Morphological Study of Bacillus thuringiensis Crystals and Spores
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Spore/Crystal Preparation
2.2. Scanning Electron Microscopy (SEM) Image Acquisition
2.3. Spore/Crystal Size Measurement Protocol
2.4. Statistical Analysis of the Data
3. Results
3.1. Anti-Lepidopteran Strains: Collection of Bipyramidal and Cubic Crystals
3.2. Anti-Dipteran Strains: Spherical Crystals by Israelensis and Kin
3.3. B. thuringiensis Spore Morphology and Size Distribution
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Fayad, N.; Kallassy Awad, M.; Mahillon, J. Diversity of Bacillus cereus sensu lato mobilome. BMC Genom. 2019, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Biological Hazards (BIOHAZ). Risks for public health related to the presence of Bacillus cereus and other Bacillus spp. including Bacillus thuringiensis in foodstuffs. EFSA J. 2016, 14, e04524. [Google Scholar]
- Doganay, M.; Demiraslan, H. Human Anthrax as a Re-Emerging Disease. Recent Pat. Anti-Infect. Drug Discov. 2015, 10, 10–29. [Google Scholar] [CrossRef] [PubMed]
- Damalas, C.A.; Koutroubas, S.D. Current status and recent developments in biopesticide use. Agriculture 2018, 8, 13. [Google Scholar] [CrossRef]
- Palma, L.; Muñoz, D.; Berry, C.; Murillo, J.; Caballero, P. Bacillus thuringiensis Toxins: An Overview of Their Biocidal Activity. Toxins 2014, 6, 3296–3325. [Google Scholar] [CrossRef]
- Bravo, A.; Gill, S.S.; Soberon, M. Mode of action of Bacillus thuringiensis Cry and Cyt toxins and their potential for insect control. Toxicon 2007, 49, 423–435. [Google Scholar] [CrossRef]
- Bravo, A.; Likitvivatanavong, S.; Gill, S.S.; Soberón, M. Bacillus thuringensis: A story of a sucessful bioinsecticide. Insect Biochem. Mol. Biol. 2013, 41, 423–431. [Google Scholar] [CrossRef]
- Crickmore, N.; Berry, C.; Panneerselvam, S.; Mishra, R.; Connor, T.R.; Bonning, B.C. A structure-based nomenclature for Bacillus thuringiensis and other bacteria-derived pesticidal proteins. J. Invertebr. Pathol. 2020, 107438. [Google Scholar] [CrossRef]
- El khoury, M.; Azzouz, H.; Chavanieu, A.; Abdelmalak, N.; Chopineau, J.; Kallassy Awad, M. Isolation and characterization of a new Bacillus thuringiensis strain Lip harboring a new cry1Aa gene highly toxic to Ephestia kuehniella (Lepidoptera: Pyralidae) larvae. Arch. Microbiol. 2014, 196, 435–445. [Google Scholar] [CrossRef]
- Elleuch, J.; Jaoua, S.; Darriet, F.; Chandre, F.; Tounsi, S.; Zghal, R.Z. Cry4Ba and Cyt1Aa proteins from Bacillus thuringiensis israelensis: Interactions and toxicity mechanism against Aedes aegypti. Toxicon 2015, 104, 83–90. [Google Scholar] [CrossRef]
- de Barjac, H.; Frachon, E. Classification of Bacillus thuringiensis strains. Entomophaga 1990, 35, 233–240. [Google Scholar] [CrossRef]
- Xu, D.; Côté, J.-C. Sequence Diversity of the Bacillus thuringiensis and B. cereus sensu lato flagellin (H Antigen) Protein: Comparison with H Serotype Diversity. Appl. Environ. Microbiol. 2006, 72, 4653–4662. [Google Scholar] [CrossRef]
- Berry, C.; O’Neil, S.; Ben-dov, E.; Jones, A.F.; Murphy, L.; Quail, M.A.; Holden, M.T.G.; Harris, D.; Zaritsky, A.; Parkhill, J. Complete Sequence and Organization of pBtoxis, the Toxin-Coding Plasmid of Bacillus thuringiensis subsp. israelensis. Appl. Environ. Microbiol. 2002, 68, 5082–5095. [Google Scholar] [CrossRef] [PubMed]
- Gillis, A.; Fayad, N.; Makart, L.; Bolotin, A.; Sorokin, A.; Kallassy, M.; Mahillon, J. Role of plasmid plasticity and mobile genetic elements in the entomopathogen Bacillus thuringiensis serovar israelensis. FEMS Microbiol. Rev. 2018, 1–28. [Google Scholar] [CrossRef]
- Fayad, N.; Patiño-Navarrete, R.; Kambris, Z.; Antoun, M.; Osta, M.; Chopineau, J.; Mahillon, J.; El Chamy, L.; Sanchis, V.; Kallassy Awad, M. Characterization and Whole Genome Sequencing of AR23, a Highly Toxic Bacillus thuringiensis Strain Isolated from Lebanese Soil. Curr. Microbiol. 2019, 76, 1503–1511. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Song, L.; Shu, C.; Wang, P.; Deng, C.; Peng, Q.; Lereclus, D.; Wang, X.; Huang, D.; Zhang, J.; et al. Complete Genome Sequence of Bacillus thuringiensis subsp. kurstaki Strain HD73. Genome Announc. 2013, 1, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Méric, G.; Mageiros, L.; Pascoe, B.; Woodcock, D.J.; Mourkas, E.; Lamble, S.; Bowden, R.; Jolley, K.A.; Raymond, B.; Sheppard, S.K. Lineage-specific plasmid acquisition and the evolution of specialized pathogens in Bacillus thuringiensis and the Bacillus cereus group. Mol. Ecol. 2018, 27, 1524–1540. [Google Scholar] [CrossRef] [PubMed]
- Loutfi, H.; Pellen, F.; Le Jeune, B.; Lteif, R.; Kallassy, M.; Le Brun, G.; Abboud, M. Real-time monitoring of bacterial growth kinetics in suspensions using laser speckle imaging. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Loutfi, H.; Pellen, F.; Le Jeune, B.; Lteif, R.; Kallassy, M.; Le Brun, G.; Abboud, M. Interpretation of the bacterial growth process based on the analysis of the speckle field generated by calibrated scattering media. Opt. Express 2020, 28, 28648–28655. [Google Scholar] [CrossRef]
- Nassif, R.; Abou Nader, C.; Rahbany, J.; Pellen, F.; Salameh, D.; Lteif, R.; Le Brun, G.; Le Jeune, B.; Kallassy Awad, M.; Abboud, M. Characterization of Bacillus thuringiensis parasporal crystals using laser speckle technique: Effect of crystal concentration and dimension. Appl. Opt. 2015, 54, 3725–3731. [Google Scholar] [CrossRef]
- Manasherob, R.; Zaritsky, A.; Ben-Dov, E.; Saxena, D.; Barak, Z.; Einav, M. Effect of accessory proteins P19 and P20 on cytolytic activity of Cyt1Aa from Bacillus thuringiensis subsp. israelensis in Escherichia coli. Curr. Microbiol. 2001, 43, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.X.; Zeng, S.L.; Yuan, M.J.; Sun, F.; Pang, Y. Influence of accessory protein P19 from Bacillus thuringiensis on insecticidal crystal protein Cry11Aa. Wei Sheng Wu Xue Bao 2006, 46, 353–357. [Google Scholar]
- Diaz-Mendoza, M.; Bideshi, D.K.; Ortego, F.; Farinós, G.P.; Federici, B.A. The 20-kDa chaperone-like protein of Bacillus thuringiensis ssp. israelensis enhances yield, crystal size and solubility of Cry3A. Lett. Appl. Microbiol. 2012, 54, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Ennourri, K.; Hassen, H.B.; Zouari, N. Optimization of bioinsecticides overproduction by Bacillus thuringiensis subsp. kurstaki using linear regression. Pol. J. Microbiol. 2013, 62, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Perani, M.; Bishop, A.H. Effects of media composition of delta-endotoxin production and morphology of Bacillus thuringiensis in wild types and spontaneously mutated strains. Microbios 2000, 101, 47–66. [Google Scholar]
- Yezza, A.; Tyagi, R.D.; Valéro, J.R.; Surampalli, R.Y. Bioconversion of industrial wastewater and wastewater sludge into Bacillus thuringiensis based biopesticides in pilot fermentor. Bioresour. Technol. 2006, 97, 1850–1857. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.A. Effect of strain and medium variation on mosquito toxin production by Bacillus thuringiensis var. israelensis. Can. J. Microbiol. 1982, 28, 1089–1092. [Google Scholar] [CrossRef]
- Day, M.; Ibrahim, M.; Dyer, D.; Bulla, L. Genome sequence of Bacillus thuringiensis subsp. kurstaki strain HD-1. Genome Announc. 2014, 2, 613–627. [Google Scholar] [CrossRef]
- Saadaoui, I.; Rouis, S.; Jaoua, S. A new Tunisian strain of Bacillus thuringiensis kurstaki having high insecticidal activity and δ-endotoxin yield. Arch. Microbiol. 2009, 191, 341–348. [Google Scholar] [CrossRef]
- Bolotin, A.; Gillis, A.; Sanchis, V.; Nielsen-LeRoux, C.; Mahillon, J.; Lereclus, D.; Sorokin, A. Comparative genomics of extrachromosomal elements in Bacillus thuringiensis subsp. israelensis. Res. Microbiol. 2017, 168, 331–344. [Google Scholar] [CrossRef]
- Zribi Zghal, R.; Kharrat, M.; Rebai, A.; Ben Khedher, S.; Jallouli, W.; Elleuch, J.; Ginibre, C.; Chandre, F.; Tounsi, S. Optimization of bio-insecticide production by Tunisian Bacillus thuringiensis israelensis and its application in the field. Biol. Control. 2018, 124, 46–52. [Google Scholar] [CrossRef]
- Fayad, N. Analysis of Mobile Genetic Elements in the Genomes of Bacillus thuringiensis and Bacillus cereus. Ph.D. Thesis, Université Saint-Joseph de Beyrouth and Université Catholique de Louvain, Beirut, Liban, 2020. [Google Scholar]
- Travers, R.S.; Martin, P.A.W.; Reichelderfer, C.F. Selective Process for Efficient Isolation of Soil Bacillus spp. Appl. Environ. Microbiol. 1987, 53, 1263–1266. [Google Scholar] [CrossRef] [PubMed]
- Rahbani Mounsef, J.; Salameh, D.; Kallassy Awad, M.; Chamy, L.; Brandam, C.; Lteif, R. A simple method for the separation of Bacillus thuringiensis spores and crystals. J. Microbiol. Methods 2014, 107, 147–149. [Google Scholar] [CrossRef] [PubMed]
- Carrera, M.; Zandomeni, R.; Fitzgibbon, J.; Sagripanti, J.-L. Difference between the spore sizes of Bacillus anthracis and other Bacillus species. J. Appl. Microbiol. 2007, 102, 303–312. [Google Scholar] [CrossRef]
- Lane, D.M. Project Leader, Online Statistics Education: A Multimedia Course of Study. Rice University (Chapter 2 “Graphing Distributions”, Section “Histograms”). Available online: http://onlinestatbook.com/ (accessed on 25 December 2020).
- Scherrer, P.; Lüthy, P.; Trumpi, B. Production of -endotoxin by Bacillus thuringiensis as a function of glucose concentrations. Appl. Microbiol. 1973, 25, 644–646. [Google Scholar] [CrossRef]
- Guicheteau, J.; Argue, L.; Emge, D.; Hyre, A.; Jacobson, M.; Christesen, S. Bacillus spore classification via surface-enhanced Raman spectroscopy and principal component analysis. Appl. Spectrosc. 2008, 62, 267–272. [Google Scholar] [CrossRef]
- Tufts, J.A.; Calfee, M.W.; Lee, S.D.; Ryan, S.P. Bacillus thuringiensis as a surrogate for Bacillus anthracis in aerosol research. World J. Microbiol. Biotechnol. 2014, 30, 1453–1461. [Google Scholar] [CrossRef]
- Stewart, G.C. The Exosporium Layer of Bacterial Spores: A connection to the Environment and the Infected Host. Microbiol. Mol. Biol. Rev. 2015, 79, 437–457. [Google Scholar] [CrossRef]
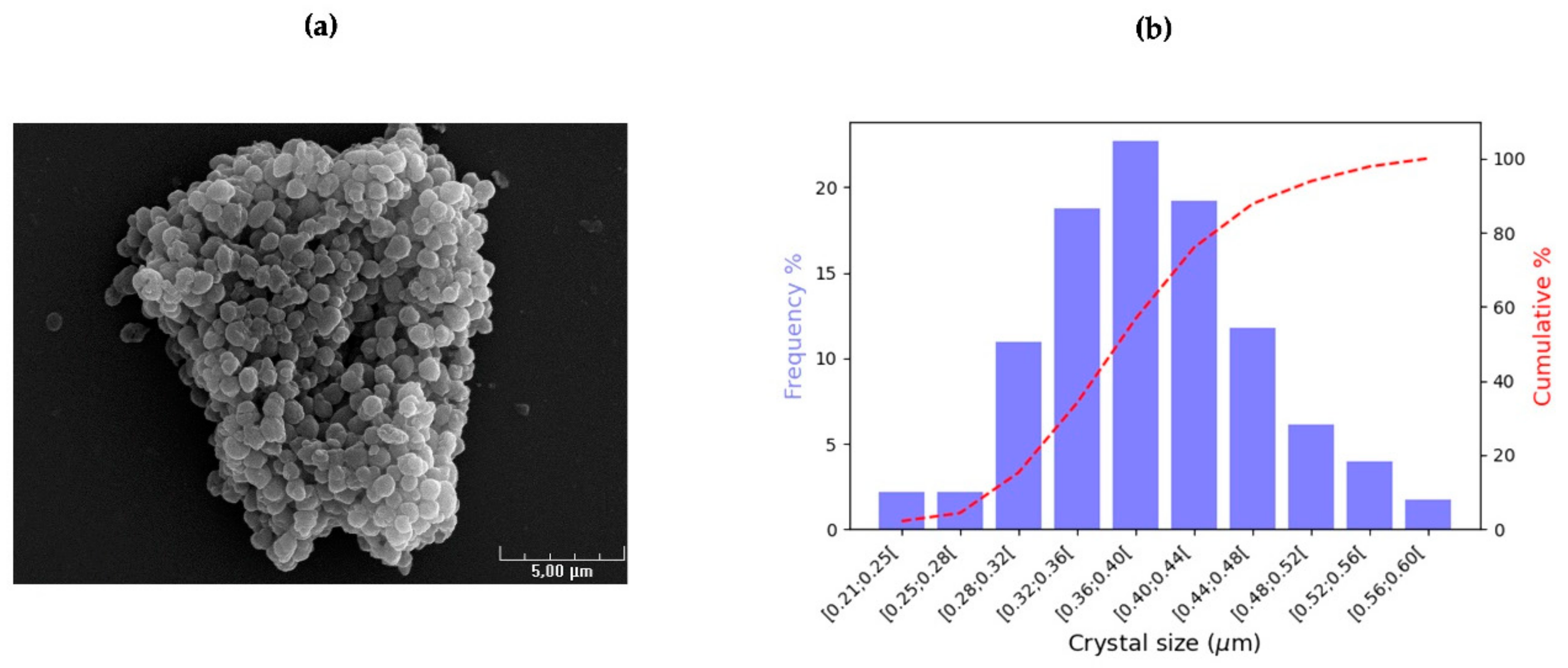

| Strain. | Origin | Serovar | Crystal Shape | Crystal Protein Genes ( ) * | Reference |
|---|---|---|---|---|---|
| HD1 | Thuricide® | kurstaki | B and C | 6:00 | [28] |
| cry1 (4) | |||||
| cry2 (2) | |||||
| LIPMKA | Lebanon | kurstaki | B and C | 6:00 | [9] |
| cry1 (4) | |||||
| cry2 (2) | |||||
| BLB1 | Tunisia | kurstaki | B and C | 6:00 | [29] |
| cry1 (4) | |||||
| cry2 (2) | |||||
| AM65-52 | Vectobac® | israelensis | S | cry4aa, cry4ba, cry10aa, cry11aa, cyt1aa, cyt1ca, cyt2ba | [30] |
| AR23 | Lebanon | israelensis | S | cry4aa, cry4ba (2), cry10aa, cry11aa, cyt1aa, cyt1ca, cyt2ba | [15] |
| BUPM98 | Tunisia | israelensis | S | cry4aa, cry4ba (2), cry10aa, cry11aa, cyt1aa, cyt1ca, cyt2ba | [31] |
| H3 | Lebanon | ND ** | S | 11 novel cry genes | [32] |
| (a) | |||||||||||
| Bipyramidal Crystals | |||||||||||
| Strain | N | Bipyramidal Height (2 h) (μm) | Side of the Base (μm) | Volume * (μm3) | Aspect ratio | ||||||
| Average | Unc. | Range | Average | Unc. | Range | Average | Unc. | Average | Unc. | ||
| HD1 | 1198 | 1.508 | 0.009 | 0.766–2.267 | 0.611 | 0.096 | 0.433–0.898 | 0.188 | 0.002 | 0.416 | 0.006 |
| LIPMKA | 1335 | 1.549 | 0.016 | 0.597–2.930 | 0.554 | 0.119 | 0.305–0.782 | 0.158 | 0.002 | 0.371 | 0.004 |
| BLB1 | 1400 | 1.531 | 0.014 | 0.723–2.685 | 0.582 | 0.116 | 0.369–0.993 | 0.173 | 0.002 | 0.393 | 0.004 |
| (b) | |||||||||||
| Cubic Crystals | |||||||||||
| Strain | N | Side of the Square (μm) | Volume ** (μm3) | ||||||||
| Average | Unc. | Range | Average | Unc. | |||||||
| HD1 | 574 | 0.573 | 0.009 | 0.264–0.886 | 0.21 | 0.009 | |||||
| LIPMKA | 248 | 0.478 | 0.014 | 0.222–0.860 | 0.129 | 0.013 | |||||
| BLB1 | 1598 | 0.614 | 0.006 | 0.285–1.157 | 0.262 | 0.008 | |||||
| Spherical Crystals | ||||||
|---|---|---|---|---|---|---|
| Strain | N | Radius (μm) | Volume * (μm3) | |||
| Average | Unc. | Range | Average | Unc. | ||
| AM65-52 | 229 | 0.394 | 0.009 | 0.206–0.683 | 0.283 | 0.021 |
| AR23 | 941 | 0.381 | 0.003 | 0.228–0.530 | 0.242 | 0.006 |
| BUPM98 | 1500 | 0.37 | 0.003 | 0.143–0.524 | 0.225 | 0.005 |
| H3 | 1350 | 0.411 | 0.003 | 0.217–0.615 | 0.307 | 0.007 |
| Spores | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Strain | N | Length (μm) | Width (μm) | Volume ** (μm3) | Aspect Ratio | ||||||
| Average | Unc. | Range | Average | Unc. | Range | Average | Unc. | Average | Unc. | ||
| HD1 | 44 | 1.343 | 0.092 | 0.824–2.203 | 0.789 | 0.050 | 0.459–1.326 | 0.476 | 0.104 | 0.595 | 0.030 |
| LIPMKA | 365 | 1.649 | 0.023 | 0.870–2.767 | 0.8269 | 0.009 | 0.445–1.112 | 0.603 | 0.018 | 0.508 | 0.008 |
| BLB1 | 212 | 1.459 | 0.053 | 0.395–2.259 | 0.722 | 0.025 | 0.221–1.069 | 0.465 | 0.032 | 0.501 | 0.009 |
| AM65-52 | 118 | 1.257 | 0.04 | 0.706–1.928 | 0.783 | 0.026 | 0.465–1.142 | 0.426 | 0.036 | 0.636 | 0.026 |
| AR23 | 823 | 1.503 | 0.014 | 0.673–2.118 | 0.863 | 0.0113 | 0.605–1.884 | 0.584 | 0.012 | 0.605 | 0.014 |
| BUPM98 | 263 | 1.55 | 0.019 | 1.123–1.957 | 0.8093 | 0.009 | 0.598–1.054 | 0.540 | 0.015 | 0.525 | 0.007 |
| H3 | 212 | 1.387 | 0.042 | 0.676–2.520 | 0.921 | 0.043 | 0.402–1.463 | 0.620 | 0.049 | 0.754 | 0.072 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loutfi, H.; Fayad, N.; Pellen, F.; Le Jeune, B.; Chakroun, M.; Benfarhat, D.; Lteif, R.; Kallassy, M.; Le Brun, G.; Abboud, M. Morphological Study of Bacillus thuringiensis Crystals and Spores. Appl. Sci. 2021, 11, 155. https://doi.org/10.3390/app11010155
Loutfi H, Fayad N, Pellen F, Le Jeune B, Chakroun M, Benfarhat D, Lteif R, Kallassy M, Le Brun G, Abboud M. Morphological Study of Bacillus thuringiensis Crystals and Spores. Applied Sciences. 2021; 11(1):155. https://doi.org/10.3390/app11010155
Chicago/Turabian StyleLoutfi, Hadi, Nancy Fayad, Fabrice Pellen, Bernard Le Jeune, Maissa Chakroun, Dalel Benfarhat, Roger Lteif, Mireille Kallassy, Guy Le Brun, and Marie Abboud. 2021. "Morphological Study of Bacillus thuringiensis Crystals and Spores" Applied Sciences 11, no. 1: 155. https://doi.org/10.3390/app11010155
APA StyleLoutfi, H., Fayad, N., Pellen, F., Le Jeune, B., Chakroun, M., Benfarhat, D., Lteif, R., Kallassy, M., Le Brun, G., & Abboud, M. (2021). Morphological Study of Bacillus thuringiensis Crystals and Spores. Applied Sciences, 11(1), 155. https://doi.org/10.3390/app11010155





