Online Database for Retrieval Information about Prebiotics and Their Activity
Abstract
1. Introduction
2. Materials and Methods
3. Results
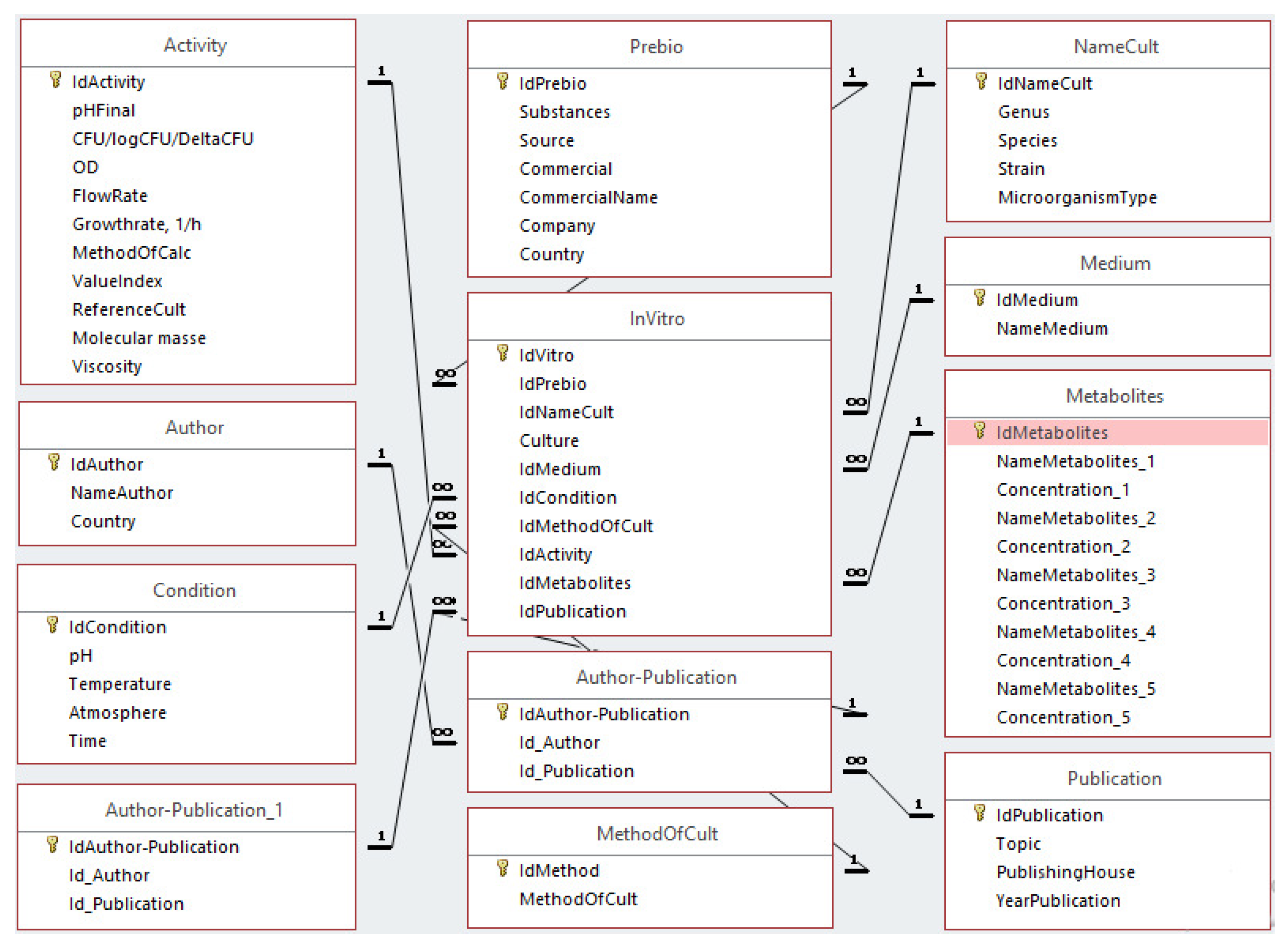
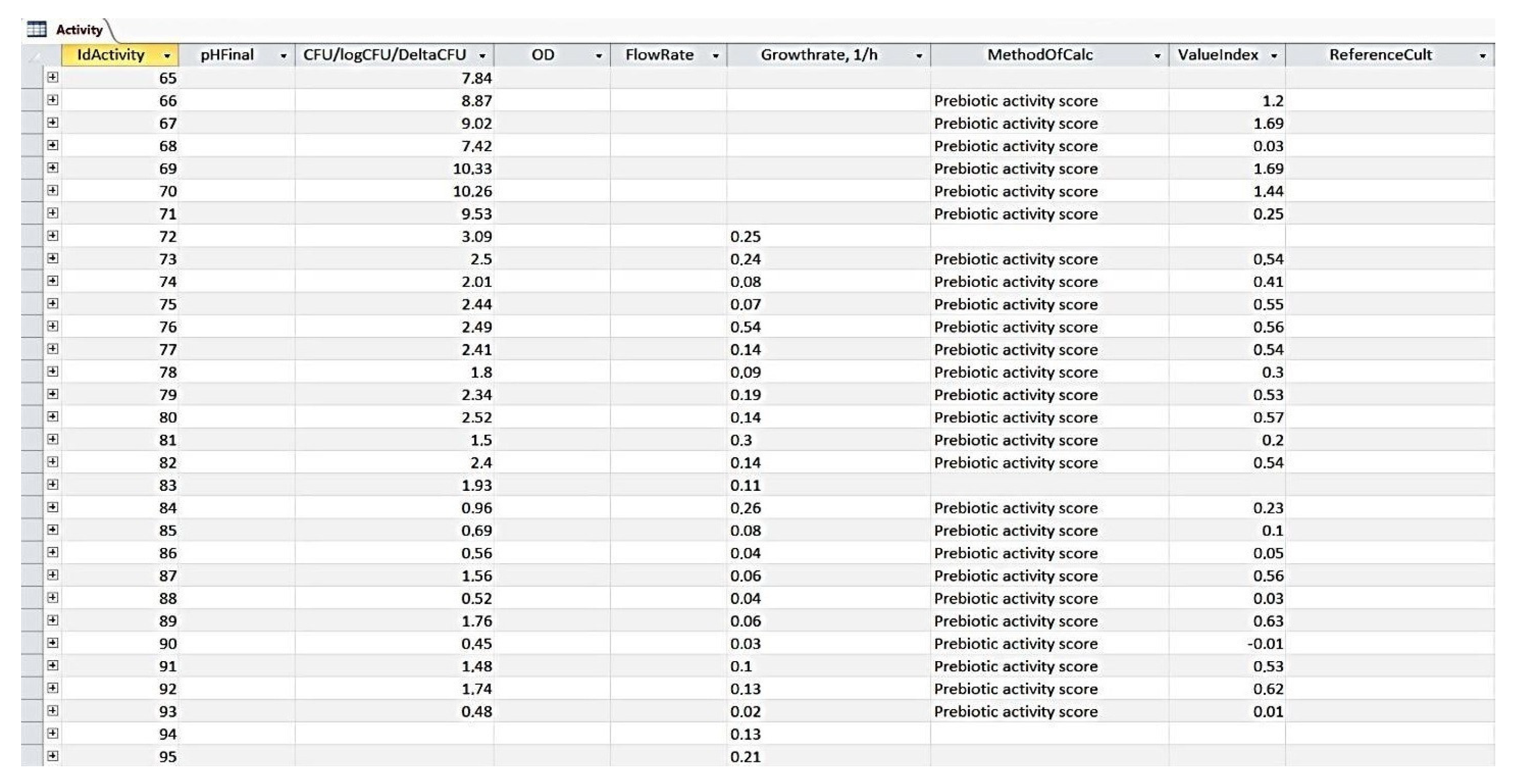
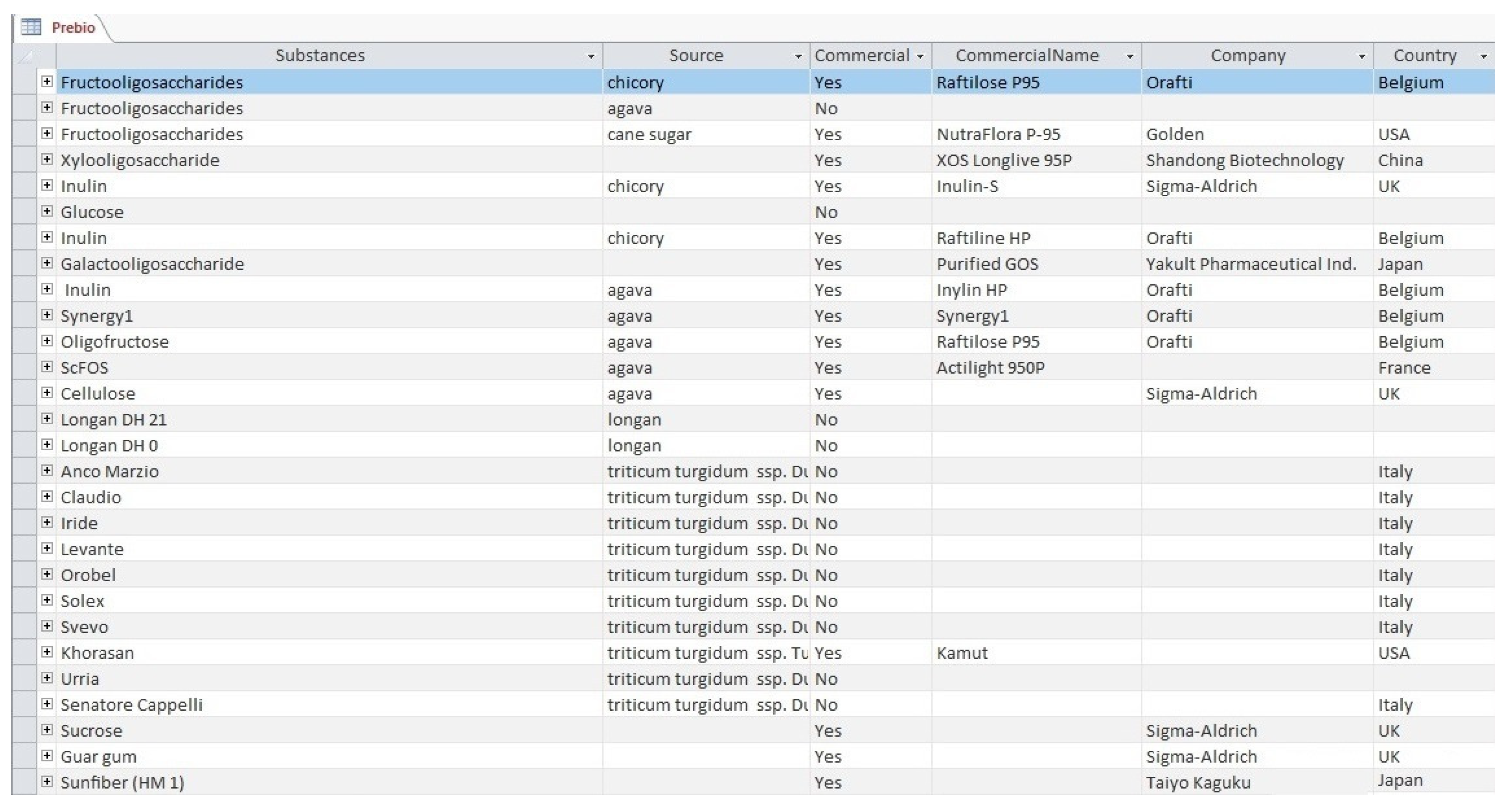
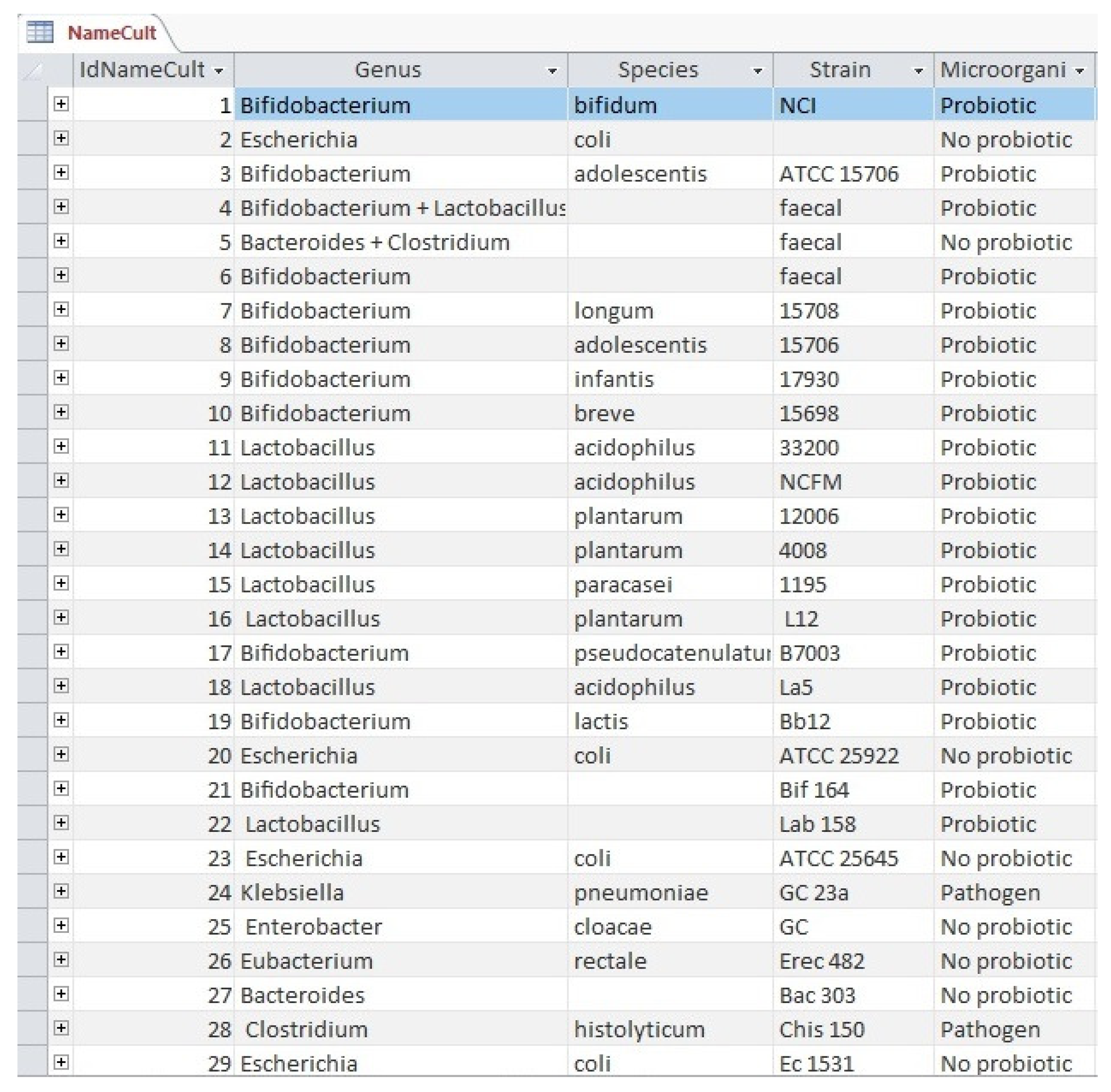
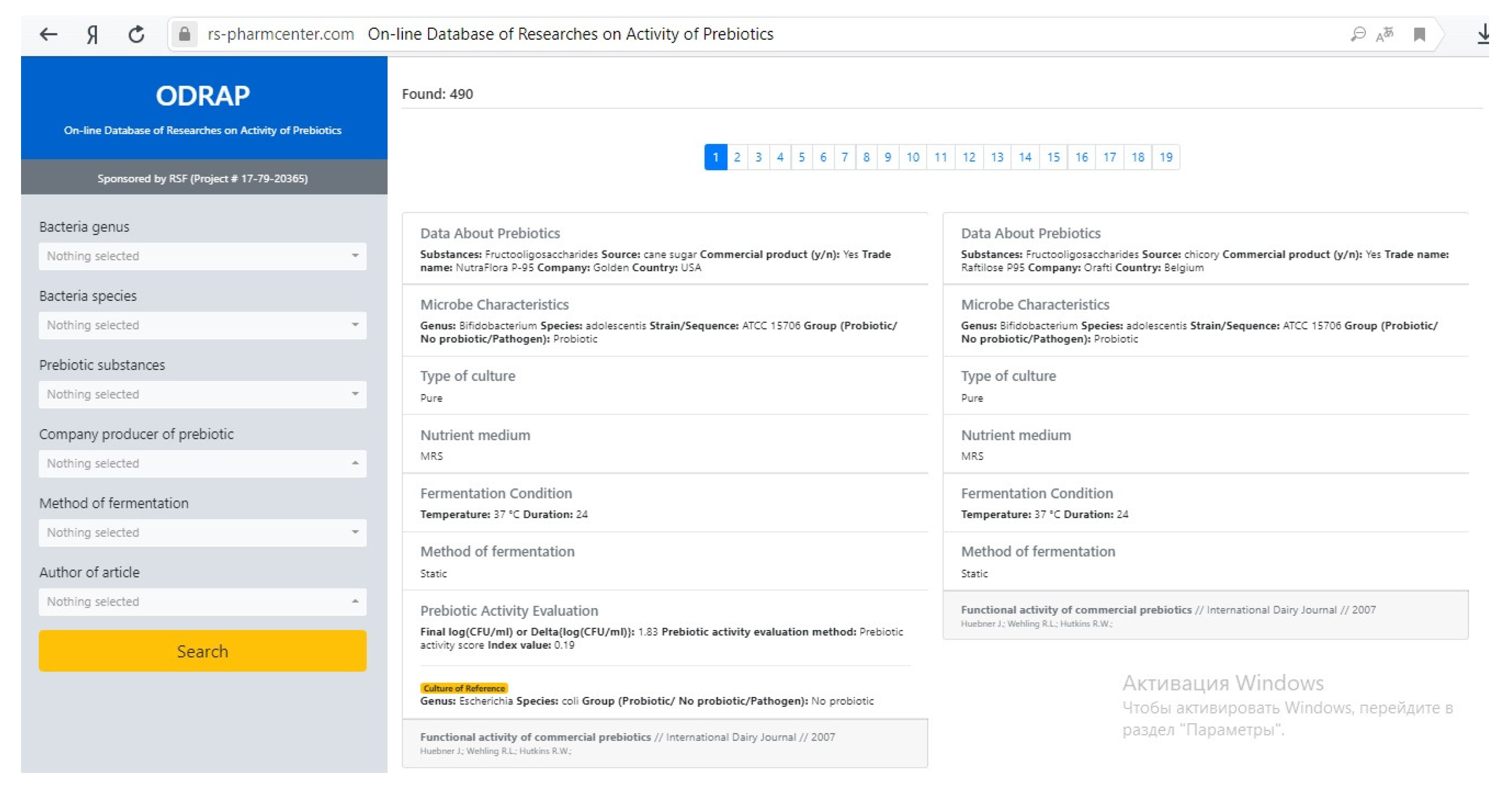
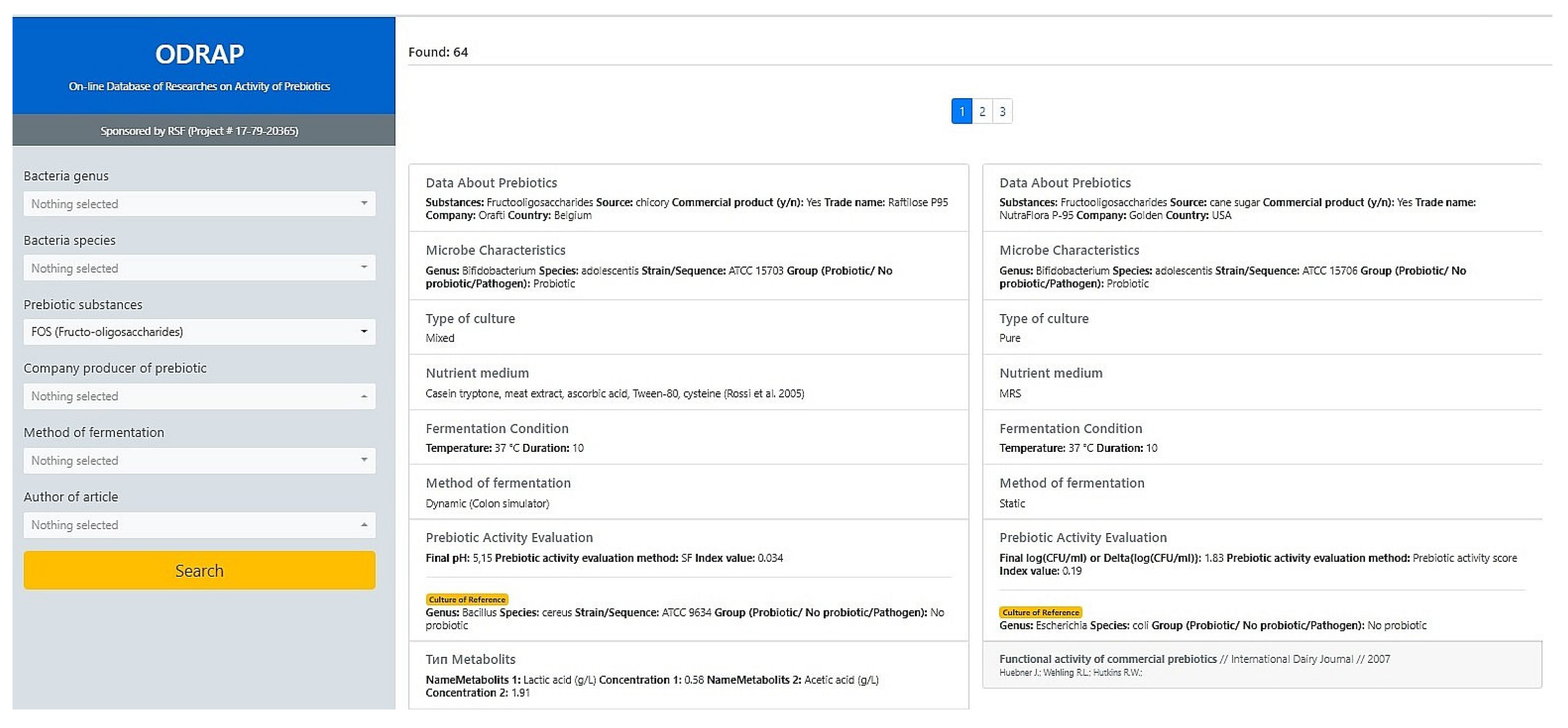
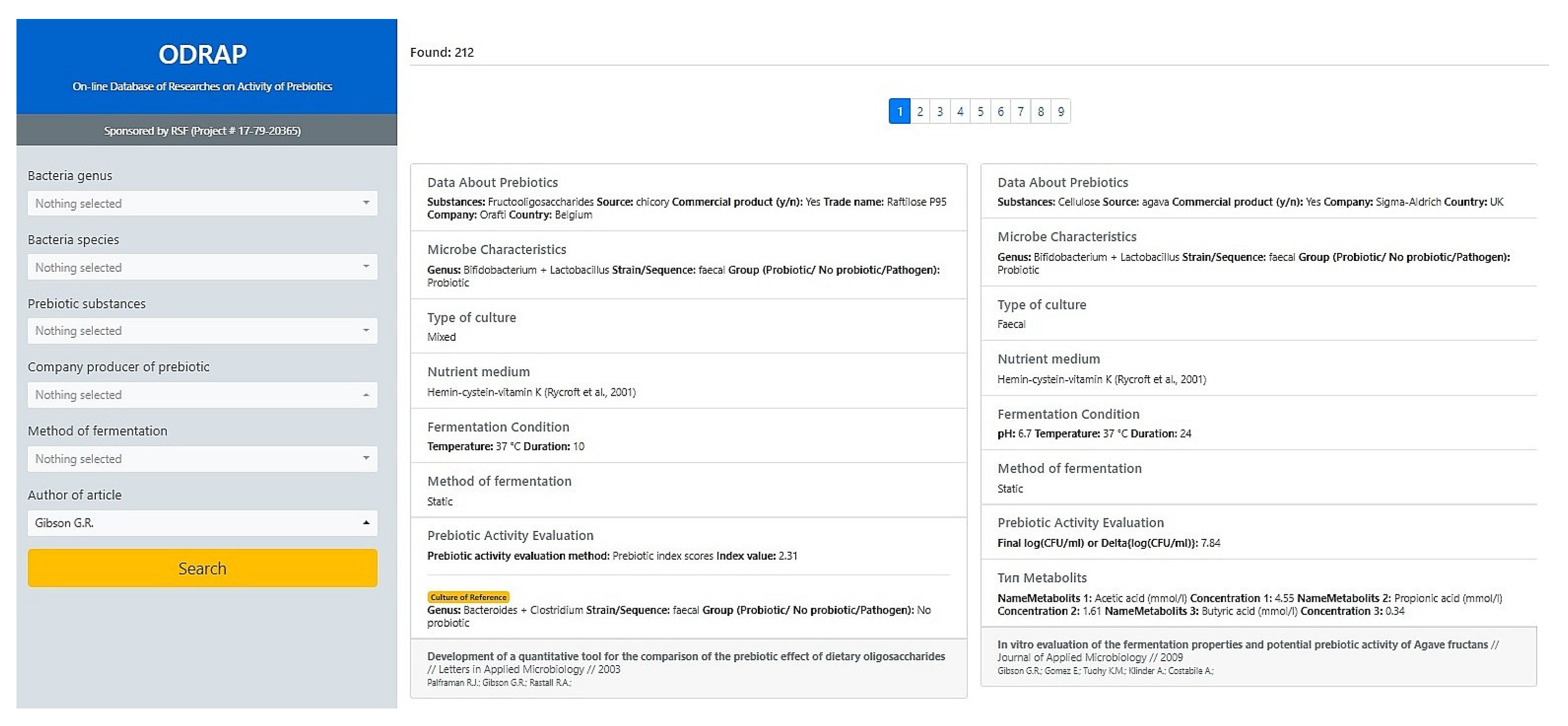
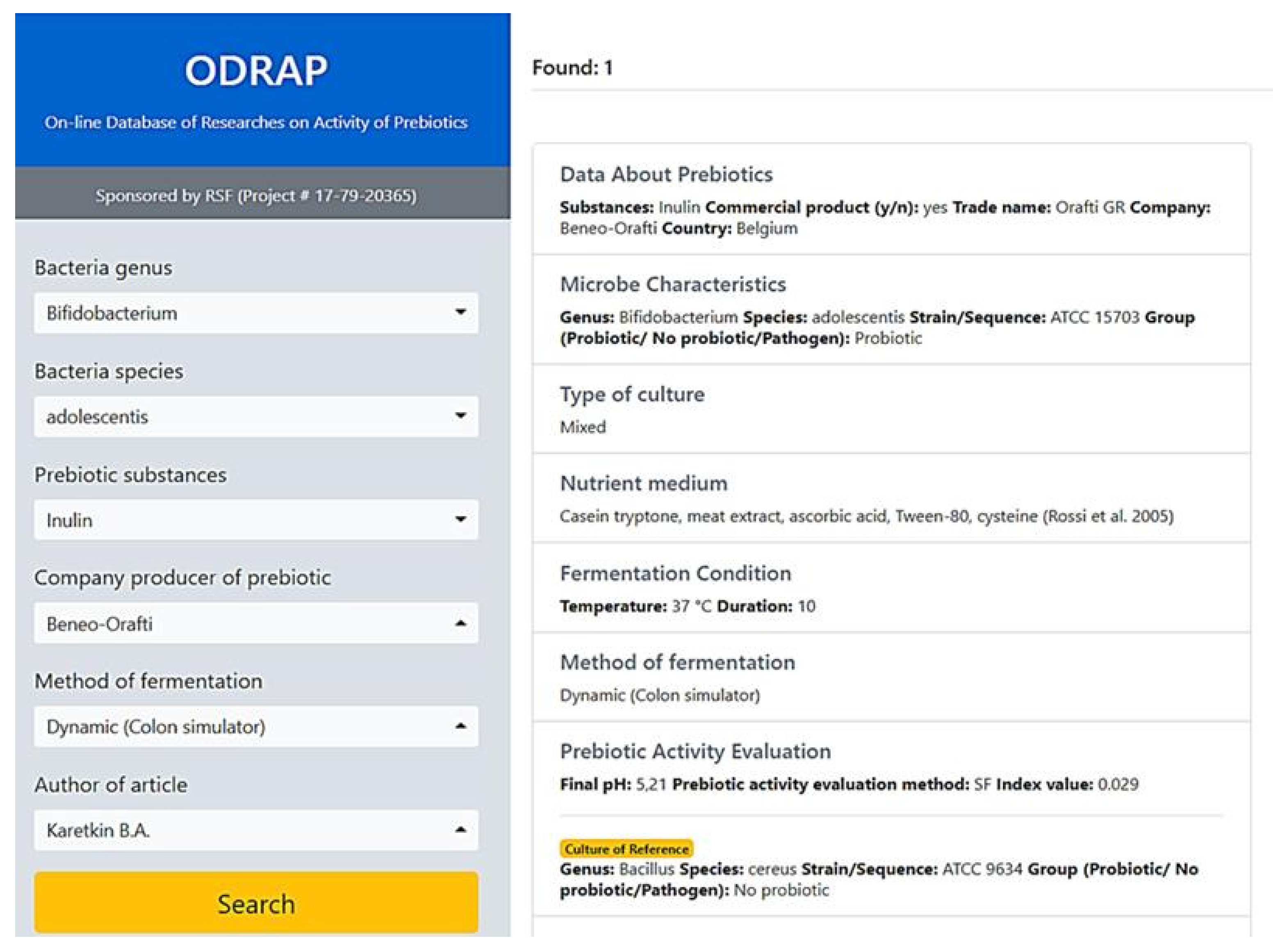
3.1. The ODRAP Database
3.2. The Web-Interface
- bacteria genus;
- bacteria species;
- prebiotic substances;
- company producer of prebiotic;
- method of fermentation; and
- author of article.
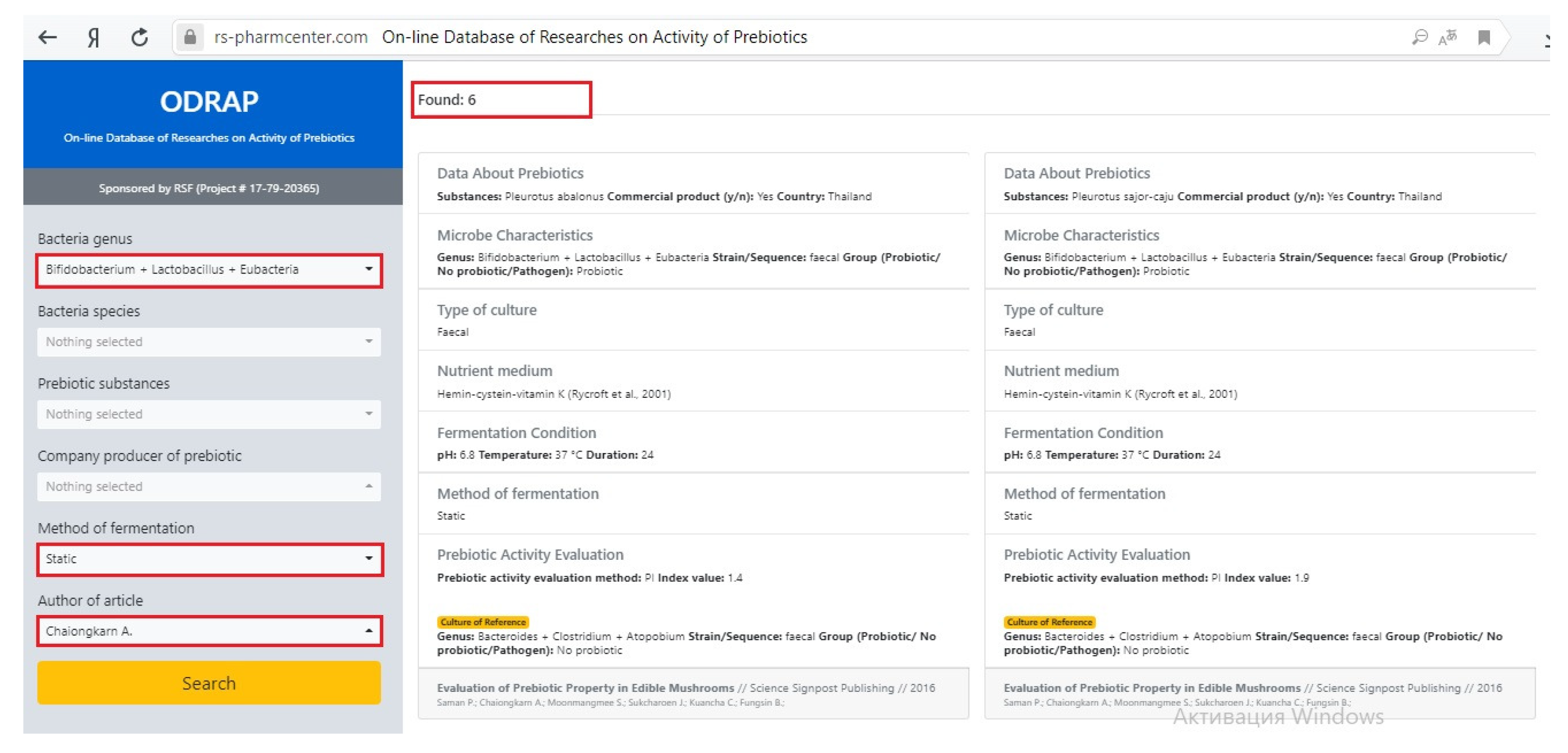
- bacteria genus: Bifidobacterium+Lactobacillus+Eubacteria;
- author of article: Chaiongharn A.;
- method of fermentation: Static.
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| PAS | Prebiotic activity score |
| MPE | Measure of the prebiotic effect |
| PI | Prebiotic Index |
| SI | Synbiotic index |
| SF | Synbiotic factor |
References
- Enam, F.; Mansell, T.J. Prebiotics: Tools to manipulate the gut microbiome and metabolome. J. Ind. Microbiol. Biotechnol. 2019, 46, 1445–1459. [Google Scholar] [CrossRef] [PubMed]
- Tuohy, K.M.; Probert, H.M.; Smejkal, C.W.; Gibson, G.R. Using probiotics and prebiotics to improve gut health. Drug Discov. Today 2003, 8, 692–700. [Google Scholar] [CrossRef]
- Bindels, L.B.; Delzenne, N.M.; Cani, P.D.; Walter, J. Towards a more comprehensive concept for prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 303. [Google Scholar] [CrossRef] [PubMed]
- Chaluvadi, S.; Hotchkiss, A.T.; Yam, K.L. Gut Microbiota. In Probiotics, Prebiotics, and Synbiotics; Elsevier: Amsterdam, The Netherlands, 2016; pp. 515–523. [Google Scholar] [CrossRef]
- Shokrvash, B.; Homayouni, A.; Payahoo, L.; Biglu, M.H.; Mehrabany, E.V.; Jafarabadi, M.A. Probiotics and Health. In Probiotics, Prebiotics, and Synbiotics; Elsevier: Amsterdam, The Netherlands, 2016; pp. 691–698. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef]
- Bello, M.G.D.; Knight, R.; Gilbert, J.A.; Blaser, M.J. Preserving microbial diversity. Science 2018, 362, 33–34. [Google Scholar] [CrossRef]
- Diminic, J.; Zucko, J.; Ruzic, I.T.; Gacesa, R.; Hranueli, D.; Long, P.F.; Cullum, J.; Starcevic, A. Databases of the thiotemplate modular systems (CSDB) and their in silico recombinants (r-CSDB). J. Ind. Microbiol. Biotechnol. 2013, 40, 653–659. [Google Scholar] [CrossRef]
- Kosina, S.M.; Greiner, A.M.; Lau, R.K.; Jenkins, S.; Baran, R.; Bowen, B.P.; Northen, T.R. Web of microbes (WoM): A curated microbial exometabolomics database for linking chemistry and microbes. BMC Microbiol. 2018, 18, 1–10. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, L.; Wu, L.; Li, W.; Liu, Q.; Zhang, J.; Liu, D.; Ma, J. Web resources for microbial data. Genom. Proteom. Bioinform. 2015, 13, 69–72. [Google Scholar] [CrossRef]
- Microbesonline. Technical Report. Available online: http://meta.microbesonline.org/ (accessed on 8 April 2020).
- Venkataramana, P. Online Databases and Other Internet Resources for Earth Science; Chandos Information Professional Series; Elsevier Science: Amsterdam, The Netherlands, 2007. [Google Scholar]
- What Is the Key Best Practice for Collaborating with a Computational Biologist? Cell Syst. 2016, 3, 7–11. [CrossRef]
- Tao, L.; Wang, B.; Zhong, Y.; Pow, S.H.; Zeng, X.; Qin, C.; Zhang, P.; Chen, S.; He, W.; Tan, Y.; et al. Database and bioinformatics studies of probiotics. J. Agric. Food Chem. 2017, 65, 7599–7606. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, Y.; Latta, M.; Ma, W.; Wu, Z.; Chen, P. Probiotics database: A potential source of fermented foods. Int. J. Food Prop. 2019, 22, 198–217. [Google Scholar] [CrossRef]
- Probiotics Database. Technical Report. Available online: http://www.optibacprobiotics.com/uk/professionals/probiotics-database (accessed on 8 April 2020).
- Prebioticassociation. Technical report. Available online: http://prebioticassociation.org/ (accessed on 8 April 2020).
- Wang, X.; Gibson, G. Effects of the in vitro fermentation of oligofructose and inulin by bacteria growing in the human large intestine. J. Appl. Bacteriol. 1993, 75, 373–380. [Google Scholar] [CrossRef]
- Mao, Y.H.; Song, A.X.; Li, L.Q.; Siu, K.C.; Yao, Z.P.; Wu, J.Y. Effects of exopolysaccharide fractions with different molecular weights and compositions on fecal microflora during in vitro fermentation. Int. J. Biol. Macromol. 2020, 144, 76–84. [Google Scholar] [CrossRef]
- Amorim, C.; Silvério, S.C.; Cardoso, B.B.; Alves, J.I.; Pereira, M.A.; Rodrigues, L.R. In vitro assessment of prebiotic properties of xylooligosaccharides produced by Bacillus subtilis 3610. Carbohydr. Polym. 2020, 229, 115460. [Google Scholar] [CrossRef]
- Charoensiddhi, S.; Conlon, M.A.; Vuaran, M.S.; Franco, C.M.; Zhang, W. Impact of extraction processes on prebiotic potential of the brown seaweed Ecklonia radiata by in vitro human gut bacteria fermentation. J. Funct. Foods 2016, 24, 221–230. [Google Scholar] [CrossRef]
- Palframan, R.; Gibson, G.; Rastall, R. Development of a quantitative tool for the comparison of the prebiotic effect of dietary oligosaccharides. Lett. Appl. Microbiol. 2003, 37, 281–284. [Google Scholar] [CrossRef]
- Vulevic, J.; Rastall, R.A.; Gibson, G.R. Developing a quantitative approach for determining the in vitro prebiotic potential of dietary oligosaccharides. FEMS Microbiol. Lett. 2004, 236, 153–159. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, J.; Yan, Q.; You, X.; Yang, S.; Jiang, Z. In vitro digestibility and prebiotic potential of curdlan (1→ 3)-β-d-glucan oligosaccharides in Lactobacillus species. Carbohydr. Polym. 2018, 188, 17–26. [Google Scholar] [CrossRef]
- Ariestanti, C.A.; Seechamnanturakit, V.; Harmayani, E.; Wichienchot, S. Optimization on production of konjac oligo-glucomannan and their effect on the gut microbiota. Food Sci. Nutr. 2019, 7, 788–796. [Google Scholar] [CrossRef]
- Huebner, J.; Wehling, R.; Hutkins, R.W. Functional activity of commercial prebiotics. Int. Dairy J. 2007, 17, 770–775. [Google Scholar] [CrossRef]
- De Melo, F.H.C.; Menezes, F.N.D.D.; de Sousa, J.M.B.; dos Santos Lima, M.; da Silva Campelo Borges, G.; de Souza, E.L.; Magnani, M. Prebiotic activity of monofloral honeys produced by stingless bees in the semi-arid region of Brazilian Northeastern toward Lactobacillus acidophilus LA-05 and Bifidobacterium lactis BB-12. Food Res. Int. 2020, 128, 108809. [Google Scholar] [CrossRef]
- Praveen, M.A.; Parvathy, K.K.; Jayabalan, R.; Balasubramanian, P. Dietary fiber from Indian edible seaweeds and its in-vitro prebiotic effect on the gut microbiota. Food Hydrocoll. 2019, 96, 343–353. [Google Scholar] [CrossRef]
- NithyaBalaSundari, S.; Nivedita, V.; Chakravarthy, M.; Srisowmeya, G.; Usha, A.; Nandhini, D.G. Characterization of microbial polysaccharides and prebiotic enrichment of wheat bread with pullulan. LWT Food Sci. Technol. 2020, 109002. [Google Scholar] [CrossRef]
- Shalini, R.; Abinaya, G.; Saranya, P.; Antony, U. Growth of selected probiotic bacterial strains with fructans from Nendran banana and garlic. LWT Food Sci. Technol. 2017, 83, 68–78. [Google Scholar] [CrossRef]
- Macfarlane, G.; Macfarlane, S.; Gibson, G. Validation of a three-stage compound continuous culture system for investigating the effect of retention time on the ecology and metabolism of bacteria in the human colon. Microb. Ecol. 1998, 35, 180–187. [Google Scholar] [CrossRef]
- Grimaldi, R.; Cela, D.; Swann, J.R.; Vulevic, J.; Gibson, G.R.; Tzortzis, G.; Costabile, A. In vitro fermentation of B-GOS: Impact on faecal bacterial populations and metabolic activity in autistic and non-autistic children. FEMS Microbiol. Ecol. 2017, 93. [Google Scholar] [CrossRef]
- Astó, E.; Méndez, I.; Rodríguez-Prado, M.; Cuñé, J.; Espadaler, J.; Farran-Codina, A. Effect of the Degree of Polymerization of Fructans on Ex Vivo Fermented Human Gut Microbiome. Nutrients 2019, 11, 1293. [Google Scholar] [CrossRef]
- Herrera, P.; O’Bryan, C.; Crandall, P.; Ricke, S. Growth response of Salmonella enterica Typhimurium in co-culture with ruminal bacterium Streptococcus bovis is influenced by time of inoculation and carbohydrate substrate. Food Res. Int. 2012, 45, 1054–1057. [Google Scholar] [CrossRef]
- Likotrafiti, E.; Valavani, P.; Argiriou, A.; Rhoades, J. In vitro evaluation of potential antimicrobial synbiotics using Lactobacillus kefiri isolated from kefir grains. Int. Dairy J. 2015, 45, 23–30. [Google Scholar] [CrossRef]
- Valdés-Varela, L.; Hernández-Barranco, A.M.; Ruas-Madiedo, P.; Gueimonde, M. Effect of Bifidobacterium upon Clostridium difficile growth and toxicity when co-cultured in different prebiotic substrates. Front. Microbiol. 2016, 7, 738. [Google Scholar] [CrossRef]
- Medina, D.A.; Pinto, F.; Ovalle, A.; Thomson, P.; Garrido, D. Prebiotics mediate microbial interactions in a consortium of the infant gut microbiome. Int. J. Mol. Sci. 2017, 18, 2095. [Google Scholar] [CrossRef]
- Karetkin, B.A.; Guseva, E.V.; Evdokimova, S.A.; Mishchenko, A.S.; Khabibulina, N.V.; Grosheva, V.D.; Menshutina, N.V.; Panfilov, V.I. A quantitative model of Bacillus cereus ATCC 9634 growth inhibition by bifidobacteria for synbiotic effect evaluation. World J. Microbiol. Biotechnol. 2019, 35, 89. [Google Scholar] [CrossRef]
- Oude Blenke, E.; Evers, M.J.; Baumann, V.; Winkler, J.; Storm, G.; Mastrobattista, E. Critical evaluation of quantification methods for oligonucleotides formulated in lipid nanoparticles. Int. J. Pharm. 2018, 548, 793–802. [Google Scholar] [CrossRef]
- Gu, J.; Roberts, K. Probiotics and Prebiotics. In Adult Short Bowel Syndrome; Elsevier: Amsterdam, The Netherlands, 2019; pp. 67–80. [Google Scholar] [CrossRef]
- Harris, S.; Powers, S.; Monteagudo-Mera, A.; Kosik, O.; Lovegrove, A.; Shewry, P.; Charalampopoulos, D. Determination of the prebiotic activity of wheat arabinogalactan peptide (AGP) using batch culture fermentation. Eur. J. Nutr. 2020, 59, 297–307. [Google Scholar] [CrossRef]
- Thitiratsakul, B.; Anprung, P. Prebiotic activity score and bioactive compounds in longan (Dimocarpus longan Lour.): Influence of pectinase in enzyme-assisted extraction. J. Food Sci. Technol. 2014, 51, 1947–1955. [Google Scholar] [CrossRef]
- Manisseri, C.; Gudipati, M. Prebiotic activity of purified xylobiose obtained from Ragi (Eleusine coracana, Indaf-15) Bran. Indian J. Microbiol. 2012, 52, 251–257. [Google Scholar] [CrossRef]
- Barengolts, E. Gut microbiota, prebiotics, probiotics, and synbiotics in management of obesity and prediabetes: Review of randomized controlled trials. Endocr. Pract. 2016, 22, 1224–1234. [Google Scholar] [CrossRef]
- Liu, Y.; He, J.; Xu, Q.; Zhang, C.; Chen, N.; Xie, X. Enhanced Adenosine Production by Bacillus subtilis at Condition with Comprehensively Controlled Dissolved Oxygen and pH During Fermentation. In Advances in Applied Biotechnology; Springer: Berlin, Germany, 2015; pp. 439–452. [Google Scholar]
- Patel, A.K.; Singhania, R.R.; Pandey, A. Production, Purification, and Application of Microbial Enzymes. In Biotechnology of Microbial Enzymes; Elsevier: Amsterdam, The Netherlands, 2017; pp. 13–41. [Google Scholar] [CrossRef]
- Connelly, S.; Shin, S.G.; Dillon, R.J.; Ijaz, U.Z.; Quince, C.; Sloan, W.T.; Collins, G. Bioreactor Scalability: Laboratory-Scale Bioreactor Design Influences Performance, Ecology, and Community Physiology in Expanded Granular Sludge Bed Bioreactors. Front. Microbiol. 2017, 8, 664. [Google Scholar] [CrossRef]
- Coletta, A.; Molter, C.; Duqué, R.; Steenhoff, D.; Taminau, J.; de Schaetzen, V.; Lazar, C.; Meganck, S.; Nowé, A.; Bersini, H.; et al. InSilico DB: An online platform to collaboratively structure and export publicly available datasets from the Gene Expression Omnibus database. Genome Biol. 2011, 12, P33. [Google Scholar] [CrossRef]
- Cannone, J.J.; Subramanian, S.; Schnare, M.N.; Collett, J.R.; D’Souza, L.M.; Du, Y.; Feng, B.; Lin, N.; Madabusi, L.V.; Müller, K.M.; et al. The comparative RNA web (CRW) site: An online database of comparative sequence and structure information for ribosomal, intron, and other RNAs. BMC Bioinform. 2002, 3, 2. [Google Scholar]
- Tsai, C.Y.; Huang, W.L. Design and performance modeling of an efficient remote collaboration system. Int. J. Grid Distrib. Comput. 2015, 8, 11–26. [Google Scholar] [CrossRef]
- Ram, B.K.; Kumar, S.A.; Prathap, S.; Mahesh, B.; Sarma, B.M. Remote laboratories: For real time access to experiment setups with online session booking, utilizing a database and online interface with live streaming. In Online Engineering & Internet of Things; Springer: Berlin, Germany, 2018; pp. 190–204. [Google Scholar]
- Tang, B.; Wang, Y.; Zhu, J.; Zhao, W. Web Resources for Model Organism Studies. Genom. Proteom. Bioinform. 2015, 13, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Singh, O.; Chang, N.W.; Dai, H.J.; Jonnagaddala, J. Translational Bioinformatics Databases. In Encyclopedia of Bioinformatics and Computational Biology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1058–1062. [Google Scholar] [CrossRef]
- Herce-Zelaya, J.; Porcel, C.; Bernabé-Moreno, J.; Tejeda-Lorente, L.; Herrera-Viedma, E. Web platform for learning distributed databases’ queries processing. Procedia Comput. Sci. 2019, 162, 827–834. [Google Scholar] [CrossRef]
- Kritikos, K.; Magoutis, K.; Papoutsakis, M.; Ioannidis, S. A survey on vulnerability assessment tools and databases for cloud-based web applications. Array 2019, 3, 100011. [Google Scholar] [CrossRef]
- Vathy-Fogarassy, G.; Hugyák, T. Uniform data access platform for SQL and NoSQL database systems. Inf. Syst. 2017, 69, 93–105. [Google Scholar] [CrossRef]
- Markert, K.N.; Pulla, S.T.; Lee, H.; Markert, A.M.; Anderson, E.R.; Okeowo, M.A.; Limaye, A.S. AltEx: An open source web application and toolkit for accessing and exploring altimetry datasets. Environ. Model. Softw. 2019, 117, 164–175. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guseva, E.; Karetkin, B.; Batyrgazieva, D.; Menshutina, N.; Panfilov, V. Online Database for Retrieval Information about Prebiotics and Their Activity. Appl. Sci. 2020, 10, 3328. https://doi.org/10.3390/app10093328
Guseva E, Karetkin B, Batyrgazieva D, Menshutina N, Panfilov V. Online Database for Retrieval Information about Prebiotics and Their Activity. Applied Sciences. 2020; 10(9):3328. https://doi.org/10.3390/app10093328
Chicago/Turabian StyleGuseva, Elena, Boris Karetkin, Diana Batyrgazieva, Natalia Menshutina, and Victor Panfilov. 2020. "Online Database for Retrieval Information about Prebiotics and Their Activity" Applied Sciences 10, no. 9: 3328. https://doi.org/10.3390/app10093328
APA StyleGuseva, E., Karetkin, B., Batyrgazieva, D., Menshutina, N., & Panfilov, V. (2020). Online Database for Retrieval Information about Prebiotics and Their Activity. Applied Sciences, 10(9), 3328. https://doi.org/10.3390/app10093328






