M. FGF5 is a crucial regulator of hair length in humans. Proc. Natl. Acad. Sci. USA 2014, 111, 10648–10653. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, H.; Pan, H.; Wang, X.; Zhang, Y.; Yao, B.; Li, N.; Lai, L.; Li, Z. CRISPR/Cas9-mediated disruption of Fibroblast Growth Factor 5 in rabbits results in a systemic long hair phenotype by prolonging anagen. Genes 2020, 11, 297. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Cheng, G.; Leng, J.; Yang, Y.; Zhao, X.; Wang, X.; Qi, Y.; Huang, D.; Zhao, H. Analysis of histological and microRNA profiles changes in rabbit skin development. Sci. Rep. 2020, 10, 454. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Chen, Y.; Hu, S.; Yang, N.; Wang, M.; Liu, M.; Li, J.; Xiao, Y.; Wu, X. Systematic analysis of non-coding RNAs involved in the Angora rabbit (Oryctolagus cuniculus) hair follicle cycle by RNA sequencing. Front. Genet. 2019, 10, 407. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Bao, Z.; Zhao, B.; Zhou, T.; Li, J.; Liu, M.; Hu, S.; Yang, N.; Chen, Y.; Wu, X. Characterization and functional analysis of Krtap11-1 during hair follicle development in Angora rabbits (Oryctolagus cuniculus). Genes Genomics 2020, 42, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Chen, Y.; Hu, S.; Yang, N.; Liu, M.; Li, J.; Bao, Z.; Wu, X. Characterization of HTATIP2 and its role during hair follicle cycles in Angora rabbit. Genome 2020, 63, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Greene, H.S.; Hu, C.K.; Brown, W.H. A lethal dwarf mutation in the rabbit with stigmata of endocrine abnormality. Science 1934, 79, 487–488. [Google Scholar] [CrossRef]
- Greene, H.S. A dwarf mutation in the rabbit: The constitutional influence on homozygous and heterozygous individuals. J. Exp. Med. 1940, 71, 839–856. [Google Scholar] [CrossRef]
- Carneiro, M.; Hu, D.; Archer, J.; Feng, C.; Afonso, S.; Chen, C.; Blanco-Aguiar, J.A.; Garreau, H.; Boucher, S.; Ferreira, P.G.; et al. Dwarfism and altered craniofacial development in rabbits is caused by a 12.1 kb deletion at the HMGA2 locus. Genetics 2017, 205, 955–965. [Google Scholar] [CrossRef]
- Létard, E. Une mutation nouvelle chez le lapin. Bull. Acad. Vét. Fr. 1935, 8, 608–610. [Google Scholar]
- Létard, E. Troubles de la locomotion et de la vision chez le lapin, liaison hereditaire. Bull. Acad. Vét. Fr. 1943, 16, 184–192. [Google Scholar]
- Boucher, S. Le lapin Sauteur d’Alfort: Un symbole au service de la pathologie cunicole. La Sem. Vet. 1994, 746, 17–18. [Google Scholar]
- Audigier, I.; Renous, S. Les allures du lapin normal peuvent-elles expliquer la marche acrobatique du lapin sauteur d’Alfort? Mammalia 2002, 66, 563–578. [Google Scholar] [CrossRef]
- Carneiro, M.; Vieillard, J.; Andrade, P.; Boucher, S.; Afonso, S.; Blanco-Aguiar, J.A.; Santos, N.; Branco, J.; Esteves, P.J.; Ferrand, N.; et al. Retinoid-related orphan nuclear receptor RORB is required for saltatorial locomotion in rabbits. Curr. Biol. 2019. [Google Scholar] [CrossRef]
- Pease, M. Yellow fat in rabbits, a linked character? Z. für Indukt. Abstamm. und Vererb. 1928, 2, 1153–1156. [Google Scholar]
- Wilson, W.K.; Dudley, F.J. Fat colour and fur colour in different varieties of rabbit. J. Genet. 1946, 47, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Berry, S.D.; Davis, S.R.; Beattie, E.M.; Thomas, N.L.; Burrett, A.K.; Ward, H.E.; Stanfield, A.M.; Biswas, M.; Ankersmit-Udy, A.E.; Oxley, P.E.; et al. Mutation in bovine beta-carotene oxygenase 2 affects milk color. Genetics 2009, 182, 923–926. [Google Scholar] [CrossRef]
- Tian, R.; Pitchford, W.S.; Morris, C.A.; Cullen, N.G.; Bottema, C.D. Genetic variation in the beta, beta-carotene-9′, 10′-dioxygenase gene and association with fat colour in bovine adipose tissue and milk. Anim. Genet. 2010, 41, 253–259. [Google Scholar] [CrossRef]
- Våge, D.I.; Boman, I.A. A nonsense mutation in the beta-carotene oxygenase 2 (BCO2) gene is tightly associated with accumulation of carotenoids in adipose tissue in sheep (Ovis aries). BMC Genet. 2010, 11, 10. [Google Scholar] [CrossRef]
- Fallahshahroudi, A.; Sorato, E.; Altimiras, J.; Jensen, P. The domestic BCO2 allele buffers low-carotenoid diets in chickens: Possible fitness increase through species hybridization. Genetics 2019, 212, 1445–1452. [Google Scholar] [CrossRef]
- Strychalski, J.; Brym, P.; Czarnik, U.; Gugołek, A. A novel AAT-deletion mutation in the coding sequence of the BCO2 gene in yellow-fat rabbits. J. Appl. Genet. 2015, 56, 535–537. [Google Scholar] [CrossRef] [PubMed]
- Strychalski, J.; Gugołek, A.; Antoszkiewicz, Z.; Kowalska, D.; Konstantynowicz, M. Biologically active compounds in selected tissues of white-fat and yellow-fat rabbits and their production performance parameters. Livest. Sci. 2016, 183, 92–97. [Google Scholar] [CrossRef]
- Strychalski, J.; Gugołek, A.; Brym, P.; Antoszkiewicz, Z. Effect of the β-carotene oxygenase 2 genotype on the content of carotenoids, retinol and α-tocopherol in the liver, fat and milk of rabbit does, reproduction parameters and kitten growth. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1585–1593. [Google Scholar] [CrossRef] [PubMed]
- Strychalski, J.; Gugołek, A.; Brym, P.; Antoszkiewicz, Z.; Chwastowska-Siwiecka, I. Polymorphism of the BCO2 gene and the content of carotenoids, retinol, and α-tocopherol in the liver and fat of rabbits. Rev. Bras. Zootec. 2019, 48, e20180243. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).