The Effects of Antioxidant Supplements on the Inflammatory Gene Expression of Osteoarthritis-Like Chondrocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Establishment of Osteoarthritis (OA)-Like Chondrocyte Cell Model
2.2. Cell Viability Analysis
2.3. Inflammatory Gene Expression Analysis
2.4. Statistical Analysis
3. Results
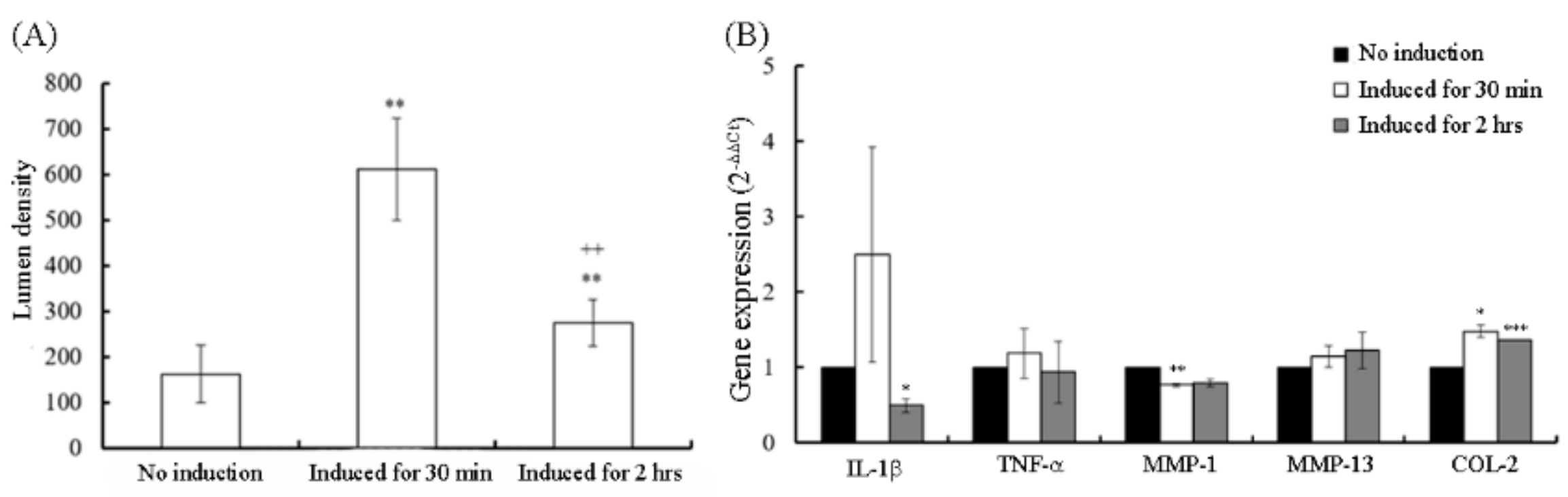
3.1. Inflammation Gene Expression of OA-Like Cells
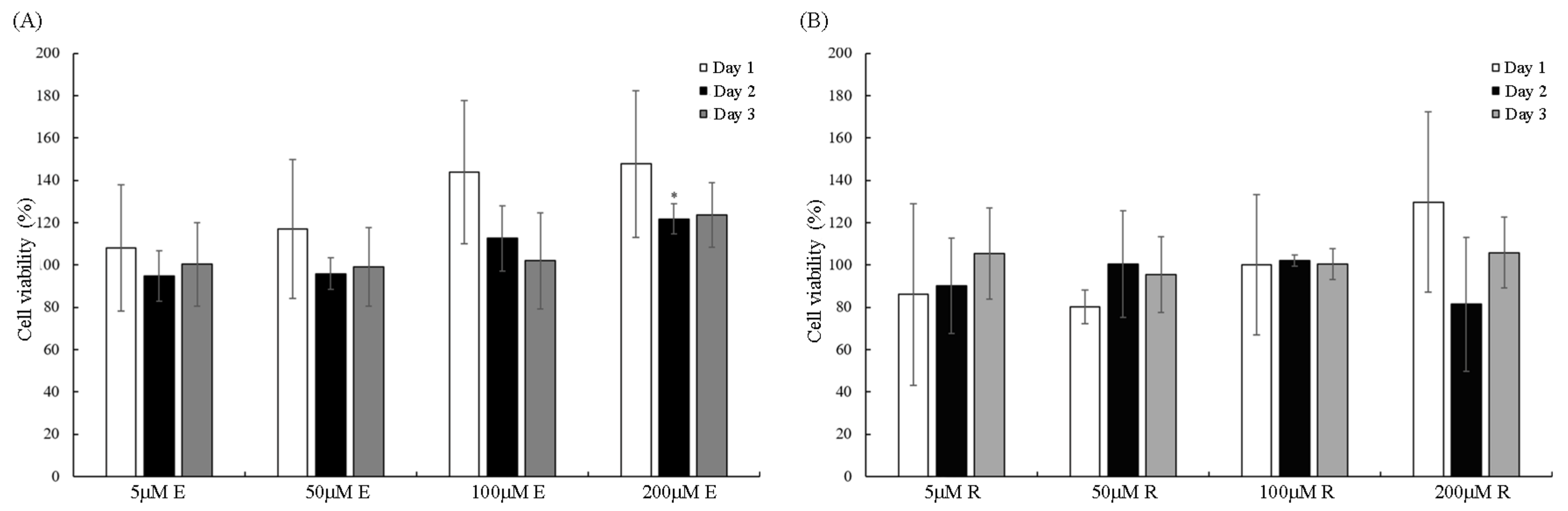
3.2. Cell Viability of Vitamin E and Resveratrol
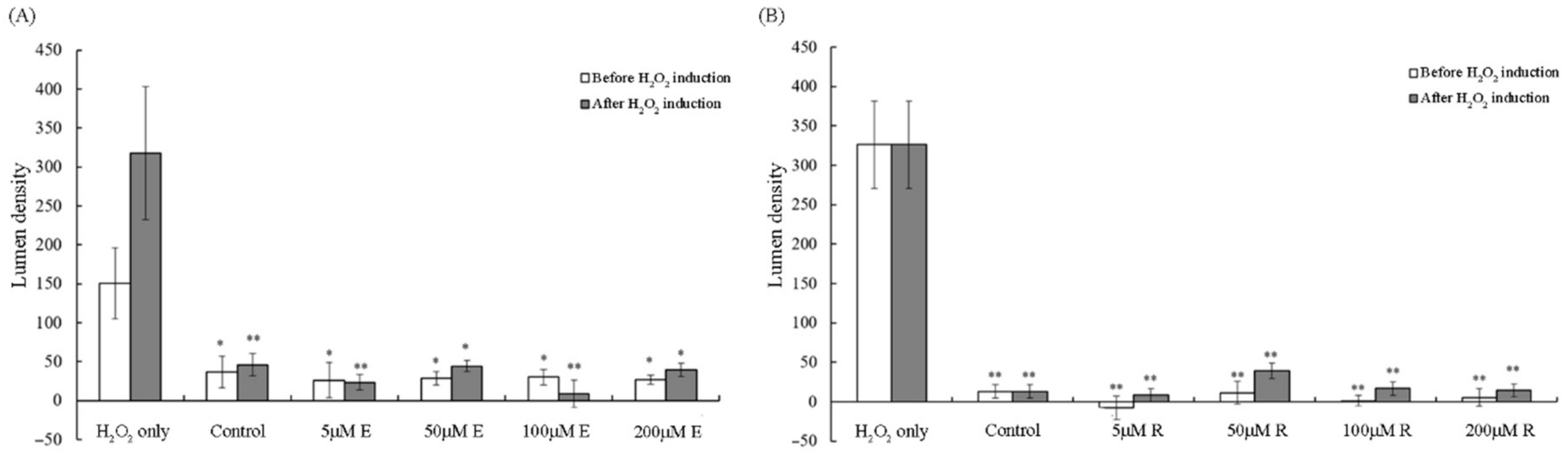
3.3. Reduction in Lumen Density by Vitamin E and Resveratrol
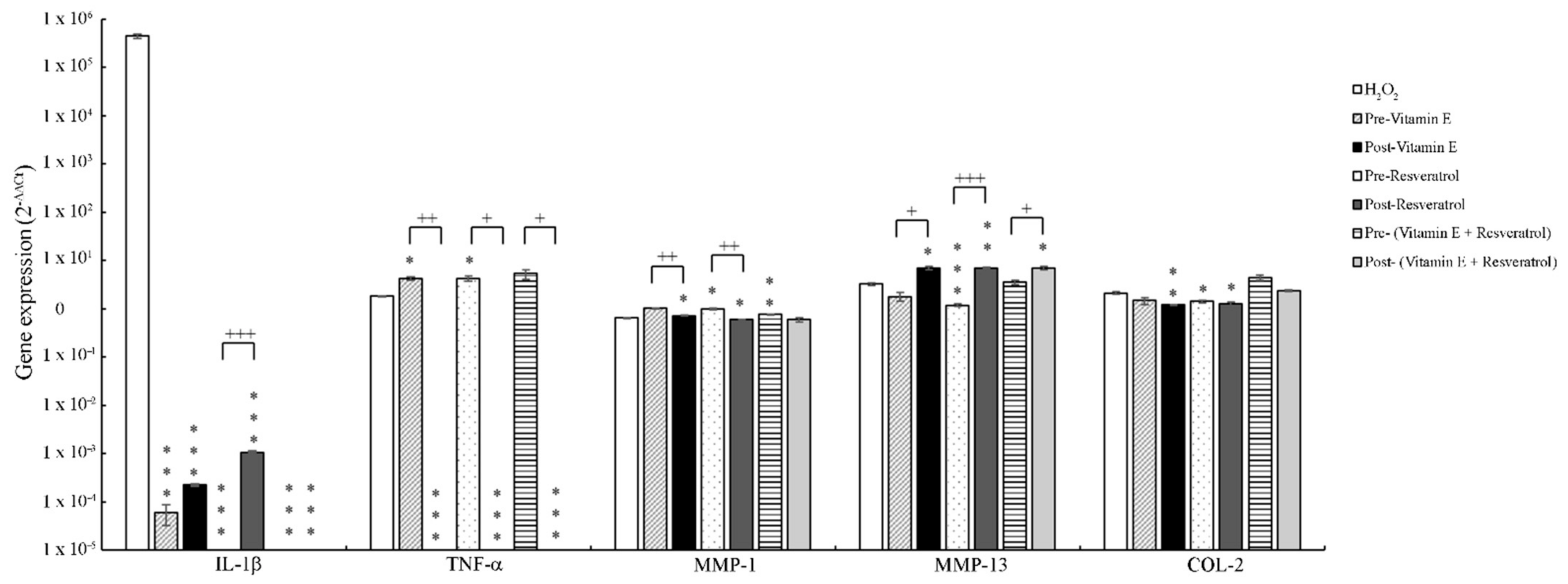
3.4. The Effect of Vitamin E and Resveratrol on Inflammatory Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grover, A.K.; Samson, S.E. Benefits of antioxidant supplements for knee osteoarthritis: Rationale and reality. Nutr. J. 2016, 15, 1. [Google Scholar] [CrossRef] [PubMed]
- Adatia, A.; Rainsford, K.D.; Kean, W.F. Osteoarthritis of the knee and hip. Part 2: Therapy with ibuprofen and a review of clinical trials. J. Pharm. Pharmacol. 2012, 64, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Juhl, C.; Christensen, R.; Roos, E.M.; Zhang, W.; Lund, H. Impact of exercise type and dose on pain and disability in knee osteoarthritis: A systematic review and meta-regression analysis of randomized controlled trials. Arthritis Rheumatol. 2014, 66, 622–636. [Google Scholar] [CrossRef] [PubMed]
- McCarberg, B.; Tenzer, P. Complexities in the pharmacologic management of osteoarthritis pain. Curr. Med. Res. Opin. 2013, 29, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Wojdasiewicz, P.; Poniatowski, L.A.; Szukiewicz, D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediat. Inflamm. 2014, 2014, 561459. [Google Scholar] [CrossRef] [PubMed]
- Melchiorri, C.; Meliconi, R.; Frizziero, L.; Silvestri, T.; Pulsatelli, L.; Mazzetti, I.; Borzi, R.M.; Uguccioni, M.; Facchini, A. Enhanced and coordinated in vivo expression of inflammatory cytokines and nitric oxide synthase by chondrocytes from patients with osteoarthritis. Arthritis Rheum. 1998, 41, 2165–2174. [Google Scholar] [CrossRef]
- van de Loo, F.A.; Joosten, L.A.; van Lent, P.L.; Arntz, O.J.; van den Berg, W.B. Role of interleukin-1, tumor necrosis factor alpha, and interleukin-6 in cartilage proteoglycan metabolism and destruction. Effect of in situ blocking in murine antigen—and zymosan-induced arthritis. Arthritis Rheum. 1995, 38, 164–172. [Google Scholar]
- Shakibaei, M.; Schulze-Tanzil, G.; John, T.; Mobasheri, A. Curcumin protects human chondrocytes from il-l1beta-induced inhibition of collagen type ii and beta1-integrin expression and activation of caspase-3: An immunomorphological study. Ann. Anat. 2005, 187, 487–497. [Google Scholar] [CrossRef]
- Stove, J.; Huch, K.; Gunther, K.P.; Scharf, H.P. Interleukin-1beta induces different gene expression of stromelysin, aggrecan and tumor-necrosis-factor-stimulated gene 6 in human osteoarthritic chondrocytes in vitro. Pathobiology 2000, 68, 144–149. [Google Scholar] [CrossRef]
- Vincenti, M.P.; Brinckerhoff, C.E. Transcriptional regulation of collagenase (mmp-1, mmp-13) genes in arthritis: Integration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Res. 2002, 4, 157–164. [Google Scholar] [CrossRef]
- Fridovich, I. Oxygen: How do we stand it? Med. Princ. Pract. 2013, 22, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Walia, M.; Kwan, C.Y.; Grover, A.K. Effects of free radicals on coronary artery. Med. Princ. Pract. 2003, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Altindag, O.; Erel, O.; Aksoy, N.; Selek, S.; Celik, H.; Karaoglanoglu, M. Increased oxidative stress and its relation with collagen metabolism in knee osteoarthritis. Rheumatol. Int. 2007, 27, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Sutipornpalangkul, W.; Morales, N.P.; Charoencholvanich, K.; Harnroongroj, T. Lipid peroxidation, glutathione, vitamin e, and antioxidant enzymes in synovial fluid from patients with osteoarthritis. Int. J. Rheum. Dis. 2009, 12, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Chang, Y.W.; Tan, K.P.; Shen, Y.S.; Wang, Y.H.; Chang, C.H. Can mesenchymal stem cells and their conditioned medium assist inflammatory chondrocytes recovery? PLoS ONE 2018, 13, e0205563. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Ding, H.; Zhuang, C.; Fan, W. Effects of hesperidin on H2O2-treated chondrocytes and cartilage in a rat osteoarthritis model. Med. Sci. Monit. 2018, 24, 9177–9186. [Google Scholar] [CrossRef]
- Hochberg, M.C. New directions in symptomatic therapy for patients with osteoarthritis and rheumatoid arthritis. Semin. Arthritis Rheum. 2002, 32, 4–14. [Google Scholar] [CrossRef]
- Crofford, L.J. Use of nsaids in treating patients with arthritis. Arthritis Res. Ther. 2013, 15, S2. [Google Scholar] [CrossRef]
- Dworski, R.; Han, W.; Blackwell, T.S.; Hoskins, A.; Freeman, M.L. Vitamin e prevents nrf2 suppression by allergens in asthmatic alveolar macrophages in vivo. Free Radic. Biol. Med. 2011, 51, 516–521. [Google Scholar] [CrossRef]
- Peh, H.Y.; Tan, W.S.; Liao, W.; Wong, W.S. Vitamin e therapy beyond cancer: Tocopherol versus tocotrienol. Pharmacol. Ther. 2016, 162, 152–169. [Google Scholar] [CrossRef]
- Bhatti, F.U.; Mehmood, A.; Wajid, N.; Rauf, M.; Khan, S.N.; Riazuddin, S. Vitamin e protects chondrocytes against hydrogen peroxide-induced oxidative stress in vitro. Inflamm. Res. 2013, 62, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Csaki, C.; Keshishzadeh, N.; Fischer, K.; Shakibaei, M. Regulation of inflammation signalling by resveratrol in human chondrocytes in vitro. Biochem. Pharmacol. 2008, 75, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Shakibaei, M.; Csaki, C.; Nebrich, S.; Mobasheri, A. Resveratrol suppresses interleukin-1beta-induced inflammatory signaling and apoptosis in human articular chondrocytes: Potential for use as a novel nutraceutical for the treatment of osteoarthritis. Biochem. Pharmacol. 2008, 76, 1426–1439. [Google Scholar] [CrossRef] [PubMed]
- Shakibaei, M.; John, T.; Seifarth, C.; Mobasheri, A. Resveratrol inhibits il-1 beta-induced stimulation of caspase-3 and cleavage of parp in human articular chondrocytes in vitro. Ann. N.Y. Acad. Sci. 2007, 1095, 554–563. [Google Scholar] [CrossRef]
- Elmali, N.; Esenkaya, I.; Harma, A.; Ertem, K.; Turkoz, Y.; Mizrak, B. Effect of resveratrol in experimental osteoarthritis in rabbits. Inflamm. Res. 2005, 54, 158–162. [Google Scholar] [CrossRef]
- Deng, Z.; Li, Y.; Liu, H.; Xiao, S.; Li, L.; Tian, J.; Cheng, C.; Zhang, G.; Zhang, F. The role of sirtuin 1 and its activator, resveratrol in osteoarthritis. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef]
- Lei, M.; Wang, J.G.; Xiao, D.M.; Fan, M.; Wang, D.P.; Xiong, J.Y.; Chen, Y.; Ding, Y.; Liu, S.L. Resveratrol inhibits interleukin 1beta-mediated inducible nitric oxide synthase expression in articular chondrocytes by activating sirt1 and thereby suppressing nuclear factor-kappab activity. Eur. J. Pharmacol. 2012, 674, 73–79. [Google Scholar] [CrossRef]
- Moon, M.H.; Jeong, J.K.; Lee, Y.J.; Seol, J.W.; Jackson, C.J.; Park, S.Y. Sirt1, a class iii histone deacetylase, regulates tnf-alpha-induced inflammation in human chondrocytes. Osteoarthr. Cartil. 2013, 21, 470–480. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, C.-Y.; Luo, Y.; Fang, C.-H.; Fang, H.-W. The Effects of Antioxidant Supplements on the Inflammatory Gene Expression of Osteoarthritis-Like Chondrocytes. Appl. Sci. 2021, 11, 239. https://doi.org/10.3390/app11010239
Su C-Y, Luo Y, Fang C-H, Fang H-W. The Effects of Antioxidant Supplements on the Inflammatory Gene Expression of Osteoarthritis-Like Chondrocytes. Applied Sciences. 2021; 11(1):239. https://doi.org/10.3390/app11010239
Chicago/Turabian StyleSu, Chen-Ying, Yongxiang Luo, Chi-Hau Fang, and Hsu-Wei Fang. 2021. "The Effects of Antioxidant Supplements on the Inflammatory Gene Expression of Osteoarthritis-Like Chondrocytes" Applied Sciences 11, no. 1: 239. https://doi.org/10.3390/app11010239
APA StyleSu, C.-Y., Luo, Y., Fang, C.-H., & Fang, H.-W. (2021). The Effects of Antioxidant Supplements on the Inflammatory Gene Expression of Osteoarthritis-Like Chondrocytes. Applied Sciences, 11(1), 239. https://doi.org/10.3390/app11010239




