Synthesis of Silver Nanoparticles with Gemini Surfactants as Efficient Capping and Stabilizing Agents
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Used
2.2. Synthesis
2.2.1. Synthesis of Gemini Surfactants
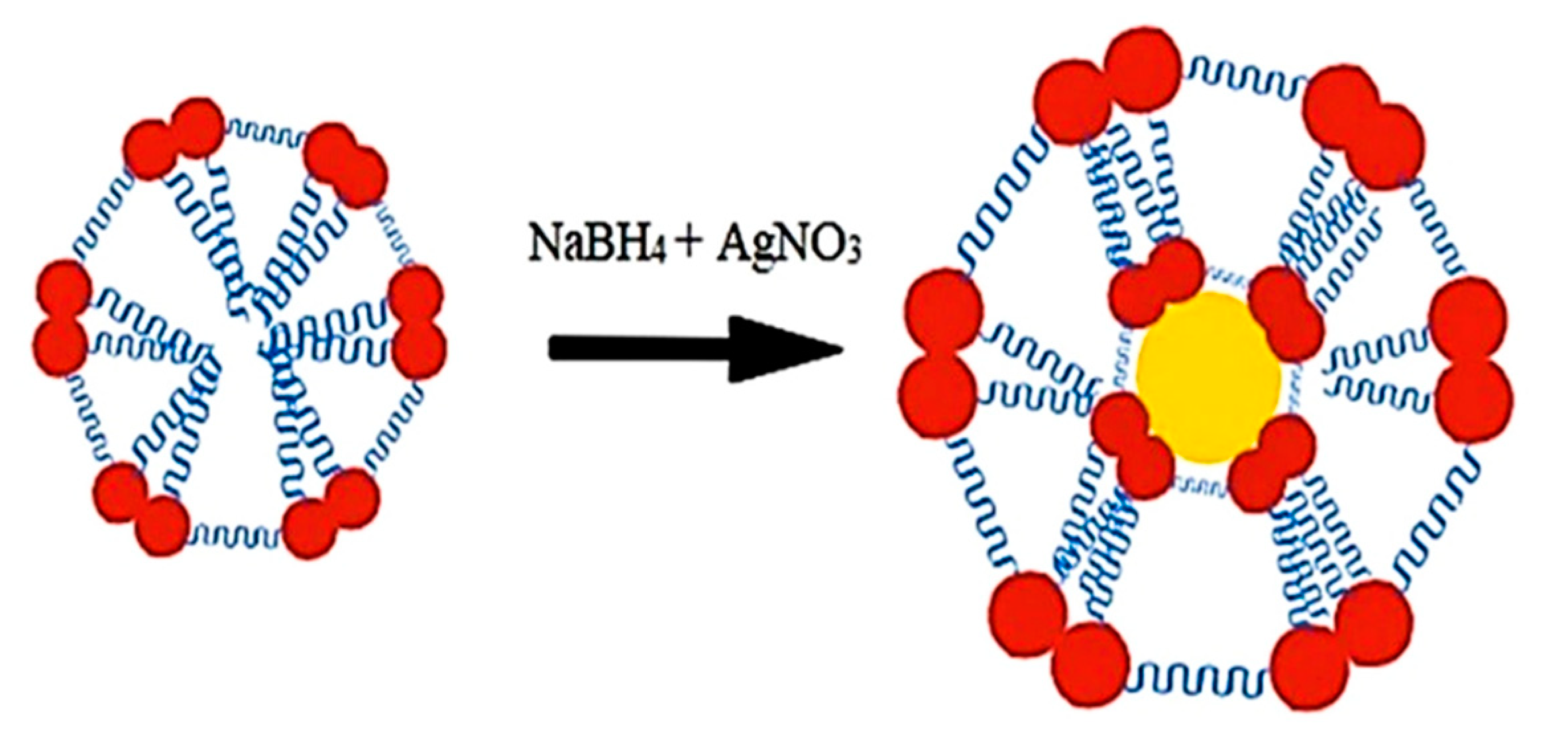
2.2.2. Synthesis of Silver Nanoparticles
2.3. Experimental Methods
2.3.1. Analysis of Dimeric Alkylammonium Salts
2.3.2. Analysis of Silver Nanoparticles
3. Results and Discussion
3.1. Synthesis and Spectroscopic Characterization of Gemini Surfactants
3.2. Surface Properties of Gemini Surfactants
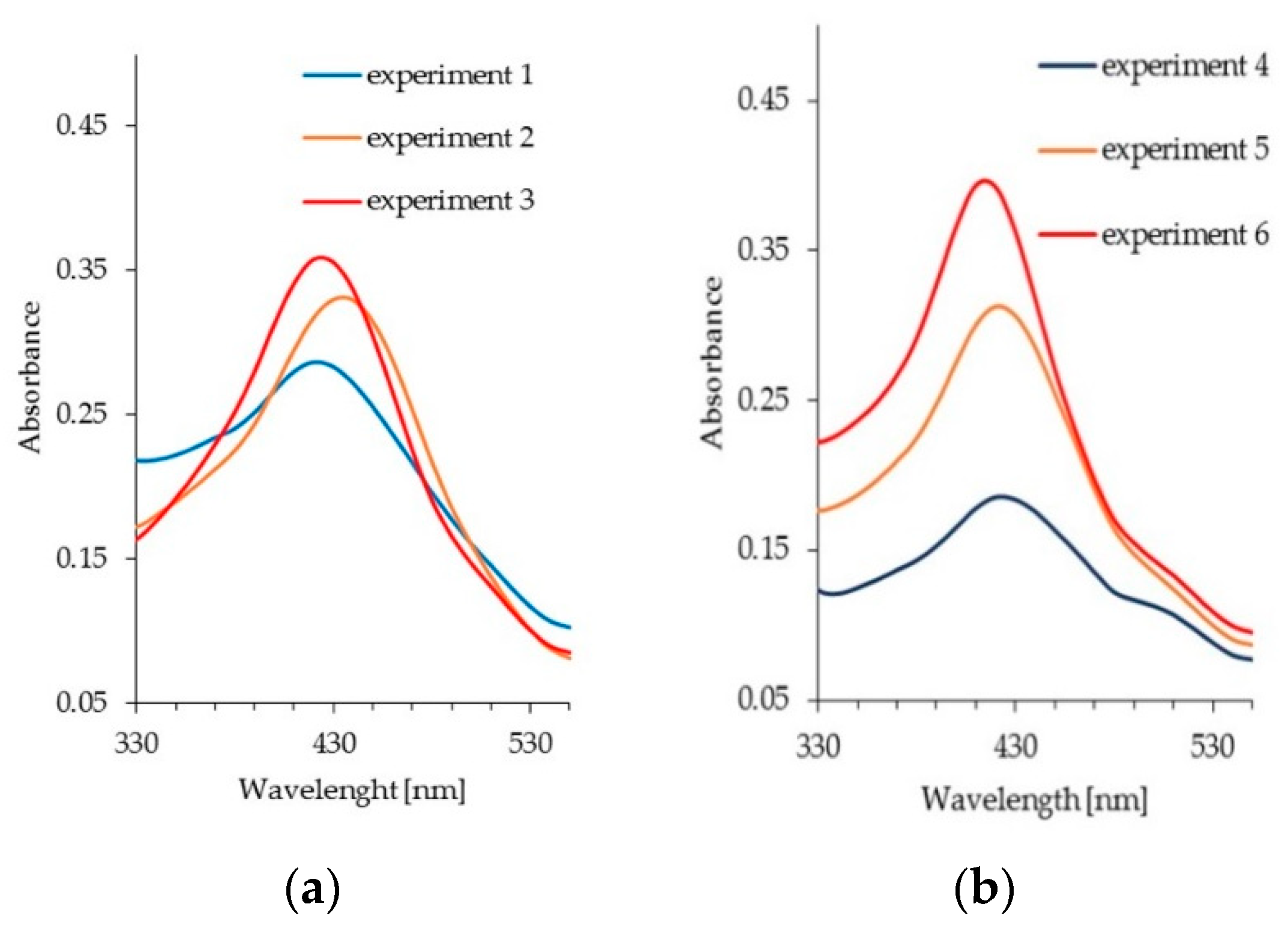
3.3. Preparation and Characterization of Silver Nanoparticles
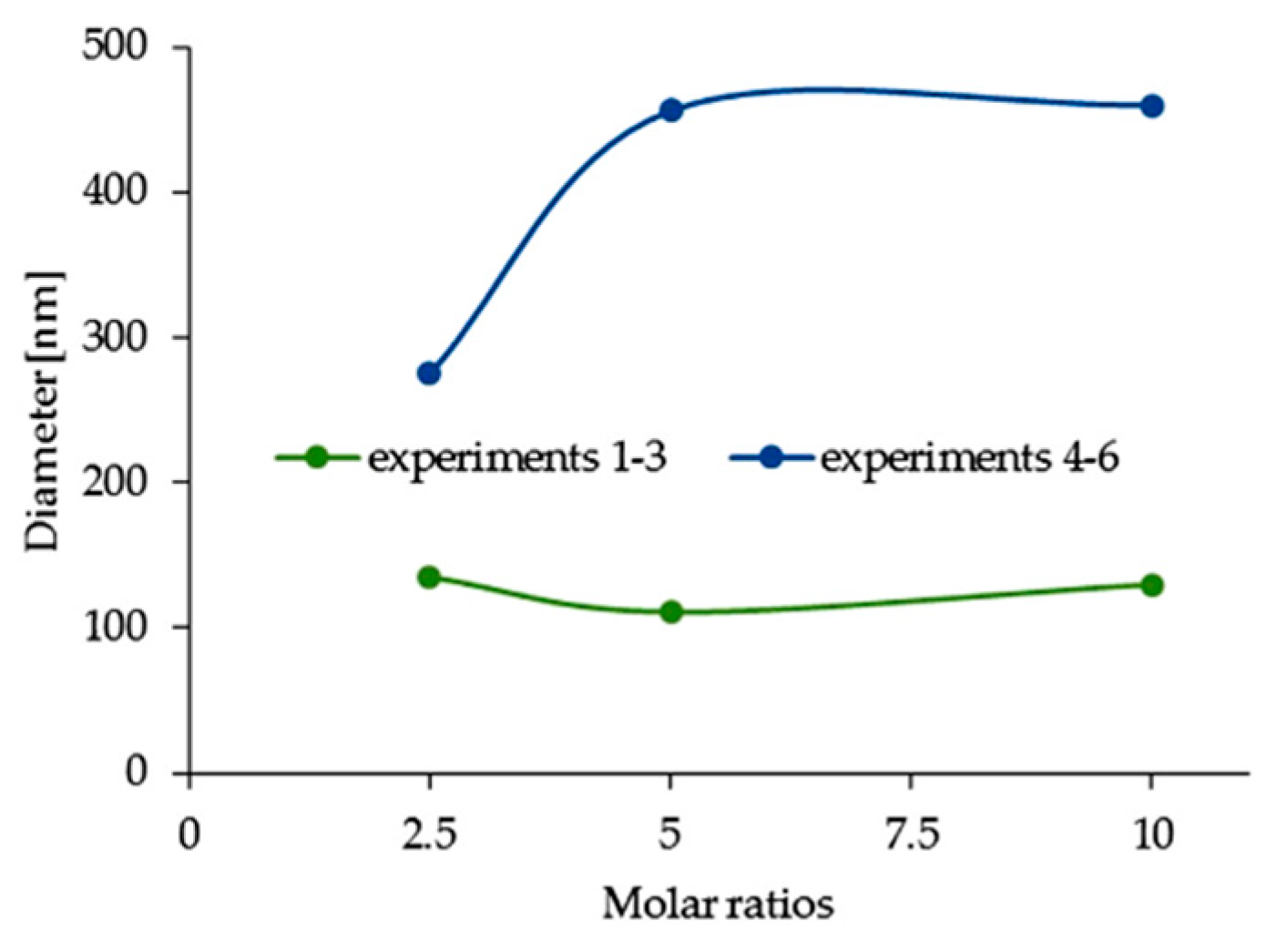
3.4. Effect of Spacer Structure on the Size of Silver Nanoparticles
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, V.K.; Yngard, R.A.; Lin, Y. Silver nanoparticles: Green synthesis and their antimicrobial activities. Adv. Colloid Interface Sci. 2009, 145, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Helmlinger, J.; Sengstock, C.; Groß-Heitfeld, C.; Mayer, C.; Schildhauer, T.A.; Köller, M.; Epple, M. Silver nanoparticles with different size and shape: Equal cytotoxicity, but different antibacterial effects. RSC Adv. 2016, 6, 18490–18501. [Google Scholar] [CrossRef]
- Ulubayram, K.; Calamak, S.; Shahbazi, R.; Eroglu, I. Nanofibers Based Antibacterial Drug Design, Delivery and Applications. Curr. Pharm. Des. 2015, 21, 1930–1943. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Ikram, S. Silver Nanoparticles: One Pot Green Synthesis Using Terminalia arjuna Extract for Biological Application. J. Nanomed. Nanotechnol. 2015, 6. [Google Scholar] [CrossRef]
- Yaqoob, S.B.; Adnan, R.; Rameez Khan, R.M.; Rashid, M. Gold, Silver, and Palladium Nanoparticles: A Chemical Tool for Biomedical Applications. Front. Chem. 2020, 8, 376. [Google Scholar] [CrossRef]
- Panáček, A.; Kvítek, L.; Prucek, R.; Kolář, M.; Večeřová, R.; Pizúrová, N.; Sharma, V.K.; Nevěčná, T.; Zbořil, R. Silver Colloid Nanoparticles: Synthesis, Characterization, and Their Antibacterial Activity. J. Phys. Chem. B 2006, 110, 16248–16253. [Google Scholar] [CrossRef]
- Quang, D.V.; Sarawade, P.B.; Hilonga, A.; Kim, J.-K.; Chai, Y.G.; Kim, S.H.; Ryu, J.-Y.; Kim, H.T. Preparation of silver nanoparticle containing silica micro beads and investigation of their antibacterial activity. Appl. Surf. Sci. 2011, 257, 6963–6970. [Google Scholar] [CrossRef]
- Martínez-Gutierrez, F.; Thi, E.P.; Silverman, J.M.; de Oliveira, C.C.; Svensson, S.L.; Hoek, A.V.; Sánchez, E.M.; Reiner, N.E.; Gaynor, E.C.; Pryzdial, E.L.G.; et al. Antibacterial activity, inflammatory response, coagulation and cytotoxicity effects of silver nanoparticles. Nanomedicine 2012, 8, 328–336. [Google Scholar] [CrossRef]
- Salleh, A.; Naomi, R.; Utami, N.D.; Mohammad, A.W.; Mahmoudi, E.; Mustafa, N.; Fauzi, M.B. The Potential of Silver Nanoparticles for Antiviral and Antibacterial Applications: A Mechanism of Action. Nanomaterials 2020, 10, 1566. [Google Scholar] [CrossRef] [PubMed]
- Oluwaniyi, O.O.; Adegoke, H.I.; Adesuji, E.T.; Alabi, A.B.; Bodede, S.O.; Labulo, A.H.; Oseghale, C.O. Biosynthesis of silver nanoparticles using aqueous leaf extract of Thevetia peruviana Juss and its antimicrobial activities. Appl. Nanosci. 2016, 6, 903–912. [Google Scholar] [CrossRef]
- Youssef, F.S.; El-Banna, H.A.; Elzorba, H.Y.; Galal, A.M. Application of some nanoparticles in the field of veterinary medicine. Int. J. Vet. Sci. Med. 2019, 7, 78–93. [Google Scholar] [CrossRef] [PubMed]
- Burdușel, A.-C.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical Applications of Silver Nanoparticles: An Up-to-Date Overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef] [PubMed]
- Vankar, P.S.; Shukla, D. Biosynthesis of silver nanoparticles using lemon leaves extract and its application for antimicrobial finish on fabric. Appl. Nanosci. 2012, 2, 163–168. [Google Scholar] [CrossRef]
- Xia, Z.-K.; Ma, Q.-H.; Li, S.-Y.; Zhang, D.-Q.; Cong, L.; Tian, Y.-L.; Yang, R.-Y. The antifungal effect of silver nanoparticles on Trichosporon asahii. J. Microbiol. Immunol. Infect. 2016, 49, 182–188. [Google Scholar] [CrossRef]
- Panáček, A.; Kolář, M.; Večeřová, R.; Prucek, R.; Soukupová, J.; Kryštof, V.; Hamal, P.; Zbořil, R.; Kvítek, L. Antifungal activity of silver nanoparticles against Candida spp. Biomaterials 2009, 30, 6333–6340. [Google Scholar] [CrossRef]
- Elgorban, A.M.; El-Samawaty, A.E.-R.M.; Yassin, M.A.; Sayed, S.R.; Adil, S.F.; Elhindi, K.M.; Bakri, M.; Khan, M. Antifungal silver nanoparticles: Synthesis, characterization and biological evaluation. Biotechnol. Biotechnol. Equip. 2016, 30, 56–62. [Google Scholar] [CrossRef]
- Usman, M.; Farooq, M.; Wakeel, A.; Nawaz, A.; Cheema, S.A.; Rehman, H.; Ashraf, I.; Sanaullah, M. Nanotechnology in agriculture: Current status, challenges and future opportunities. Sci. Total Environ. 2020, 721, 137778. [Google Scholar] [CrossRef]
- Galdiero, S.; Falanga, A.; Vitiello, M.; Cantisani, M.; Marra, V.; Galdiero, M. Silver Nanoparticles as Potential Antiviral Agents. Molecules 2011, 16, 8894–8918. [Google Scholar] [CrossRef]
- Thi Ngoc Dung, T.; Nang Nam, V.; Thi Nhan, T.; Ngoc, T.T.B.; Minh, L.Q.; Nga, B.T.T.; Phan Le, V.; Viet Quang, D. Silver nanoparticles as potential antiviral agents against African swine fever virus. Mater. Res. Express 2020, 6, 1250–1259. [Google Scholar] [CrossRef]
- Mori, Y.; Ono, T.; Miyahira, Y.; Nguyen, V.Q.; Matsui, T.; Ishihara, M. Antiviral activity of silver nanoparticle/chitosan composites against H1N1 influenza A virus. Nanoscale Res. Lett. 2013, 8, 93. [Google Scholar] [CrossRef]
- Bekele, A.Z.; Gokulan, K.; Williams, K.M.; Khare, S. Dose and Size-Dependent Antiviral Effects of Silver Nanoparticles on Feline Calicivirus, a Human Norovirus Surrogate. Foodborne Pathog. Dis. 2016, 13, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Singh, L.; Kruger, H.G.; Maguire, G.E.M.; Govender, T.; Parboosing, R. The role of nanotechnology in the treatment of viral infections. Ther. Adv. Infect. Dis. 2017, 4, 105–131. [Google Scholar] [CrossRef] [PubMed]
- Seino, S.; Imoto, Y.; Kosaka, T.; Nishida, T.; Nakagawa, T.; Yamamoto, T.A. Antiviral Activity of Silver Nanoparticles Immobilized onto Textile Fabrics Synthesized by Radiochemical Process. MRS Adv. 2016, 1, 705–710. [Google Scholar] [CrossRef]
- Balagna, C.; Perero, S.; Percivalle, E.; Nepita, E.V.; Ferraris, M. Virucidal effect against coronavirus SARS-CoV-2 of a silver nanocluster/silica composite sputtered coating. Open Ceram. 2020, 1, 100006. [Google Scholar] [CrossRef]
- Nikaeen, G.; Abbaszadeh, S.; Yousefinejad, S. Application of nanomaterials in treatment, anti-infection and detection of coronaviruses. Nanomedicine 2020, 15, 1501–1512. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Jun, B.-H. Silver Nanoparticles: Synthesis and Application for Nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef]
- Sotiriou, G.A.; Pratsinis, S.E. Engineering nanosilver as an antibacterial, biosensor and bioimaging material. Curr. Opin. Chem. Eng. 2011, 1, 3–10. [Google Scholar] [CrossRef]
- Marassi, V.; Cristo, L.D.; Smith, G.J.; Ortelli, S.; Blosi, M.; Costa, A.L.; Reschiglian, P.; Volkov, Y.; Prina-Mello, A. Silver nanoparticles as a medical device in healthcare settings: A five-step approach for candidate screening of coating agents. R. Soc. Open Sci. 2018, 5, 171113. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Y.; Guo, Q.; Wang, Z.; Wang, H.; Yang, Y.; Huang, Y. TAT-modified nanosilver for combating multidrug-resistant cancer. Biomaterials 2012, 33, 6155–6161. [Google Scholar] [CrossRef]
- Ardhendu, K.M. Silver Nanoparticles as Drug Delivery Vehicle. Glob. J. Nanomed. 2017, 3, 555607. [Google Scholar] [CrossRef]
- Lee, K.-S.; El-Sayed, M.A. Gold and Silver Nanoparticles in Sensing and Imaging: Sensitivity of Plasmon Response to Size, Shape, and Metal Composition. J. Phys. Chem. B 2006, 110, 19220–19225. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sun, Y.; Che, G.; Li, Z. Self-assembled silver nanoparticle films at an air–liquid interface and their applications in SERS and electrochemistry. App. Surf. Sci. 2011, 257, 7150–7155. [Google Scholar] [CrossRef]
- Alvarez-Puebla, R.A.; Aroca, R.F. Synthesis of Silver Nanoparticles with Controllable Surface Charge and Their Application to Surface-Enhanced Raman Scattering. Anal. Chem. 2009, 81, 2280–2285. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Wang, Q.; Gu, Z.; Fang, K.; Marriott, G.; Gu, N. Silver Nanoparticle-Embedded Microbubble as a Dual-Mode Ultrasound and Optical Imaging Probe. ACS Appl. Mater. Interfaces 2013, 5, 9217–9223. [Google Scholar] [CrossRef] [PubMed]
- Varghese Alex, K.; Tamil Pavai, P.; Rugmini, R.; Shiva Prasad, M.; Kamakshi, K.; Sekhar, K.C. Green Synthesized Ag Nanoparticles for Bio-Sensing and Photocatalytic Applications. ACS Omega 2020, 5, 13123–13129. [Google Scholar] [CrossRef]
- Manno, D.; Filippo, E.; Di Giulio, M.; Serra, A. Synthesis and characterization of starch-stabilized Ag nanostructures for sensors applications. J. Non-Cryst. Solids 2008, 354, 5515–5520. [Google Scholar] [CrossRef]
- Dong, X.-Y.; Gao, Z.-W.; Yang, K.-F.; Zhang, W.-Q.; Xu, L.-W. Nanosilver as a new generation of silver catalysts in organic transformations for efficient synthesis of fine chemicals. Catal. Sci. Technol. 2015, 5, 2554–2574. [Google Scholar] [CrossRef]
- Treshchalov, A.; Erikson, H.; Puust, L.; Tsarenko, S.; Saar, R.; Vanetsev, A.; Tammeveski, K.; Sildos, I. Stabilizer-free silver nanoparticles as efficient catalysts for electrochemical reduction of oxygen. J. Colloid Interface Sci. 2017, 491, 358–366. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Umar, K.; Ibrahim, M.N.M. Silver nanoparticles: Various methods of synthesis, size affecting factors and their potential applications—A review. Appl. Nanosci. 2020, 10, 1369–1378. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Abou El-Nour, K.M.M.; Eftaiha, A.; Al-Warthan, A.; Ammar, R.A.A. Synthesis and applications of silver nanoparticles. Arab. J. Chem. 2010, 3, 135–140. [Google Scholar] [CrossRef]
- Chouhan, N. Silver Nanoparticles: Synthesis, Characterization and Applications. In Silver Nanoparticles—Fabrication, Characterization and Applications; Maaz, K., Ed.; InTech: Rijeka, Croatia, 2018; ISBN 978-1-78923-478-7. [Google Scholar]
- Khodashenas, B.; Ghorbani, H.R. Synthesis of silver nanoparticles with different shapes. Arab. J. Chem. 2019, 12, 1823–1838. [Google Scholar] [CrossRef]
- AL-Thabaiti, S.A.; Al-Nowaiser, F.M.; Obaid, A.Y.; Al-Youbi, A.O.; Khan, Z. Formation and characterization of surfactant stabilized silver nanoparticles: A kinetic study. Colloids Surf. B 2008, 67, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Menger, F.M.; Littau, C.A. Gemini-surfactants: Synthesis and properties. J. Am. Chem. Soc. 1991, 113, 1451–1452. [Google Scholar] [CrossRef]
- Alami, E.; Beinert, G.; Marie, P.; Zana, R. Alkanediyl-α,ω-bis(dimethylalkylammonium bromide) surfactants. 3. Behavior at the air-water interface. Langmuir 1993, 9, 1465–1467. [Google Scholar] [CrossRef]
- Brycki, B.; Kowalczyk, I.; Kozirog, A. Synthesis, Molecular Structure, Spectral Properties and Antifungal Activity of Polymethylene-α,ω-bis(N,N- dimethyl-N-dodecyloammonium Bromides). Molecules 2011, 16, 319–335. [Google Scholar] [CrossRef]
- Brycki, B.E.; Kowalczyk, I.H.; Szulc, A.; Kaczerewska, O.; Pakiet, M. Multifunctional Gemini Surfactants: Structure, Synthesis, Properties and Applications. In Application and Characterization of Surfactants; Najjar, R., Ed.; InTech: Rijeka, Croatia, 2017; ISBN 978-953-51-3325-4. [Google Scholar]
- Menger, F.M.; Keiper, J.S. Gemini Surfactants. Angew. Chem. Int. Ed. 2000, 39, 1906–1920. [Google Scholar] [CrossRef]
- Brycki, B.; Szulc, A.; Koenig, H.; Kowalczyk, I.; Pospieszny, T.; Górka, S. Effect of the alkyl chain length on micelle formation for bis(N-alkyl-N,N-dimethylethylammonium)ether dibromides. C. R. Chim. 2019, 22, 386–392. [Google Scholar] [CrossRef]
- Chlebicki, J.; Węgrzyńska, J.; Wilk, K.A. Surface-active, micellar, and antielectrostatic properties of bis-ammonium salts. J. Colloid Interface Sci. 2008, 323, 372–378. [Google Scholar] [CrossRef]
- Das, S.; Mukherjee, I.; Paul, B.K.; Ghosh, S. Physicochemical Behaviors of Cationic Gemini Surfactant (14-4-14) Based Microheterogeneous Assemblies. Langmuir 2014, 30, 12483–12493. [Google Scholar] [CrossRef]
- Pisárčik, M.; Devínsky, F. Surface tension study of cationic gemini surfactants binding to DNA. Cent. Eur. J. Chem. 2014, 12, 577–585. [Google Scholar] [CrossRef]
- Devínsky, F.; Masárová, Ľ.; Lacko, I. Surface activity and micelle formation of some new bisquaternary ammonium salts. J. Colloid Interface Sci. 1985, 105, 235–239. [Google Scholar] [CrossRef]
- Kowalczyk, I.; Pakiet, M.; Szulc, A.; Koziróg, A. Antimicrobial Activity of Gemini Surfactants with Azapolymethylene Spacer. Molecules 2020, 25, 4054. [Google Scholar] [CrossRef] [PubMed]
- Koziróg, A.; Brycki, B. Monomeric and gemini surfactants as antimicrobial agents—Influence on environmental and reference strains. Acta Biochim. Pol. 2015, 62, 879–883. [Google Scholar] [CrossRef] [PubMed]
- Kaczerewska, O.; Leiva-Garcia, R.; Akid, R.; Brycki, B.; Kowalczyk, I.; Pospieszny, T. Heteroatoms and π electrons as favorable factors for efficient corrosion protection. Mater. Corros. 2019, 70, 1099–1110. [Google Scholar] [CrossRef]
- Garcia, M.T.; Kaczerewska, O.; Ribosa, I.; Brycki, B.; Materna, P.; Drgas, M. Biodegradability and aquatic toxicity of quaternary ammonium-based gemini surfactants: Effect of the spacer on their ecological properties. Chemosphere 2016, 154, 155–160. [Google Scholar] [CrossRef]
- Kaczerewska, O.; Brycki, B.; Ribosa, I.; Comelles, F.; Garcia, M.T. Cationic gemini surfactants containing an O-substituted spacer and hydroxyethyl moiety in the polar heads: Self-assembly, biodegradability and aquatic toxicity. J. Ind. Eng. Chem. 2018, 59, 141–148. [Google Scholar] [CrossRef]
- Brycki, B.; Waligórska, M.; Szulc, A. The biodegradation of monomeric and dimeric alkylammonium surfactants. J. Hazard. Mater. 2014, 280, 797–815. [Google Scholar] [CrossRef]
- Akram, M.; Anwar, S.; Ansari, F.; Bhat, I.A.; Kabir-ud-Din, K.-D. Bio-physicochemical analysis of ethylene oxide-linked diester-functionalized green cationic gemini surfactants. RSC Adv. 2016, 6, 21697–21705. [Google Scholar] [CrossRef]
- Bergero, M.F.; Liffourrena, A.S.; Opizzo, B.A.; Fochesatto, A.S.; Lucchesi, G.I. Immobilization of a microbial consortium on Ca-alginate enhances degradation of cationic surfactants in flasks and bioreactor. Int. Biodeterior. Biodegrad. 2017, 117, 39–44. [Google Scholar] [CrossRef]
- Mondal, M.H.; Roy, A.; Malik, S.; Ghosh, A.; Saha, B. Review on chemically bonded geminis with cationic heads: Second-generation interfactants. Res. Chem. Intermed. 2016, 42, 1913–1928. [Google Scholar] [CrossRef]
- Mondal, M.H.; Malik, S.; Roy, A.; Saha, R.; Saha, B. Modernization of surfactant chemistry in the age of gemini and bio-surfactants: A review. RSC Adv. 2015, 5, 92707–92718. [Google Scholar] [CrossRef]
- Martín, V.I.; de la Haba, R.R.; Ventosa, A.; Congiu, E.; Ortega-Calvo, J.J.; Moyá, M.L. Colloidal and biological properties of cationic single-chain and dimeric surfactants. Colloids Surf. B 2014, 114, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Brycki, B.; Szulc, A. Gemini Alkyldeoxy-D-Glucitolammonium Salts as Modern Surfactants and Microbiocides: Synthesis, Antimicrobial and Surface Activity, Biodegradation. PLoS ONE 2014, 9, e84936. [Google Scholar] [CrossRef]
- Dani, U.; Bahadur, A.; Kuperkar, K. Micellization, Antimicrobial Activity and Curcumin Solubilization in Gemini Surfactants: Influence of Spacer and Non-Polar Tail. Colloids Interface Sci. Commun. 2018, 25, 22–30. [Google Scholar] [CrossRef]
- Jennings, M.C.; Buttaro, B.A.; Minbiole, K.P.C.; Wuest, W.M. Bioorganic Investigation of Multicationic Antimicrobials to Combat QAC-Resistant Staphylococcus aureus. ACS Infect. Dis. 2015, 1, 304–309. [Google Scholar] [CrossRef]
- Zhang, S.; Ding, S.; Yu, J.; Chen, X.; Lei, Q.; Fang, W. Antibacterial Activity, in Vitro Cytotoxicity, and Cell Cycle Arrest of Gemini Quaternary Ammonium Surfactants. Langmuir 2015, 31, 12161–12169. [Google Scholar] [CrossRef]
- Kuperkar, K.; Modi, J.; Patel, K. Surface-Active Properties and Antimicrobial Study of Conventional Cationic and Synthesized Symmetrical Gemini Surfactants. J. Surfactants Deterg. 2012, 15, 107–115. [Google Scholar] [CrossRef]
- Obłąk, E.; Piecuch, A.; Krasowska, A.; Łuczyński, J. Antifungal activity of gemini quaternary ammonium salts. Microbiol. Res. 2013, 168, 630–638. [Google Scholar] [CrossRef]
- Koziróg, A.; Kręgiel, D.; Brycki, B. Action of Monomeric/Gemini Surfactants on Free Cells and Biofilm of Asaia lannensis. Molecules 2017, 22, 2036. [Google Scholar] [CrossRef]
- Labena, A.; Hegazy, M.A.; Sami, R.M.; Hozzein, W.N. Multiple Applications of a Novel Cationic Gemini Surfactant: Anti-Microbial, Anti-Biofilm, Biocide, Salinity Corrosion Inhibitor, and Biofilm Dispersion (Part II). Molecules 2020, 25, 1348. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Tyagi, R. Industrial Applications of Dimeric Surfactants: A Review. J. Dispers. Sci. Technol. 2014, 35, 205–214. [Google Scholar] [CrossRef]
- Brycki, B.; Drgas, M.; Bielawska, M.; Zdziennicka, A.; Jańczuk, B. Synthesis, spectroscopic studies, aggregation and surface behavior of hexamethylene-1,6-bis(N,N-dimethyl-N-dodecylammonium bromide). J. Mol. Liq. 2016, 221, 1086–1096. [Google Scholar] [CrossRef]
- Han, Y.; Wang, Y. Aggregation behavior of gemini surfactants and their interaction with macromolecules in aqueous solution. Phys. Chem. Chem. Phys. 2011, 13, 1939–1956. [Google Scholar] [CrossRef]
- Zana, R. Dimeric (Gemini) Surfactants: Effect of the Spacer Group on the Association Behavior in Aqueous Solution. J. Colloid Interface Sci. 2002, 248, 203–220. [Google Scholar] [CrossRef]
- Singh, V.; Tyagi, R. Unique Micellization and CMC Aspects of Gemini Surfactant: An Overview. J. Dispers. Sci. Technol. 2014, 35, 1774–1792. [Google Scholar] [CrossRef]
- ud din Parray, M.; Maurya, N.; Ahmad Wani, F.; Borse, M.S.; Arfin, N.; Ahmad Malik, M.; Patel, R. Comparative effect of cationic gemini surfactant and its monomeric counterpart on the conformational stability of phospholipase A2. J. Mol. Struct. 2019, 1175, 49–55. [Google Scholar] [CrossRef]
- Egorova, E.M.; Kaba, S.I. The effect of surfactant micellization on the cytotoxicity of silver nanoparticles stabilized with aerosol-OT. Toxicol. In Vitro 2019, 57, 244–254. [Google Scholar] [CrossRef]
- Zana, R. Critical Micellization Concentration of Surfactants in Aqueous Solution and Free Energy of Micellization. Langmuir 1996, 12, 1208–1211. [Google Scholar] [CrossRef]
- Menger, F.M.; Littau, C.A. Gemini surfactants: A new class of self-assembling molecules. J. Am. Chem. Soc. 1993, 115, 10083–10090. [Google Scholar] [CrossRef]
- Zana, R.; Levy, H. Alkanediyl-α,ω-bis(dimethylalkylammonium bromide) surfactants (dimeric surfactants) Part 6. CMC of the ethanediyl- 1,2-bis(dimethylalkylammonium bromide) series. Colloids Surf. A 1997, 127, 229–232. [Google Scholar] [CrossRef]
- Dam, T.; Engberts, J.B.F.N.; Karthäuser, J.; Karaborni, S.; van Os, N.M. Synthesis, surface properties and oil solubilisation capacity of cationic gemini surfactants. Colloids Surf. A 1996, 118, 41–49. [Google Scholar] [CrossRef]
- Oda, R.; Candau, S.J.; Oda, R.; Huc, I. Gemini surfactants, the effect of hydrophobic chain length and dissymmetry. Chem. Commun. 1997, 2105–2106. [Google Scholar] [CrossRef]
- Chang, H.; Cui, Y.; Wang, Y.; Li, G.; Gao, W.; Li, X.; Zhao, X.; Wei, W. Wettability and adsorption of PTFE and paraffin surfaces by aqueous solutions of biquaternary ammonium salt Gemini surfactants with hydroxyl. Colloids Surf. A 2016, 506, 416–424. [Google Scholar] [CrossRef]
- Pisárčik, M.; Jampílek, J.; Lukáč, M.; Horáková, R.; Devínsky, F.; Bukovský, M.; Kalina, M.; Tkacz, J.; Opravil, T. Silver Nanoparticles Stabilised by Cationic Gemini Surfactants with Variable Spacer Length. Molecules 2017, 22, 1794. [Google Scholar] [CrossRef]
- Siddiq, A.M.; Parandhaman, T.; Begam, A.F.; Das, S.K.; Alam, M.S. Effect of gemini surfactant (16-6-16) on the synthesis of silver nanoparticles: A facile approach for antibacterial application. Enzym. Microb. Technol. 2016, 95, 118–127. [Google Scholar] [CrossRef]
- Xu, J.; Han, X.; Liu, H.; Hu, Y. Synthesis and optical properties of silver nanoparticles stabilized by gemini surfactant. Colloids Surf. A 2006, 273, 179–183. [Google Scholar] [CrossRef]
- He, S.; Chen, H.; Guo, Z.; Wang, B.; Tang, C.; Feng, Y. High-concentration silver colloid stabilized by a cationic gemini surfactant. Colloids Surf. A 2013, 429, 98–105. [Google Scholar] [CrossRef]
- Guzman, M.; Dille, J.; Godet, S. Synthesis and antibacterial activity of silver nanoparticles against gram-positive and gram-negative bacteria. Nanomedicine 2012, 8, 37–45. [Google Scholar] [CrossRef]
- He, S.; Yao, J.; Jiang, P.; Shi, D.; Zhang, H.; Xie, S.; Pang, S.; Gao, H. Formation of Silver Nanoparticles and Self-Assembled Two-Dimensional Ordered Superlattice. Langmuir 2001, 17, 1571–1575. [Google Scholar] [CrossRef]
- Njagi, E.C.; Huang, H.; Stafford, L.; Genuino, H.; Galindo, H.M.; Collins, J.B.; Hoag, G.E.; Suib, S.L. Biosynthesis of Iron and Silver Nanoparticles at Room Temperature Using Aqueous Sorghum Bran Extracts. Langmuir 2011, 27, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Fang, W.; Feng, Y.; Geng, Q.; Song, M. Stability properties of water-based gold and silver nanofluids stabilized by cationic gemini surfactants. J. Taiwan Inst. Chem. Eng. 2019, 97, 458–465. [Google Scholar] [CrossRef]
- Siddiq, A.M.; Thangam, R.; Madhan, B.; Alam, M.S. Counterion coupled (COCO) gemini surfactant capped Ag/Au alloy and core–shell nanoparticles for cancer therapy. RSC Adv. 2019, 9, 37830–37845. [Google Scholar] [CrossRef]
- Haque, M.N.; Kwon, S.; Cho, D. Formation and stability study of silver nano-particles in aqueous and organic medium. Korean J. Chem. Eng. 2017, 34, 2072–2078. [Google Scholar] [CrossRef]
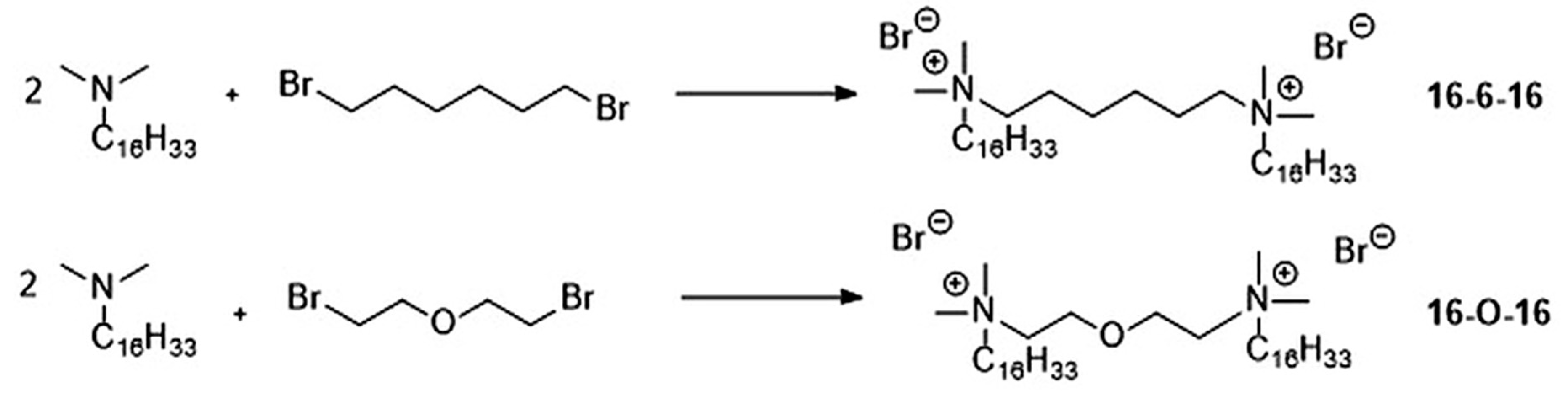

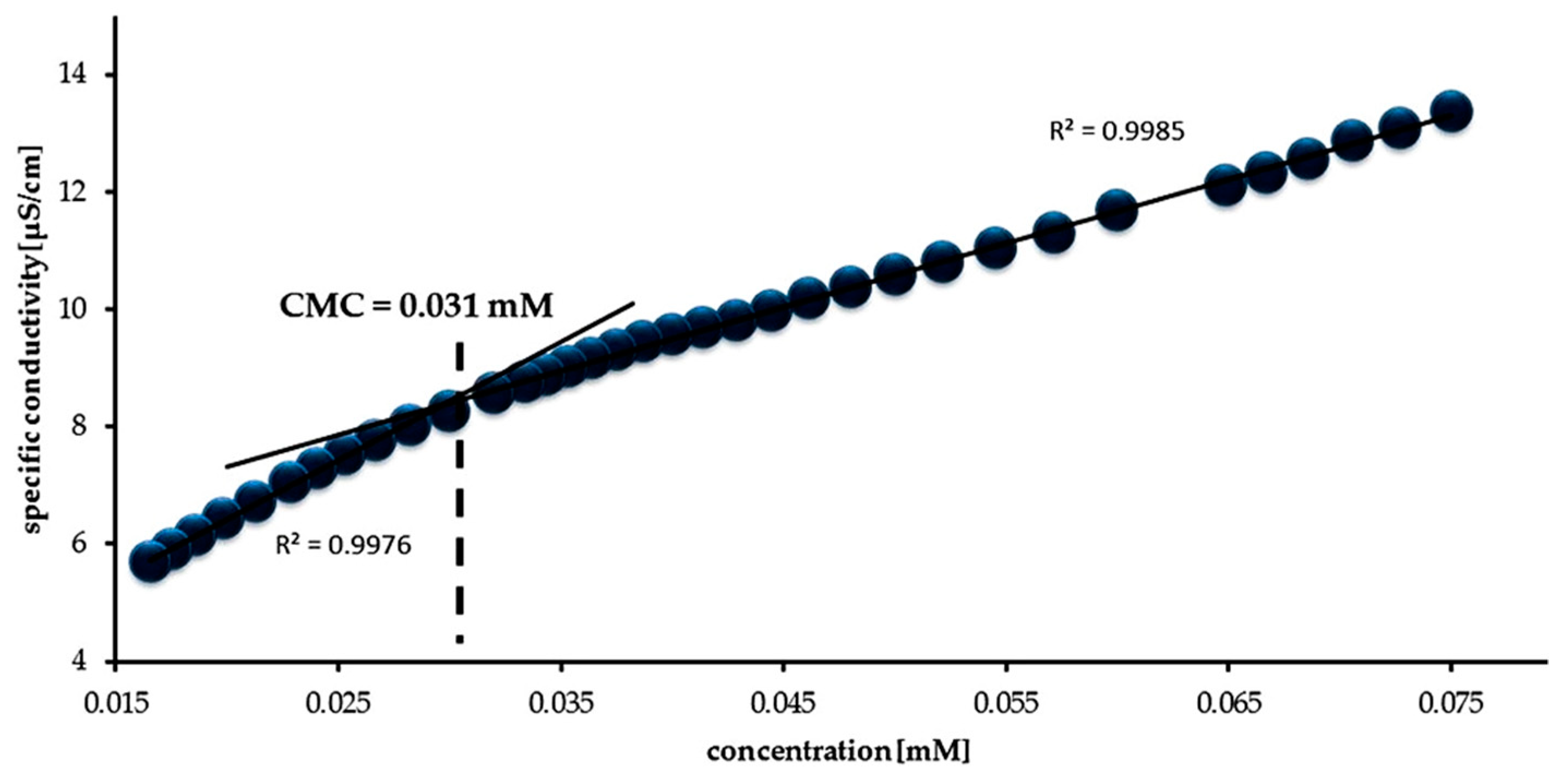


| Surfactant | CMC (mM) | α | β | ΔGomic (kJ/mol) |
|---|---|---|---|---|
| 16-6-16 | 0.034 | 0.55 | 0.45 | −50.16 |
| 16-O-16 | 0.031 | 0.40 | 0.60 | −58.32 |
| Experiment | Gemini Surfactant | Molar Ratio nAg/nGemini | Concentration of AgNO3 [mM] |
|---|---|---|---|
| 1 | 16-6-16 | 2.5 | 0.75 |
| 2 | 16-6-16 | 5 | 0.75 |
| 3 | 16-6-16 | 10 | 0.75 |
| 4 | 16-O-16 | 2.5 | 0.75 |
| 5 | 16-O-16 | 5 | 0.75 |
| 6 | 16-O-16 | 10 | 0.75 |
| 7 | 16-6-16 | 5 | 2 |
| 8 | 16-6-16 | 5 | 1 |
| Experiment | Wavelength [nm] | Absorbance |
|---|---|---|
| 1 | 420 | 0.286 |
| 2 | 435 | 0.332 |
| 3 | 425 | 0.359 |
| 4 | 420 | 0.185 |
| 5 | 420 | 0.313 |
| 6 | 415 | 0.397 |
| 7 | 410 | 0.690 |
| 8 | 419 | 0.596 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brycki, B.; Szulc, A.; Babkova, M. Synthesis of Silver Nanoparticles with Gemini Surfactants as Efficient Capping and Stabilizing Agents. Appl. Sci. 2021, 11, 154. https://doi.org/10.3390/app11010154
Brycki B, Szulc A, Babkova M. Synthesis of Silver Nanoparticles with Gemini Surfactants as Efficient Capping and Stabilizing Agents. Applied Sciences. 2021; 11(1):154. https://doi.org/10.3390/app11010154
Chicago/Turabian StyleBrycki, Bogumił, Adrianna Szulc, and Mariia Babkova. 2021. "Synthesis of Silver Nanoparticles with Gemini Surfactants as Efficient Capping and Stabilizing Agents" Applied Sciences 11, no. 1: 154. https://doi.org/10.3390/app11010154
APA StyleBrycki, B., Szulc, A., & Babkova, M. (2021). Synthesis of Silver Nanoparticles with Gemini Surfactants as Efficient Capping and Stabilizing Agents. Applied Sciences, 11(1), 154. https://doi.org/10.3390/app11010154







