Abstract
The paper aimed to compare the quality of rapeseed and honeydew honey powders, obtained by two methods of spray drying—traditional at a high temperature (inlet air 180 °C) and innovative low-temperature spray drying with the use of dehumidified air as a drying medium (inlet air 75 °C). Total polyphenol content, antioxidant activity, and the content and types of aromatic compounds were determined. In addition, Fourier-transform infrared spectroscopy (FTIR) coupled with chemometrics analyses was done. Powders obtained by the low-temperature spray drying method (with dehumidified air) were characterized by a higher content of polyphenols, antioxidant activity, and aromatic compounds, compared to powders obtained by the traditional method. Honeydew honey compared to rapeseed honey was characterized by a higher content of polyphenols, antioxidant activity, and composition of aromatic compounds. The results proved that the production method had a higher impact on the final properties of powders than the type of honey.
1. Introduction
Honey is a natural raw material produced by Apis mellifera bees from collected flower nectar or secretions of living parts of plants or excreta of insects sucking the juices of living parts of plants (honeydew). It has not only the aroma characteristics but can improve the human health. Honey is not a medicine but, as food, can have a supportive application in the prevention of diseases [1]. There are many types of honey, their chemical composition is significantly different, as well as the taste and smell. Honeydew honey contains many more minerals, polysaccharides, and dextrins than nectar honey. It is also rich in resins and essential oils and is used against diseases of the upper respiratory tract, asthma, pneumonia, and tuberculosis. Different types of honey have different susceptibility for crystallization, depending on the sugar profile. Rape honey contains a higher content of glucose as compared to fructose, crystallizes very quickly, and obtains a consistency of lard and white color [2,3].
Honey is added to cakes, biscuits, dietary supplements, and it goes well with milk-based products such as yogurts [4]. However, the use of honey on an industrial scale is limited due to its high viscosity, causing sticking of devices and handling problems [5,6]. On the contrary, the powdered form is more convenient, offering many advantages, including easy storage, transport, dosing, and combining with other powder products [7,8,9].
Spray drying is still the technique most commonly used to obtain a powder forms of food. The advantages of spray drying are relatively low cost, good availability of equipment, and suitability for sensitive products with high potential of producing powders characterized by high quality [10,11]. However, during traditional spray drying high temperature (usually above 180 °C) is applied. In principle, the decrease in drying temperature allows the preservation of bioactive compounds and sensory characteristics of dried materials. Decreasing the inlet temperature of spray drying below 100 °C, an innovation, which was described recently by Jedlińska et al., 2019, Samborska et al., 2019, Samborska et al., 2019, has not been achieved by other researchers before [12,13,14]. Drying is the simultaneous exchange in heat and mass. However, the resistance of mass movement far exceeds the resistance of heat movement. At the same time, the driving force of the process is the difference between the water vapor pressure of the dried material and the vapor pressure of the drying air. Thus, by reducing the humidity of the air, it is possible to evaporate water from the drying material at a lower temperature [14].
FTIR spectroscopy is currently gaining popularity in the context of analyzing food products concerning their potential health benefits. Its value stems from the speed, non-invasiveness, and, above all, reliability of the results.
The aim of the paper was to compare the quality of rapeseed and honeydew honey powders, after spray drying by two approaches—traditional high temperature spray drying (inlet air 180 °C) and innovative low-temperature spray drying with the use of dehumidified air as a drying medium (inlet air 75 °C). It was assumed that both the type of honey and the drying temperature could influence the bioactive (polyphenol content and antioxidant activity) and sensory properties (content and types of aromatic compounds) of the obtained powders.
2. Materials and Methods
2.1. Materials and Feed Solution Preparation
Both raw materials: rapeseed honey (RH) and honeydew honey (HH) were purchased from a local market (Pasieka Warmińska, Gietrzwałd, Poland). Moisture content in honey was measured by refractometry and was 15.2 and 14.4%, RH and HH, respectively. Nutriose FM06 (Roquette, Lestrem, France), a water-soluble compound produced from partially hydrolyzed (by heating, in the presence of food-grade acid) maize starch, with recognized prebiotic properties was used as a carrier material in this study. Nutriose fits well in the "clean label" trend, contrary to typical carriers such as maltodextrin or gum Arabic, which are not perceived well by consumers [15,16,17].
The types of feed solutions (and obtained powders) are presented in Table 1. In all feed solutions, the same ratio of the honey to the carrier solids (1:1) was utilized. The powders differed in the concentration of the feed solution. The reduction in the amount of water in the feed solution (increment of solids concentration from 30 to 60%) was required in the case of the process performed by dehumidified air, at a reduced temperature (100 °C), as it was observed in the preliminary experiments.

Table 1.
Experimental variants during traditional spray drying (SD) and dehumidified air spray drying (DASD) of rapeseed honey (RH) and honeydew honey (HH).
2.2. Spray Drying
The spray drying process was performed using a Mobile Minor dryer (GEA, Skanderborg, Denmark), coupled with an air dehumidification system that consisted of cooling (TAEevo TECH020, MTA, Italy) and condensation-adsorption device (ML270, Munters, Sweden). Inlet and outlet temperatures were 75/55 and 180/85 °C, during dehumidified air and traditional process, respectively. The absolute humidity of the air entering the spray dryer was ≤ 1 g∙m−3. The atomizing disk rotated with a speed of 26,000 rpm, and feed flow was equal to 0.22 mL∙s−1. Single drying of took on average of 45 min, the procedure was repeated twice for each experimental variant.
2.3. Total Phenolic Content
Before analysis, honey samples (5 g) or rehydrated powders (5 g) were diluted in 10 mL of water and made up to 50 mL with ethanol (Avantor Performance Materials, Radnor, PA, USA). In turn, to prepare an appropriate blank, the carrier (2 g) was dissolved with 50 mL of water, due to low solubility in alcohol. After such a preparation, mixtures were shaken at room temperature for 30 min. Afterward, the samples were exposed to static extraction at 4 °C that lasted 24 h and filtered. Such extracts were also used for antioxidant activity determinations.
Total phenolic content (TPC) was analyzed using the Folin-Ciocalteu assay for an alkaline environment. Extracts were mixed with distilled water, Folin-Ciocalteu reagent (Avantor Performance Materials, Radnor, PA, USA), and 20% Na2CO3 solution (Avantor Performance Materials, Radnor, PA, USA) according to the procedure described previously by Singleton and Rossi, 1965 [18]. After the incubation, the absorption of samples was measured spectrophotometrically (UV-mini 1240 spectrophotometer, Shimadzu, Japan) at 700 nm. The results were expressed as gallic acid equivalents per 100 g of sample solids, and per 100 g of honey present in sample solids (in the case of powders, results were corrected for respective carrier blank).
2.4. Antioxidant Activity
The reducing activity of antioxidants extracted from the material was measured based on the reduction in Cu(II) to Cu(I). The procedure depended on mixing 1 mL of CuCl2 solution (Sigma-Aldrich, St. Louis, MO, USA, 10 mmol∙L−1), 1 mL of neocuproine solution (Sigma-Aldrich, St. Louis, MO, USA, 7.5 mmol∙L−1), and 1 mL NH4CH3COO buffer solution (Avantor Performance Materials, Radnor, PA, USA, 1 mol∙L−1, pH 7) with analyzed extracts (0.2–1.1 mL; made up to 1.1 mL with 80% ethanol). The absorbance of Cu(I)-neocuproine complex was monitored at 450 nm (UV-mini 1240 spectrophotometer, Shimadzu, Japan) against a reagent blank after incubation that lasted 30 min.
The scavenging activity against 2,2-diphenyl-1-picrylhydrazyl radical (DPPH•, Sigma-Aldrich, St. Louis, MO, USA) was assessed using the modified method reported previously by Brand-Williams, Cuvelier, and Berset, 1995 [19]. An appropriate volume of extracts (1 mL) was mixed with 3 mL of methanol and subsequently 1 mL of 0.5 mol∙L−1 DPPH• solution was added. The absorbance of the mixture was checked after 30 min of incubation at room temperature at a wavelength of 517 nm (UV-mini 1240 spectrophotometer, Shimadzu, Japan).
The calibration curves were prepared using Trolox (Sigma-Aldrich, St. Louis, MO, USA) diluted in the appropriate solvent. The results were calculated as mg of Trolox equivalents per 100 g of sample solids, and per 100 g of honey present in sample solids (results were corrected for respective carrier blank). Additionally, the activity introduced into the powders by the carrier (AC) was also calculated.
2.5. Analysis of Aromatic Compounds
To prepare a sample for headspace extraction feed solution (2 g) and honey powder (1.2 g) were mixed with distilled water (0.8 g) to obtain the same concentration of solids. Afterward, the mixtures were put into 20 mL vials and incubated in the oven at a temperature of 60 °C for 5 min. Absorption of volatile aroma compounds was done by headspace solid-phase microextraction method (HS-SPME) using divinylbenzene/carboxene/polydimtheylsiloxane (DVB/CAR/PDMS from Supelco (Bellefonte, PA, USA) fiber. Absorbed volatiles were afterward transferred to a GC-MS injector.
Before the closure of vials, 1 μL of internal standard (1,2-dichlorobenzene, 0.01% solution in methanol) was added. The injection was done at 250 °C for 2 min. GC-MS device (Shimadzu QP2010S equipped with ZB WAX plus (30 m × 0.25 mm × 0.25 μm) capillary column Phenomenex (Torrance, CA, USA) was used to obtain chromatograms of volatile compounds. The flow rate of helium, which was used as a carrier gas, was equal to 1.59 mL min−1. The following pattern of column temperature was used: starting temperature 60 °C, subsequent increase to 200 °C at the rate of 3 °C/min, second increase to 250 °C at the rate of 3 °C/min. The interface temperature for GC-MS was 230 °C. Ion source temperature was set to 250 °C, while ionization energy was equal 70eV. The total ion monitoring (TIC) was used to detect volatile compounds (m/z ranged from 35 to 500). The identification of separated compounds was performed based on mass spectral libraries (NIST 47, NIST 147, and Wiley 175). The internal standard 1,2-dichlorobenzene was used to semi-quantify volatile compounds. The analysis was done in triplicate for each material.
2.6. FTIR Spectroscopy
The IR(ATR) measurements were done at of the facilities of the University of Life Sciences in Lublin using a 670-IR spectrometer (Agilent, Santa Clara, CA, USA) equipped with ZnSe crystal-containing ATR adaptor. The measurement chamber was kept in a N2 atmosphere. All spectra were recorded with 16 scans with the subsequent software averaging. All solvents used for cleaning the crystals before each measurement were ultrapure grade (Sigma-Aldrich, St. Louis, MO, USA) The resolution for spectra recording, which was performed at 23 °C, was set to 1 cm−1, while the utilized range was equal to 4000–500 cm−1. Processing of the recorded spectra was carried out by Grams/AI software (ThermoGalactic Industries, Waltham, MA, USA).
2.7. Principal Component Analysis (PCA) and Hierarchical Cluster Analysis (HCA)
FTIR spectra recorded within the range of 1850–700 cm−1 were used for PCA and HCA analysis.
PCA was based on the data array of the fingerprints of FTIR spectra of each considered samples. The first several scores of the PCA results were used to make a projection plot that provided a visual determination of the similarity among the fingerprints of FTIR spectra. HCA is a cluster analysis method that seeks to build a hierarchy of clusters. In this method, any valid metric can be used as a measure of similarity. The samples in the same clusters are characterized by many similarities. Samples in different clusters are not similar. In this study, HCA with Euclidean distance were applied. In order to establish the distance matrix for samples for cluster analysis the absorbencies were selected as a measurement for the cluster analysis in the FTIR. The analyses were done using OriginPro software (OriginLab, Northampton, MA, USA) [20,21,22,23].
2.8. Statistical Methods
One-way analysis of variance (ANOVA) and a multiple range test (p < 0.05) were used. To determine the significance of the differences between the mean values for volatiles in particular samples, Tukey’s test was used with the significance level of p = 0.05. Statistica v.13 (Dell Inc., Austin, TX, USA) software was applied to performs the statistical analysis.
3. Results and Discussion
3.1. Total Phenolic Content
The content of phenols (Table 2) in honeydew honey (HH) (122.7 ± 14.8 mg/100 g honey solids) was higher than in rapeseed honey (86.1 ± 6.4 mg / 100 g honey solids). Powders obtained from these kinds of honey reached from 16.5 ± 3.7 to 53.3 ± 3.3 mg GAE/powder solids. Lower contents (from 16.5 ± 3.7 to 34.1 ± 4.6 mg GAE/powder solids) were noted for powders dried by traditional spray drying technique (SD). Such contents of phenolics in raw honey samples and after spray drying may be found typical after comparison with literature data [13,14,15,16,17,18,19,24]. The use of a modified technique with dehumidified air application (DASD) in each case resulted in greater retention of these compounds in the powdered products. Phenolics are often degraded upon thermal processing such as spray drying [25,26]. The content of phenolic compounds in dried products results both from the influence of the process (temperature action, oxidation) and from the need to introduce the carrier into the product. After converting the results to honey solids, changes in honey-derived phenolic levels could be better compared. Much higher retention of phenolic compounds in powders obtained by the new drying technique proposed in the study (80 and 91%, RH and HH, respectively) compared to typical spray drying conditions (around 41 and 59%, RH and HH, respectively) was confirmed.

Table 2.
Total phenolic content (TPC) and antioxidant activity (CUPRAC, DPPH) of rapeseed honey (RH) and honeydew honey (HH) powders obtained by traditional spray drying (SD) and dehumidified air spray drying (DASD); AC–activity introduced into the powders by the carrier; in brackets–relative retention in comparison to raw honey. a–f: differences between mean values followed by different letters in rows were statistically significant (P<0.05).
3.2. Antioxidant Activity
The reducing potential (CUPRAC) of powders obtained in the current work was in the range from 13.2 ± 0.2 to 65.7 ± 2.1mg Trolox/100g powder solids (Table 2). Lower values (from 13.2 ± 0.2 to 31.8 ± 0.9 mg Trolox/100g powder solids) were found in powders obtained for rapeseed honey (RH), which as a raw material was also a lower source of compounds with a reducing effect (38.7 ± 0.8 mg Trolox/100 g honey solids). The RH powder obtained by the new spray drying method (DASD) showed twice the reduction power compared to that obtained by the conventional process (RH-SD). In contrast, drying of honeydew (HH-274.6 ± 9.7 mg Trolox/100 g honey solids), which was very rich in compounds with reducing effects, resulted in obtaining powders with similar antioxidant effects for both drying methods. The activity shown by the carrier was presented, expressed per 100 g powder solids. It was small (2.9 mg Trolox/100 g), and its variability resulting from values of raw material dry matter differed only on the 3rd decimal place. However, due to the unequal activities of the powders, the proportion of carrier in the total powder activity was different. The presence of the carrier had a small impact (below 5%) on the activity of the HH powders, on the contrary to the RH, in which this impact was 9 and 22%, after SD and DASD, respectively. The activity of the honey itself (without taking into account the carrier) in both HH powders was equal and accounted for about half of the strong activity of the raw material honey. In the case of weaker performing (38.7 ± 0.8 mg Trolox/100 g honey solids) rapeseed honey (RH), the proposed new spray drying method (DASD) showed a significant increase in activity (67.4 ± 1.9 mg Trolox/100 g honey solids). This was probably due to beneficial changes in antioxidants, including some phenolic compounds. The method used to determine phenols is sensitive to their composition (and the possible presence of other reducing compounds reacting under the conditions of the assay) [27]; hence, the structural changes in the components of phenol mixture (not necessarily associated with their breakdown to other compounds) may have resulted in determining their lower content using the Folin-Ciocalteu method. In the case of rapeseed honey dried by the traditional method, a significantly greater proportion of the carrier in the reducing potential of the powder (22%) was also noted than in the case of drying by the dehumidified air spray drying with (9%). The beneficial effect of the use of dehumidified air on the reducing potential of rapeseed honey during spray drying using various carriers, including Nutriose®, was also observed in our previous studies [13].
The activity of extracted antioxidants against DPPH radical expressed in Trolox equivalents was lower than their reduction capacity (Table 2). The antiradical activity of each of the powders (expressed per 100 g of powder solids) was also clearly lower than the raw material honey used for their production. The activity of powder is a result of raw material activity, the impact of the process and the presence of the carrier that has a very low activity (0.2 mg Trolox/100 g). Powders produced with the spray drying method at low temperatures were again a source of compounds with better activity against DPPH⦁. The activity of both honeydew honey (HH) powders was higher than rapeseed honey (RH) powders. The negative impact of spray drying temperature on antioxidant activity against DPPH radicals was also observed by Bazaria and Kumar, 2016 on the example of beet juice [28]. Without taking into account the activity, the source of which was the carrier used in the research, antiradical activities were obtained, whose source was the dried material itself. In this experiment, the activity of both kinds of honey dried by the new spray drying method was higher than the activity of raw materials. This was probably the result of a very low loss of phenolic compounds (9–20%) as a consequence of spray drying at a lower temperature with dehumidified air (DASD), as well as favorable transformations of these compounds under proposed conditions, which has already been observed in the reducing potential tests. The proportion of the carrier activity in the whole activity of powders was low (below 3%), except RH-SD of the lowest activity (carrier share in the activity was almost 7%).
Antioxidant activity against DPPH radicals was positively correlated with the content of phenolic compounds (r = 0.83; p <0.05). Such a correlation is known and confirmed in many studies [25,26]. The fact that the correlation obtained in this paper was quite weak may confirm the concept of beneficial structural changes in some phenolic compounds in the process of honey spray drying. In the case of such changes, the activity would not be strongly related to the total content, taking into account the raw material and the samples processed in a different way. In contrast, no correlation between the reduction capacity measured by CUPRAC with phenolic compound content or antiradical activity against DPPH⦁ was noted. The action of bioactive compounds leading to deactivate the DPPH radicals is based on both basic mechanisms of antioxidant activity, single electron transfer (i.e., through the reducing potential of the molecule), and hydrogen atom transfer, although the mechanism is believed to be much more complex [29]. The lack of correlation in both experiments of antioxidant activity testing indicates the importance of this second mechanism for the action of antioxidants contained in honey against DPPH radicals.
3.3. Aroma Compounds
Table 3 presents the results of aroma compounds in reconstituted honey samples of feed solutions. Powders were reconstituted in water to the concentration of the feed solutions. Analyzed samples were characterized by the occurrence of different volatiles in the aroma. The overall count of different aroma compounds of rapeseed powders and solution was 101 and in honeydew honey was 109 (Table 3).

Table 3.
Aroma compounds content in rapeseed and honeydew honey powders and solutions: SD—traditional spray drying, DASD—dehumidified air spray drying, RH—rapeseed honey, HH—honeydew honey. a–c: differences between mean values followed by different letters in rows were statistically significant (p < 0.05).
In all analyzed rapeseed samples, the following four compounds were detected: nonanal, bezaldehyde, 2-methylbutyl 2-methylbutanoate, and methyl hexadecanoate (Table 3). Nonanal is known as an aroma compound found in kinds of honey, which gives citrus, fatty, floral green, and spiny odor [30]. Another volatile component of rapeseed honey aroma as benzaldehyde, which contributes to the formation of sweetness and almond aroma of the honey [30]. In our previous studies, we discussed the aroma profile of reconstituted rapeseed honey powders obtained due to low-temperature spray drying with dehumidified air [13]. The most abundant aroma compounds in those profiles were also nonanal, benzaldehyde, furfural, acetic acid, phenylmethanol. Plutowska et al., 2011 described the aroma compounds present in rapeseed honey and other kinds of honey [31]. It was reported that the highest peak belonged to benzoic acid and benzoic alcohol (phenylmethanol) [31]. These components were also found in our studies; benzoic acid was found in RH-DASD and feed solution samples and phenylmethanol only in the feed solution.
In all analyzed honeydew honey samples, the following twelve compounds were detected: octanoic acid, nonanoic acid, 2-methylpropanoic aid, octanal, nonanal, benzeneacetaldehyde, butane-2,3-diol, 1-dodecanol, 2-(2-ethoxyethoxy)ethanol, propan-2-yl tetradecanoate, methyl hexadecanoate, and oxime-methoxy-phenyl (Table 4). Jánošková et al., 2014, characterized Slovakian honeydew honey aroma compounds and listed acetic acid, butane-2,3-diol, 3-hydroxy-2-butanone, and methyl ester of 2-hydrobenzoic acid as markers of honeydew honey samples [32]. In our investigation, acetic acid was also identified but only in feed solution samples and in reconstituted honeydew honey samples dehumidified with air spray drying (HH-DASD). Moreover, butane-2,3-diol was also reported in all samples of honeydew honey reconstituted from powders. Escriche et al., 2009, also described the volatile components of the honeydew honey aroma and some of them were identified in our honeydew samples, mostly in the feed solution; these were: 3-methyl-3-buten-1-ol, acetic acid, 2-methylpropanoic acid, 1-hexanol, butane-2,3-diol, phenylethanol, and D-limonene, linalool oxide [33]. Karabagias et al., 2019, investigated the aroma profile of honeydew honey. In the group of aldehydes, they identified: octanal, nonanal, and decanal, and their content was 0.032, 0.14, and 0.19 mg/100 g of solids, respectively. Additionally, Karabagias et al., 2019, showed that the content of furfural in honeydew honey samples was 0.04 mg/100 g solids [34], while in our study, it was about 1.00 ± 0.01mg/100 g in samples, which were reconstituted from spray-dried samples in the traditional way (HH-SD). Soria, Gonz, de Lorenzo, and Martínez-Castro, 2005, performed the GC-MS analyses of 20 nectar and honeydew kinds of honey [35]. They reported that the most abundant volatiles of kinds of honey aroma was as follows: 3-methyl-3-buten-1-ol, acetic acid, furfural, benzyl alcohol, and 2-phenylethanol. Moreover, Soria et al, 2005, pointed out that one of the most characteristic aroma compounds of honeydew honey was butane-2,3-diol [35]. Our studies also indicate the major presence of butane-2,3-diol in reconstituted honeydew honey (HH) samples. The highest concentration of butane-2,3-diol was reported in reconstituted honeydew honey spray-dried with dehumidified air HH-DASD (11.10 ± 3.36 mg/100 g solids) and the lowest content in reconstituted samples, which were spray-dried by traditional method HH-SD (1.20±0.24 mg/100 g solids). These observations may suggest that spray drying with dehumidified air (HH-DASD) better preserve aroma compounds found in honeydew honey than traditional spray drying (HH-SD).

Table 4.
The location of the maxima of absorption bands FTIR with the arrangement of appropriate vibration for selected for sampling: RH-SD, RH-DASD, HH-SD, HH-DASD made in terms of spectral 3750–690 cm−1.
Comparing the total content of aroma compounds found in reconstituted samples of rapeseed (RH) and honeydew honey (HH), and feed solutions, it can be observed that the total concentration of aroma compounds in feed solutions was significantly lower than in reconstituted samples. Probably during the drying, new compounds were formed, and the macromolecular compounds were broken down into small, more volatile compounds, which were easier to determine with the use of the SPME method. Vincenzentti et al., 2018, also reported the increase in aroma compounds in spray-dried donkey milk compared to fresh milk [36]. They described that the volatile’s profile of the reconstituted spray-dried donkey milk was the most complex and probably because of some irreversible changes, e.g., the Maillard-type reactions, which probably have occurred also in honey samples investigated in our study. Rapeseed feed solution contained 11.31 ± 4.43 mg of volatiles in 100 g solids and honeydew honey 85.59 ± 2.80 mg/100 g of solids, respectively. The most intensive aroma profile was observed in reconstituted samples prepared from powders obtained with the use of dehumidified air spray drying (DASD). In rapeseed honey reconstituted samples, the total content of volatiles was estimated for 156.68 ± 5.70 mg/100 g solids and in honeydew honey for 339.88 ± 49.08mg/100 g of solids. It shows that the aroma profile of honeydew honey was abundant in volatile compounds. Additionally, Plutowska et al., 2011, found that the rape honey aroma profile was not as rich in different volatile compounds as the honeydew honey aroma profile [31]. The lower total content of volatiles was investigated in reconstituted samples of rapeseed honey (RH-SD) and honeydew honey (HH-SD), which were traditionally spray-dried. The total content of volatiles identified in rapeseed honey (RH-SD) samples was 51.12 ± 1.96 mg/100 g solids, and in honeydew honey (HH-SD) samples, was 31.90 ± 1.40 mg/100 g solids (Table 3). The higher content of aromatic compounds found in reconstituted samples from powders obtained by the new method (DASD) at a lower temperature (75 °C), compared to powders prepared using the traditional method at temperature 180 °C (SD), can be explained by the evaporation of aromatic compounds at higher drying temperature. For instance, the total concentration of aroma compounds founds in reconstituted rapeseed honey samples was 52.12 ± 1.96 mg/100 g of solids, and in honeydew honey samples, was almost three-fold higher and was accounted for 181.25 ± 20.78 mg/100g solids. The above-mentioned results have proven, on the one hand, that the most intensive aroma had samples prepared with powders obtained with the use of dehumidified air spray drying both kinds of honey, and on the other hand, it was observed that feed solution and samples made of traditional spray drying powders were characterized by scanty aroma profiles. This observation proves that the new method of honey powder preparation is very effective and can also be used also in the case of samples, whose aroma profile is very rich and intensive like honeydew honey.
3.4. FTIR Spectroscopy
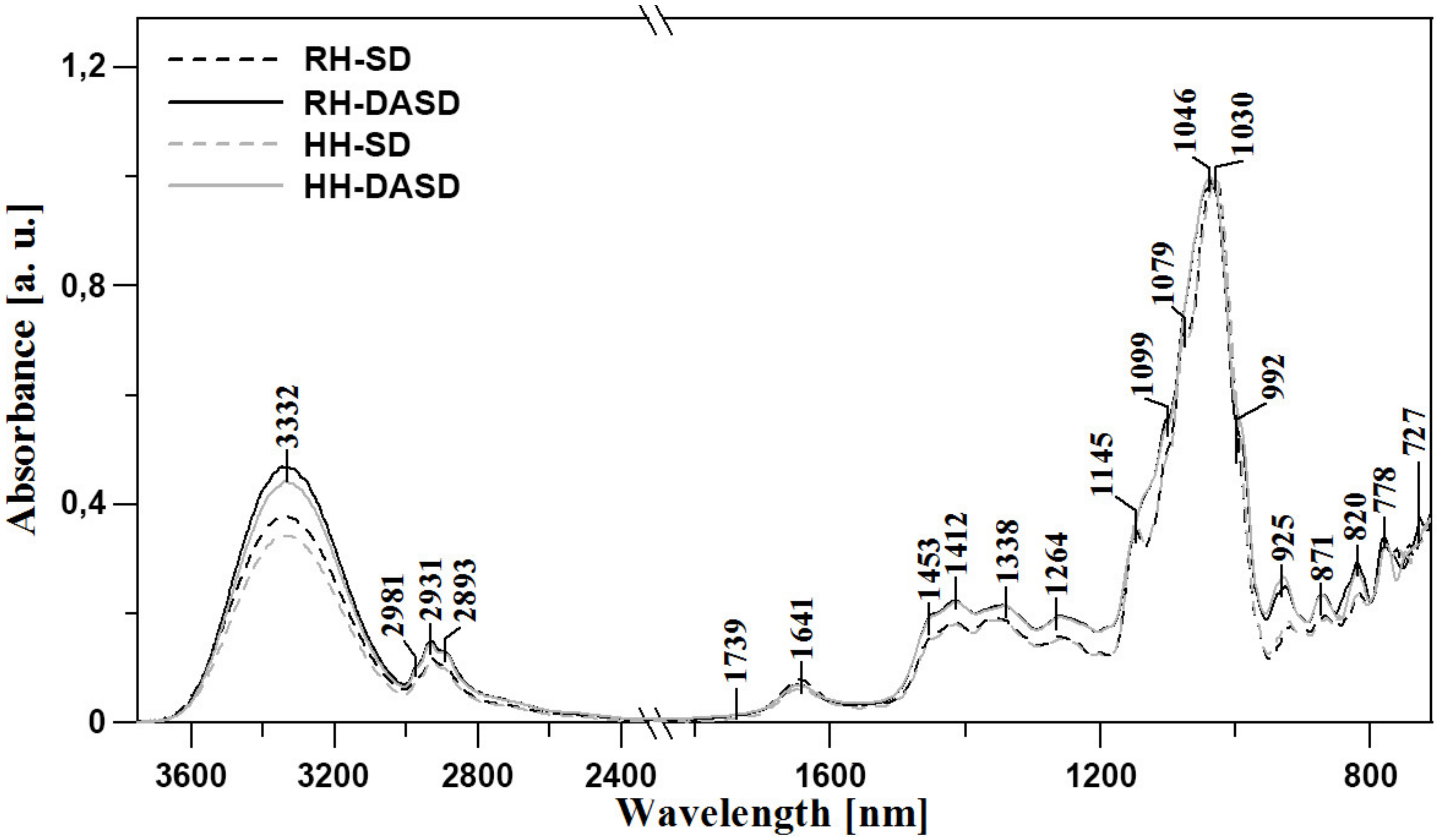
The first series of clearly visible bands are present in all samples, at 3650–3000 cm−1, and are consistent with the findings of Anjos et al., 2015, Kozłowicz et al., 2020, and Svečnjak et al., 2015 [37,38,39]. This region is characteristic of –OH stretching, which may originate from carbohydrates, organic acids, or water. Moreover, the NH3 stretching band of free amino acids may also be present in this range. The region of 3000–2800 cm−1 is dominated by a series of bands that may be assigned to stretching vibrations of sugar C–H skeleton. The band, with its maximum at ~3332 cm−1, represents carboxylic acids, whose irregular absorption enhances the stretching vibrations of C–H groups. Additionally, the strong hydrogen bonding of carboxylic acid dimers broadens the ν (–OH) vibrations band [37]. The fingerprint region starts with a moderate intensity band with the maximum at ~1715 cm−1, which most likely originates from the stretching of carbonyl C=O functionalities present in fructose (ketone) and glucose (aldehyde) [39,40].
Another characteristic series of bands occurs at lower wavenumbers, namely in the range of 1480–700 cm−1. This part of the fingerprint region is characteristic of C-O, C-C, and C-H stretching vibrations, as well as the bending vibrations of C-H present in the chemical structure of carbohydrates [35]. In some cases, these bands may also originate from organic acids and carotenes. The most prominent bands in this region are present at 1453, 1412, and 1338–1264 cm−1. These signals most likely originate from the deformative vibrations of O-CH and C-C-H groups in carbohydrate, as well as from bending of -OH groups of the C-OH structure. Other significant bands within the range from 1245 to 940 cm−1 are assigned to the stretching vibrations of C-H groups or (C-O) in carbohydrates. The vibrations of C-O groups in C-O-C are represented by the bands at 1145 and 1030 cm−1, while the C-OH group or C-C stretching in the carbohydrate structure generate bands between 1030–940 cm−1 and below 900 cm−1 [38,40,41,42,43].
Finally, the region of 900–700 cm−1 reflects the vibrations of the anomeric region in carbohydrates or C–H and C-C deformation [42,44]. Changes in this range often evidence relatively strong modifications of sugar fraction bonds. It is also noteworthy that the considerable differences between bands observed at 1200–940 cm−1 point towards differences between powders obtained using traditional methods and those obtained with the use of dehumidified air. This in turn suggests a strong impact of the modifications on the vibrations of the carbohydrate structure and bonds existing between said groups in the sugar skeleton. Further differences between samples in the analyzed context are evidenced in the spectral range of 3600–3000 cm−1 (Figure 1). To recap this part of FTIR studies, it should be underlined that primary differences in terms of the obtained spectral bands are observed in the regions of approx.: 3600–3000, 1440–1250, and 1140–980 cm−1 (Figure 1). The regions primarily reflect the content of polyphenols and antioxidative components. Differences in terms of water content may also be a factor, but these are mainly related to the method of sample preparation.
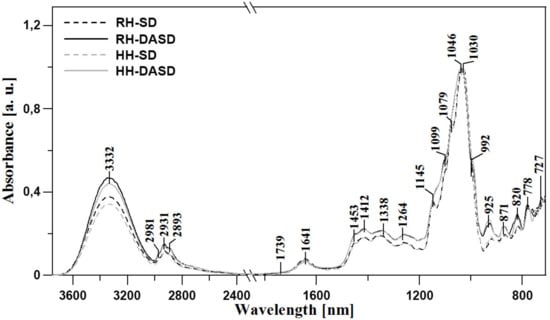
Figure 1.
ATR-FTIR absorption spectra of the analyzed samples RH-SD, RH-DASD, HH-SD, HH-DASD in the spectra range 730–3750 cm−1.
3.5. Cluster Analysis of FTIR Fingerprint of Honey Samples
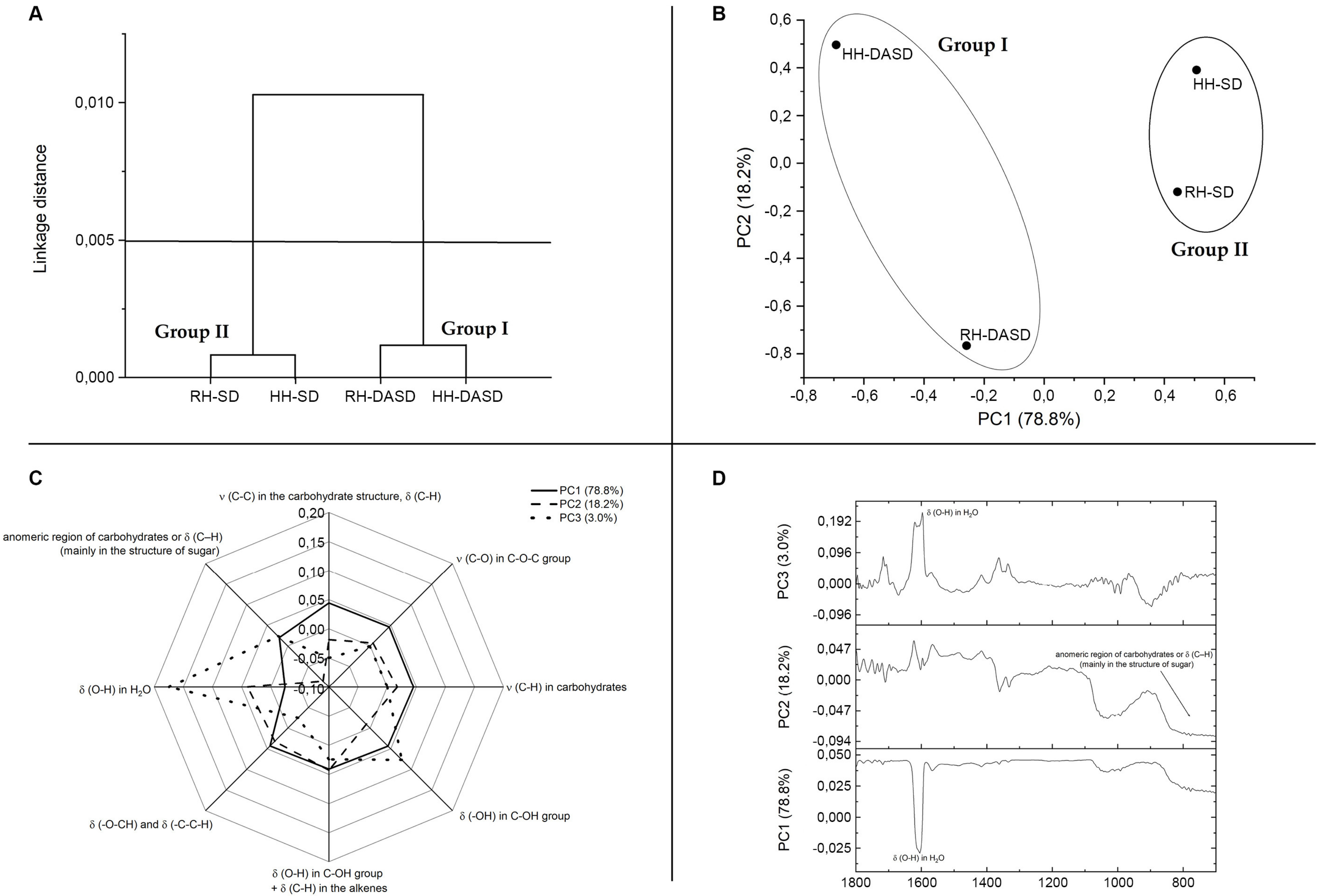
Figure 2A presents the classification of results for honey samples using FTIR spectroscopy done by chemometrics. The obtained results are presented in the dendrogram structure. Considering the cut-off of 0.005 dissimilarity units, two clusters could be distinguished. The first cluster (Group I) is the cluster aggregated on the far-right arm of the dendrogram. This cluster is formed by powder samples obtained by spray drying using dehumidified air (DASD powders). The second cluster (Group II) is composed of honey powders obtained after the traditional spray drying method. It could be noticed that powders after the traditional spray drying process (SD powders, Group 2) were characterized by more similar chemical properties than others. Thus, a more significant impact of the production method than the type of honey on the chemical properties of powders was found.
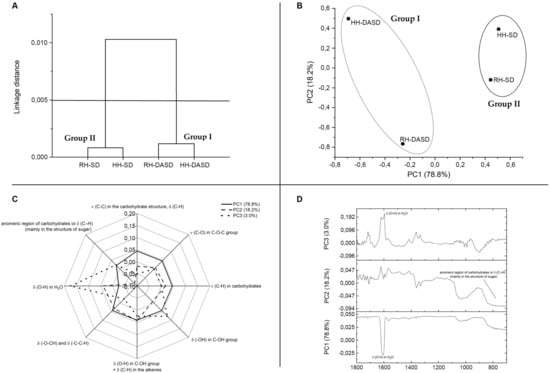
Figure 2.
(A)—Hierarchical cluster dendrogram constructed from the FTIR data for powders. (B)—The scores plot of PCA (PC1xPC2) based on FTIR spectra of powders. (C)—Spider web plot of the main type and origin of vibrations FTIR spectra in the terms of principal components PC1, PC2, PC3 for powders. (D)—The loading factors of PCA. RH-SD, RH-DASD, HH-SD, HH-DASD powders.
3.6. PCA of FTIR Spectra
Figure 2B shows that PC1 and PC2 represented 78.8 and 18.2% of the total variance, respectively. Therefore, PC1 and PC2 were characterized by high values, the score plot of the two first PCs provided sufficient information to reveal the relationship between samples. The score plot of PC1 versus PC2 (Figure 2A) shows that samples were grouped into two different clusters related to hierarchical cluster dendrogram (Figure 2A). Created groups were differentiated by the drying method, not the type of honey. Figure 2B, similarly to Figure 2A, also confirms that powders after traditional drying had more common characteristics (HH-SD and RH-SD points are closer together) than powders after drying with dehumidified air. This relationship was also observed when the content of polyphenols and antioxidant activity was discussed.
3.7. Loading Analysis of FTIR Fingerprint of Honey Samples
The loadings or weighted factors indicate the presence or absence of functional groups found in the datasets and show the spectral differences that are responsible for grouping within the selected PC. In other words, the loading analysis shows which functional groups have the greatest impact on PC separation [45]. PC1 was characterized by the smallest share of the band ~1641 cm−1, corresponding to deformation vibrations of OH groups (Figure 2C and 2D). The peak with the lowest range in PC2 included ~720–~860 cm−1. This peak was closely related to the anomeric carbohydrate region, δ (C-H), (mainly in the sugar structure). The peak with the highest share in PC3 (~1641 cm−1, corresponding to deformation vibrations of OH groups) is the peak with a smaller share in PC1.
4. Conclusions
The article describes a significant impact of the type of drying method (traditional spray drying or spray drying with dehumidified air) on the quality characteristics of the obtained honey powders. Powders obtained by the low-temperature spray drying method (with dehumidified air) were characterized by a higher content of polyphenols, aromatic compounds, and antioxidant activity, compared to powders obtained by the traditional method.
A significant impact of the type of honey on the quality characteristics of honey powders was found. Honeydew honey compared to rapeseed honey was characterized by a higher content of polyphenols, antioxidant activity, and aromatic compounds. HCA and PCA analysis divided the powders into two groups, according to the method of preparation. Thus, a more significant impact of the production method than the type of honey on the chemical properties of powders was found.
Author Contributions
Conceptualization, A.J. and K.S.; methodology, A.J., D.D., R.W., A.M., A.N.; analysis, A.J., K.S., A.W., D.D.; writing—original draft preparation A.J., A.M., A.N.; writing—review and editing, A.J., D.W.-R.; visualization, A.J., A.M., A.N. All authors have read and agreed to the published version of the manuscript.
Funding
The research of Agnieszka Niemczynowicz was partially supported by the grant of University of Warmia and Mazury in Olsztyn no. POWER.03.05.00-00-Z310/17. The authors Agnieszka Niemczynowicz and Arkadiusz Matwijczuk acknowledge the Cost project CA 15126. The work was also co-financed by a statutory activity subsidy from the Polish Ministry of Science and Higher Education for the Institute of Food Sciences of Warsaw University of Life Sciences.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy restriction.
Acknowledgments
We would like to thank Agata Wieczorek for her participation in obtaining the results. We thank MASPEX-GMW Sp. Z o.o. Sp. K for technical support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Samarghandian, S.; Farkhondeh, T.; Samini, F. Honey and Health: A Review of Recent Clinical Research. Pharmacogn. Res. 2017, 9, 121–127. [Google Scholar] [CrossRef]
- Popescu, R.; Soria, A.C.; Geana, E.I.; Dinca, O.R.; Sandru, C.; Costinel, D.; Ionete, R.E. Characterization of the Quality and Floral Origin of Romanian Honey. Anal. Lett. 2015, 49, 411–422. [Google Scholar] [CrossRef]
- Sanza, M.L.; Gonzalezb, M.; Lorenzob, C.; Sanza, J.; Martınez-Castro, I. A contribution to the differentiation between nectar honeyand honeydew. Food Chem. 2005, 91, 113–119. [Google Scholar] [CrossRef]
- Samborska, K.; Langa, E.; Kamińska-Dwórznicka, A.; Witrowa-Rajchert, D. The influence of sodium caseinate on the physical properties of spray dried honey. Int. J. Food Sci. Technol. 2015, 50, 256–262. [Google Scholar] [CrossRef]
- Cui, Z.; Sun, L.; Chen, W.; Sun, D. Preparation of dry honey by microwave–vacuum drying. J. Food Eng. 2008, 84, 582–590. [Google Scholar] [CrossRef]
- Samborska, K.; Czelejewska, M. The influence of thermal treatment and spray drying on the physico-chemical properties of Polish honeys. J. Food Process. Preserv. 2014, 38, 413–419. [Google Scholar] [CrossRef]
- Hebbar, H.U.; Rastogi, N.K.; Subramanian, R. Properties of dried and intermediate moisture honey products: A review. Int. J. Food Prop. 2008, 11, 804–819. [Google Scholar] [CrossRef]
- Samborska, K. Powdered honey—Drying methods and parameters, types of carriers and drying aids, physicochemical properties and storage stability. Trends Food Sci. Technol. 2019, 88, 133–142. [Google Scholar] [CrossRef]
- Shi, Q.; Fang, Z.; Bhandari, B. Effect of addition of whey protein isolate on spray drying behavior of honey with maltodextrin as a Carrier material. Dry. Technol. 2013, 31, 1681–1692. [Google Scholar] [CrossRef]
- Janiszewska, E.; Jedlińska, A.; Witrowa-Rajchert, D. Effect of homogenization parameters on selected physical properties of lemon aroma powder. Food Bioprod. Process. 2014, 94, 405–413. [Google Scholar] [CrossRef]
- Desai, K.G.H.; Park, H.J. Recent developments in microencapsulation of food ingredients. Dry. Technol. 2005, 23, 1361–1394. [Google Scholar] [CrossRef]
- Jedlińska, A.; Samborska, K.; Wieczorek, A.; Wiktor, A.; Ostrowska-Ligęza, E.; Jamróz, W.; Skwarczyńska-Maj, K.; Kiełczewski, D.; Błażowski, Ł.; Tułodziecki, M.; et al. The application of dehumidified air in rapeseed and honeydew honey spray drying—Process performance and powders properties considerations. J. Food Eng. 2019, 245, 80–87. [Google Scholar] [CrossRef]
- Samborska, K.; Jedlińska, A.; Wiktor, A.; Derewiaka, D.; Wołosiak, R.; Matwijczuk, A.; Jamróz, W.; Skwarczyńska-Maj, K.; Kiełczewski, D.; Błażowski, Ł.; et al. The effect of low-temperature spray drying with dehumidified air on phenolic compounds, antioxidant activity, and aroma compounds of rapeseed honey powders. Food Bioprocess Technol. 2019, 12, 919–932. [Google Scholar] [CrossRef]
- Samborska, K.; Wiktor, A.; Jedlińska, A.; Matwijczuk, A.; Jamróz, W.; Skwarczyńska-Maj, K.; Kiełczewski, D.; Tułodziecki, M.; Błażowski, Ł.; Witrowa-Rajchert, D. Development and characterization of physical properties of honey-rich powder. Food Bioprod. Process. 2019, 115, 78–86. [Google Scholar] [CrossRef]
- Lefranc-Millot, C. NUTRIOSE 06: A useful soluble dietary fibre for added nutritional value. Nutr. Bull. 2008, 33, 234–239. [Google Scholar] [CrossRef]
- Chambers, E.; Chambers, E.; Castro, M. What Is “Natural”? Consumer Responses to Selected Ingredients. Foods 2018, 7, 65. [Google Scholar] [CrossRef]
- Lefranc-Millot, C.; Guerin-Deremaux, L.; Wils, D. Impact a resistant dextrin on intestinal ecology: How altering the digestive ecosystem with Nutriose, a soluble fiber with prebiotics properties, may be beneficial for health. J. Int. Med Res. 2012, 40, 211–224. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acis reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Lebensm.-Wiss. Und Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Brereton, R.G. Chemometrics: Data Analysis for the Laboratory and Chemical Plant; John Wiley: Chichester, UK, 2003. [Google Scholar]
- Xu, C.J.; Liang, Y.Z.; Chau, F.T.; Heyden, Y.V. Pretreatments of chromatographic fingerprints for quality control of herbal medicines. J. Chromatogr. A 2006, 1134, 253–259. [Google Scholar] [CrossRef]
- Li, Y.Q.; Kong, D.X.; Wu, H. Analysis and evaluation of essential oil components of cinnamon barks using GC–MS and FTIR spectroscopy. Ind. Crop. Prod. 2013, 41, 269–278. [Google Scholar] [CrossRef]
- Abdi, H.; Williams, L.J. Principal component analysis. In Wiley Interdisciplinary Reviews: Computational Statistics; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2010; Volume 2, pp. 433–459. [Google Scholar]
- Ciappini, M.C.; Stoppani, F.S. Determination of antioxidant capacity, flavonoids, and total phenolic content in eucalyptus and clover honeys. J. Agric. Sci. 2014, 58, 103–111. [Google Scholar] [CrossRef]
- Suhag, Y.; Nanda, V. Optimization of spray drying processparametersof nutritionally rich honey powderusing responce Surface methodology. Cogent Food Agric. 2016, 2, 1–12. [Google Scholar] [CrossRef]
- Fang, Z.; Bhandari, B. Effect of spray drying and storage on the stability of bayberry poliphenols. Food Chem. 2011, 129, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Bazaria, B.; Kumar, P. Effect of whey protein concentrate as drying aid and drying parameters on physicochemical and functional properties of spray dried betroot juice concentrate. Food Biosci. 2016, 14, 21–27. [Google Scholar] [CrossRef]
- Musialik, M.; Litwinienko, G. Scavenging of DPPH• radicals by vitamin E is accelerated by its partial ionization: The role of sequential proton loss electron transfer. Org. Lett. 2005, 22, 4951–4954. [Google Scholar] [CrossRef]
- Manyi-Loh, C.E.; Ndip, R.N.; Clarke, A.M. Volatile compounds in honey: A review on their involvement in aroma, botanical origin determination and potential biomedical activities. Int. J. Mol. Sci. 2011, 12, 9514–9532. [Google Scholar] [CrossRef]
- Plutowska, B.; Chmiel, T.; Dymerski, T.; Wardenecki, W. A headspace solid-phase microextraction method development and its application in the determination of volatiles in honey by gas chromatography. Food Chem. 2011, 126, 1288–1298. [Google Scholar] [CrossRef]
- Jánošková, N.; Vyviurska, O.; Špánik, I. Identification of volatile organic compounds in honeydew honeys using comprehensive gas chromatography. J. Food Nutr. Res. 2014, 53, 353–362. [Google Scholar]
- Escriche, I.; Visquert, M.; Juan-Borrás, M.; Fito, P. Influence of simulated industrial thermal treatments on the volatile fractions of different varieties of honey. Food Chem. 2009, 112, 329–338. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Nikolaou, C.; Karabagias, V.K. Volatile fingerprints of common and rare honeys produced in Greece: In search of PHVMs with implementation of the honey code. Eur. Food Res. Technol. 2019, 245, 23–39. [Google Scholar] [CrossRef]
- Soria, A.C.; Gonz, M.; de Lorenzo, C.; Martínez-Castro, I. Estimation of the honeydew ratio in honey samples from their physicochemical data and from their volatile composition obtained by SPME and GC-MS. J. Sci. Food Agric. 2005, 85, 817–824. [Google Scholar] [CrossRef]
- Vincenzetti, S.; Cecchi, T.; Perinelli, D.R.; Pucciarelli, S.; Polzonetti, V.; Bonacucina, G.; Ariani, A.; Parrocchia, L.; Spera, D.M.; Ferretti, E.; et al. Effects of freeze-drying and spray-drying on donkey milk volatile compounds and whey proteins stability. LWT—Food Sci. Technol. 2018, 88, 189–195. [Google Scholar] [CrossRef]
- Anjos, O.; Campos, M.G.; Ruiz, P.C.; Antunes, P. Application of FTIR-ATR spectroscopy to the quantification of sugar in honey. Food Chem. 2015, 169, 218–223. [Google Scholar] [CrossRef]
- Svečnjak, L.; Bubalo, D.; Baranović, G.; Novosel, H. Optimization of FTIR-ATR spectroscopy for botanical authentication of unifloral honey types and melissopalynological data prediction. Eur. Food Res. Technol. 2015, 240, 1101–1115. [Google Scholar]
- Kozłowicz, K.; Różyło, R.; Gładyszewska, B.; Matwijczuk, A.; Gładyszewski, G.; Chocyk, D.; Samborska, K.; Piekut, J.; Smolewska, M. Identification of sugars and phenolic compounds in honey powders with the use of GC–MS, FTIR spectroscopy, and X-ray diffraction. Sci. Rep. 2020, 10, 16269. [Google Scholar] [CrossRef]
- Samborska, K.; Suszek, J.; Hać-Szymańczuk, E.; Matwijczuk, A.; Gładyszewska, B.; Chocyk, D.; Gładyszewski, G.; Gondek, E. Characterization of membrane processed honey and the effect of ultrafiltration with diafiltration on subsequent spray drying. J. Food Process Eng. 2018, 41, e12818. [Google Scholar] [CrossRef]
- Gallardo-Velázquez, T.; Osorio-Revilla, G.; Zuñiga-de Loa, M.; Rivera-Espinoza, Y. Application of FTIR-HATR spectroscopy and multivariate analysis to the quantification of adulterants in Mexican honeys. Food Res. Int. 2009, 42, 313–318. [Google Scholar] [CrossRef]
- Svečnjak, L.; Prđun, S.; Rogina, J.; Bubalo, D.; Jerković, I. Characterization of Satsuma mandarin (Citrus unshiu Marc.) nectar-to-honey transformation pathway using FTIR-ATR spectroscopy. Food Chem. 2017, 232, 286–294. [Google Scholar] [CrossRef]
- Samborska, K.; Kamińska, P.; Jedlińska, A.; Matwijczuk, A.; Kamińska-Dwórznicka, A. Membrane processing in the sustainable production of low-sugar apple-cranberry cloudy juice. Appl. Sci. 2018, 8, 1082. [Google Scholar] [CrossRef]
- Elzey, B.; Pollard, D.; Fakayode, S.O. Determination of adulterated neem and flaxseed oil compositions by FTIR spectroscopy and multivariate regression analysis. Food Control 2016, 68, 303–309. [Google Scholar] [CrossRef]
- Mohsin, G.F.; Schmitt, F.J.; Kanzler, C.; Hoehl, A. PCA-based indentification and differentiation of FTIR data from model melanoidins with spectric molecular composition. Food Chem. 2019, 281, 106–113. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).