Featured Application
The aim of this study was to evaluate the application of ultrasound (US) as a processing aid for salt reduction in brined pork meat.
Abstract
Meat samples (Longissimus dorsi) were processed using an ultrasonic (US) probe system (20 kHz) and a US bath (33 kHz), in brine solutions of 15% NaCl or NaCl/KCl. Selected quality parameters, namely hardness (Warner–Bratzler shear force, WBSF), secondary lipid oxidation products (thiobarbituric acid reactive substances, TBARs) and total colour difference (TCD) were analysed at day 0 and day 60. Inoculated E. coli and L. innocua cells, total viable counts and lactic acid bacteria were also monitored for 60 days on meat stored at 4 and 10 °C. US brining could achieve a 25% sodium reduction in a shorter processing time. No changes were observed for WBSF and TBARs values; noticeable colour differences (ΔΕ > 5) were measured in US-treated samples at the end of storage. Whilst no differences were observed in the levels of inoculated and spoilage bacteria on the meat surface, a significant reduction in E. coli in the brine subjected to US treatment indicates the potential of US as a hurdle technology to prevent cross contamination during meat processing. These results suggest that US processing, in combination with KCl, could assist current sodium reduction strategies improving processing time. In addition, the potential effects for decontamination of brining tanks increasing the shelf-life of the brine and preventing processing losses are highlighted.
1. Introduction
A target of 30% decrease in dietary sodium intake has been set by the World Health Organization by 2025 (WHO, 2013). Sodium chloride (NaCl) is one of the most widely used ingredients in the food processing sector, where processed meats contribute to about 20% of the total salt/sodium dietary intake [1,2]. The general concern about sodium intake from the diet has led to an increased interest in the development of methods to achieve sodium reduction targets, particularly in processed meat products where salt is used to prolong shelf-life, enhance flavour and increase juiciness and tenderness of the products [3,4,5]. To tackle salt reduction in meat products a variety of approaches have been proposed and reviewed [1,3]. Among these approaches, the use of metallic salt, salt reducers, salt replacers and flavour enhancers are the most common ways. When reducing salt, increasing the spices or acidity can help to improve flavour, but it may reduce the quality, yield and texture of the products; concerns about the upper limits of use of these ingredients in formulations and their effectivity to maintain the safety of the product are in addition, some of their limitations. The partial substitution of NaCl by other chloride salts (e.g., KCl, CaCl2 and MgCl2) has been suggested as one of the best alternatives to reduce sodium content in meat and dairy products [1,6,7]. In particular, with salt being an important preservative agent against bacterial growth, preliminary tests on the use of potassium chloride (KCl) as its substitute, have confirmed that KCl has an equivalent antimicrobial effect to NaCl and could therefore be a good candidate for salt replacement [8]. The major issue when lower salt levels are used is being able to maintain product quality characteristics without affecting shelf-life and safety of the products [3,9]. In addition, to achieve the required salt concentrations within the meat, longer brining times are required, which poses higher microbial contamination risks in the case of raw meat [10].
Development of novel technologies for meat processing has attracted attention in the scientific and research community for decades, leading to an increased interest in helping the transition from research to full commercial applications of new technologies in the meat processing sector. The increased demand for “less processed” meat products, with a reduced ingredients list and ingredients with less chemical sounding names, has also significantly reduced the number of options for processors and has led the food industry to look for alternative processing treatments [11]. Among the novel technologies, application of ultrasonic (US) processing (20–100 kHz) to meat products has been shown to improve meat tenderisation, meat functional properties and salt diffusion mechanisms, suggesting its potential use to speed up processing time and compensate for quality losses caused by sodium reduction in meat products [10,12,13,14,15]. Different studies have reported the possible applications of ultrasound technology for microbial inactivation, due to the effects of the sonochemical reactions happening in the liquid media [16,17,18,19]. However, most of the literature available focuses on US applications for bacterial inactivation in liquid foods, and limited information is available on the effects of US processing on bacteria attached to the meat matrix.
Thus, the aim of this study was to evaluate the effects of US-assisted brining on selected quality parameters such as texture, colour and lipid oxidation on reduced sodium pork meat. Given the potential of US for bacterial decontamination, its effects on spoilage microorganisms and on the survival of inoculated bacteria on the meat and in the brine after processing were also examined.
2. Materials and Methods
2.1. Sample and Brine Preparation
Pork loin (Longissimus dorsi) muscle, obtained from a local supermarket, was used for all experiments. Muscles were stored (vacuum packed) at 4 °C prior to being processed. Before curing, the connective tissue was carefully trimmed from the surface of the meat, and the muscles were cut into slabs of similar weight (200 ± 5 g) and thickness (4 cm). From each slab, 8 cubes of ~25 g were obtained. Two brine solutions were used in this experiment: 15% (w/w) NaCl (salt), and a mixture of 15% NaCl/KCl (w/w) in a 1:2 ratio. Meat samples brined in 15% NaCl were labelled as NS (normal sodium content), while the samples brined in 15% NaCl/KCl, were labelled as RS (reduced sodium content). Brines were prepared in bulk and stored in a plastic container at 4 °C for the duration of the experiment. A summary of the experiment procedure plan is presented in Figure 1.
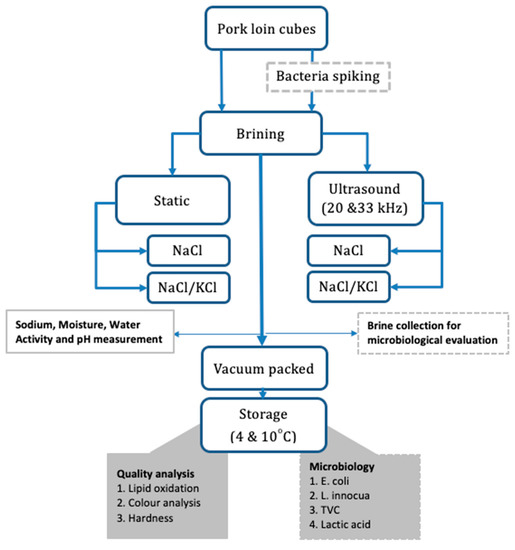
Figure 1.
Experimental procedure plan.
2.2. Bacterial Cultures and Meat Spiking
Non-pathogenic strains Escherichia coli K12 TEAG 1133 and Listeria innocua NCTC 11288, obtained from the microbiology stock cultures at Teagasc Food Research Centre Ashtown, were used for this study as model organisms to observe potential effects of US-assisted brining against key Gram-negative and Gram-positive pathogens in food products. Listeria innocua is a non-pathogenic species found in similar environments to L. monocytogenes with physiological characteristics very similar to the pathogenic bacterium and therefore, is often used as a surrogate bacterium [20]. The bacteria were maintained as frozen stocks at −80 °C on protective beads, which were plated onto tryptic soy agar (TSA: Oxoid Ltd., Basingstoke, Hampshire, UK) and incubated overnight at 37 °C to obtain single colonies. Working cultures were prepared by inoculating a single colony from TSA into 20 mL of tryptic soy broth (TSB: Oxoid Ltd., Basingstoke, Hampshire, UK) and incubated at 37 °C for 18–22 h or until a concentration of ~log10 7 CFU/mL was reached. Cells were collected by centrifugation (2000× g, 15 min), and the pellet was re-suspended in sterile phosphate-buffered saline (PBS, 10 mM, pH 7.2). Surface inoculation of each cube was performed to a target level of approximately log10 5 CFU/mL and allowed to dry at room temperature for 30 min.
2.3. Ultrasound Treatment
Meat samples were placed in a sterile metal basket and immersed in a glass beaker filled with 300 mL of either of the brine solutions. Brining was carried out in an ultrasonic bath operating at 33 kHz (96 W), in combination with an US probe operating at 20 kHz (75 W) for 1 h. Meat samples were treated four at a time and randomly assigned to each storage time. As a control, non-sonicated meat samples were brined without any agitation for 4 h, based on a previous optimisation study [14]. After brining, meat samples were drained of excess salt solution, rinsed, patted dry and vacuum packed for storage at 4 °C and 10 °C. Treatments were performed on separate sets, used for meat quality analyses, detection of total viable counts (TVC) and lactic acid bacteria (LAB), and to enumerate the inoculated microorganisms, each with three independent replicates.
2.4. Physicochemical Properties
For sodium (Na) measurements, ~10 g of meat was blended for 30 s using a standard food blender; samples were weighed into porcelain dishes, dried overnight and place on a Gallenkamp hot plate until completely burnt. Burned samples were ashed in a muffle furnace at 525 °C for approximately 8–10 h. Sodium was quantified with an Atomic Absorption Spectrometer 3110 (Perkin Elmer, Waltham, MA, USA) using the corresponding standard solutions and calibration curves to correlate the relative absorbance. Samples were measured in triplicate. Measurements of the pH were performed in duplicate in a 10 g sample mixed with 90 mL distilled water using a pH meter (ThermoFisher Scientific, Waltham, MA, USA). Moisture content was determined using the hot air oven drying method at 105 °C for 20–24 h. Water activity, aw, was measured using an AquaLab water activity meter (Decagon Devices Inc., Pullman, WA, USA). Physicochemical properties including hardness (Warner–Bratzler shear force (WBSF)), colour (L*, a*, b*) and total colour differences (TCD) were measured in triplicates. WBSF was performed on samples cooked in a water bath set to 77 °C until a core temperature of 72 °C was reached; shear force was measured by Warner–Bratzler test performed on cylindrical cores (17 ϕ × 20 mm) taken parallel to the longitudinal orientation of the muscle fibres. Samples underwent a double axial compression (70%) at a speed of 50 mm/min with a 35 mm flat circular anvil attached to a 5 kN load cell on an Instron Universal testing machine (Model No. 5543, Instron, Bucks, UK). Shear and penetration force were taken as the maximum recorded force on the output expressed as Newton (N) [14]. Instrumental colour analysis was measured in the transparent packaging material on both sides of the meat surfaces on days 0, 3, 7, 14, 21, 35 and 60 using a dual beam spectrometer Hunter Lab system (UltraScan XE, Hunter Lab., Reston, VA, USA). Hunter L* a* and b* were determined as indicator of lightness, redness, and yellowness, respectively. Illumination was matched to daylight (D65, 10°) with an 8° viewing angle and a 25 mm port size. Standardisation was performed using a light trap and a white tile. Total colour difference expressed as was numerically calculated using the colour difference at the beginning and at the end of the shelf life using Equation (1):
Formation of secondary lipid oxidation products at the beginning and the end of the storage time were estimated as thiobarbituric acid reactive substances (TBARS), as described by Botsoglou, Fletouris [21]. Results were expressed as mg of malondialdehyde (MDA) per kg of meat.
2.5. Bacterial Enumeration
Microbiological analyses were carried out on vacuum packed pork samples after 0, 3, 7, 14, 21, 35 and 60 days of storage at 4 °C and 10 °C. Each cube of ~25 g was aseptically taken, diluted 10-fold in Maximum Recovery Diluent (MRD, Oxoid Ltd., Basingstoke, Hampshire, UK) and homogenised for 2 min in a stomacher. Plate count agar (PCA, Oxoid Ltd., Basingstoke, Hampshire, UK) incubated at 30 °C, 72 h was used to determine total viable counts (TVC); while De Man, Rogosa, Sharpe Agar (MRS) incubated anaerobically at 30 °C for 72 h was used to enumerate lactic acid bacteria (LAB). To follow the growth of the inoculated microorganisms, from each dilution a 0.1 mL sample was plated on selective agar, namely MacConkey agar and Listeria Selective Agar Base (Oxford) (Oxoid Ltd., Basingstoke, Hampshire, UK), for E. coli and L. innocua, respectively.
2.6. Scanning Electron Microscopy
The microstructure of the meat samples before and after ultrasound-assisted brining was observed with a scanning electron microscope (SEM). The meat samples of size 1 × 1 × 0.25 cm were fixed in 3% glutaraldehyde for 1h; samples were dehydrated in a series of ethanol solutions (10, 20, 30, 50, 70, 90, 100%) and dried at critical point CO2 (Critical Point Dryer (CPD) Quorum E3100, Quorum Technologies Ltd., Lewes, UK). Samples were observed under a Zeiss Ultra Plus Field Emission SEM (Carl Zeiss Ltd., Jena, Germany).
2.7. Statistical Analysis
Average values and standard deviations of the replicates were determined from the microbiological data. The normal distribution of the data was assessed through Shapiro–Wilk normality tests. A three-way ANOVA was run to examine the effects of the independent variables (processing, brine used and storage temperature) over the dependant factors; where no significant interaction was found, the main effects on the means were evaluated as significant at p < 0.05, followed by Tukey post hoc test. Statistical analyses were performed using Minitab 17. Principal component analysis (PCA) was based on correlation analysis of the data matrix between responses (columns) and treatments (rows) and was performed using Statistica 13.3 (TIBCO Software, Palo Alto, CA, USA). Data were autoscaled before PCA analysis and factor loadings higher than 0.60 were used to project the treatments on the factor plane (principal component 1 vs. principal component 2) [22].
3. Results and Discussion
3.1. Sodium Uptake and Moisture
The sodium content of pork brined in 15% NaCl (NS) or 15% NaCl/KCl (RS) with or without ultrasound processing is presented in Table 1. As expected from the brine formulations, significant differences (p < 0.05) in the amount of sodium (Na) were observed between the samples. The sodium content of the RS meat, was significantly lower (p < 0.05) than the one brined in NaCl, containing 0.62 g and 0.90 g Na/100 g meat, respectively. No differences (p > 0.05) were observed between the level of sodium uptake in the control and sonicated samples using the optimized processing times of 1 h and 4 h, respectively, for ultrasound or immersion curing.

Table 1.
Sodium content, moisture, water activity, and pH of pork meat brined with or without ultrasound. Sodium content is express as g of sodium/100 g of meat. Comparisons are between the processing conditions (static brining and ultrasonic (US)) and the brines used for curing NaCl (normal sodium content, NS) and NaCl/KCl (reduced sodium, RS).
The effect of ultrasound processing on the meat surface is shown in Figure 2. The SEM pictures clearly show the formation of micro-channels on the meat surface, when processed using ultrasound in comparison to untreated meat [10]. The visible formation of micro-channels on the meat surface allow for a faster and more homogenous distribution of the brine on the meat; this physical disruption at the cellular level in response to acoustic cavitation, is the main reason for the shorter processing time achieved by US in comparison to standard methods. At the measured sodium levels, no differences in expressible moisture content, water activity and pH of the meat were observed, Table 1. Similarly, a recent work from Stanley et al. [23] has shown that no significant effects on moisture and pH were found in pork sausage patties when salt was replaced with potassium chloride. In regard to the effects of using US for meat processing, different studies have looked at its effect on pH in beef muscles, reporting no or small differences when ultrasound was applied with a frequency between 24 and 45 kHz [24,25,26].

Figure 2.
Scanning electron microscopy (SEM) images of pork meat surface subjected to conventional immersion brining, control (a) or ultrasound-assisted brining (b).
3.2. Formation of Secondary Lipid Oxidation Products
Evaluating lipid oxidation status is particularly important when using novel processing technologies like high power US, since the formation of radicals and oxidative compounds due to acoustic cavitation can trigger further oxidative reactions in the food matrix [27,28]. Although other studies have shown that the use of US on meat products can increase lipid oxidation [29,30], in this experiment no significant increase in the oxidation levels of ultrasound processed samples compared to the control was observed, Figure 3.
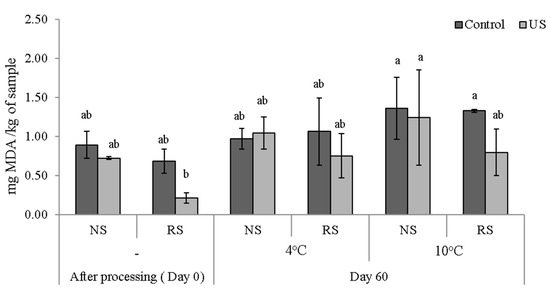
Figure 3.
Secondary lipid oxidation values of meat samples with standard sodium content (NS) or reduced sodium content (RS) at Day 0 and after 60 days storage at 4 and 10 °C. Values represent means ± standard deviation (SD, N = 3). Different letters are significantly different at p < 0.05.
Overall, studies reporting negative effects of ultrasound are scarce [10,26]. In a study from Stadnik et al. [26], samples treated with ultrasound (45 kHz, for 120 s) follow by refrigerated storage for 4 days, were not compromised in terms of oxidative stability of lipids. Similarly, other studies [31,32] have shown that US did not cause an increase in lipid oxidation in cooked meat emulsion and brined pork, respectively. Moreover, Cheng and Wang [33] investigated the effect of low sodium content on lipid oxidation and the colour of pork patties, observing that TBARS values of fresh pork patties were reduced by the replacement of NaCl with KCl. Patties with a 1:1 w/w substitution of NaCl with KCl had significantly lower TBARS values compared to patties with a 2:1 ratio of NaCl to KCl; therefore, the replacement of NaCl caused a significantly lower TBARS values compared to the control [33]. This may explain why we observed lower levels of oxidation in the RS sample after US processing, Figure 3; after the 60 days of storage however, no significant differences (p > 0.05) were observed in the oxidation level of the fatty acids of the control and sonicated samples, suggesting that during storage, despite the initial differences, the US–RS lipid oxidation values increased by a higher rate than those of the other groups. These differences in the oxidation rate could be the results of the formation of free radicals by US, leading to an increased speed of the chemical reactions [19].
3.3. Colour Values
Different studies have reported that ultrasound treatment can cause changes in meat appearance due to the dissipation of ultrasound energy on the upper surface of the meat [14,34]. Results of the colour parameters and total colour difference in this study are presented in Table 2. No significant interactions (p > 0.05) were found between the independent variables. The only significant effect on the colour parameters was caused by the storage days: the main effect on the mean of lightness (L*) and redness (a*) of the samples over storage time, showed no statistically significant differences among the treatments at p = 0.078 and 0.275, respectively. Significant differences (p < 0.001) were however observed for the b* (yellowness) values, which increased during storage in all samples, regardless of the processing treatment used. As some studies suggest, the lack of colour changes after US treatment can happen if the heat generated by US processing is insufficient to denature proteins and pigments [35]. Given the conditions used in this study where the system was constantly maintained cool, this factors could have played an important role. Similarly, a recent study [36] reported no statistically significant effect of ultrasound intensity, ultrasound duration, and storage time on meat redness, lightness and yellowness of bovine Longissimus dorsi. The total colour difference (ΔE) parameter is used to indicate the degree of colour difference between samples; values can be ranked as not noticeable (0–0.5), slightly noticeable (0.5–1.5), noticeable (1.5–3.0), well visible (3.0–6.0) and great (6.0–12.0) [37]. According to this scale, noticeable colour differences (ΔE > 2) could be observed between all the samples processed with US, becoming well visible (ΔE > 5) after storage when compared to the initial colour of the pork brined by immersion (Table 2). However, well visible changes (ΔE > 3) were also measured for the control samples after 60 days storage, suggesting that natural changes in colour happen during storage but, if ultrasound is used for processing, the total colour changes could be more easily identified by a visual assessment.

Table 2.
Colour parameters and total colour difference (TCD) of brined pork at day 0 and after 60 days of storage: values represent means ± standard deviation (SD, N = 3) of ultrasound-assisted (US) meat containing standard (NS) or reduced sodium content (RS).
3.4. Shear Force Measurments
Consumers’ responses have shown that texture is the most important palatability factor when determining meat quality, and a variety of results have been reported on the potential effects of ultrasound for meat tenderization [1,13,38]. The general understanding is that US treatment can effectively improve meat tenderness by the mechanical rupture of the myofibrillar protein structure, leading to faster proteolysis, as well as by acting on the thermal properties of collagen [10]. In the results presented in Figure 4, neither the storage temperature (p = 0.855), the US treatment (p = 0.638) nor the salt (p = 0.125) alone or in combination, significantly influenced the average hardness values. With the conditions used in this study, we observed a greater effect caused by the storage time (p < 0.001), with an increase in the hardness of the meat between day 0 and day 60, more pronounced for the NS samples. A non-significant tendency towards a decrease in the toughness of the muscle due to US was similarly observed in beef [36]. In general, effects of US on meat tenderness vary greatly depending on the US system used, power, temperature and meat cut; some studies showed significant effect of ultrasound to improve tenderness [25,39,40], while others seem to indicate that ultrasound has no effect on shear force of meat [32,41]. Studies on beef cured with NaCl and exposure times of 30 and 120 min with ultrasound (150 and 300 W, 20 kHz) reported changes in the water retention capacity and the tenderness of the meat compared to traditional curing (p < 0.05) [42]. Despite the same US frequency used, differences in the chosen processing time as well as a lower ultrasonic power, could explain why no differences were observed in our study. It has to be addressed that in this experiment, good care was taken to ensure that the samples were obtained from the same part of the Longissimus dorsi muscle; as a very long muscle, thus with a lot of variability, different outcomes in the effect of the treatment could be observed based on the section of the muscle used.

Figure 4.
Hardness values of meat samples with standard sodium content (NS) or reduced sodium content (RS) at Day 0 and after 60 days storage at 4 and 10 °C. Values represent means ± standard deviation (SD, N = 3). Different letters are significantly different at p < 0.05.
3.5. Bacterial Enumeration on Treated Pork
Understanding bacterial growth is an important element to assess the spoilage and safety profile of a product, especially in sodium-reduced foods where the impact of salt reduction can be unclear. The addition of ultrasound processing can cause diverse effects on bacterial populations depending on control parameters such as power, frequency and treatment time [43,44]. At the moment, not many studies have been looking at the use of ultrasound for bacteria inactivation on solid foods and, the majority of the literature available provides report on liquid/semi solid application [45]. At the conditions used in this study, the application of US processing showed no significant effects on bacterial inactivation on the meat; no changes in the growth of the inoculated bacteria populations were observed among samples containing high or low sodium content (>0.88 g and 0.65 g/100 g of meat, respectively) between all the conditions used (data not shown). A similar lack of antimicrobial effects of ultrasound was previously described by Pohlman et al. [46] who observed that ultrasound exposures reduced the total plate count during storage, but it was impossible to produce significant difference from the control muscle samples on chicken skin. The hypothesis is that the meat layers can provide some degree of protection from cavitation for bacteria [38].
Similarly, the use of a mix of NaCl/KCl as a salt replacer, did not have a negative impact on the samples (Control NS vs. Control RS), showing no differences in the microbiological profile of SR and NS and supporting previous studies highlighting the antimicrobial efficacy of KCl [8,47].
An increase in bacterial population following US treatment was observed in the samples stored at 10 °C, showing significantly higher counts for TVC and LAB (p = 0.001 and 0.002, respectively) in NS–US meat in comparison to the control, Figure 5.
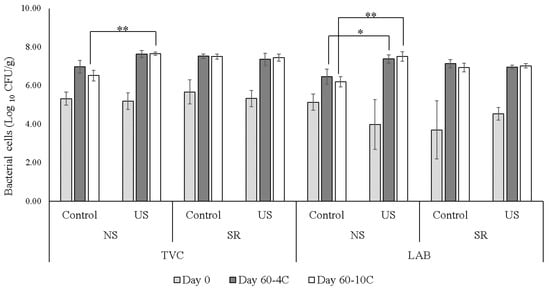
Figure 5.
Total viable counts (TVC) and lactic acid bacteria (LAB) counts at day 0 and after 60 days of storage at 4 °C and 10 °C. Comparison between samples contained normal sodium levels (NS) and reduced sodium levels (RS) and processed with ultrasound (US) or without (control). Differences between treatments are significant at * p-value < 0.05, ** p-value < 0.01.
Lactic acid bacteria (LAB), at maximum numbers of ~log10 8 CFU/g, are usually the predominant bacteria present on meat stored under anaerobic conditions such as vacuum-packed or modified atmosphere [48]. In general, for all the treatments used in this study, the population of lactic acid bacteria remained below log10 8 CFU/g, Figure 5. The only significantly higher counts (p = 0.026) were observed in NS meat samples after 60 days of storage at 4 °C in comparison to the other samples at the same salt level. Similar observations were reported [49], where it was shown that low-frequency ultrasound (70 kHz) increased the growth rate of bacterial cells on polyethylene surface compared to growth without ultrasound; the authors suggested that by increasing the rate of transport of oxygen and nutrients to the cells, US processing could influence the growth of bacteria [49]. The authors reported that a treatment of 10 min was the best US condition (37 kHz, 90 W) to achieve microbial reduction in beef, while increased treatment time led to increased bacterial growth [50]. On the other hand, Kang et al. [17] have reported a significant (p < 0.05) increase in E. coli cell numbers in beef samples after US processing, suggesting that extended ultrasound treatment could be needed to inactivate the microorganisms present on the meat surface. It is evident from the literature that the effects of US treatment on the microorganisms in the meat matrix differ greatly based on the specific experimental conditions, matrix, treatment time and US frequency. Further studies are therefore needed to determine the ideal treatment time to achieve bacterial inactivation, while also considering the potential effects on meat quality.
3.6. Bacterial Enumeration in Brine
Even though meat itself is an implicit source of bacterial contamination, other sources such as improperly cleaned equipment or brine recirculation during processing could also be cause of contamination. An investigation on the bacterial contamination levels of recirculating brine used in the production of pork, found that the number of L. monocytogenes increased with time, reaching a maximum of log10 2.34 after 2.5 h [51]. The potential use of ultrasound for decontamination purposes is based on the effect of acoustic cavitation that generates strong oxidizing agents, such as ●OH radicals, H2O2 and ozone, initiating and enhancing redox reactions with the consequential effect of bacterial inactivation [16,52]. The decontamination efficiency of US for the brining environment was evaluated by enumerating the number of E. coli and L. innocua present in the liquid after processing the inoculated meat, Figure 6.
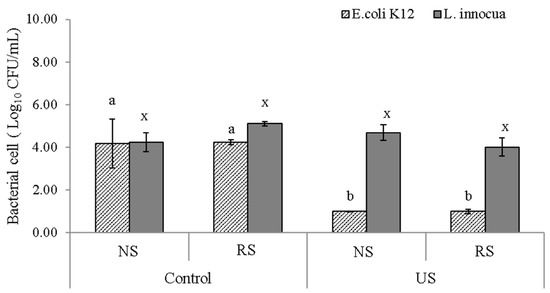
Figure 6.
Bacterial inactivation in brine; values represents means ± standard deviation (SD, N = 3) of the log10 CFU/mL for E. coli and L. innocua present in NaCl or NaCl/KCl brines after the inoculated meat was processed with (US) or without ultrasound (Control) for 1 and 4 h, respectively. Different letters represent significant differences at p < 0.05.
In meat samples brined with US, a significant reduction of 4 log10 CFU/mL (p = 0.005) was observed for E. coli in both the brines used. In agreement with these results, previous studies have shown that the Gram-negative bacteria in particular E. coli, could be easily inactivated by sonication treatment in the presence of salts, due to its thinner peptidoglycan layer [17,18,53]. On the other hand, the L. innocua population in the brine did not show a significant reduction for both US-assisted and static brining with both salt solutions, Figure 6. Generally, Listeria spp. tends to be more resistant to high salt concentrations and ultrasound treatments than Gram-negative bacteria are; as observed by Faleiro et al. [54], this organism could survive in a cheese brining system with a salt concentration up to 10%. Therefore, its persistency even under ultrasound treatment is not surprising; in order to be inactivated, it may require additional stressors such as temperature (e.g., thermo-sonication) or addition of antimicrobials [55,56,57].
3.7. Principal Component Analysis
Principal component analysis (PCA) was used to identify the most important elements of variability in the physiochemical parameters of brined pork treated by ultrasound. The factor loading and the score plot with the location of the objects in the multivariate space of two principal component score vectors are presented in Figure 7. The analysis shows that about 73.25% of the total variation is explained by the first principal component (PC1), and 19.05% by the second principal component (PC2), therefore explaining up to 93% of data variability using only two factors.
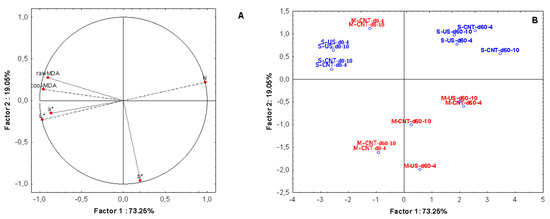
Figure 7.
Principal component analyses (PCA) of pork meat brined with/without ultrasound and with NaCl or NaCl/KCl based on physicochemical data; (A) factor loadings and (B) projection of the four groups of treatments in the plane. Salt type: S (normal sodium content, blue colour), M (reduced sodium content, red colour); processing: CNT (Control), US (ultrasound); Day of storage: d0, d60; Temperature of storage: 4 °C, 10 °C.
The most important variables for PC1 were meat hardness, lightness, and the levels of lipid oxidation; hardness was positively correlated with PC1 with factor loading of 0.98, while L* and lipid oxidation were negatively correlated (r = −0.96, p < 0.05) as shown by their opposite position in the loading plot (Figure 7). The PC2 was mostly negatively characterized by the b* colour values (−0.96). Based on the results, two groups of samples could be clearly separated by PCA: samples brined using the reduced sodium mixture (M) that were all located in the lower side of the graph and samples brined using salt (S) that were placed on the upper right side of the plot. Regardless of the salt type, processing conditions or storage temperature, a third group could be observed on the top left side, grouping together all the physiochemical parameters of the samples at day 0. The results indicate that the hardness of the samples explained a considerable proportion of the total variance when PCA was used to select between the qualities parameters used.
4. Conclusions
Ultrasound-assisted brining could successfully be used in combination with KCl, to achieve salt reduction with a shorter processing time than standard curing. No changes in pH, moisture and water activity of the meat were observed. Quality parameters such as tenderness and lipid oxidation were not affected by the use of ultrasound. Further development of this technology could provide insights on the use of ultrasound processing to reduce processing time for meat brining. In addition, in support of other studies demonstrating the use of ultrasound technology for liquid decontamination, US processing significantly reduced the number of E. coli cells present in the brine after meat processing, but was not successful at reducing the microbial load on the meat surface. In this regard, we suggest the role of US as a hurdle technology to prevent possible cross-contamination in brining tanks during meat processing, increasing the shelf-life of the brine solution, and thereby preventing unnecessary economic and processing losses. However, future sensory studies are needed to examine the acceptability of US processed meat as a tool to assist sodium reduction strategies, without compromising product palatability and consumer acceptance.
Author Contributions
Conceptualization, E.S.I., B.K.T. and C.M.B.; methodology, E.S.I.; formal analysis, E.S.I. and D.G.; writing—original draft preparation, D.G., E.S.I.; writing—review and editing, E.S.I., D.G., J.P.K., B.K.T. and C.M.B. All authors have read and agreed to the published version of the manuscript.
Funding
The support of the Teagasc Walsh Fellowship Programme is acknowledged.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Inguglia, E.S.; Zhang, Z.; Tiwari, B.K.; Kerry, J.P.; Burgess, C.M. Salt reduction strategies in processed meat products—A review. Trends Food Sci. Technol. 2017, 59, 70–78. [Google Scholar] [CrossRef]
- Ruusunen, M.; Puolanne, E. Reducing sodium intake from meat products. Meat Sci. 2005, 70, 531–541. [Google Scholar] [CrossRef]
- Desmond, E. Reducing salt: A challenge for the meat industry. Meat Sci. 2006, 74, 188–196. [Google Scholar] [CrossRef]
- Terrell, R.N. Reducing the sodium content of processed meats. Food Technol. 1983, 37, 66–71. [Google Scholar]
- Taormina, P.J. Implications of Salt and Sodium Reduction on Microbial Food Safety. Crit. Rev. Food Sci. Nutr. 2011, 51, 477. [Google Scholar] [CrossRef] [PubMed]
- Israr, T.; Rakha, A.; Sohail, M.; Rashid, S.; Shehzad, A. Salt reduction in baked products: Strategies and constraints. Trends Food Sci. Technol. 2016, 51, 98–105. [Google Scholar] [CrossRef]
- Grummer, J.; Bobowski, N.; Karalus, M.; Vickers, Z.; Schoenfuss, T. Use of potassium chloride and flavor enhancers in low sodium Cheddar cheese. J. Dairy Sci. 2013, 96, 1401–1418. [Google Scholar] [CrossRef] [PubMed]
- Bidlas, E.; Lambert, R.J.W. Comparing the antimicrobial effectiveness of NaCl and KCl with a view to salt/sodium replacement. Int. J. Food Microbiol. 2008, 124, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Baer, A.A.; Miller, M.J.; Dilger, A.C. Pathogens of interest to the pork industry: A review of Research on interventions to assure food safety. Compr. Rev. Food Sci. Food Saf. 2013, 12, 183–217. [Google Scholar] [CrossRef]
- Alarcon-Rojo, A.D.; Carrillo-Lopez, L.; Villagrana, R.A.R.; Huerta-Jimenez, M.; Garcia-Galicia, I.A. Ultrasound and meat quality: A review. Ultrason. Sonochem. 2019, 55, 369–382. [Google Scholar] [CrossRef]
- Pinton, M.B.; Dos Santos, B.A.; Lorenzo, J.M.; Cichoski, A.J.; Boeira, C.P.; Campagnol, P.C.B. Green technologies as a strategy to reduce NaCl and phosphate in meat products: An overview. Curr. Opin. Food Sci. 2020, 40, 1–5. [Google Scholar] [CrossRef]
- González-González, L.; Luna-Rodríguez, L.; Carrillo-Lopez, L.M.; Alarcón-Rojo, A.D.; García-Galicia, I.; Villagrana, R.A.R. Ultrasound as an alternative to conventional marination: Acceptability and mass transfer. J. Food Qual. 2017, 2017, 1–8. [Google Scholar] [CrossRef]
- Alarcon-Rojo, A.D.; Janacua, H.; Rodriguez, J.; Paniwnyk, L.; Mason, T.J. Power ultrasound in meat processing. Meat Sci. 2015, 107, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Inguglia, E.S.; Zhang, Z.; Burgess, C.M.; Kerry, J.P.; Tiwari, B.K. Influence of extrinsic operational parameters on salt diffusion during ultrasound assisted meat curing. Ultrasonics 2018, 83, 164–170. [Google Scholar] [CrossRef]
- Troy, D.J.; Ojha, K.S.; Kerry, J.P.; Tiwari, B.K. Sustainable and consumer-friendly emerging technologies for application within the meat industry: An. overview. Meat Sci. 2016, 120, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Hemar, Y.; AshokKumar, M.; Paturel, S.; Lewis, G.D. Inactivation of bacteria and yeast using high-frequency ultrasound treatment. Water Res. 2014, 60, 93–104. [Google Scholar] [CrossRef]
- Inguglia, E.S.; Tiwari, B.K.; Kerry, J.P.; Burgess, C.M. Effects of high intensity ultrasound on the inactivation profiles of Escherichia coli K12 and Listeria innocua with salt and salt replacers. Ultrason Sonochem. 2018, 48, 492–498. [Google Scholar] [CrossRef]
- Kang, D.; Jiang, Y.; Xing, L.; Zhou, G.; Zhang, W. Inactivation of Escherichia coli O157:H7 and Bacillus cereus by power ultrasound during the curing processing in brining liquid and beef. Food Res. Int. 2017, 102, 717–727. [Google Scholar] [CrossRef]
- Awad, T.; Moharram, H.; E Shaltout, O.; Asker, D.; Youssef, M. Applications of ultrasound in analysis, processing and quality control of food: A review. Food Res. Int. 2012, 48, 410–427. [Google Scholar] [CrossRef]
- Hu, M.; Gurtler, J.B. Selection of surrogate bacteria for use in food safety challenge studies: A review. J. Food Prot. 2017, 80, 1506–1536. [Google Scholar] [CrossRef]
- Botsoglou, N.A.; Fletouris, D.J.; Papageorgiou, G.E.; Vassilopoulos, V.N.; Mantis, A.J.; Trakatellis, A.G. Rapid, sensitive, and specific thiobarbituric acid method for measuring lipid peroxidation in animal tissue, food, and feedstuff samples. J. Agric. Food Chem. 1994, 42, 1931–1937. [Google Scholar] [CrossRef]
- Granato, D.; Santos, J.S.; Escher, G.B.; Ferreira, B.L.; Maggio, R.M. Use of principal component analysis (PCA) and hierarchical cluster analysis (HCA) for multivariate association between bioactive compounds and functional properties in foods: A critical perspective. Trends Food Sci. Technol. 2018, 72, 83–90. [Google Scholar] [CrossRef]
- Stanley, R.E.; Bower, C.G.; Sullivan, G.A. Influence of sodium chloride reduction and replacement with potassium chloride based salts on the sensory and physico-chemical characteristics of pork sausage patties. Meat Sci. 2017, 133, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Got, F.; Culioli, J.; Berge, P.; Vignon, X.; Astruc, T.; Quideau, J.; Lethiecq, M. Effects of high-intensity high-frequency ultrasound on ageing rate, ultrastructure and some physico-chemical properties of beef. Meat Sci. 1999, 51, 35–42. [Google Scholar] [CrossRef]
- Stadnik, J.; Dolatowski, Z.J. Influence of sonication on Warner-Bratzler shear force, colour and myoglobin of beef (m. semimembranosus). Eur. Food Res. Technol. 2011, 233, 553–559. [Google Scholar] [CrossRef]
- Stadnik, J.; Dolatowski, Z.J.; Baranowska, H.M. Effect of ultrasound treatment on water holding properties and microstructure of beef (m. semimembranosus) during ageing. LWT Food Sci. Tehnol. 2008, 41, 2151–2158. [Google Scholar] [CrossRef]
- Pérez-Andrés, J.M.; Charoux, C.M.G.; Cullen, P.; Tiwari, B.K.; Pérez, J. Chemical Modifications of Lipids and Proteins by Nonthermal Food Processing Technologies. J. Agric. Food Chem. 2018, 66, 5041–5054. [Google Scholar] [CrossRef]
- Pingret, D.; Tixier, A.-S.; Chemat, F. Degradation during application of ultrasound in food processing: A review. Food Control. 2013, 31, 593–606. [Google Scholar] [CrossRef]
- Kang, D.-C.; Zou, Y.-H.; Cheng, Y.-P.; Xing, L.-J.; Zhou, G.; Zhang, W. Effects of power ultrasound on oxidation and structure of beef proteins during curing processing. Ultrason Sonochem. 2016, 33, 47–53. [Google Scholar] [CrossRef]
- Chang, H.C.; Wong, R.X. Textural and biochemical properties of cobia (Rachycentron canadum) sashimi tenderised with the ultrasonic water bath. Food Chem. 2012, 132, 1340–1345. [Google Scholar] [CrossRef]
- Pinton, M.B.; Correa, L.P.; Facchi, M.M.X.; Heck, R.T.; Leães, Y.S.V.; Cichoski, A.J.; Lorenzo, J.M.; Dos Santos, M.; Pollonio, M.A.R.; Campagnol, P.C.B.; et al. Ultrasound: A new approach to reduce phosphate content of meat emulsions. Meat Sci. 2019, 152, 88–95. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, C.K.; Lyng, J.G.; Allen, P. The use of power ultrasound for accelerating the curing of pork. Meat Sci. 2014, 98, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.H.; Wang, S.T.; Ockerman, H.W. Lipid oxidation and color change of salted pork patties. Meat Sci. 2007, 75, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Pohlman, F.; Dikeman, M.; Kropf, D. Effects of high intensity ultrasound treatment, storage time and cooking method on shear, sensory, instrumental color and cooking properties of packaged and unpackaged beef pectoralis muscle. Meat Sci. 1997, 46, 89–100. [Google Scholar] [CrossRef]
- Sikes, A.L.; Mawson, R.; Stark, J.; Warner, R. Quality properties of pre- and post-rigor beef muscle after interventions with high frequency ultrasound. Ultrason Sonochem. 2014, 21, 2138–2143. [Google Scholar] [CrossRef]
- Carrillo-Lopez, L.M.; Huerta-Jimenez, M.; Garcia-Galicia, I.A.; Alarcon-Rojo, A.D. Bacterial control and structural and physicochemical modification of bovine Longissimus dorsi by ultrasound. Ultrason Sonochem. 2019, 58, 104608. [Google Scholar] [CrossRef]
- Cserhalmi, Z.; Sass-Kiss, Á.; Tóth-Markus, M.; Lechner, N. Study of pulsed electric field treated citrus juices. Innov. Food Sci. Emerg. Technol. 2006, 7, 49–54. [Google Scholar] [CrossRef]
- Jayasooriya, S.D.; Bhandari, B.R.; Torley, P.; D’Arcy, B.R. Effect of high power ultrasound waves on properties of meat: A review. Int. J. Food Prop. 2004, 7, 301–319. [Google Scholar] [CrossRef]
- Peña-Gonzalez, E.; Alarcón-Rojo, A.D.; Rentería, A.; García, I.; Santellano, E.; Quintero, A.; Luna, L. Quality and sensory profile of ultrasound-treated beef. Ital. J. Food Sci. 2017, 29. [Google Scholar] [CrossRef]
- Alves, L.D.L.; Da Silva, M.S.; Flores, D.R.M.; Athayde, D.R.; Ruviaro, A.R.; Brum, D.D.S.; Batista, V.S.F.; Mello, R.D.O.; De Menezes, C.R.; Campagnol, P.C.B.; et al. Effect of ultrasound on the physicochemical and microbiological characteristics of Italian salami. Food Res. Int. 2018, 106, 363–373. [Google Scholar] [CrossRef]
- Lyng, J.G.; Allen, P.; McKenna, B. The influence of high intensity ultrasound baths on aspects of beef tenderness. J. Muscle Foods 1997, 8, 237–249. [Google Scholar] [CrossRef]
- Kang, D.C.; Gao, X.Q.; Ge, Q.; Zhou, G.; Zhang, W. Effects of ultrasound on the beef structure and water distribution during curing through protein degradation and modification. Ultrason Sonochem. 2017, 38, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Ojha, K.S.; Kerry, J.P.; Alvarez, C.; Walsh, D.; Tiwari, B.K. Effect of high intensity ultrasound on the fermentation profile of Lactobacillus sakei in a meat model system. Ultrason Sonochem. 2016, 31, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.M.P.; Lee, Y.K.; Zhou, W. Effect of high intensity ultrasound on carbohydrate metabolism of bifidobacteria in milk fermentation. Food Chem. 2012, 130, 866–874. [Google Scholar] [CrossRef]
- Piyasena, P.; Mohareb, E.; McKellar, R. Inactivation of microbes using ultrasound: A review. Int. J. Food Microbiol. 2003, 87, 207–216. [Google Scholar] [CrossRef]
- Pohlman, F.; Dikeman, M.; Zayas, J. The effect of low-intensity ultrasound treatment on shear properties, color stability and shelf-life of vacuum-packaged beef semitendinosus and biceps femoris muscles. Meat Sci. 1997, 45, 329–337. [Google Scholar] [CrossRef]
- Boziaris, I.; Skandamis, P.; Anastasiadi, M.; Nychas, G.-J.E. Effect of NaCl and KCl on fate and growth/no growth interfaces of Listeria monocytogenes Scott A at different pH and nisin concentrations. J. Appl. Microbiol. 2007, 102, 796–805. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hazards. Growth of spoilage bacteria during storage and transport of meat. EFSA J. 2016, 14, 04523.
- Pitt, W.G.; Ross, S.A. Ultrasound Increases the rate of bacterial cell growth. Biotechnol. Prog. 2003, 19, 1038–1044. [Google Scholar] [CrossRef]
- Diaz-Almanza, S.; Reyes-Villagrana, R.; Alarcon-Rojo, A.D.; Huerta-Jimenez, M.; Carrillo-Lopez, L.M.; Estepp, C.; Urbina-Perez, J.; Garcia-Galicia, I.A. Time matters when ultrasonicating beef: The best time for tenderness is not the best for reducing microbial counts. J. Food Process. Eng. 2019, 42, 13210. [Google Scholar] [CrossRef]
- Greer, G.G.; Nattress, F.; Dilts, B.; Baker, L. Bacterial Contamination of recirculating brine used in the commercial production of moisture-enhanced pork. J. Food Prot. 2004, 67, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Jambrak, A.R.; Herceg, Z. Application of ultrasonics in food preservation and processing. In Conventional and Advanced Food Processing Technologies; Bhattacharya, S., Ed.; Wiley: Hoboken, NJ, USA, 2014; pp. 515–536. [Google Scholar]
- Monsen, T.; Lövgren, E.; Widerström, M.; Wallinder, L. In vitro effect of ultrasound on bacteria and suggested protocol for sonication and diagnosis of prosthetic infections. J. Clin. Microbiol. 2009, 47, 2496–2501. [Google Scholar] [CrossRef] [PubMed]
- Faleiro, M.L.; Andrew, P.W.; Power, D.M. Stress response of Listeria monocytogenes isolated from cheese and other foods. Int. J. Food Microbiol. 2003, 84, 207–216. [Google Scholar] [CrossRef]
- Pennisi, L.; Di Clerico, D.; Costantini, L.; Festino, A.R.; Vergara, A. Ultrasonic decontamination in smoked salmon experimentally contaminated with Listeria monocytogenes: Preliminary results. Ital. J. Food Saf. 2020, 9, 8398. [Google Scholar] [CrossRef] [PubMed]
- Baumann, A.R.; Martin, S.E.; Feng, H. Power ultrasound treatment of Listeria monocytogenes in apple cider. J. Food Prot. 2005, 68, 2333–2340. [Google Scholar] [CrossRef]
- Dolan, H.L.; Bastarrachea, L.J.; Tikekar, R.V. Inactivation of Listeria innocua by a combined treatment of low-frequency ultrasound and zinc oxide. LWT 2018, 88, 146–151. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).