Bonding Strength Characteristics of FA-Based Geopolymer Paste as a Repair Material When Applied on OPC Substrate
Abstract
1. Introduction
2. Experimental Program and Procedure
2.1. Materials
2.1.1. Mix Proportions of Fly Ash (FA)-Based Geopolymer Paste
2.1.2. Mix Proportion of Ordinary Portland Cement (OPC) Concrete Substrate
2.2. Experimental Testing
2.2.1. Setting Time
2.2.2. Compressive Strength Test
2.2.3. Bonding Test
2.2.4. Phase Characterization
2.2.5. Microstructure Characterization
3. Results and Discussion
3.1. Setting Time of FA-Based Geopolymer Repair Material
3.2. Compressive Strength of FA-Based Geopolymer Repair Material
3.3. Bonding Strength between FA-Based Geopolymer Paste and OPC Concrete Substrate
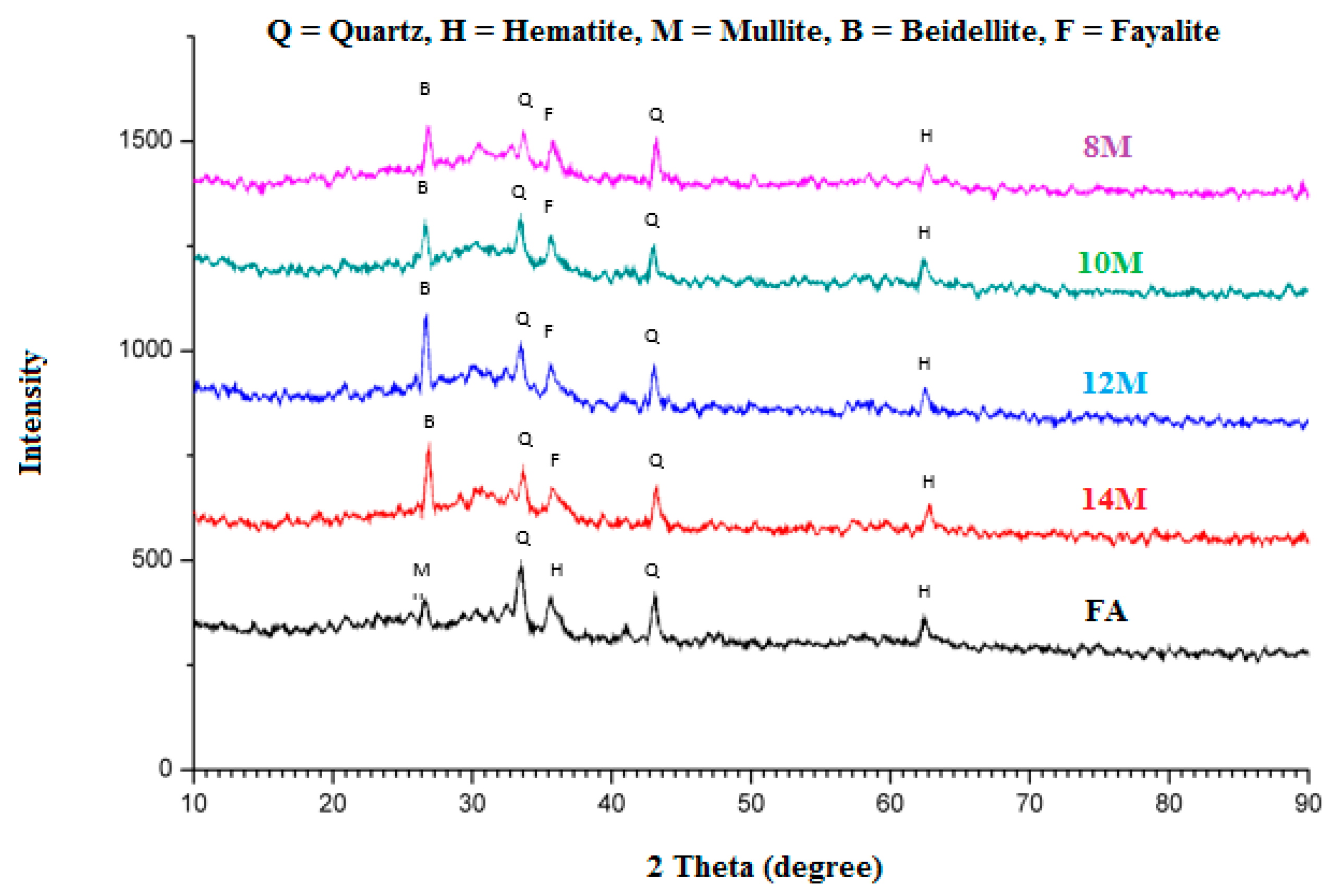
3.4. Phase Analysis
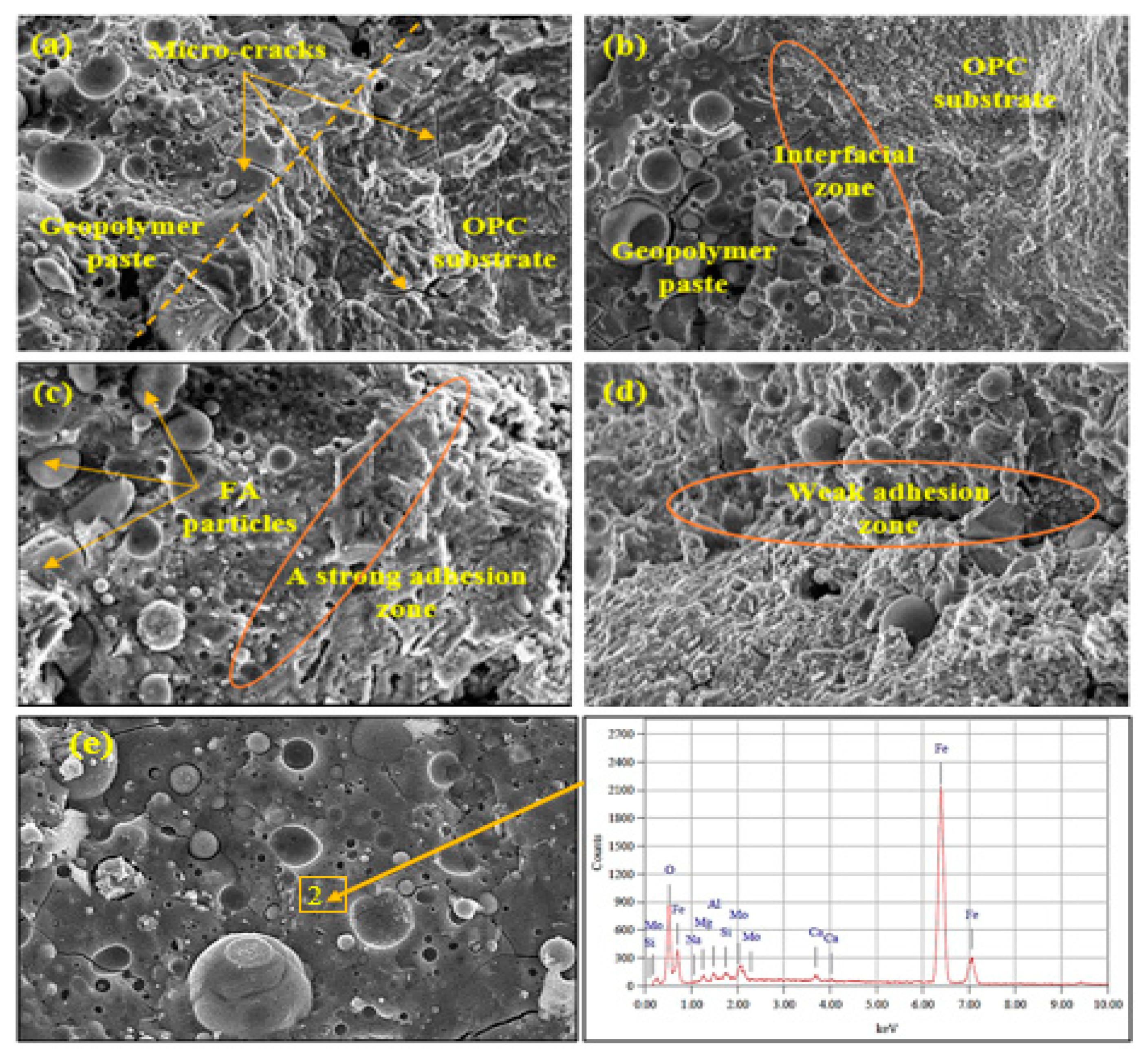
3.5. Scanning Electron Microscope (SEM) Images at the Bonding Zone (BZ) between Geopolymer and Concrete Substrate
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bouaissi, A.; Li, Y.L.; Abdullah, M.M.A.B.; Bui, Q.B. Mechanical properties and microstructure analysis of FA-GGBS-HMNS based geopolymer concrete. Constr. Build. Mater. 2019, 210, 198–209. [Google Scholar] [CrossRef]
- Malhotra, V.M. Introduction: Sustainable development and concrete technology. Concr. Int. 2002, 24, 22. [Google Scholar]
- Davidovits, J. Geopolymers—Inorganic polymeric new materials. J. Therm. Anal. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- Ranjbar, N.; Mehrali, M.; Alengaram, U.J.; Metselaar, H.S.C.; Jumaat, M.Z. Compressive strength and microstructural analysis of fly ash/palm oil fuel ash based geopolymer mortar under elevated temperatures. Constr. Build. Mater. 2014, 65, 114–121. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Castro-Gomes, J.P.; Jalali, S. Adhesion characterization of tungsten mine waste geopolymeric binder. Influence of OPC concrete substrate surface treatment. Constr. Build. Mater. 2008, 22, 154–161. [Google Scholar] [CrossRef]
- Part, W.K.; Ramli, M.; Cheah, C.B. An Overview on the Influence of Various Factors on the Properties of Geopolymer Concrete Derived From Industrial Byproducts. Constr. Build. Mater. 2015, 77, 370–395. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Abdollahnejad, Z.; Miraldo, S.; Baklouti, S.; Ding, Y. An overview on the potential of geopolymers for concrete infrastructure rehabilitation. Constr. Build. Mater. 2012, 36, 1053–1058. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D. Geopolymerisation: A review and prospects for the minerals industry. Miner. Eng. 2007, 20, 1261–1277. [Google Scholar] [CrossRef]
- Provis, J.L.; van Deventer, J.S.J. Geopolymerisation kinetics. 2. Reaction kinetic modelling. Chem. Eng. Sci. 2007, 62, 2318–2329. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A.; Criado, M. Microstructure development of alkali-activated fly ash cement: A descriptive model. Cem. Concr. Res. 2005, 35, 1204–1209. [Google Scholar] [CrossRef]
- Phoo-ngernkham, T.; Maegawa, A.; Mishima, N.; Hatanaka, S.; Chindaprasirt, P. Effects of sodium hydroxide and sodium silicate solutions on compressive and shear bond strengths of FA–GBFS geopolymer. Constr. Build. Mater. 2015, 91, 1–8. [Google Scholar] [CrossRef]
- Alanazi, H.; Yang, M.; Zhang, D.; Gao, Z. Bond strength of PCC pavement repairs using metakaolin-based geopolymer mortar. Cem. Concr. Compos. 2016, 65, 75–82. [Google Scholar] [CrossRef]
- Laskar, S.M.; Talukdar, S. Preparation and tests for workability, compressive and bond strength of ultra-fine slag based geopolymer as concrete repairing agent. Constr. Build. Mater. 2017, 154, 176–190. [Google Scholar] [CrossRef]
- Collins, F.; Sanjayan, J.G. Effects of ultra-fine materials on workability and strength of concrete containing alkali-activated slag as the binder. Cem. Concr. Res. 1999, 29, 459–462. [Google Scholar] [CrossRef]
- ASTM C618. Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use as a Mineral Admixture in Concrete. In Annual Book of ASTM Standards; American Society for Testing and Materials: West Conshohocken, PA, USA, 2001; Volume 04.02. [Google Scholar]
- Mustafa Al Bakri, A.M.; Kamarudin, H.; Bnhussain, M.; Abd Razak, R.; Yahya, Z. Effect of Na2SiO3/NaOH ratios and NaOH molarities on compressive strength of fly-ash-based geopolymer. ACI Mater. J. 2012, 109, 503–508. [Google Scholar]
- Malkawi, A.B.; Fadhil, M.; Fauzi, A.; Almattarneh, H. Effects of Alkaline Solution on Properties of the HCFA Geopolymer Mortars. Procedia Eng. 2016, 148, 710–717. [Google Scholar] [CrossRef]
- Abdullah, M.M.A.B.; Kamarudin, H.; Bnhussain, M.; Ismail, K.N.; Ibrahim, W.M. Mechanism and Chemical Reaction of Fly Ash Geopolymer Cement- A Review. Int. J. Pure Appl. Sci. Technol. 2001, 6, 35–44. [Google Scholar]
- ASTM C403. Standard Test Method for Time of Setting of Concrete Mixtures by Penetration Resistance. In Annual Book of ASTM Standards; American Society for Testing and Materials: West Conshohocken, PA, USA, 2003; Volume 04.02. [Google Scholar]
- Williamson, T.; Juenger, M.C.G. The role of activating solution concentration on alkali–silica reaction in alkali-activated fly ash concrete. Cem. Concr. Res. 2016, 83, 124–130. [Google Scholar] [CrossRef]
- ASTM C109. Standard Test Method for Compressive Strength of Hydraulic Cement Mortars. In Annual Book of ASTM Standards; American Society for Testing and Materials: West Conshohocken, PA, USA, 2016; Volume 04.01. [Google Scholar]
- ASTM C882. Standard Test Method for Bond Strength of Epoxy-Resin Systems Used With Concrete. In Annual Book of ASTM Standards; American Society for Testing and Materials: West Conshohocken, PA, USA, 2005; Volume 04.02. [Google Scholar]
- Ismail, I.; Bernal, S.A.; Provis, J.L.; San Nicolas, R.; Brice, D.G.; Kilcullen, A.R.; Hamdan, S.; van Deventer, J.S. Influence of fly ash on the water and chloride permeability of alkali-activated slag mortars and concretes. Constr. Build. Mater. 2013, 48, 1187–1201. [Google Scholar] [CrossRef]
- Hu, S.; Wang, H.; Zhang, G.; Ding, Q. Bonding and abrasion resistance of geopolymeric repair material made with steel slag. Cem. Concr. Compos. 2008, 30, 239–244. [Google Scholar] [CrossRef]
- Phoo-ngernkham, T.; Sata, V.; Hanjitsuwan, S.; Ridtirud, C.; Hatanaka, S.; Chindaprasirt, P. High calcium fly ash geopolymer mortar containing Portland cement for use as repair material. Constr. Build. Mater. 2015, 98, 482–488. [Google Scholar] [CrossRef]
- Al Bakri Abdullah, M.M.; Kamarudin, H.; Nizar, I.K.; Bnhussain, M.; Yahya, Z.; Abd Razak, R. Correlation between Na2SiO3/NaOH Ratio and Fly Ash / Alkaline Activator Ratio to the Strength of Geopolymer. Adv. Mater. Res. 2012, 342, 189–193. [Google Scholar]
- Hamidi, R.M.; Man, Z.; Azizli, K.A. Concentration of NaOH and the Effect on the Properties of Fly Ash Based Geopolymer. Procedia Eng. 2016, 148, 189–193. [Google Scholar] [CrossRef]
- Kaze, R.C.; à Moungam, L.M.B.; Cannio, M.; Rosa, R.; Kamseu, E.; Melo, U.C.; Leonelli, C. Microstructure and engineering properties of Fe 2 O 3 ( FeO ) -Al 2 O 3 -SiO 2 based geopolymer composites. J. Clean. Prod. 2018, 199, 849–859. [Google Scholar] [CrossRef]
- Sabitha, D.; Dattatreya, J.K.; Sakthivel, N.; Bhuvaneshwari, M.; Sathik, S.A.J. Reactivity, workability and strength of potassium versus sodium-activated high volume fly ash-based geopolymers. Curr. Sci. 2012, 103, 1320–1327. [Google Scholar]
- Najafi, E.; Allahverdi, A.; Provis, J.L. Efflorescence control in geopolymer binders based on natural pozzolan. Cem. Concr. Compos. 2012, 34, 25–33. [Google Scholar] [CrossRef]
- Zailan, S.N.; Bouaissi, A.; Mahmed, N.; Abdullah, M.M. Al Bakri. Influence of ZnO Nanoparticles on Mechanical Properties and Photocatalytic Activity of Self-cleaning ZnO-Based Geopolymer Paste. J. Inorg. Organomet. Polym. Mater. 2019, 1–10. [Google Scholar]
- Fernandez-Jimenez, A.; García-Lodeiro, I.; Palomo, A. Durability of alkali-activated fly ash cementitious materials. J. Mater. Sci. 2007, 42, 3055–3065. [Google Scholar] [CrossRef]
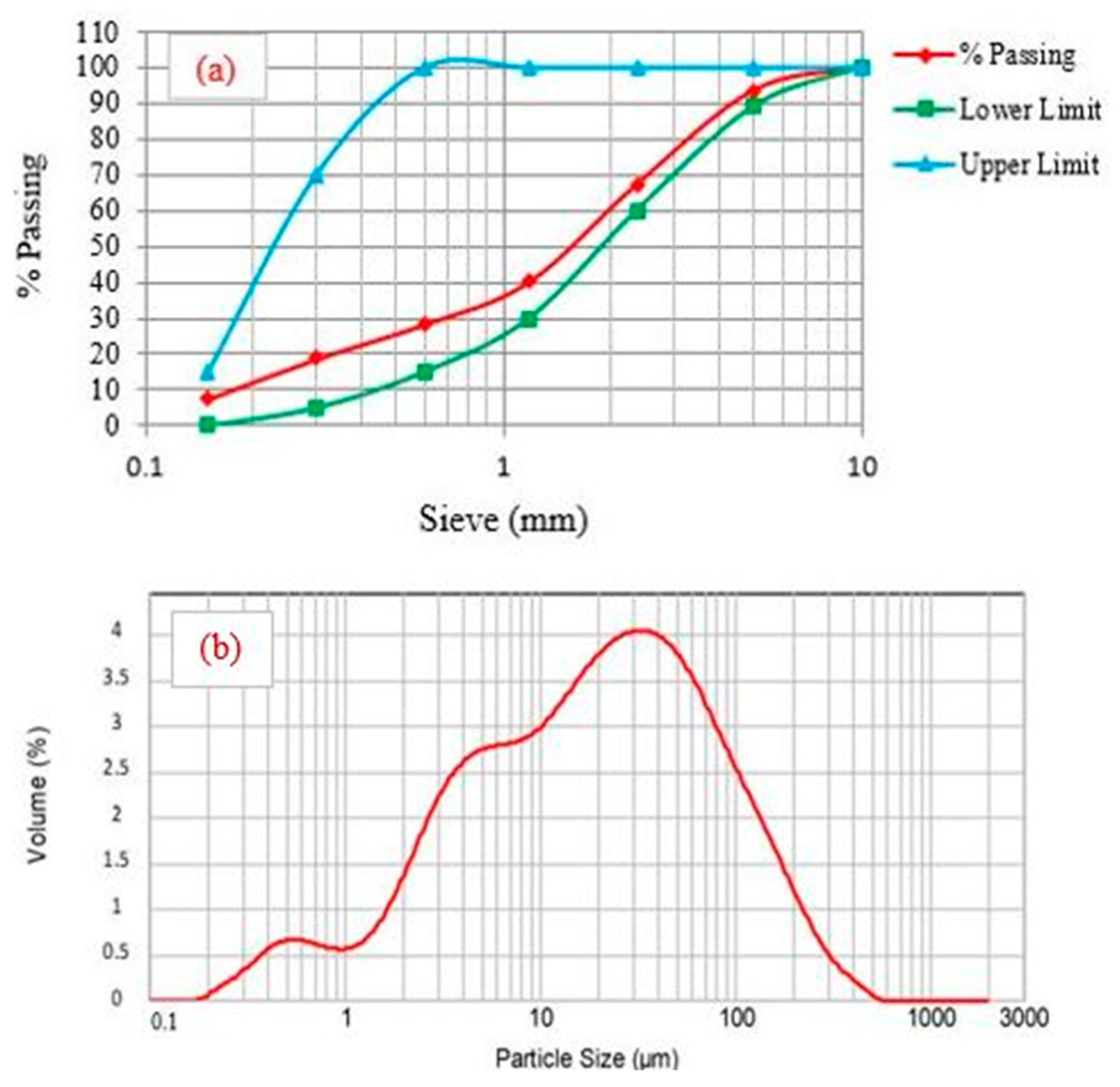
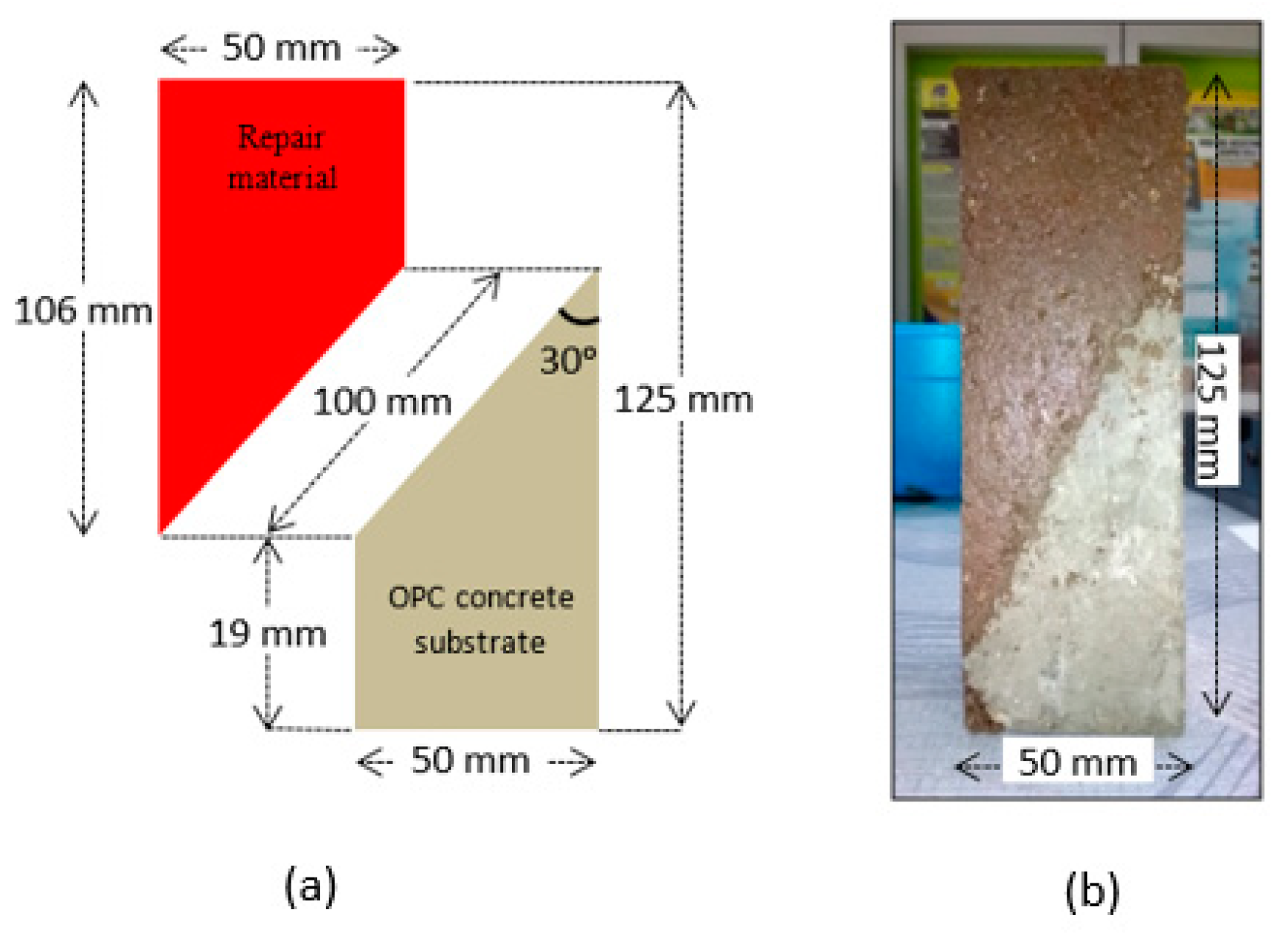


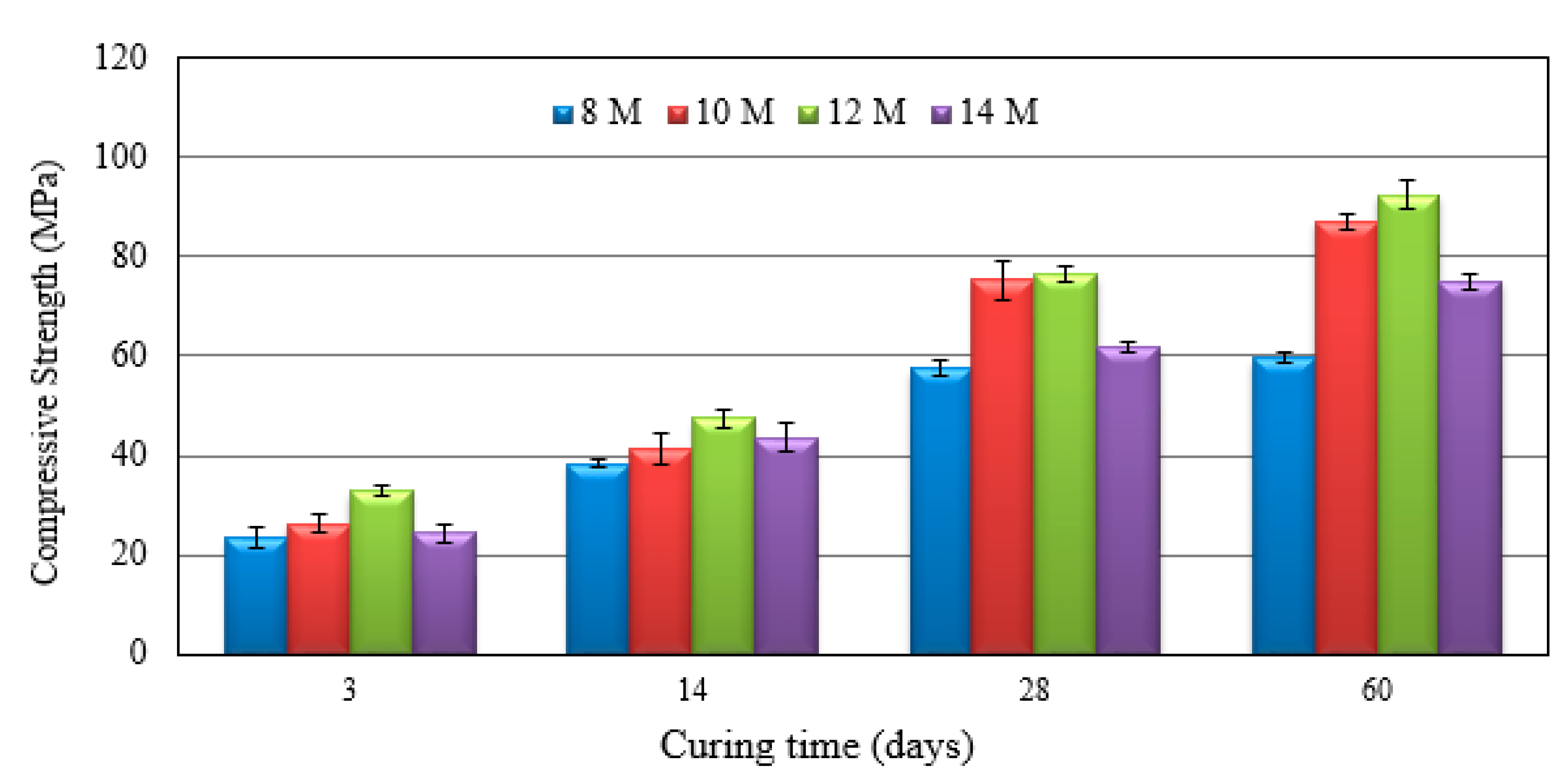
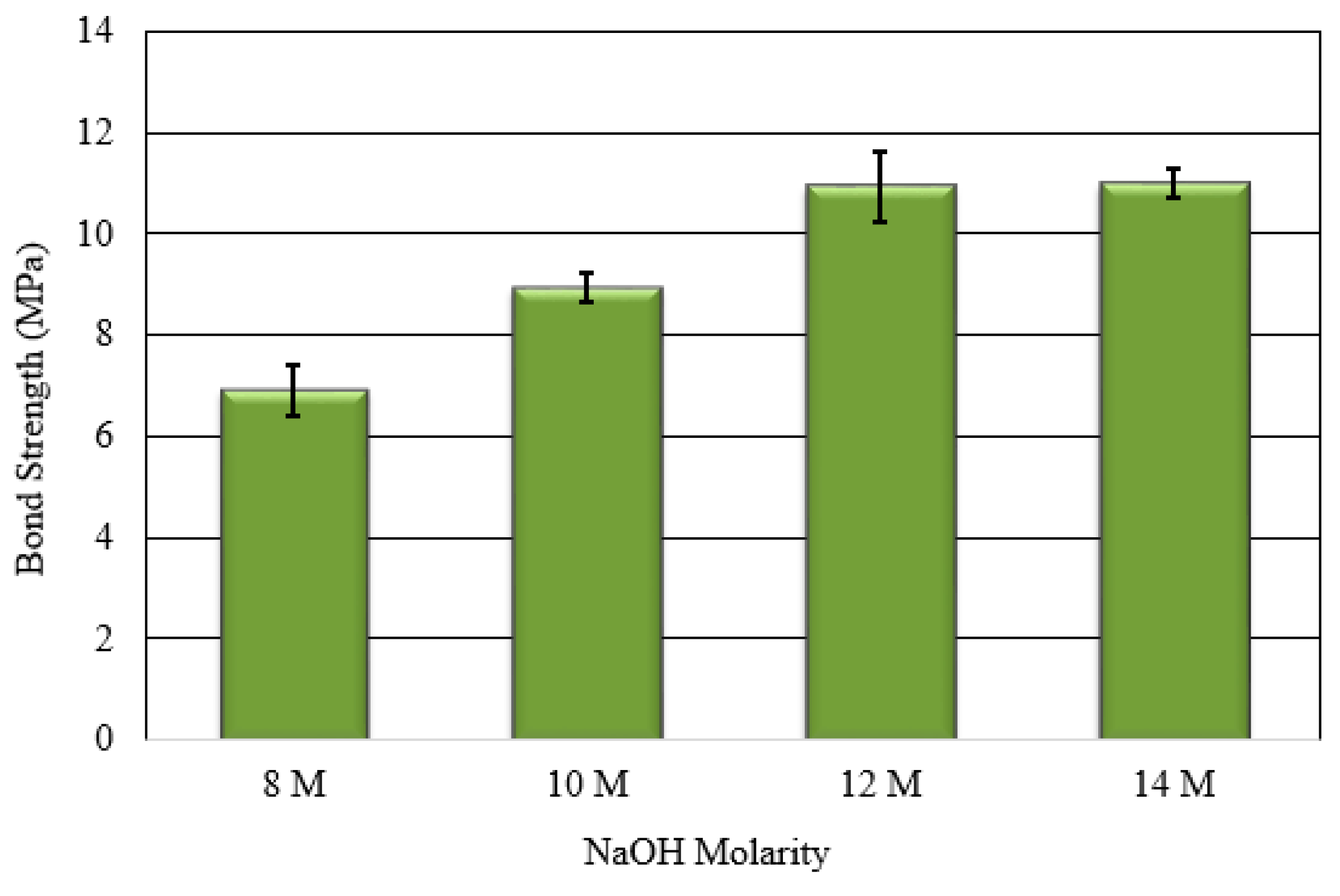


| Materials | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | TiO2 | SO3 | LOI |
|---|---|---|---|---|---|---|---|---|---|
| Fly ash (%) | 38.40 | 14.50 | 19.57 | 18.4 | 3.6 | 1.71 | 1.00 | 1.56 | 1.05 |
| OPC (%) | 14 | 3 | 4.39 | 72.24 | - | 1.01 | 0.3 | 4.52 | 0.065 |
| Components | OPC (kg m−3) | Aggregates (kg m−3) | Water (kg m−3) | Properties of OPC | |
|---|---|---|---|---|---|
| Fine | Coarse | fc (MPa) | |||
| Proportions | 410 | 648 | 1152 | 205 | 30 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad Zailani, W.W.; Bouaissi, A.; Abdullah, M.M.A.B.; Abd Razak, R.; Yoriya, S.; Mohd Salleh, M.A.A.; Rozainy M. A. Z., M.R.; Fansuri, H. Bonding Strength Characteristics of FA-Based Geopolymer Paste as a Repair Material When Applied on OPC Substrate. Appl. Sci. 2020, 10, 3321. https://doi.org/10.3390/app10093321
Ahmad Zailani WW, Bouaissi A, Abdullah MMAB, Abd Razak R, Yoriya S, Mohd Salleh MAA, Rozainy M. A. Z. MR, Fansuri H. Bonding Strength Characteristics of FA-Based Geopolymer Paste as a Repair Material When Applied on OPC Substrate. Applied Sciences. 2020; 10(9):3321. https://doi.org/10.3390/app10093321
Chicago/Turabian StyleAhmad Zailani, Warid Wazien, Aissa Bouaissi, Mohd Mustafa Al Bakri Abdullah, Rafiza Abd Razak, Sorachon Yoriya, Mohd Arif Anuar Mohd Salleh, Mohd Remy Rozainy M. A. Z., and Hamzah Fansuri. 2020. "Bonding Strength Characteristics of FA-Based Geopolymer Paste as a Repair Material When Applied on OPC Substrate" Applied Sciences 10, no. 9: 3321. https://doi.org/10.3390/app10093321
APA StyleAhmad Zailani, W. W., Bouaissi, A., Abdullah, M. M. A. B., Abd Razak, R., Yoriya, S., Mohd Salleh, M. A. A., Rozainy M. A. Z., M. R., & Fansuri, H. (2020). Bonding Strength Characteristics of FA-Based Geopolymer Paste as a Repair Material When Applied on OPC Substrate. Applied Sciences, 10(9), 3321. https://doi.org/10.3390/app10093321







