Few-Layered MoS2 Nanoparticles Covering Anatase TiO2 Nanosheets: Comparison between Ex Situ and In Situ Synthesis Approaches
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Synthesis of the TiO2 Nanosheets
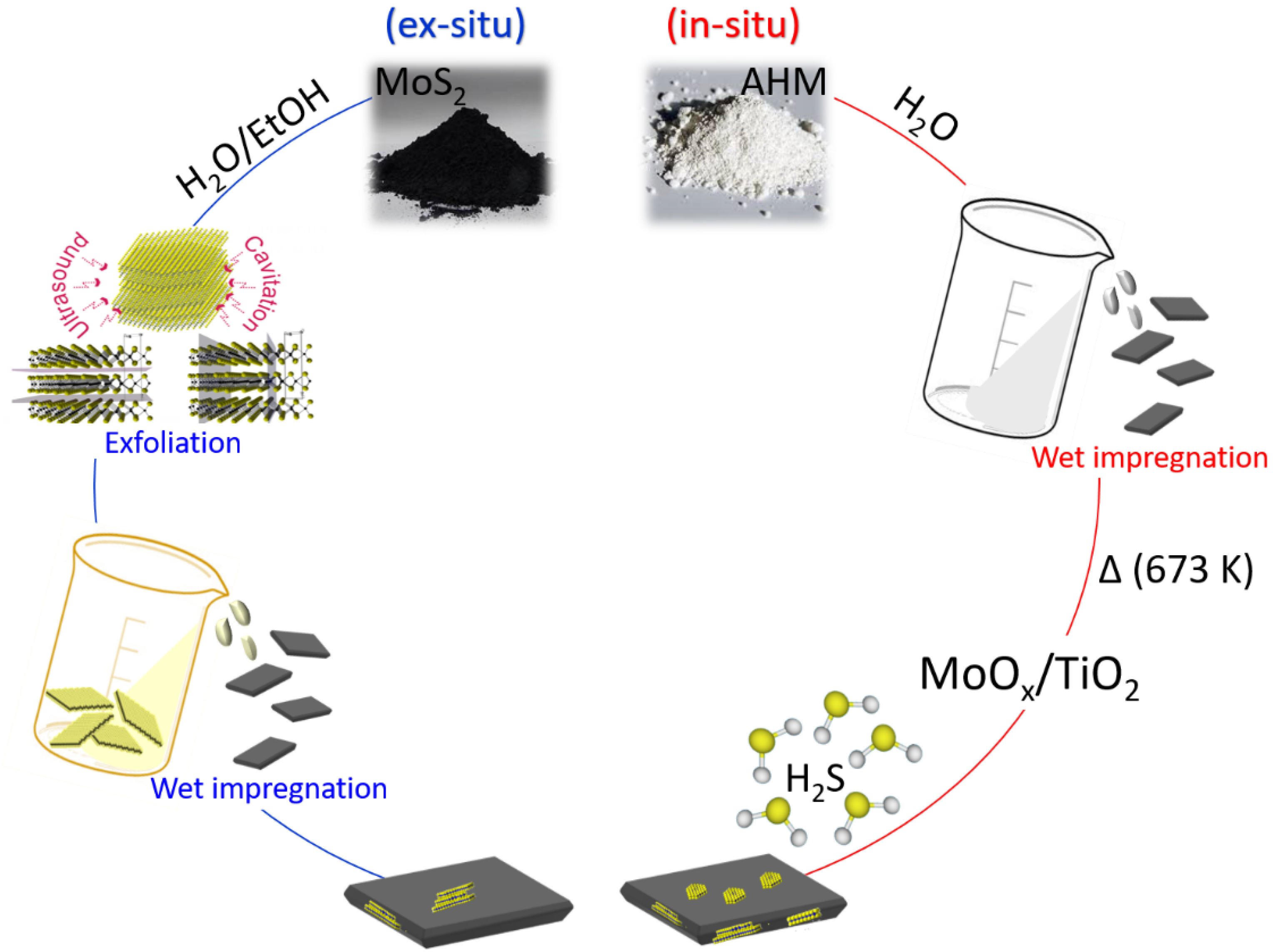
2.1.2. Synthesis of the MoS2/TiO2 Nanosheets by Ex Situ Method
2.1.3. Synthesis of the MoS2/TiO2 Nanosheets by In Situ Method
2.2. Methods
- (i)
- X-ray diffraction (XRD) patterns of samples have been collected with a PAN analytical PW3050/60 X’Pert PRO MPD diffractometer with a Cu anode and a Ni filter, in Bragg–Brentano configuration. The diffractograms were acquired in an interval equal to 10° ≤ 2θ ≤ 80° with an acquisition step of 0.02°.
- (ii)
- High-resolution transmission electron microscopy (HRTEM) images have been obtained with a JEOL 3010-UHR HRTEM microscope operating at 300 kV with a point-to-point resolution of 0.12 nm, equipped with a 2 k × 2 k pixels Gatan US1000 CCD camera.
- (iii)
- Raman spectra were acquired in backscattering mode using a Renishaw In Via Raman spectrophotometer, equipped with an Ar+ laser emitting at 514.5 nm. The backscattered light was analyzed by a grid with 1200 lines/mm and detected by a CCD detector. The effects of the radiation damage on the samples were reduced by limiting the output power to 0.5%.
- (iv)
- FTIR spectra were acquired by means of a Bruker IFS 66 FTIR spectrometer equipped with a cryogenic MCT detector with 2 cm−1 resolution. Each titania sample was pressed in the form of a self-supporting pellet with “optical thickness” of ca. 10 mg∙cm−2. To investigate the surface properties, the CO probe molecule was dosed on samples by means of a gas manifold connected to the IR cell, thus allowing us to perform thermal treatments under vacuum and gas dosage. The spectra were collected after CO dosage (70 Torr) at 77 K in an IR cell designed for liquid N2 flow conditions.
- (v)
- The optical properties of the samples dispersed in solution have been investigated by means of transmittance mode using quartz cuvettes with an optical path of 1 cm, while the properties of the powder samples have been studied by means of diffuse reflectance (DR) mode. A Varian Cary UV 5000 spectrophotometer, equipped with a diffuse reflectance sphere, was used in the 2500–190 nm wavelength range.
3. Results and Discussion
3.1. Structure and Morphology by Means of XRD, HRTEM, and Raman Analyses
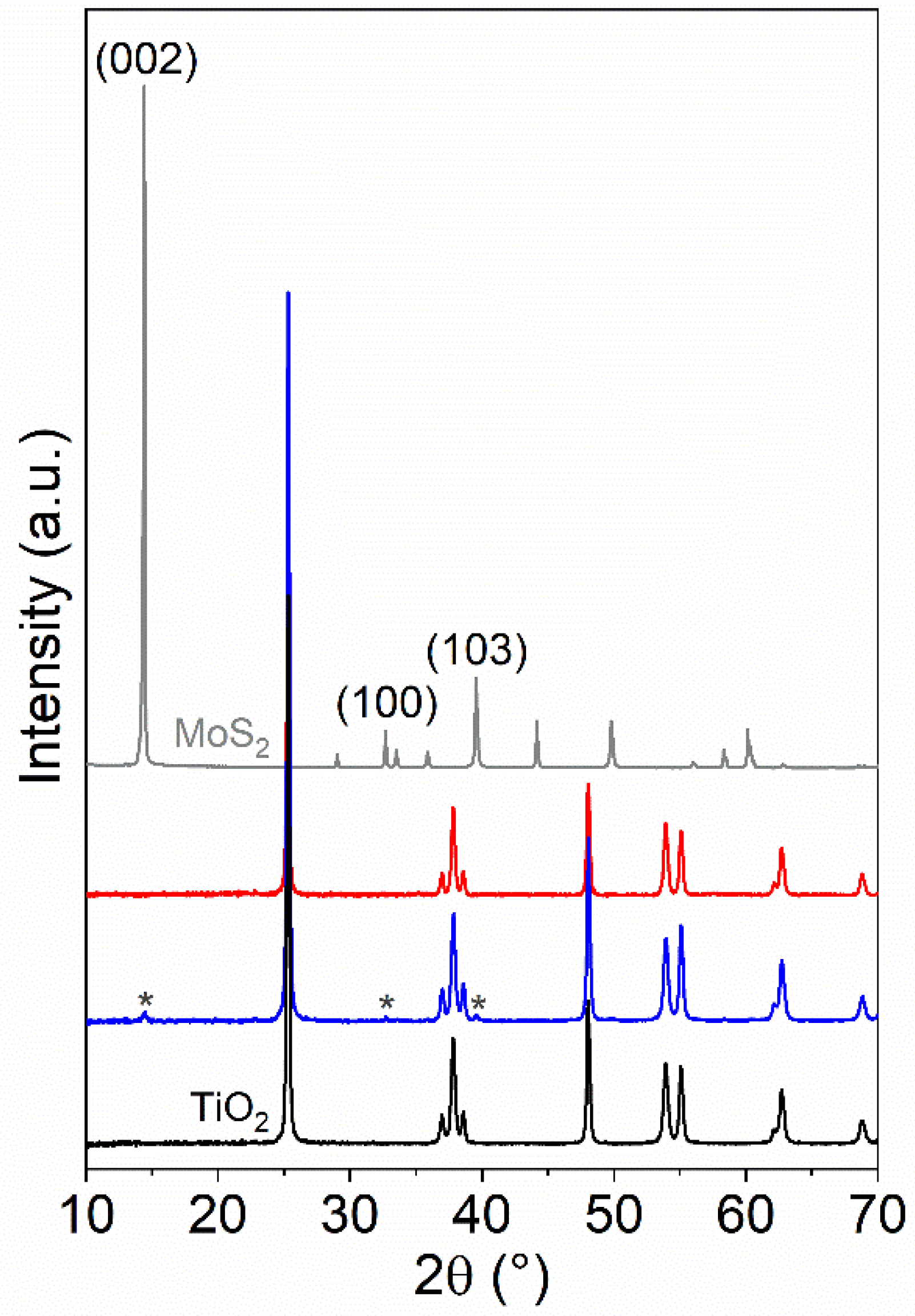
3.1.1. XRD Analysis
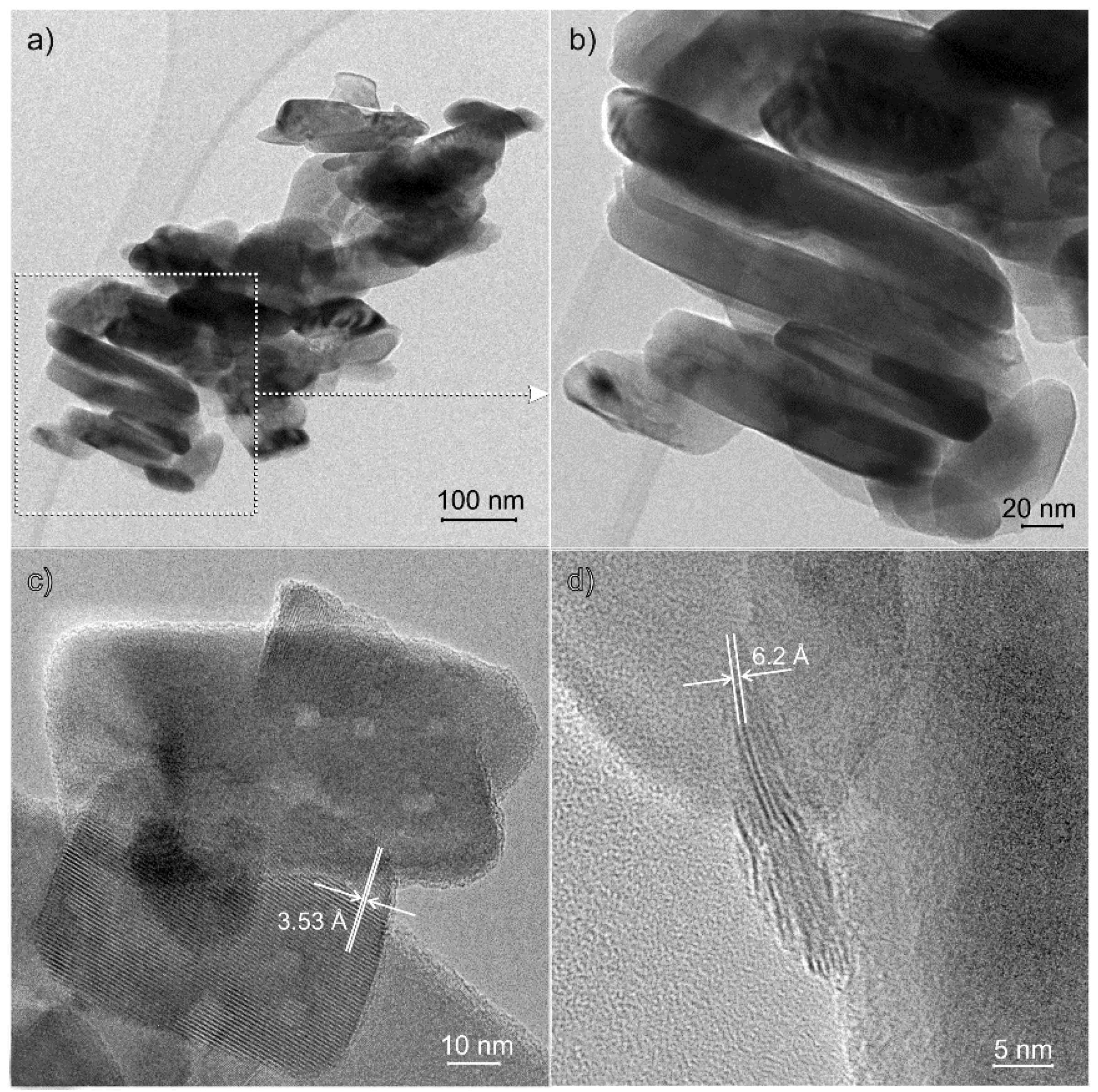
3.1.2. HRTEM Images
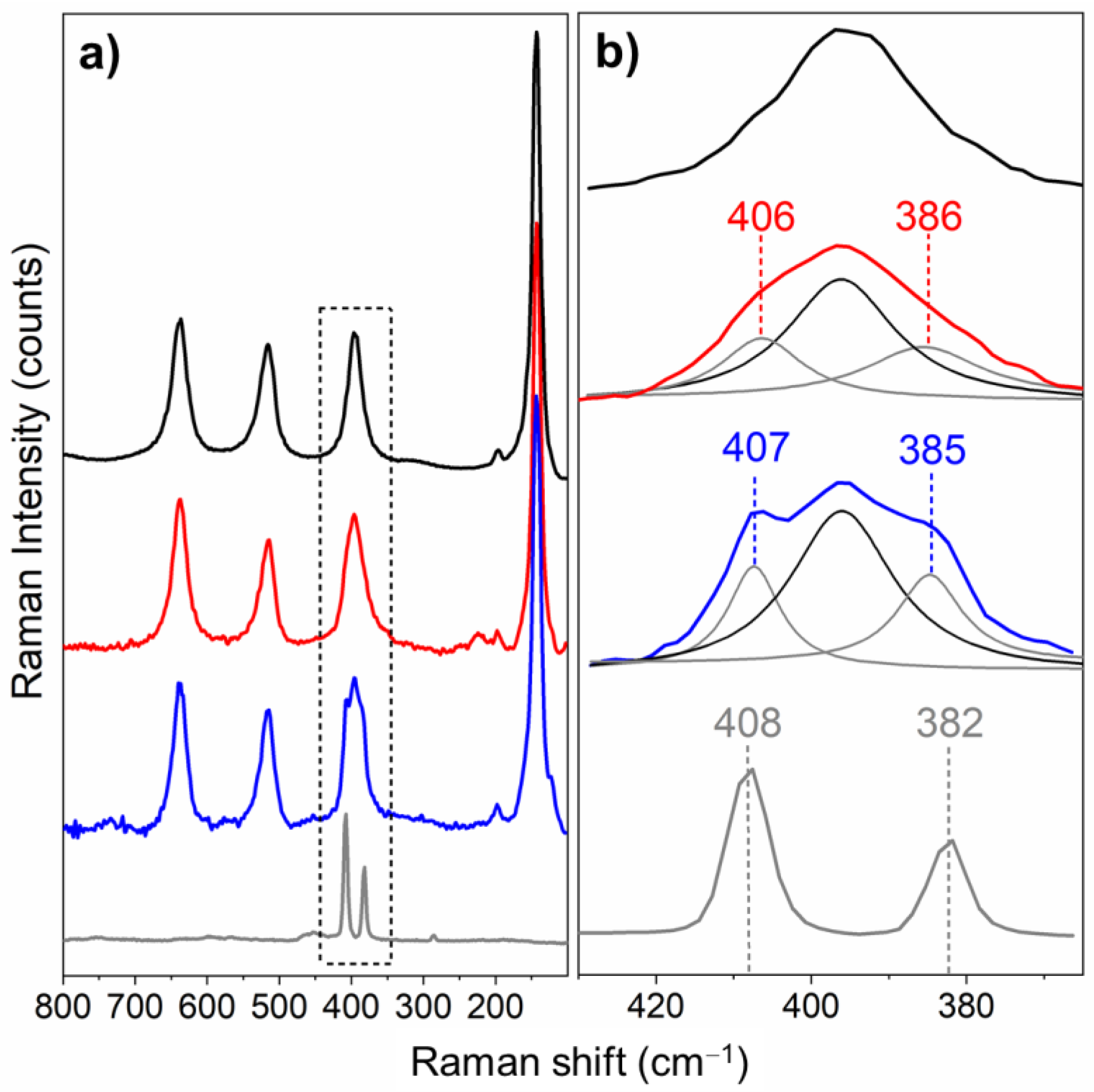
3.1.3. Raman Investigation
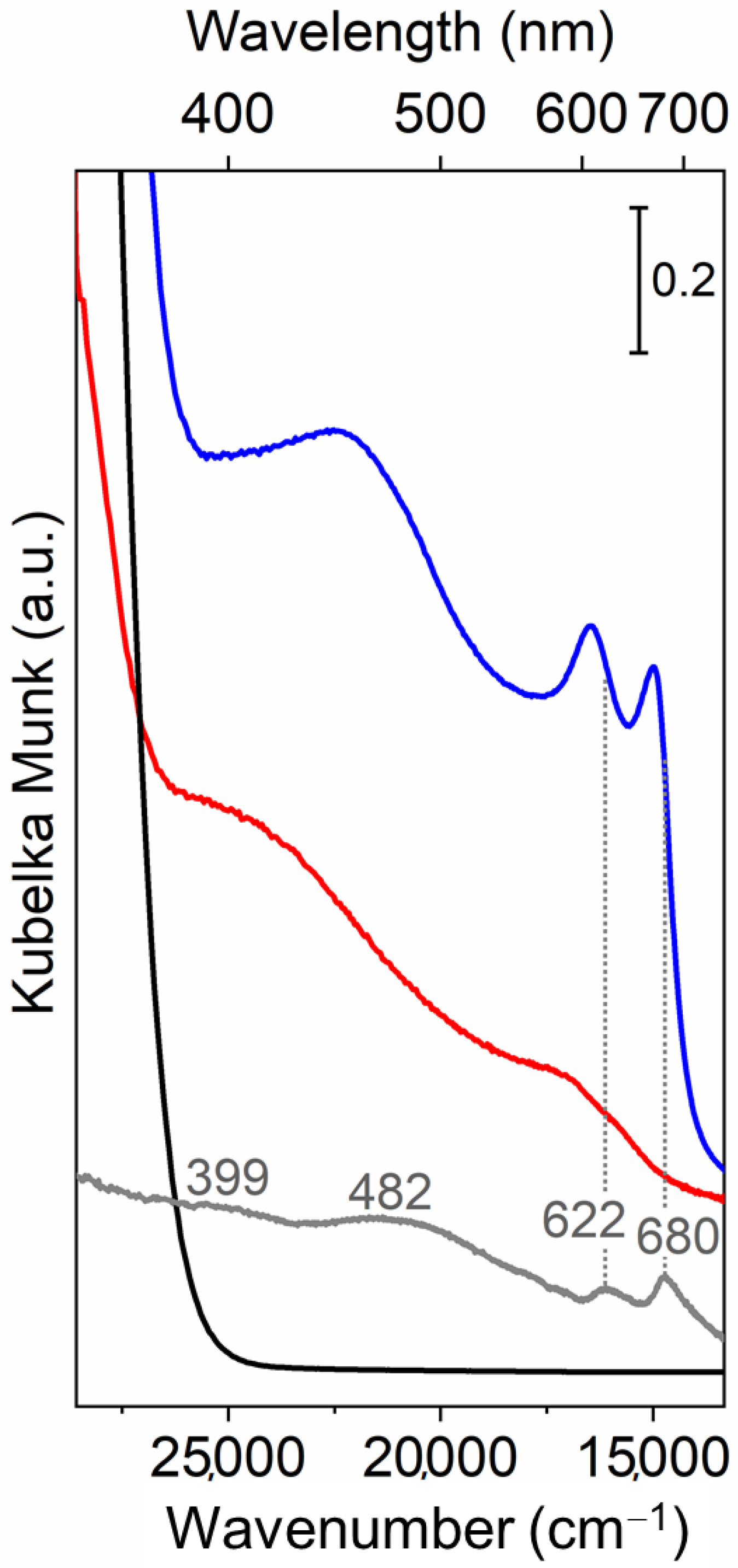
3.2. Optical Properties by UV–vis Spectroscopy
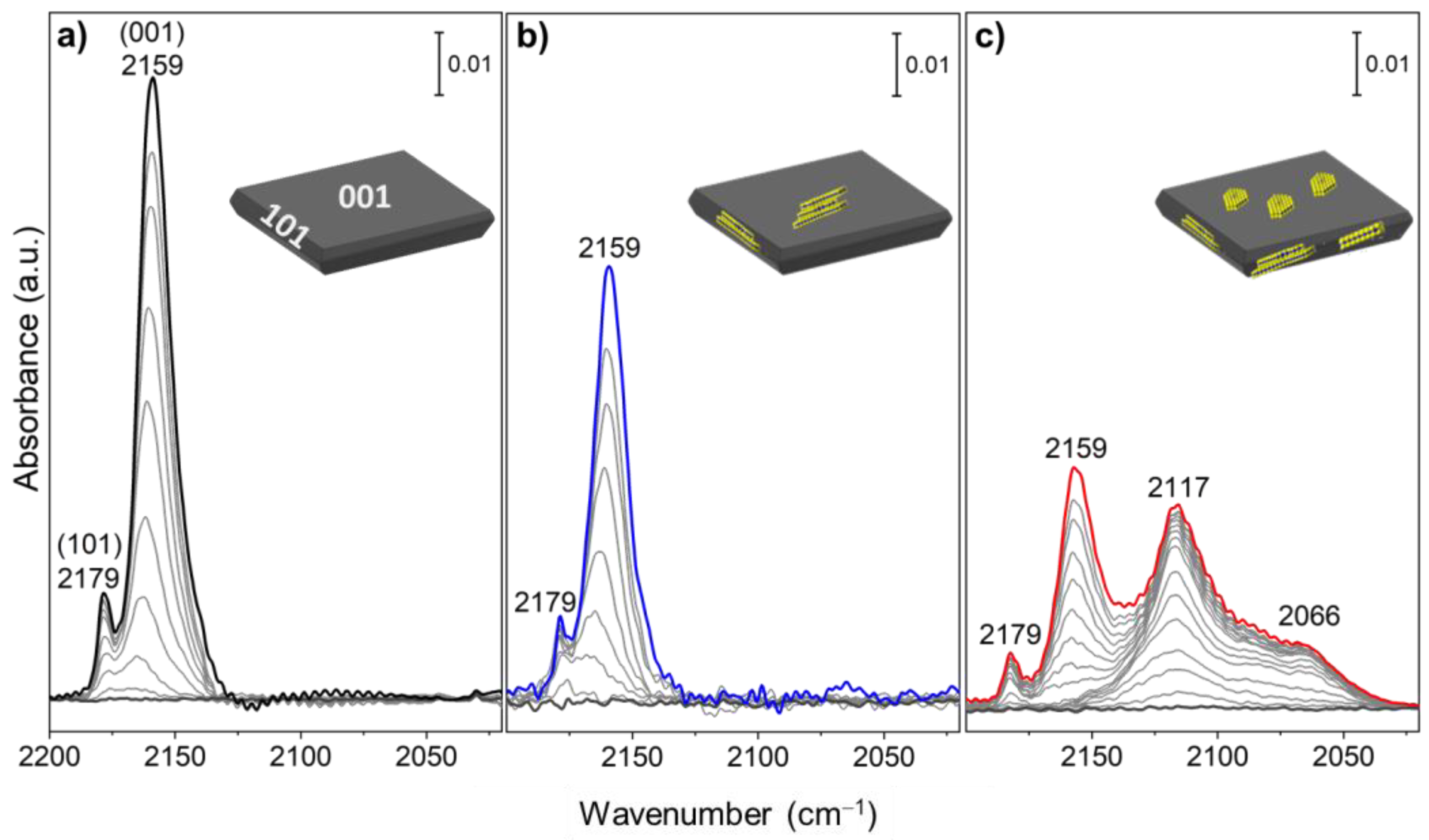
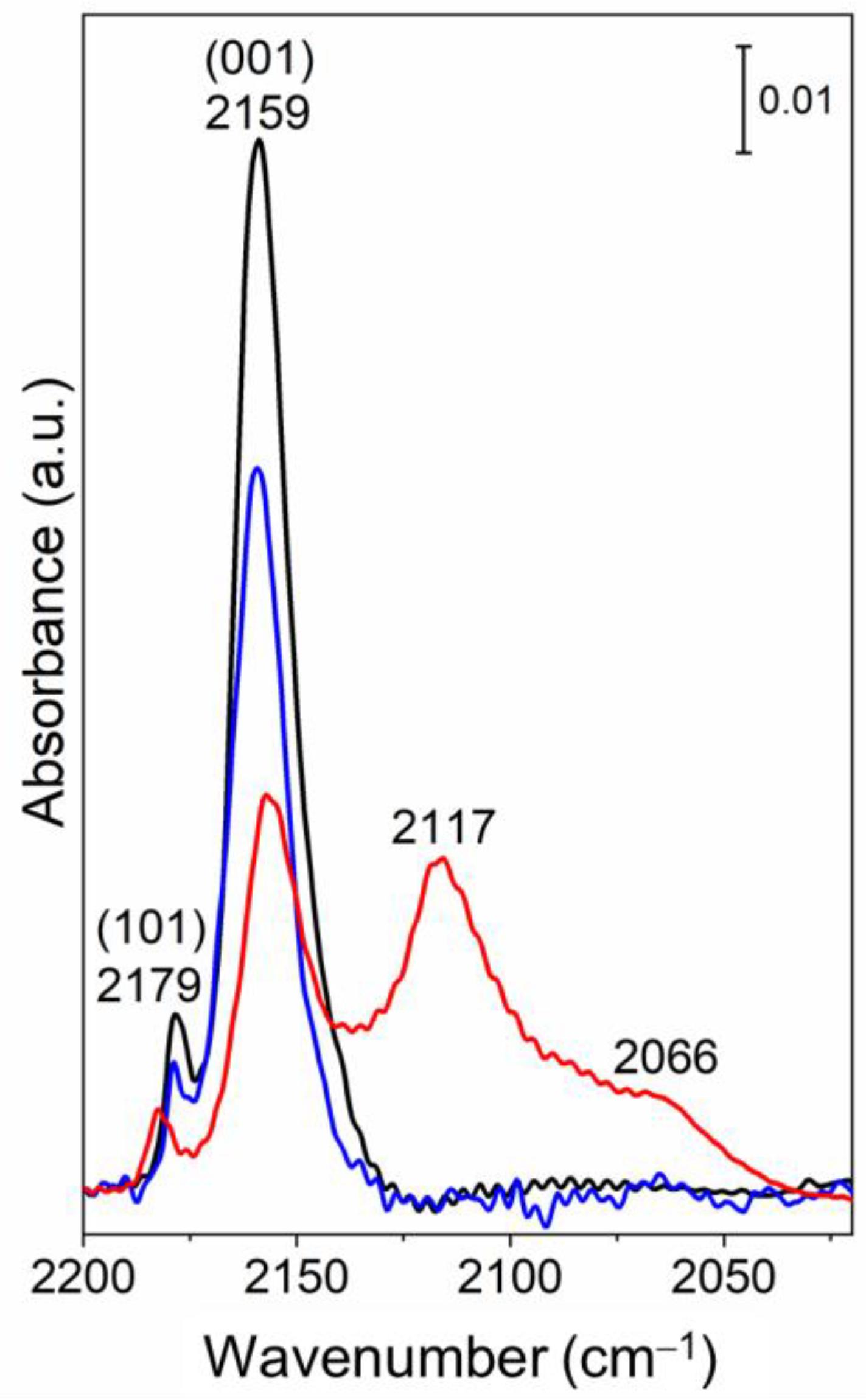
3.3. Surface Vibrational Properties by FTIR
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chatterjee, D.; Mahata, A. Visible light induced photodegradation of organic pollutants on dye adsorbed TiO2 surface. J. Photochem. Photobiol. A Chem. 2002, 153, 199–204. [Google Scholar] [CrossRef]
- Matos, J.; García, A.; Zhao, L.; Titirici, M.M. Solvothermal carbon-doped TiO2 photocatalyst for the enhanced methylene blue degradation under visible light. Appl. Catal. A Gen. 2010, 390, 175–182. [Google Scholar] [CrossRef]
- Cheng, Y.; Geng, H.; Huang, X. Advanced water splitting electrocatalysts via the design of multicomponent heterostructures. Dalton Trans. 2020, 49, 2761–2765. [Google Scholar] [CrossRef] [PubMed]
- Uddin, J.; Daramola, D.E.; Velasquez, E.; Dickens, T.J.; Yan, J.; Hammel, E.; Cesano, F.; Okoli, O.I. A high efficiency 3D photovoltaic microwire with carbon nanotubes (CNT)-quantum dot (QD) hybrid interface. Phys. Status solidi Rapid Res. Lett. 2014, 8, 898–903. [Google Scholar] [CrossRef]
- Zhao, N.; Meng, Y.; Sha, J.; Zhong, C.; Yuhuan, M.; Zhao, N. Preparation of MoS2/TiO2 based nanocomposites for photocatalysis and rechargeable batteries: Progress, challenges, and perspective. Nanoscale 2018, 10, 34–68. [Google Scholar] [CrossRef]
- Wang, H.; Liu, F.; Fu, W.; Fang, Z.; Zhou, W.; Liu, Z. Two-dimensional heterostructures: Fabrication, characterization, and application. Nanoscale 2014, 6, 12250–12272. [Google Scholar] [CrossRef]
- Wang, Z.; Mi, B. Environmental Applications of 2D Molybdenum Disulfide (MoS2) Nanosheets. Environ. Sci. Technol. 2017, 51, 8229–8244. [Google Scholar] [CrossRef]
- Yuan, Y.-J.; Ye, Z.-J.; Lu, H.-W.; Hu, B.; Li, Y.-H.; Chen, D.-Q.; Zhong, J.-S.; Yu, Z.-T.; Zou, Z.-G. Constructing Anatase TiO2 Nanosheets with Exposed (001) Facets/Layered MoS2 Two-Dimensional Nanojunctions for Enhanced Solar Hydrogen Generation. ACS Catal. 2016, 6, 532–541. [Google Scholar] [CrossRef]
- Goswami, N.; Giri, A.; Pal, S.K. MoS2 Nanocrystals Confined in a DNA Matrix Exhibiting Energy Transfer. Langmuir 2013, 29, 11471–11478. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Zhang, X.; Zhang, W.; Chang, M.-T.; Lin, C.-T.; Chang, K.-D.; Yu, Y.-C.; Wang, J.T.-W.; Chang, C.-S.; Li, L.-J.; et al. Synthesis of Large-Area MoS2 Atomic Layers with Chemical Vapor Deposition. Adv. Mater. 2012, 24, 2320–2325. [Google Scholar] [CrossRef]
- Stephenson, T.; Li, Z.; Olsen, B.C.; Mitlin, D. Lithium ion battery applications of molybdenum disulfide (MoS2) nanocomposites. Energy Environ. Sci. 2014, 7, 209–231. [Google Scholar] [CrossRef]
- Lin, Y.; Ren, P.; Wei, C. Fabrication of MoS2/TiO2 heterostructures with enhanced photocatalytic activity. CrystEngComm 2019, 21, 3439–3450. [Google Scholar] [CrossRef]
- Wan, J.; Lacey, S.D.; Dai, J.; Bao, W.; Fuhrer, M.S.; Hu, L. Tuning two-dimensional nanomaterials by intercalation: Materials, properties and applications. Chem. Soc. Rev. 2016, 45, 6742–6765. [Google Scholar] [CrossRef]
- Sreepal, V.; Yagmurcukardes, M.; Vasu, K.S.; Kelly, D.J.; Taylor, S.F.R.; Kravets, V.G.; Kudrynskyi, Z.; Kovalyuk, Z.D.; Patanè, A.; Grigorenko, A.N.; et al. Two-Dimensional Covalent Crystals by Chemical Conversion of Thin van der Waals Materials. Nano Lett. 2019, 19, 6475–6481. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Song, P.; Wang, C.; Riis-Jensen, A.C.; Fu, W.; Deng, Y.; Wan, D.; Kang, L.; Ning, S.; Dan, J.; et al. Engineering covalently bonded 2D layered materials by self-intercalation. Nat. Cell Biol. 2020, 581, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K.; Grigorieva, I.V. Van der Waals heterostructures. Nat. Cell Biol. 2013, 499, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Pumera, M.; Loo, A.H. Layered transition-metal dichalcogenides (MoS2 and WS2) for sensing and biosensing. TrAC Trends Anal. Chem. 2014, 61, 49–53. [Google Scholar] [CrossRef]
- Guo, L.; Yang, Z.; Marcus, K.; Li, Z.; Luo, B.; Zhou, L.; Wang, X.; Du, Y.; Yang, Y. MoS2/TiO2 heterostructures as nonmetal plasmonic photocatalysts for highly efficient hydrogen evolution. Energy Environ. Sci. 2018, 11, 106–114. [Google Scholar] [CrossRef]
- Scarano, D.; Cesano, F.; Zecchina, A. MoS2 Domains on TiO2-Based Nanostructures: Role of Titanate/TiO2 Transformation and Sulfur Doping on the Interaction with the Support. J. Phys. Chem. C 2019, 123, 7799–7809. [Google Scholar] [CrossRef]
- Cravanzola, S.; Sarro, M.; Cesano, F.; Calza, P.; Scarano, D. Few-Layer MoS2 Nanodomains Decorating TiO2 Nanoparticles: A Case Study for the Photodegradation of Carbamazepine. Nanomaterials 2018, 8, 207. [Google Scholar] [CrossRef]
- Kumar, S.G.; Devi, L.G. Review on Modified TiO2 Photocatalysis under UV/Visible Light: Selected Results and Related Mechanisms on Interfacial Charge Carrier Transfer Dynamics. J. Phys. Chem. A 2011, 115, 13211–13241. [Google Scholar] [CrossRef]
- Rao, C.; Maitra, U.; Waghmare, U.V. Extraordinary attributes of 2-dimensional MoS2 nanosheets. Chem. Phys. Lett. 2014, 609, 172–183. [Google Scholar] [CrossRef]
- Zhou, X.; Dong, H. A Theoretical Perspective on Charge Separation and Transfer in Metal Oxide Photocatalysts for Water Splitting. ChemCatChem 2019, 11, 3688–3715. [Google Scholar] [CrossRef]
- Selcuk, S.; Selloni, A. Surface Structure and Reactivity of Anatase TiO2 Crystals with Dominant {001} Facets. J. Phys. Chem. C 2013, 117, 6358–6362. [Google Scholar] [CrossRef]
- Yang, X.; Huang, H.; Jin, B.; Luo, J.; Zhou, X. Facile synthesis of MoS2/B-TiO2 nanosheets with exposed {001} facets and enhanced visible-light-driven photocatalytic H2 production activity. RSC Adv. 2016, 6, 107075–107080. [Google Scholar] [CrossRef]
- Chen, H.; Wen, X.; Zhang, J.Z.G.; Wu, T.; Gong, Y.; Zhang, X.; Yuan, J.; Yi, C.; Lou, J.; Ajayan, P.M.; et al. Ultrafast formation of interlayer hot excitons in atomically thin MoS2/WS2 heterostructures. Nat. Commun. 2016, 7, 12512. [Google Scholar] [CrossRef]
- Hu, X.; Lu, S.; Tian, J.; Wei, N.; Song, X.; Wang, X.; Cui, H. The selective deposition of MoS2 nanosheets onto (101) facets of TiO2 nanosheets with exposed (001) facets and their enhanced photocatalytic H2 production. Appl. Catal. B: Environ. 2019, 241, 329–337. [Google Scholar] [CrossRef]
- Chen, C.; Xin, X.; Zhang, J.; Li, G.; Zhang, Y.; Lu, H.; Gao, J.; Yang, Z.; Wang, C.; He, Z. Few-Layered MoS2 Nanoparticles Loaded TiO2 Nanosheets with Exposed {001} Facets for Enhanced Photocatalytic Activity. Nano 2018, 13, 1850129. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, L.; Lu, Z.; Jin, Z.; Wang, X.; Xu, G.; Zhang, E.; Wang, H.; Kong, Z.; Xi, J.; et al. Crystal face regulating MoS2/TiO2 (001) heterostructure for high photocatalytic activity. J. Alloy. Compd. 2016, 688, 840–848. [Google Scholar] [CrossRef]
- Cao, L.; Wang, R.; Wang, N.; Li, X.; Jia, H. MoS2-hybridized TiO2 nanosheets with exposed {001} facets to enhance the visible-light photocatalytic activity. Mater. Lett. 2015, 160, 286–290. [Google Scholar] [CrossRef]
- Wei, T.; Lau, W.-M.; An, X.; Liu, J. Interfacial Charge Transfer in MoS2/TiO2 Heterostructured Photocatalysts: The Impact of Crystal Facets and Defects. Molecules 2019, 24, 1769. [Google Scholar] [CrossRef]
- Dozzi, M.V.; Selli, E. Specific Facets-Dominated Anatase TiO2: Fluorine-Mediated Synthesis and Photoactivity. Catalysts 2013, 3, 455–485. [Google Scholar] [CrossRef]
- Gordon, T.R.; Cargnello, M.; Paik, T.; Mangolini, F.; Weber, R.T.; Fornasiero, P.; Murray, C.B. Nonaqueous Synthesis of TiO2 Nanocrystals Using TiF4 to Engineer Morphology, Oxygen Vacancy Concentration, and Photocatalytic Activity. J. Am. Chem. Soc. 2012, 134, 6751–6761. [Google Scholar] [CrossRef] [PubMed]
- Uddin, J.; Cesano, F.; Chowdhury, A.R.; Trad, T.; Cravanzola, S.; Martra, G.; Mino, L.; Zecchina, A.; Scarano, D. Surface Structure and Phase Composition of TiO2 P25 Particles After Thermal Treatments and HF Etching. Front. Mater. 2020, 7, 192. [Google Scholar] [CrossRef]
- Mino, L.; Pellegrino, F.; Rades, S.; Radnik, J.; Hodoroaba, V.-D.; Spoto, G.; Maurino, V.; Martra, G. Beyond Shape Engineering of TiO2 Nanoparticles: Post-Synthesis Treatment Dependence of Surface Hydration, Hydroxylation, Lewis Acidity and Photocatalytic Activity of TiO2 Anatase Nanoparticles with Dominant {001} or {101} Facets. ACS Appl. Nano Mater. 2018, 1, 5355–5365. [Google Scholar] [CrossRef]
- Davydov, A. Theoretical Fundamentals and Experimental Considerations of the Spectroscopic Methods Used in Surface Chemistry; Wiley: Chichester, UK, 2003; pp. 1–25. [Google Scholar]
- Pellegrino, F.; Sordello, F.; Mino, L.; Minero, C.; Hodoroaba, V.-D.; Martra, G.; Maurino, V. Formic Acid Photoreforming for Hydrogen Production on Shape-Controlled Anatase TiO2 Nanoparticles: Assessment of the Role of Fluorides, {101}/{001} Surfaces Ratio, and Platinization. ACS Catal. 2019, 9, 6692–6697. [Google Scholar] [CrossRef]
- Han, X.; Kuang, Q.; Jin, M.; Xie, Z.; Zheng, L. Synthesis of Titania Nanosheets with a High Percentage of Exposed (001) Facets and Related Photocatalytic Properties. J. Am. Chem. Soc. 2009, 131, 3152–3153. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, J.; Ma, Y.; Qi, L. Mesocrystalline TiO2 nanosheet arrays with exposed {001} facets: Synthesis via topotactic transformation and applications in dye-sensitized solar cells. Nano Res. 2017, 10, 2610–2625. [Google Scholar] [CrossRef]
- Muscuso, L.; Cravanzola, S.; Cesano, F.; Scarano, D.; Zecchina, A. Optical, Vibrational, and Structural Properties of MoS2 Nanoparticles Obtained by Exfoliation and Fragmentation via Ultrasound Cavitation in Isopropyl Alcohol. J. Phys. Chem. C 2015, 119, 3791–3801. [Google Scholar] [CrossRef]
- Cesano, F.; Bertarione, S.; Piovano, A.; Agostini, G.; Rahman, M.M.; Groppo, E.; Bonino, F.; Scarano, D.; Lamberti, C.; Bordiga, S.; et al. Model oxide supported MoS2 HDS catalysts: Structure and surface properties. Catal. Sci. Technol. 2011, 1, 123–136. [Google Scholar] [CrossRef]
- Cravanzola, S.; Cesano, F.; Magnacca, G.; Zecchina, A.; Scarano, D. Designing rGO/MoS2 hybrid nanostructures for photocatalytic applications. RSC Adv. 2016, 6, 59001–59008. [Google Scholar] [CrossRef]
- Cesano, F.; Agostini, G.; Scarano, D. Nanocrystalline TiO2 micropillar arrays grafted on conductive glass supports: Microscopic and spectroscopic studies. Thin Solid Film. 2015, 590, 200–206. [Google Scholar] [CrossRef]
- Sharma, A.A.; Mahlouji, R.; Wu, L.; Verheijen, M.A.; Vandalon, V.; Balasubramanyam, S.; Hofmann, J.P.; Kessels, W.M.M.; Bol, A.A. Large area, patterned growth of 2D MoS2 and lateral MoS2–WS2 heterostructures for nano- and opto-electronic applications. Nanotechnology 2020, 31, 255603. [Google Scholar] [CrossRef]
- Cravanzola, S.; Cesano, F.; Gaziano, F.; Scarano, D. Sulfur-Doped TiO2: Structure and Surface Properties. Catalysts 2017, 7, 214. [Google Scholar] [CrossRef]
- Kumar, S.; Shakya, J.; Mohanty, T. Probing interfacial charge transfer dynamics in MoS2/TiO2 nanocomposites using scanning Kelvin probe for improved photocatalytic response. Surf. Sci. 2020, 693, 121530. [Google Scholar] [CrossRef]
- Mignuzzi, S.; Pollard, A.J.; Bonini, N.; Brennan, B.; Gilmore, I.S.; Pimenta, M.A.; Richards, D.; Roy, D. Effect of disorder on Raman scattering of single-layer MoS2. Phys. Rev. B 2015, 91. [Google Scholar] [CrossRef]
- Cravanzola, S.; Cesano, F.; Gaziano, F.; Scarano, D. Carbon Domains on MoS2/TiO2 System via Catalytic Acetylene Oligomerization: Synthesis, Structure, and Surface Properties. Front. Chem. 2017, 5, 91. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Yan, H.; Brus, L.E.; Heinz, T.F.; Hone, J.; Ryu, S. Anomalous Lattice Vibrations of Single- and Few-Layer MoS2. ACS Nano 2010, 4, 2695–2700. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Q.; Yap, C.C.R.; Tay, B.K.; Edwin, T.H.T.; Olivier, A.; Baillargeat, D. From Bulk to Monolayer MoS2: Evolution of Raman Scattering. Adv. Funct. Mater. 2012, 22, 1385–1390. [Google Scholar] [CrossRef]
- Wilcoxon, J.P.; Newcomer, P.P.; Samara, G.A. Synthesis and optical properties of MoS2 and isomorphous nanoclusters in the quantum confinement regime. J. Appl. Phys. 1997, 81, 7934–7944. [Google Scholar] [CrossRef]
- Cravanzola, S.; Muscuso, L.; Cesano, F.; Agostini, G.; Damin, A.; Scarano, D.; Zecchina, A. MoS2 Nanoparticles Decorating Titanate-Nanotube Surfaces: Combined Microscopy, Spectroscopy, and Catalytic Studies. Langmuir 2015, 31, 5469–5478. [Google Scholar] [CrossRef] [PubMed]
- Matte, H.S.S.R.; Gomathi, A.; Manna, A.K.; Late, D.J.; Datta, R.; Pati, S.K.; Rao, C.N.R. MoS2 and WS2 Analogues of Graphene. Angew. Chem. Int. Ed. 2010, 49, 4059–4062. [Google Scholar] [CrossRef]
- Shi, H.; Yan, R.; Bertolazzi, S.; Brivio, J.; Gao, B.; Kis, A.; Jena, D.; Xing, H.G.; Huang, L. Exciton Dynamics in Suspended Monolayer and Few-Layer MoS2 2D Crystals. ACS Nano 2013, 7, 1072–1080. [Google Scholar] [CrossRef] [PubMed]
- Tsyganenko, A.A.; Can, F.; Travert, A.; Maugé, F. FTIR study of unsupported molybdenum sulfide?in situ synthesis and surface properties characterization. Appl. Catal. A Gen. 2004, 268, 189–197. [Google Scholar] [CrossRef]
- Bolis, V.; Barbaglia, A.; Bordiga, S.; Lamberti, A.C.; Zecchina, A. Heterogeneous Nonclassical Carbonyls Stabilized in Cu(I)− and Ag(I)−ZSM-5 Zeolites: Thermodynamic and Spectroscopic Features. J. Phys. Chem. B 2004, 108, 9970–9983. [Google Scholar] [CrossRef]
- Bachelier, J.; Duchet, J.; Cornet, D. On the promoting effect in sulfided Ni$z.sbnd;Mo/Al2O3 catalysts as studied by chemisorption. J. Catal. 1984, 87, 283–291. [Google Scholar] [CrossRef]
- Maugé, F.; LaMotte, J.; Nesterenko, N.; Manoilova, O.; Tsyganenko, A. FT-IR study of surface properties of unsupported MoS2. Catal. Today 2001, 70, 271–284. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santalucia, R.; Vacca, T.; Cesano, F.; Martra, G.; Pellegrino, F.; Scarano, D. Few-Layered MoS2 Nanoparticles Covering Anatase TiO2 Nanosheets: Comparison between Ex Situ and In Situ Synthesis Approaches. Appl. Sci. 2021, 11, 143. https://doi.org/10.3390/app11010143
Santalucia R, Vacca T, Cesano F, Martra G, Pellegrino F, Scarano D. Few-Layered MoS2 Nanoparticles Covering Anatase TiO2 Nanosheets: Comparison between Ex Situ and In Situ Synthesis Approaches. Applied Sciences. 2021; 11(1):143. https://doi.org/10.3390/app11010143
Chicago/Turabian StyleSantalucia, Rosangela, Tiziano Vacca, Federico Cesano, Gianmario Martra, Francesco Pellegrino, and Domenica Scarano. 2021. "Few-Layered MoS2 Nanoparticles Covering Anatase TiO2 Nanosheets: Comparison between Ex Situ and In Situ Synthesis Approaches" Applied Sciences 11, no. 1: 143. https://doi.org/10.3390/app11010143
APA StyleSantalucia, R., Vacca, T., Cesano, F., Martra, G., Pellegrino, F., & Scarano, D. (2021). Few-Layered MoS2 Nanoparticles Covering Anatase TiO2 Nanosheets: Comparison between Ex Situ and In Situ Synthesis Approaches. Applied Sciences, 11(1), 143. https://doi.org/10.3390/app11010143







