Microstructure and Photothermal Conversion Performance of Ti/(Mo-TiAlN)/(Mo-TiAlON)/Al2O3 Selective Absorbing Film for Non-Vacuum High-Temperature Applications
Abstract
1. Introduction
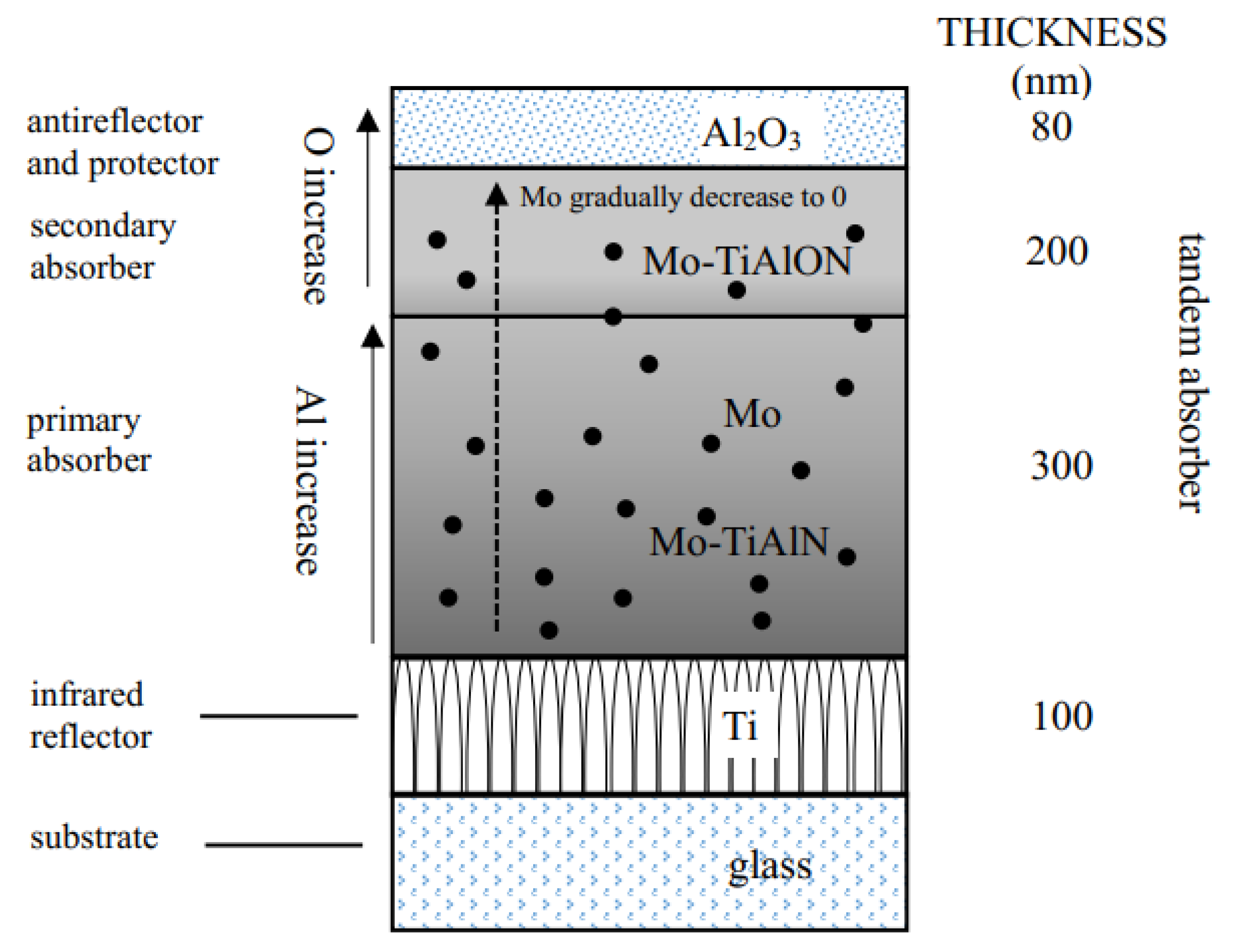
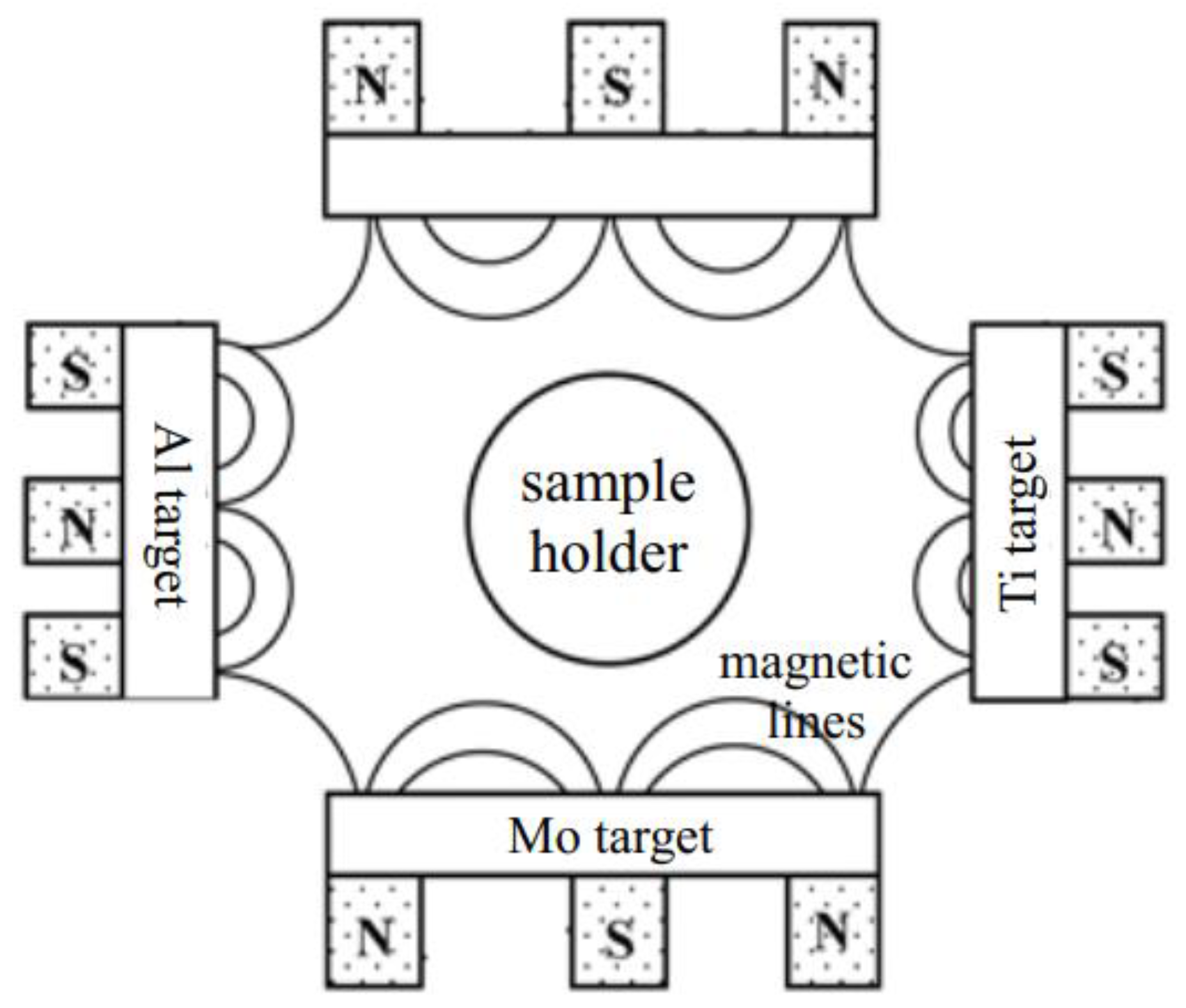
2. Experimental Details
3. Results and Discussion
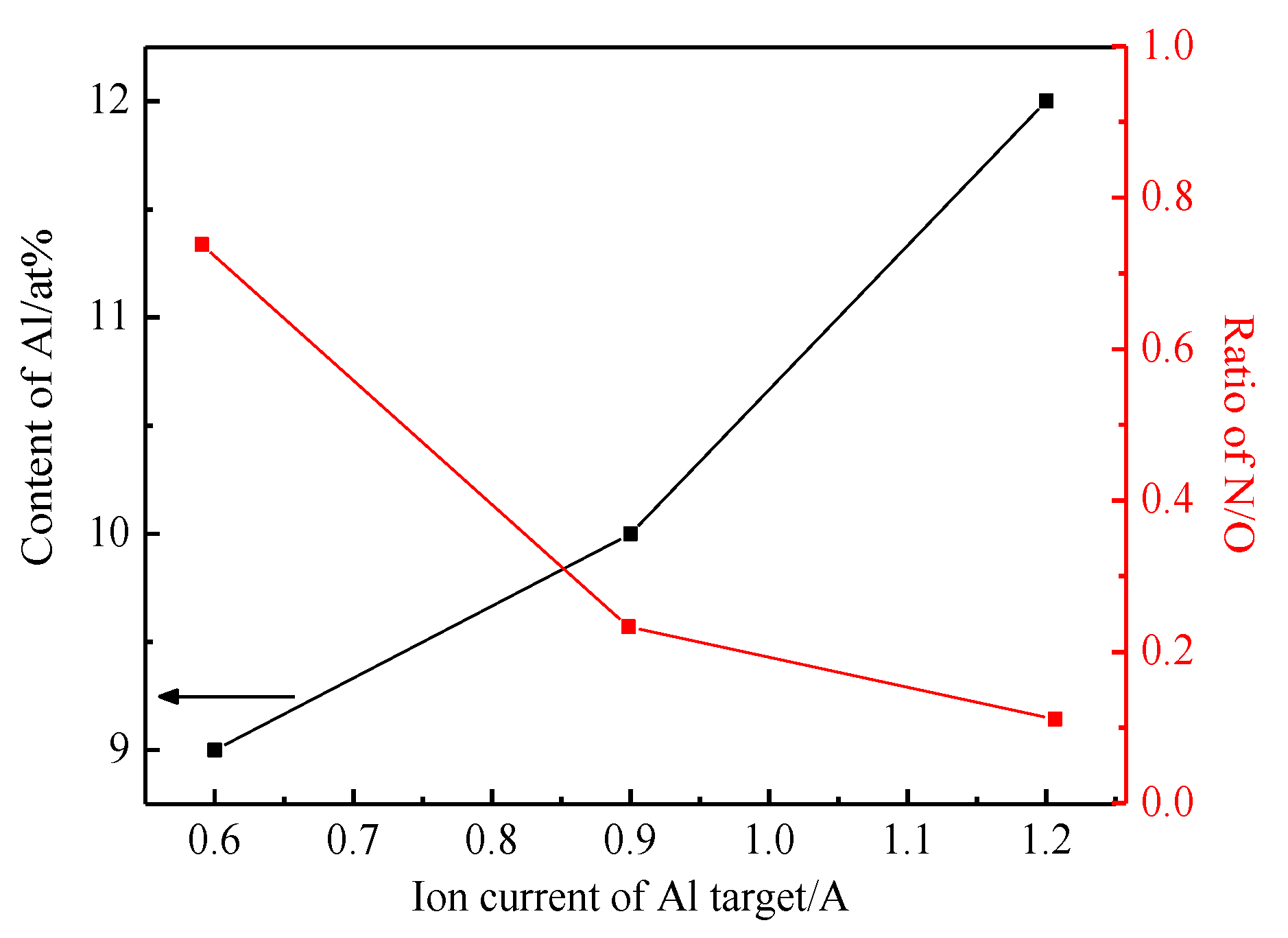
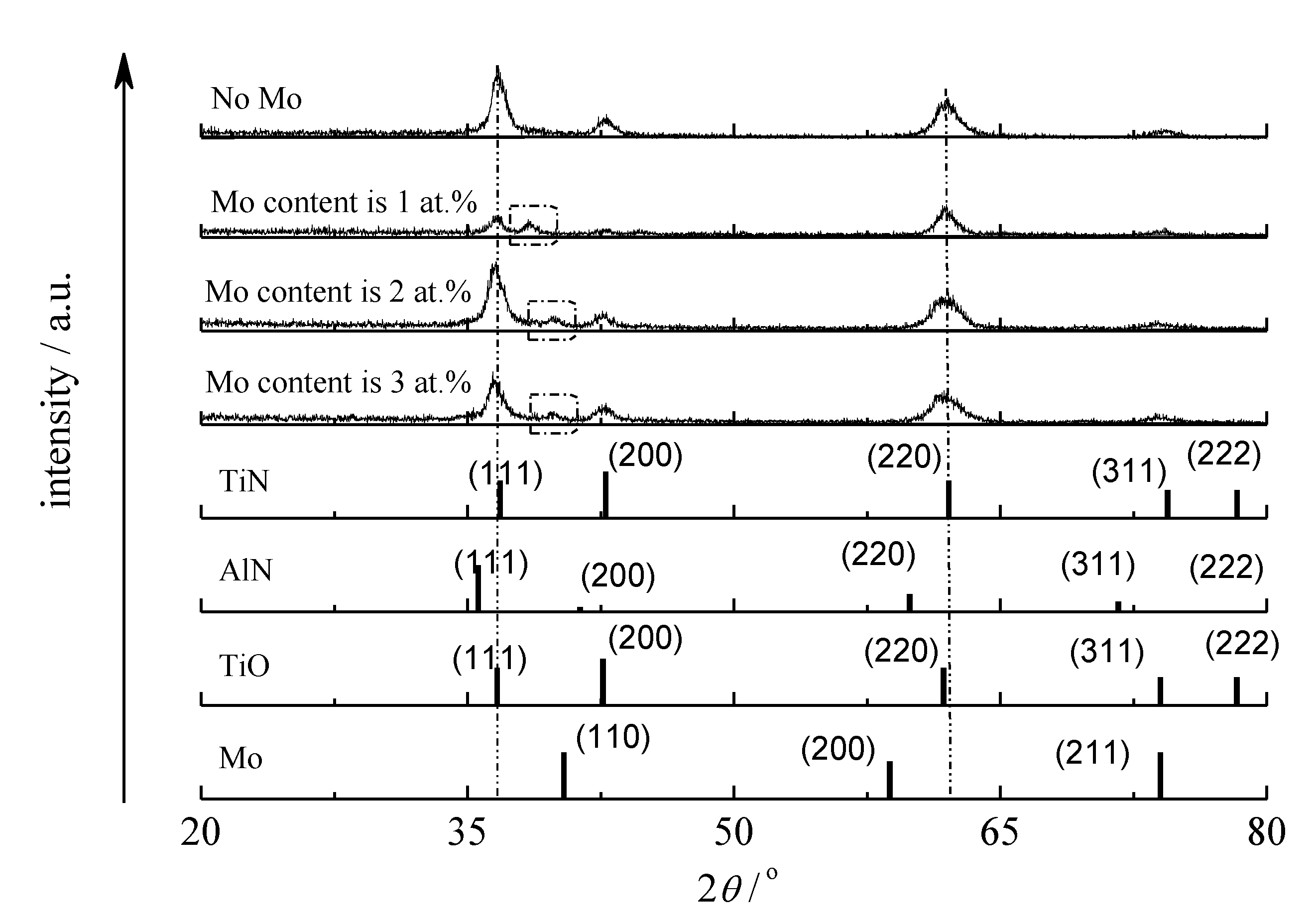
3.1. Composition and Phase Analysis of the Film
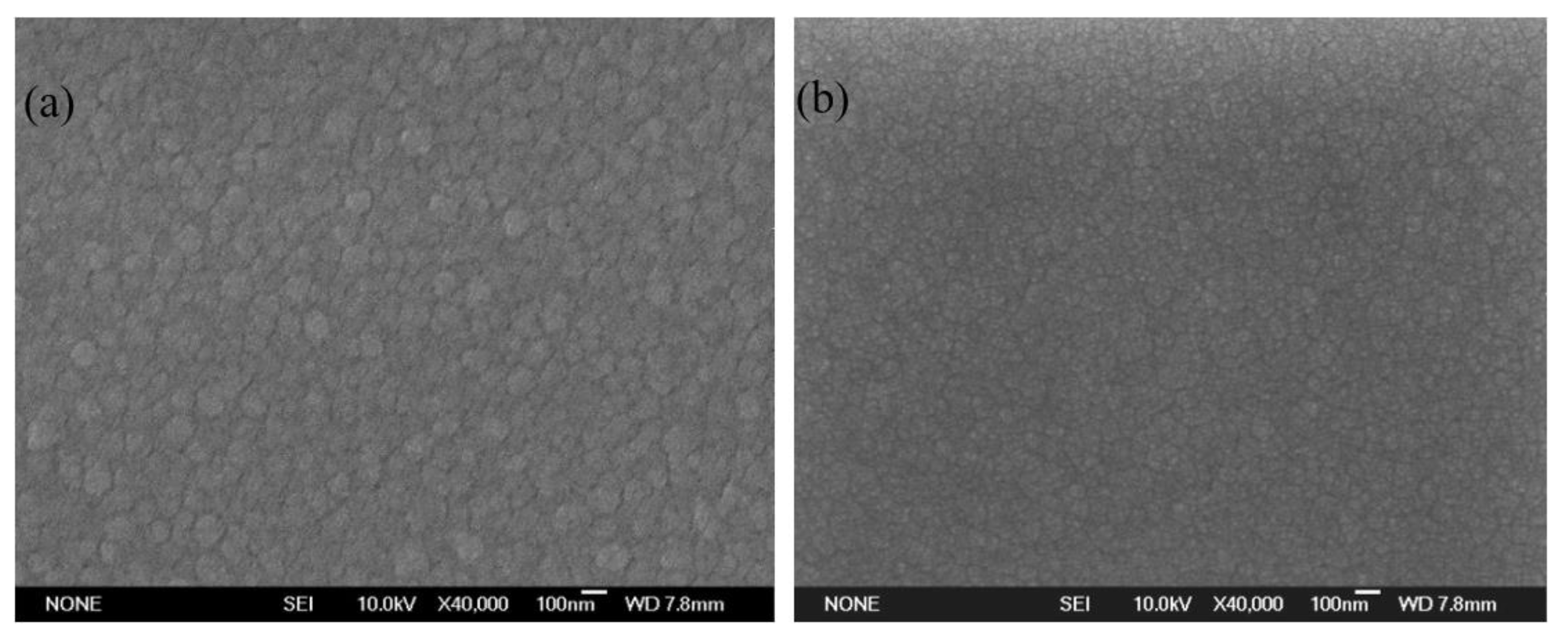
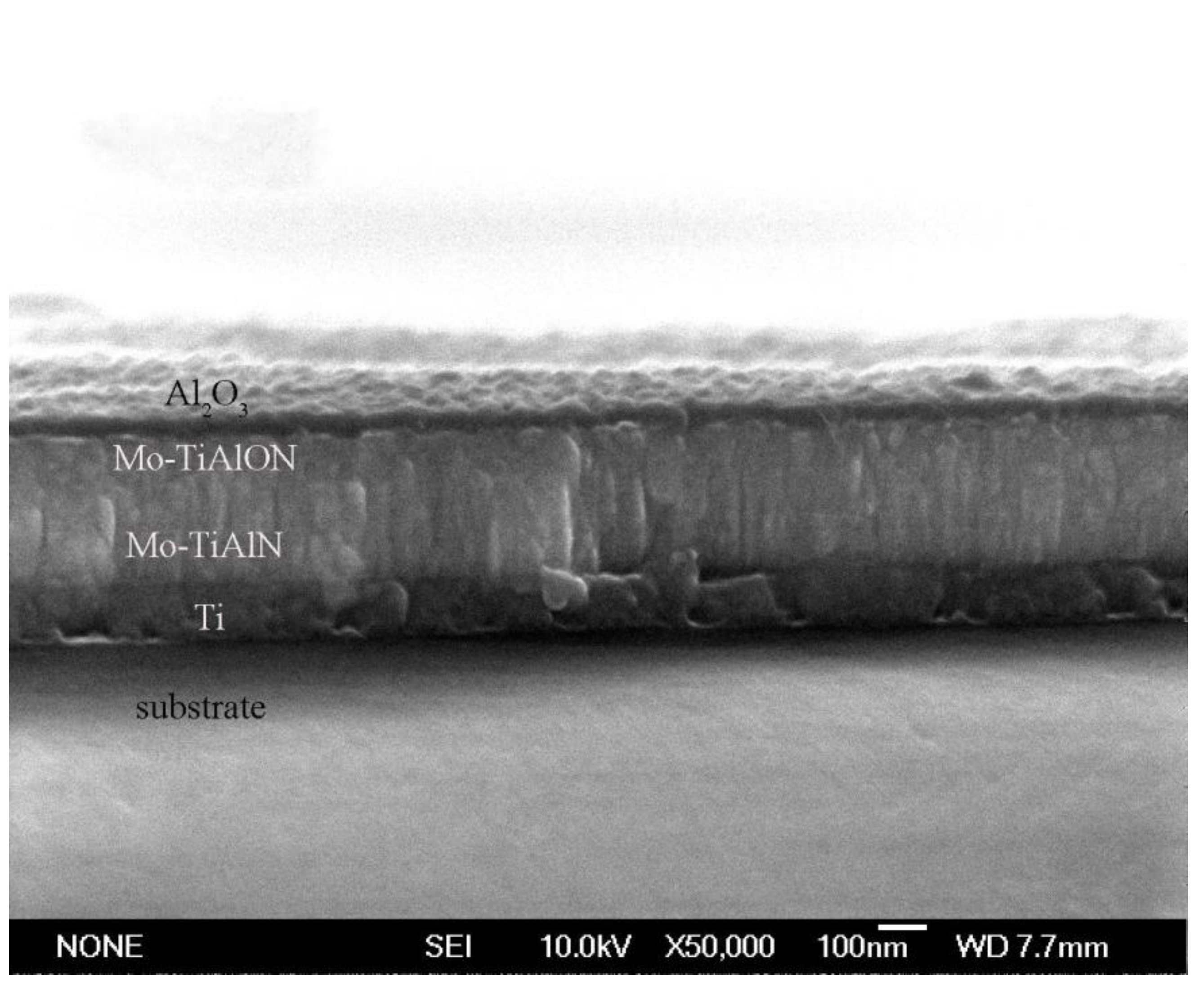
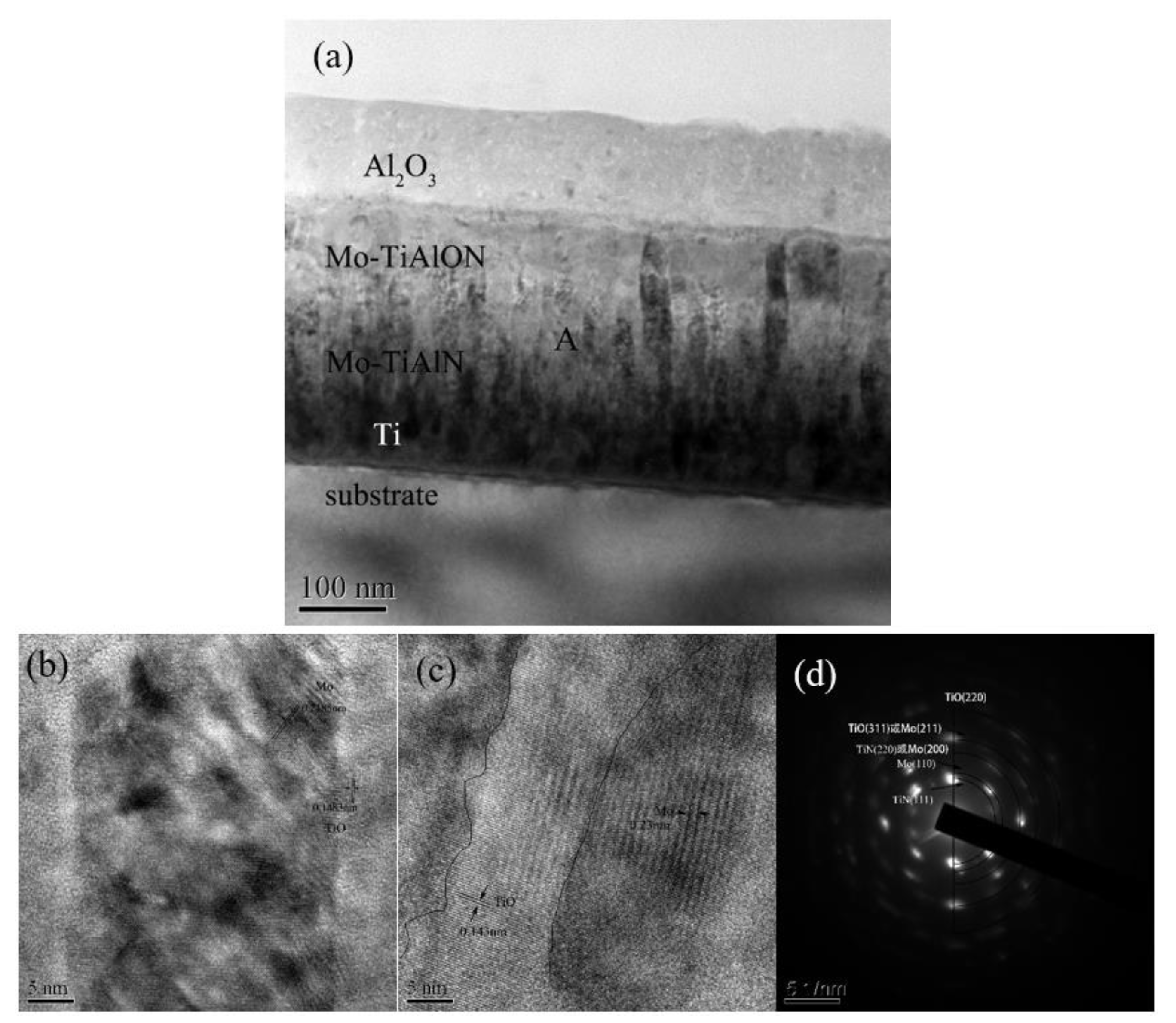
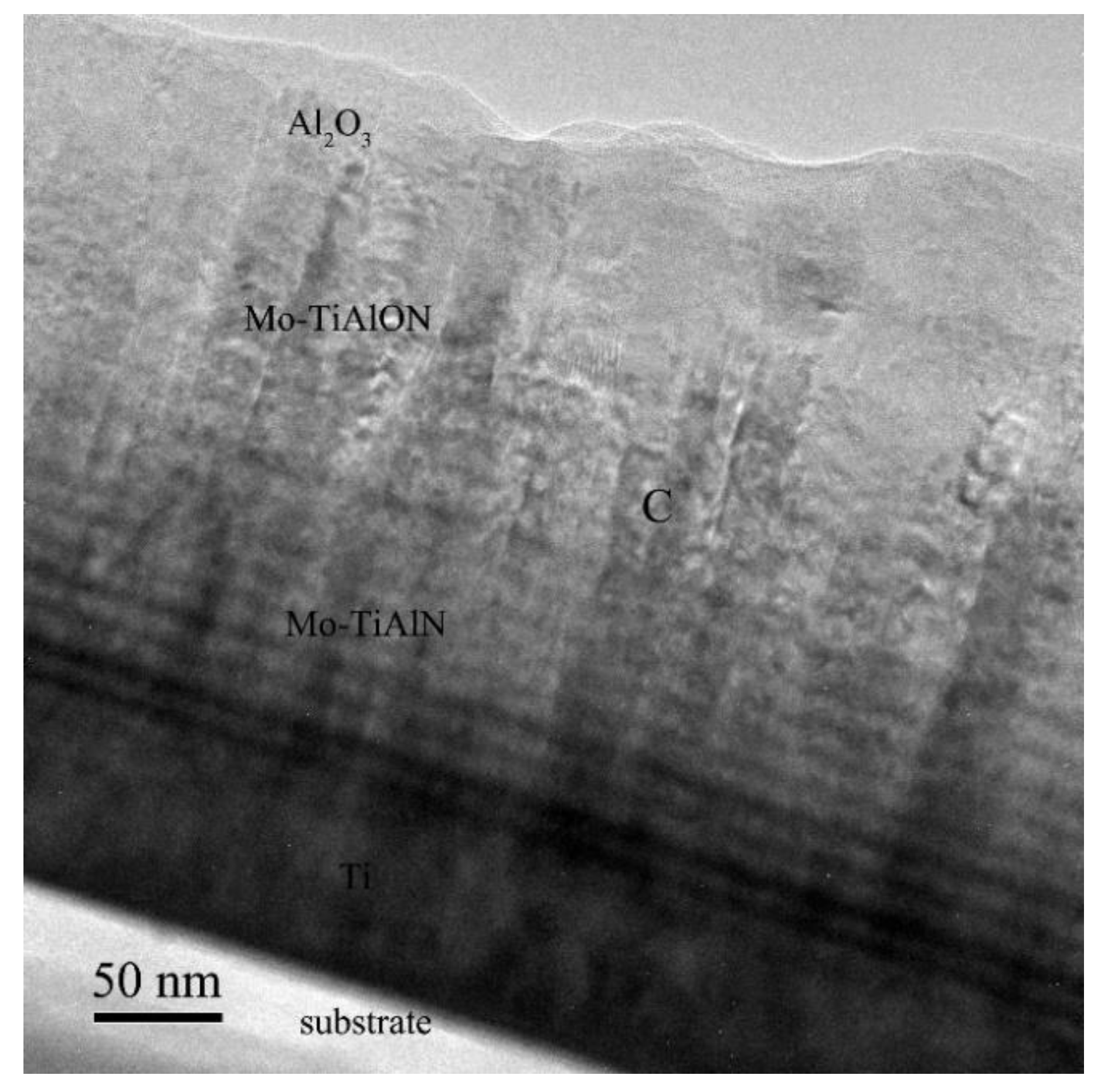
3.2. Microstructure Characterization
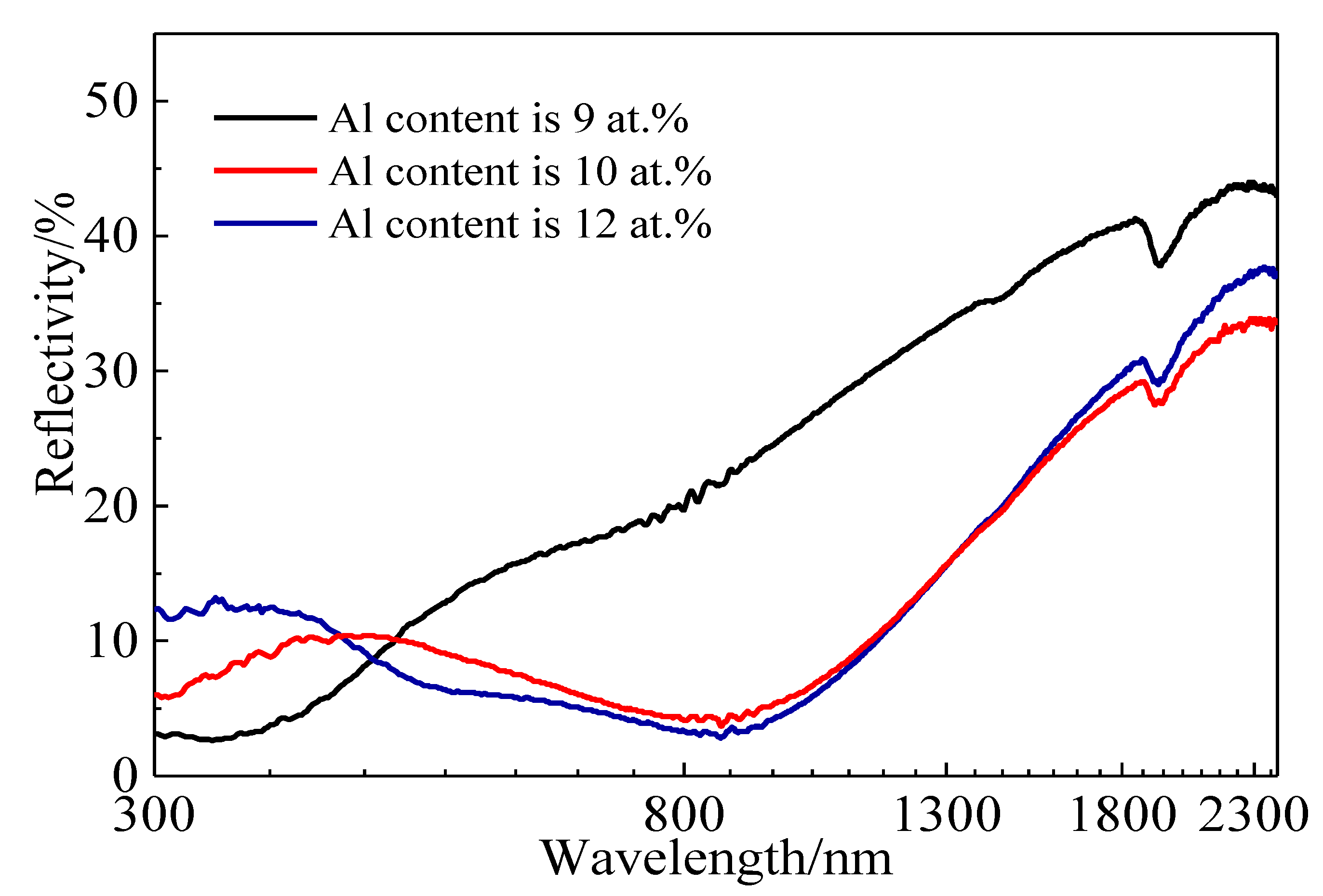
3.3. Photothermal Conversion Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meinel, A.B.; Meinel, M.P. Solar Photothermal Power Generation. Environ. Conserv. 1976, 3, 15–21. [Google Scholar] [CrossRef]
- Corona, B.; Ruiz, D.; Miguel, G.S. Environmental Assessment of a HYSOL CSP Plant Compared to a Conventional Tower CSP Plant. Procedia Comput. Sci. 2016, 83, 1110–1117. [Google Scholar] [CrossRef][Green Version]
- Pan, X.M.; Zhou, F.L. Thermostability and weatherability of TiN/TiC-Ni/Mo solar absorption coating by spray method-laser cladding hybrid deposition. Opt. Lasers Eng. 2020, 127, 105938. [Google Scholar]
- Gao, X.H.; Theiss, W.; Shen, Y.Q.; Ma, P.J.; Liu, G. Optical simulation, corrosion behavior and long term thermal stability of TiC-based spec-trally selective solar absorbers. Sol. Energy Mater. Sol. Cells 2017, 167, 150–156. [Google Scholar] [CrossRef]
- Xu, K.; Du, M.; Hao, L.; Mi, J.; Yu, Q.; Li, S. A review of high-temperature selective absorbing coatings for solar thermal applications. J. Mater. 2020, 6, 167–182. [Google Scholar] [CrossRef]
- Wang, X.B.; Zhang, X.M.; Li, Q.Y.; Min, J.; Cheng, X.D. Spectral properties of alcrno-based multi-layer solar selective absorb-ing coating during the initial stage of thermal aging upon exposure to air. Sol. Energy Mater. Sol. Cells 2018, 188, 81–92. [Google Scholar] [CrossRef]
- Usmani, B.; Dixit, A. Spectrally selective response of ZrOx/ZrC-ZrN/Zr absorber-reflector tandem structures on stainless steel and copper substrates for mid temperature solar thermal applications. Sol. Energy 2016, 134, 353–365. [Google Scholar] [CrossRef]
- Geng, H.B.; Wu, T.; Ma, C.W. Deposition & characterization of TiAlON & TiMoAlON solar absorber coatings for high tem-perature photothermal applications prepared by PEM controlled dual-gas reactive magnetron sputtering. Adv. Mater. Res. 2012, 538–541, 344–349. [Google Scholar]
- Depla, D.; Strijckmans, K.; Dulmaa, A.; Cougnon, F.; DeDoncker, R.; Schelfhout, R.; Schramm, I.; Moens, F.; De Gryse, R. Modeling reactive magnetron sputtering: Opportunities and challenges. Thin Solid Films 2019, 688, 137326. [Google Scholar] [CrossRef]
- Schiller, S.; Heisig, U.; Steinfelder, K.; Strümpfel, J.; Voigt, R.; Fendler, R.; Teschner, G. On the investigation of d.c. plasmatron discharges by optical emission spectrometry. Thin Solid Films 1982, 96, 235–240. [Google Scholar] [CrossRef]
- Burmakov, A.P.; Zaikov, V.A.; Labuda, A.A.; Chernyi, V.E. Instability of the reactive magnetron sputtering process. J. Appl. Spectrosc. 1996, 63, 901–904. [Google Scholar] [CrossRef]
- Schueler, A.; Thommen, V.; Reimann, P.; Oelhafen, P.; Francz, G.; Zehnder, T.; Düggelin, M.; Mathys, D.; Guggenheim, R. Structural and optical properties of titanium aluminum nitride films (Ti1−xAlxN). J. Vac. Sci. Technol. A 2001, 19, 922–929. [Google Scholar] [CrossRef]
- Barshilia, H.C.; Selvakumar, N.; Rajam, K.; Rao, D.S.; Muraleedharan, K. Deposition and characterization of TiAlN/TiAlON/Si3N4 tandem absorbers prepared using reactive direct current magnetron sputtering. Thin Solid Films 2008, 516, 6071–6078. [Google Scholar] [CrossRef]
- Bertóti, I.; Mohai, M.; Sullivan, J.; Saied, S. Surface characterisation of plasma-nitrided titanium: An XPS study. Appl. Surf. Sci. 1995, 84, 357–371. [Google Scholar] [CrossRef]
- Signore, M.A.; Sytchkova, A.; Rizzo, A. Sputtering deposition and characterization of tandem absorbers for photo-thermal sys-tems operating at high temperature. Opt. Mater. 2012, 34, 292–297. [Google Scholar] [CrossRef]
- Barshilia, H.C.; Selvakumar, N.; Rajam, K.S. Thermal stability of TiAlN/TiAlON/Si3N4 tandem absorbers prepared by reactive direct current magnetron sputtering. J. Vac. Sci. Technol. A Vac. Surf. Films 2007, 25, 383–390. [Google Scholar] [CrossRef]
- Barshilia, H.C.; Selvakumar, N.; Rajam, K.; Biswas, A. Optical properties and thermal stability of TiAlN/AlON tandem absorber prepared by reactive DC/RF magnetron sputtering. Sol. Energy Mater. Sol. Cells 2008, 92, 1425–1433. [Google Scholar] [CrossRef]
- Barshilia, H.C.; Selvakumar, N.; Rajam, K.S.; Rao, D.V.S.; Muraleedharan, K.; Biswas, A. TiAlN∕TiAlON∕Si3N4 tandem absorber for high temperature solar selective applications. Appl. Phys. Lett. 2006, 89, 191909. [Google Scholar] [CrossRef]



| Deposition Parameters | |||||||
| Sub-layer | Duration/s | Ion Current of Targets/A | PEM SetPoint/% | O2 Flow Rate/Sccm | |||
| Mo | Al | Ti | |||||
| infrared layer (Ti) | 300 | 0 | 0 | 4.0 | 100 | 0 | |
| main absorber (Mo-TiAlN) | 900 | 1.0 | 0→0.6/0.9/1.2 | 4.0 | 50 | 0 | |
| secondary absorber (Mo-TiAlON) | 360 | 0.6/1.0/1.2→0 | 0.9 | 4.0 | 50 | 0→8.5 | |
| antireflective layer (Al2O3) | 180 | 0 | 3.0 | 0 | OFF | 8.5 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geng, H.; Ye, H.; Chen, X.; Du, S. Microstructure and Photothermal Conversion Performance of Ti/(Mo-TiAlN)/(Mo-TiAlON)/Al2O3 Selective Absorbing Film for Non-Vacuum High-Temperature Applications. Appl. Sci. 2021, 11, 124. https://doi.org/10.3390/app11010124
Geng H, Ye H, Chen X, Du S. Microstructure and Photothermal Conversion Performance of Ti/(Mo-TiAlN)/(Mo-TiAlON)/Al2O3 Selective Absorbing Film for Non-Vacuum High-Temperature Applications. Applied Sciences. 2021; 11(1):124. https://doi.org/10.3390/app11010124
Chicago/Turabian StyleGeng, Haibin, Hanzhe Ye, Xingliang Chen, and Sibin Du. 2021. "Microstructure and Photothermal Conversion Performance of Ti/(Mo-TiAlN)/(Mo-TiAlON)/Al2O3 Selective Absorbing Film for Non-Vacuum High-Temperature Applications" Applied Sciences 11, no. 1: 124. https://doi.org/10.3390/app11010124
APA StyleGeng, H., Ye, H., Chen, X., & Du, S. (2021). Microstructure and Photothermal Conversion Performance of Ti/(Mo-TiAlN)/(Mo-TiAlON)/Al2O3 Selective Absorbing Film for Non-Vacuum High-Temperature Applications. Applied Sciences, 11(1), 124. https://doi.org/10.3390/app11010124





