Study of the Properties of Human Milk Fat Substitutes Using DSC and GC Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Enzymatic Modification
2.3. Peroxide Value and an Acid Value
2.4. Fatty Acid Composition
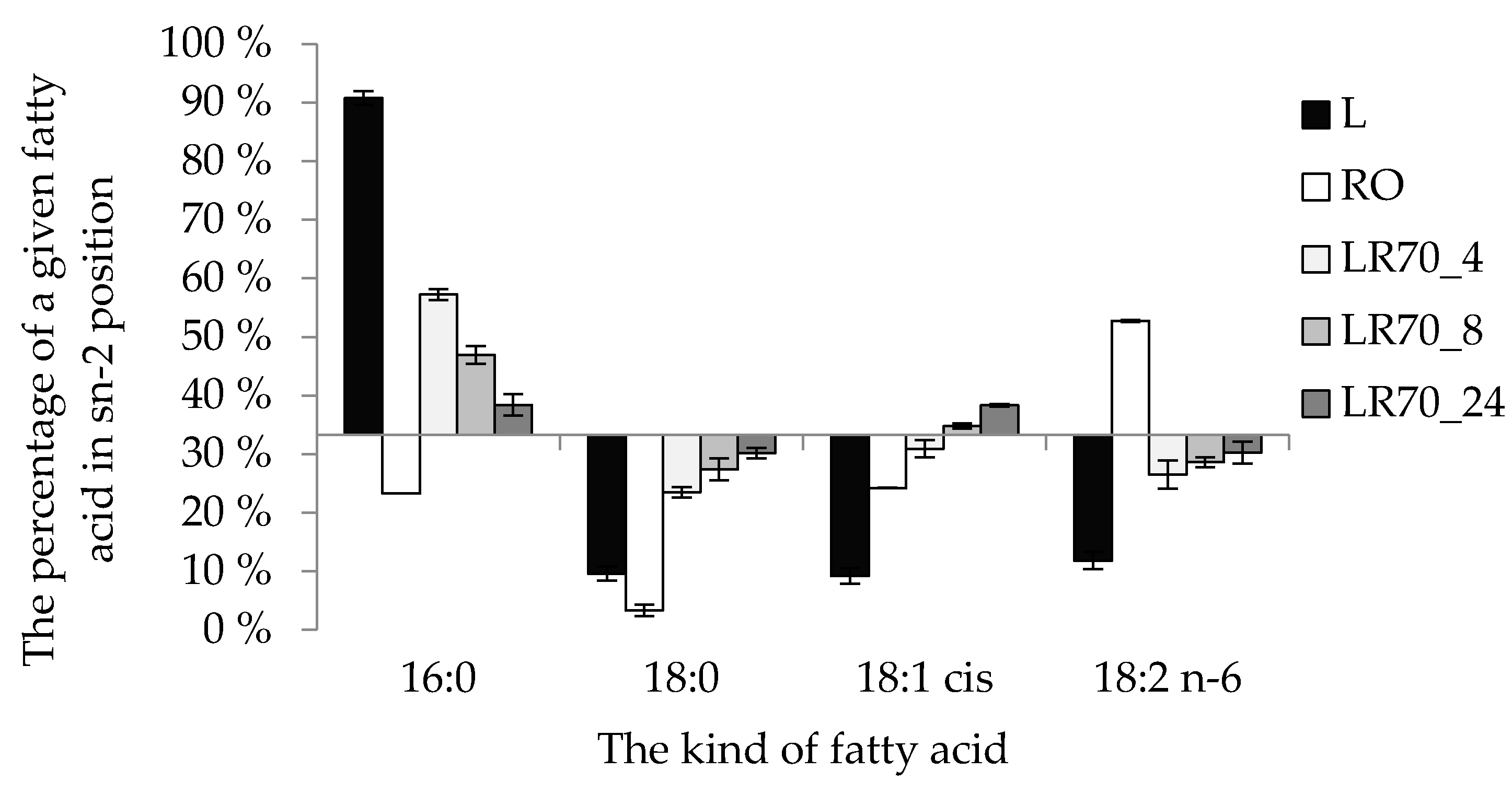
2.5. Positional Distribution of Fatty Acids in the sn-2 and sn-1,3 Positions of TAG
2.6. DSC Measurements
2.7. Statistical Analysis
3. Results
3.1. Comparison of the Composition of FAs and the Structure of TAG of Obtained SL with HMF
3.1.1. FAs Composition in Studied Fats
3.1.2. FAs Distribution in TAG in Studied Fats
3.2. The Quality Assessment of Studied Fats
3.3. Kinetic Analysis of Oxidation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations for Figures and Tables
| L | lard |
| RO | rapeseed oil |
| LR70_4 | blend of L and RO (8:2), modification for 4 h at 70 °C |
| LR70_8 | blend of L and RO (8:2), modification for 8 h at 70 °C |
| LR70_24 | blend of L and RO (8:2), modification for 24 h at 70 °C |
| AV | acid value |
| PV | peroxide value |
| IT | oxidation induction time |
References
- Sahin, N.; Akoh, C.C.; Karaali, A. Enzymatic production of human milk fat substitutes containing-linolenic acid: Optimization of reactions by response surface methodology. J. Am. Oil Chem. Soc. 2005, 82, 549–557. [Google Scholar] [CrossRef]
- Nielsen, N.S.; Yang, T.; Xu, X.; Jacobsen, C. Production and oxidative stability of a human milk fat substitute produced from lard by enzyme technology in a pilot packed-bed reactor. Food Chem. 2006, 94, 53–60. [Google Scholar] [CrossRef]
- Li, Y.; Mu, H.; Andersen, J.E.T.; Xu, X.; Meyere, O.; Ørngreene, A. New human milk fat substitutes from butterfat to improve fat absorption. Food Res. Int. 2010, 43, 739–744. [Google Scholar] [CrossRef]
- Bryś, J.; Vaz Flores, I.F.; Górska, A.; Wirkowska-Wojdyła, M.; Ostrowska-Ligęza, E.; Bryś, A. Use of GC and PDSC methods to characterize human milk fat substitutes obtained from lard and milk thistle oil mixtures. J. Therm. Anal. Calorim. 2017, 130, 319–327. [Google Scholar] [CrossRef]
- Lien, E.L. The role of fatty acid composition and positional distribution in fat absorption in infants. J. Pediatr. 1994, 125 Pt 2, 562–568. [Google Scholar] [CrossRef]
- Xu, X. Production of specific-structured triacylglycerols by lipase-catalyzed reactions: A review. Eur. J. Lipid Sci. Technol. 2000, 102, 287–303. [Google Scholar] [CrossRef]
- Uauy, R.; Castillo, C. Lipid requirements of infants: Implications for nutrient composition of fortified complementary foods. J. Nutr. 2003, 133, 2962S–2972S. [Google Scholar] [CrossRef]
- Tecelao, C.; Silva, J.; Dubreucq, E.; Ribeiro, M.; Ferreira-Dias, S. Production of human milk fat substitutes enriched in omeg-3 polyunsaturated fatty acids using immobilized commercial lipases and Candida parapsilosis lipase/acyltransferase. J. Mol. Catal. B Enzym. 2010, 65, 122–127. [Google Scholar] [CrossRef]
- Sahin, N.; Akoh, C.C.; Karaali, A. Human milk fat substitutes containing omega-3 fatty acids. J. Agric. Food Chem. 2006, 54, 3717–3722. [Google Scholar] [CrossRef]
- Claro da Silva, R.; Schaffer De Martini Soares, F.A.; Fernandes, T.G.; Castells, A.L.D.; Guimarães da Silva, K.C.; Gonçalves, M.I.A.; Ming, C.C.; Gonçalves, L.A.G.; Gioielli, L.A. Interesterification of lard and soybean oil blends catalyzed by immobilized lipase in a continuous packed bed reactor. J. Am. Oil Chem. Soc. 2011, 88, 1925–1933. [Google Scholar] [CrossRef]
- Marangoni, A.G.; Rousseau, D. Chemical and enzymatic modification of butterfat and butterfat–canola oil blends. Food Res. Int. 1998, 31, 595–599. [Google Scholar] [CrossRef]
- Wang, Y.H.; Qin, X.L.; Zhu, Q.S.; Zhou, R.; Yang, B.; Li, L. Lipase-catalyzed acidolysis of lard for the production of human milk fat substitute. Eur. Food Res. Technol. 2010, 230, 769–777. [Google Scholar] [CrossRef]
- Yang, T.; Xu, X.; He, C.; Li, L. Lipase-catalyzed modification of lard to produce human milk fat substitutes. Food Chem. 2003, 80, 473–481. [Google Scholar] [CrossRef]
- Ilyasoglu, H. Production of human fat milk analogue containing α-linolenic acid by solvent-free enzymatic interesterification. LWY Food Sci. Technol. 2013, 54, 179–185. [Google Scholar] [CrossRef]
- Wąsowicz, E.; Gramza, A.; Hęś, M.; Jeleń, H.H.; Korczak, J.; Małecka, M.; Mildner-Szkudlarz, S.; Rudzińska, M.; Samotyja, U.; Zawirska-Wojtasiak, R. Oxidation of lipids in food. Pol. J. Food Nutr. Sci. 2004, 13/54, 87–100. [Google Scholar]
- Bialek, M.; Rutkowska, J.; Bialek, A.; Adamska, A. Oxidative stability of lipid fraction of cookies enriched with chokeberry polyphenols extract. Pol. J. Food Nutr. Sci. 2016, 66, 77–84. [Google Scholar] [CrossRef]
- Tan, C.P.; Che Man, Y.B. Differential scanning calorimetric analysis for monitoring the oxidation of heated oils. Food Chem. 1999, 67, 177–184. [Google Scholar] [CrossRef]
- Thurgood, J.; Ward, R.; Martini, S. Oxidation kinetics of soybean oil/anhydrous milk fat blends: A differential scanning calorimetry study. Food Res. Int. 2007, 40, 1030–1037. [Google Scholar] [CrossRef]
- Maduko, C.O.; Park, Y.W.; Akoh, C.C. Characterization and oxidative stability of structured lipids: Infant milk fat analog. J. Am. Oil Chem. Soc. 2008, 85, 197–204. [Google Scholar] [CrossRef]
- Martin, D.; Reglero, G.; Señoráns, F.J. Oxidative stability of structured lipids. Eur. Food Res. Technol. 2010, 231, 635–653. [Google Scholar] [CrossRef]
- Bryś, J.; Wirkowska, M.; Górska, A.; Ostrowska-Ligęza, E.; Bryś, A.; Koczoń, P. The use of DSC and FT-IR spectroscopy for evaluation of oxidative stability of interesterified fats. J. Therm. Anal. Calorim. 2013, 112, 481–487. [Google Scholar] [CrossRef]
- Gray, J.I. Measurement of lipid oxidation: A review. J. Am. Oil Chem. Soc. 1978, 55, 539–546. [Google Scholar] [CrossRef]
- Bryś, J.; Wirkowska, M.; Górska, A.; Ostrowska-Ligęza, E.; Bryś, A. Application of the calorimetric and spectroscopic methods in analytical evaluation of the human milk fat substitutes. J. Therm. Anal. Calorim. 2014, 118, 841–848. [Google Scholar] [CrossRef]
- Ostrowska-Ligęza, E.; Bekas, W.; Kowalska, D.; Łobacz, M.; Wroniak, M.; Kowalski, B. Kinetics of commercial olive oil oxidation: Dynamic differential scanning calorimetry and Rancimat studies. Eur. J. Lipid Sci. Technol. 2010, 112, 268–274. [Google Scholar] [CrossRef]
- Ciemniewska-Żytkiewicz, H.; Ratusz, K.; Bryś, J.; Reder, M.; Koczoń, P. Determination of the oxidative stability of hazelnut oils by PDSC and Rancimat methods. J. Therm. Anal. Calorim. 2014, 118, 875–881. [Google Scholar] [CrossRef]
- ISO 3960:2007. Animal and Vegetable Fats and Oils—Determination of Peroxide Value—Iodometric (Visual) Endpoint Determination; International Organization for Standardization: Geneva, Switzerland, 2007. [Google Scholar]
- ISO 660:2009. Animal and Vegetable Fats and Oils—Determination of Acid Value and Acidity; International Organization for Standardization: Geneva, Switzerland, 2009. [Google Scholar]
- PN EN ISO 5509:2001. Animal and Vegetable Fats and Oils–Preparation of Methyl Esters of Fatty Acids; Polish Committee for Standardization: Warsaw, Poland, 2001. [Google Scholar]
- Danthine, A.; De Clercq, N.; Dewettinck, K.; Gibon, V. Monitoring batch lipase catalyzed interesterification of palm oil and fractions by differential scanning calorimetry. J. Therm. Anal. Calorim. 2014, 115, 2219–2229. [Google Scholar] [CrossRef]
- López-López, A.; López-Sabater, M.C.; Campoy-Folgoso, C.; Rivero-Urgell, M.; Castellote-Bargalló, A.I. Fatty acid and sn-2 fatty acid composition in human milk from Granada (Spain) and infant formulas. Eur. J. Clin. Nutr. 2002, 56, 1242–1254. [Google Scholar] [CrossRef]
- Ostrowska-Ligęza, E.; Jakubczyk, E.; Górska, A.; Wirkowska, M.; Bryś, J. The use of moisture sorption isotherms and glass transition temperature to assess the stability of powdered baby formulas. J. Therm. Anal. Calorim. 2014, 118, 911–918. [Google Scholar] [CrossRef]
- Ciemniewska-Żytkiewicz, H.; Bryś, J.; Sujka, K.; Koczoń, P. Assessment of the hazelnuts roasting process by pressure differential scanning calorimetry and MID-FT-IR spectroscopy. Food Anal. Methods 2015, 8, 2465–2473. [Google Scholar] [CrossRef]
- Kowalski, B.; Tarnowska, K.; Gruczyńska, E.; Bekas, W. Chemical and enzymatic interesterification of beef tallow and rapeseed oil blend with low content of tallow. J. Oleo Sci. 2004, 53, 479–488. [Google Scholar] [CrossRef][Green Version]
- Yüksel, A.; Yesilçubuk, N.S. Enzymatic production of human milk fat analogues containing stearidonic acid and optimization of reactions by response surface methodology. LWT J. Food Sci. Technol. 2012, 46, 210–216. [Google Scholar] [CrossRef]
- Mu, H. Production and nutritional aspects of human milk fat substitutes. Lipid Technol. 2010, 22, 126–129. [Google Scholar] [CrossRef]
- Small, D.M. The effects of glyceride structure on adsorption and metabolism. Annu. Rev. Nutr. 1991, 11, 413–434. [Google Scholar] [CrossRef] [PubMed]
- Padley, F.B.; Gunstone, F.D.; Harwood, J.L. Occurance and characteristics of oils and fats. In The Lipid Handbook, 2nd ed.; Gunstone, F.D., Harwood, J.L., Padley, F.B., Eds.; Chapman & Hall: London, UK, 1994; pp. 47–223. [Google Scholar]
- Xu, X.; Skands, A.R.H.; Adler-Nissen, J.; Høy, C.E. Production of specific structured lipids by enzymatic interesterification: Optimization of the reaction by response surface design. Fett/Lipid 1998, 100, 463–471. [Google Scholar] [CrossRef]
- Frega, N.; Mozzon, M.; Lercker, G. Effects of Free Fatty Acids on Oxidative Stability of Vegetable Oil. J. Am. Oil Chem. Soc. 1999, 76, 325–329. [Google Scholar] [CrossRef]
- Liu, L.; Lampert, D. Monitoring chemical interesterification. J. Am. Oil Chem. Soc. 1999, 76, 783–787. [Google Scholar] [CrossRef]
- Hamam, F.; Shahidi, F. Enzymatic acidolysis of an arachidonic acid single-cell oil with capric acid. J. Am. Oil Chem. Soc. 1998, 81, 887–892. [Google Scholar] [CrossRef]
- Wijesundera, C.; Ceccato, C.; Watkins, P.; Fagan, P.; Fraser, B.; Thienthong, N.; Perlmutter, P. Docosahexaenoic Acid is More Stable to Oxidation when Located at the sn-2 Position of Triacylglycerol Compared to sn-1(3). J. Am. Oil Chem. Soc. 2008, 85, 543–548. [Google Scholar] [CrossRef]

| Fatty Acid | L 1 | RO 1 | LR70_4 1 | LR70_8 1 | LR70_24 1 |
|---|---|---|---|---|---|
| C10:0 | 0.14 ± 0.01 b | - | 0.10 ± 0.01 a | 0.10 ± 0.01 a | 0.10 ± 0.01 a |
| C12:0 | 0.13 ± 0.01 b | - | 0.10 ± 0.01 a | 0.11 ± 0.01 a | 0.10 ± 0.01 a |
| C14:0 | 1.70 ± 0.04 c | - | 1.41 ± 0.01 ab | 1.49 ± 0.01 b | 1.33 ± 0.04 a |
| C16:0 | 27.65 ± 0.08 d | 4.44 ± 0.02 a | 24.2 ± 0.1 c | 24.8 ± 0.1 c | 23.2 ± 0.7 b |
| C16:1 cis | 2.17 ± 0.06 d | 0.22 ± 0.01 a | 1.73 ± 0.04 b,c | 1.81 ± 0.01 c | 1.70 ± 0.01 b |
| C17:0 | 0.38 ± 0.02 c | 0.12 ± 0.01 a | 0.32 ± 0.02 b | 0.33 ± 0.04 b,c | 0.32 ± 0.02 b |
| C17:1 cis | 0.22 ± 0.01 b | 0.06 ± 0.01 a | 0.21 ± 0.01 b | 0.21 ± 0.01 b | 0.21 ± 0.01 b |
| C18:0 | 18.00 ± 0.07 c | 1.98 ± 0.01 a | 14.4 ± 0.1 b | 14.5 ± 0.2 b | 14.5 ± 0.1 b |
| C18:1 cis | 38.0 ± 0.1 a | 61.07 ± 0.07 e | 42.4 ± 0.4 c | 41.8 ± 0.3 b | 43.4 ± 0.4 d |
| C18:2 n-6 | 9.50 ± 0.06 a | 19.21 ± 0.03 c | 11.21 ± 0.01 ab | 11.12 ± 0.02 a | 11.25 ± 0.07 b |
| C18:3 n-3 | 0.78 ± 0.01 a | 9.26 ± 0.02 d | 2.38 ± 0.04 c | 2.32 ± 0.03 b,c | 2.31 ± 0.01 b |
| C20:0 | 0.24 ± 0.01 a | 0.70 ± 0.01 c | 0.31 ± 0.01 b | 0.30 ± 0.01 b | 0.30 ± 0.01 b |
| C20:1 cis | 0.75 ± 0.04 a | 1.59 ± 0.01 d | 0.93 ± 0.04 b,c | 0.89 ± 0.01 b | 0.98 ± 0.03 c |
| C20:2 n-6 | 0.36 ± 0.03 b | 0.40 ± 0.01 ab | 0.31 ± 0.01 a | 0.31 ± 0.01 a | 0.33 ± 0.04 a |
| Type of Sample | FAs (wt%) | ||||
|---|---|---|---|---|---|
| C16:0 | C18:0 | C18:1 cis | C18:2 n-6 | ||
| sn-2 MAG | L | 74.6 ± 0.5 e 1 | 4.7 ± 0.3 b | 11.6 ± 0.8 a | 3.1 ± 0.2 a |
| RO | 2.9 ± 0.3 a | 0.15 ± 0.07 a | 45.2 ± 1.1 c | 29.7 ± 1.1 c | |
| LR70_4 | 41.6 ± 0.7 d | 10.2 ± 0.4 c | 39.3 ± 1.9 b | 8.9 ± 0.8 b | |
| LR70_8 | 34.9 ± 0.6 c | 11.9 ± 0.4 d | 43.6 ± 0.3 c | 9.6 ± 0.1 b | |
| LR70_24 | 26.8 ± 1.3 b | 13.1 ± 0.4 d | 49.9 ± 0.3 d | 10.2 ± 0.6 b | |
| sn-1,3 DAG | L | 4.2 ± 0.2 a | 24.6 ± 0.2 d | 51.2 ± 0.4 c | 12.7 ± 0.1 b |
| RO | 5.20 ± 0.07 a | 2.89 ± 0.02 a | 69.0 ± 0.3 d | 14.0 ± 0.3 c | |
| LR70_4 | 15.5 ± 0.3 b | 16.5 ± 0.2 c | 44.0 ± 0.9 b | 12.4 ± 0.4 a,b | |
| LR70_8 | 19.7 ± 1.1 c | 15.7 ± 0.8 b | 40.9 ± 0.6 a | 11.9 ± 0.3 a,b | |
| LR70_24 | 21.5 ± 0.6 d | 15.2 ± 0.2 b | 40.1 ± 0.1 a | 11.8 ± 0.3 a |
| Type of Sample | AV 1 (mg KOH/g) | PV 1 (mmol O2/kg) | IT 1 (min) |
|---|---|---|---|
| L | 0.86 ± 0.06 b | 2.7 ± 0.2 a | 70.9 ± 0.8 a |
| RO | 0.58 ± 0.05 a | 3.4 ± 0.1 b | 72.0 ± 0.6 a |
| LR70_4 | 8.7 ± 0.1 d | 5.7 ± 0.2 e | 27.2 ± 1.4 d |
| LR70_8 | 11.0 ± 0.6 e | 4.8 ± 0.3 d | 34.7 ± 0.2 c |
| LR70_24 | 5.4 ± 0.2 c | 4.1 ± 0.3 c | 53.0 ± 1.8 b |
| Heating Rates (°C/min) | Ton (°C) | ||
|---|---|---|---|
| LR70_4 | LR70_8 | LR70_24 | |
| 2.5 | 140.2 ± 1.6 | 150.9 ± 0.1 | 149.7 ± 1.3 |
| 5.0 | 147.8 ± 0.6 | 156.4 ± 1.9 | 161 ± 1 |
| 7.5 | 153.0 ± 0.4 | 166.6 ± 1.7 | 168.2 ± 0.6 |
| 10.0 | 162.0 ± 0.2 | 171.3 ± 0.9 | 172.7 ± 0.4 |
| 12.5 | 164.4 ± 1.3 | 174.9 ± 1.0 | 176.4 ± 0.2 |
| Parameter | Ton Based Value | ||
|---|---|---|---|
| LR70_4 | LR70_8 | LR70_24 | |
| a | −4912.3 | −5090.0 | −4949.3 |
| b | 12.33 | 12.46 | 12.10 |
| R2 | 0.96 | 0.96 | 0.99 |
| Ea | 89.40 | 92.63 | 90.07 |
| Z | 4.08 × 1010 | 4.41 × 1010 | 1.93 × 1010 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bryś, J.; Górska, A.; Ostrowska-Ligęza, E.; Wirkowska-Wojdyła, M.; Bryś, A.; Brzezińska, R.; Dolatowska-Żebrowska, K.; Ziarno, M.; Obranović, M.; Škevin, D. Study of the Properties of Human Milk Fat Substitutes Using DSC and GC Methods. Appl. Sci. 2021, 11, 319. https://doi.org/10.3390/app11010319
Bryś J, Górska A, Ostrowska-Ligęza E, Wirkowska-Wojdyła M, Bryś A, Brzezińska R, Dolatowska-Żebrowska K, Ziarno M, Obranović M, Škevin D. Study of the Properties of Human Milk Fat Substitutes Using DSC and GC Methods. Applied Sciences. 2021; 11(1):319. https://doi.org/10.3390/app11010319
Chicago/Turabian StyleBryś, Joanna, Agata Górska, Ewa Ostrowska-Ligęza, Magdalena Wirkowska-Wojdyła, Andrzej Bryś, Rita Brzezińska, Karolina Dolatowska-Żebrowska, Małgorzata Ziarno, Marko Obranović, and Dubravka Škevin. 2021. "Study of the Properties of Human Milk Fat Substitutes Using DSC and GC Methods" Applied Sciences 11, no. 1: 319. https://doi.org/10.3390/app11010319
APA StyleBryś, J., Górska, A., Ostrowska-Ligęza, E., Wirkowska-Wojdyła, M., Bryś, A., Brzezińska, R., Dolatowska-Żebrowska, K., Ziarno, M., Obranović, M., & Škevin, D. (2021). Study of the Properties of Human Milk Fat Substitutes Using DSC and GC Methods. Applied Sciences, 11(1), 319. https://doi.org/10.3390/app11010319












