Antioxidant Properties of Jatropha curcas L. Seed Shell and Kernel Extracts
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of Extracts
2.3. 1,1-diphenyl-2-picrylhydrazyl Radical Scavenging Assay
2.4. 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) Radical Scavenging Assay
2.5. Reducing Power Assay
2.6. Determination of the Total Phenolic Content
2.7. In Vitro Cytotoxicity Test
2.8. Statistical Analysis
3. Results and Discussion
3.1. Extraction Yield
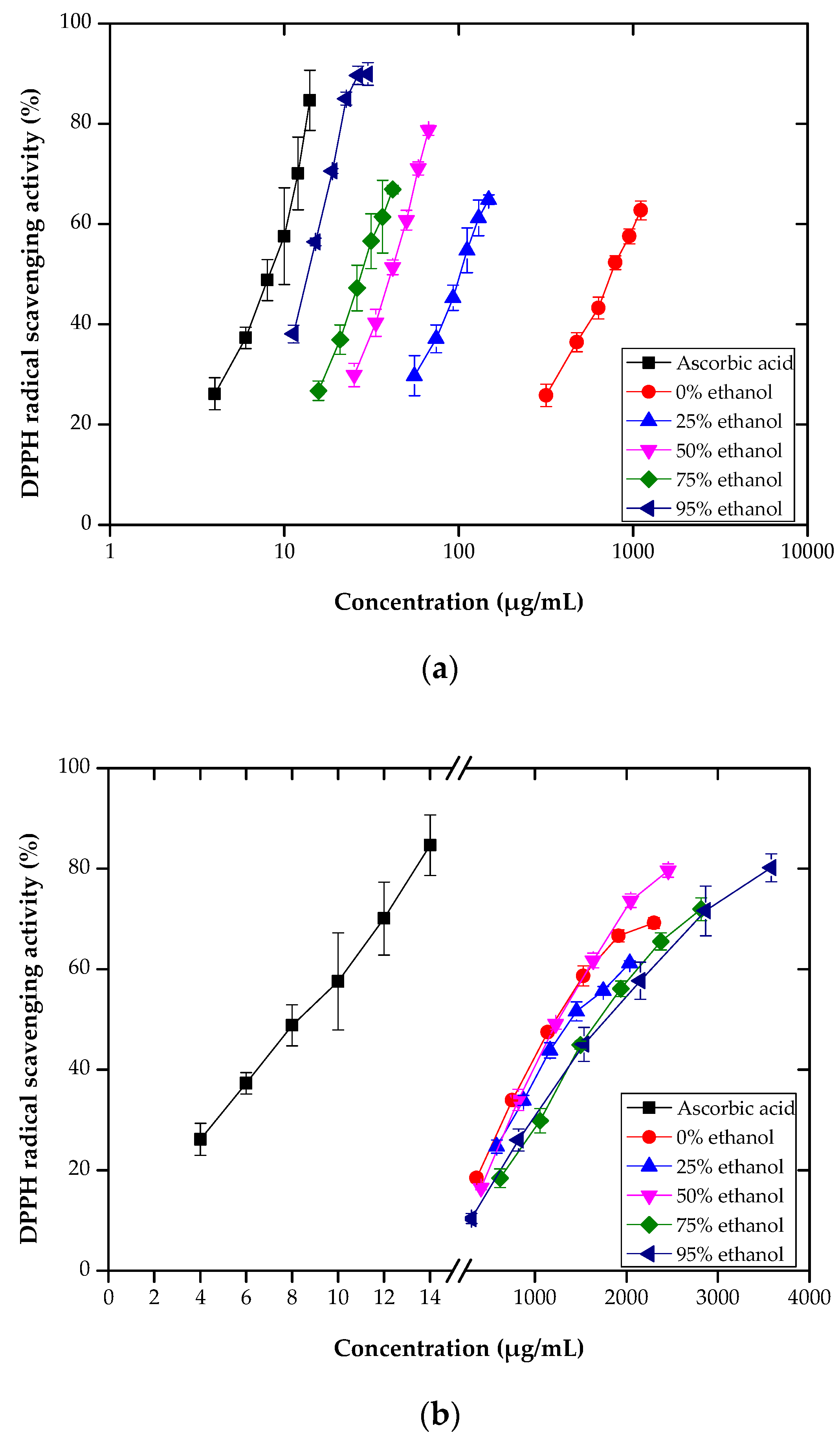
3.2. DPPH Radical Scavenging Assay
3.3. ABTS Radical Scavenging Assay
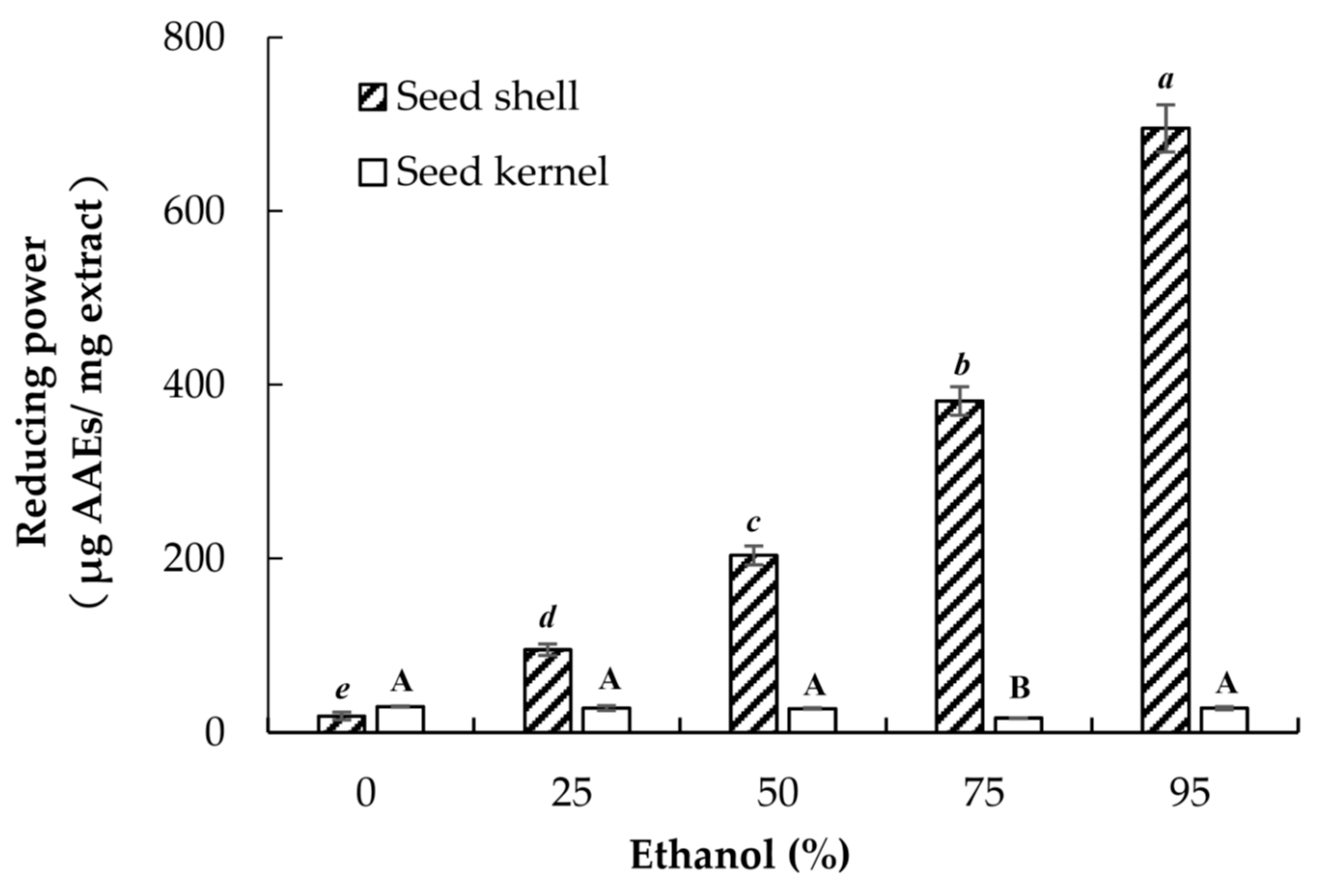
3.4. Reducing Power Assay
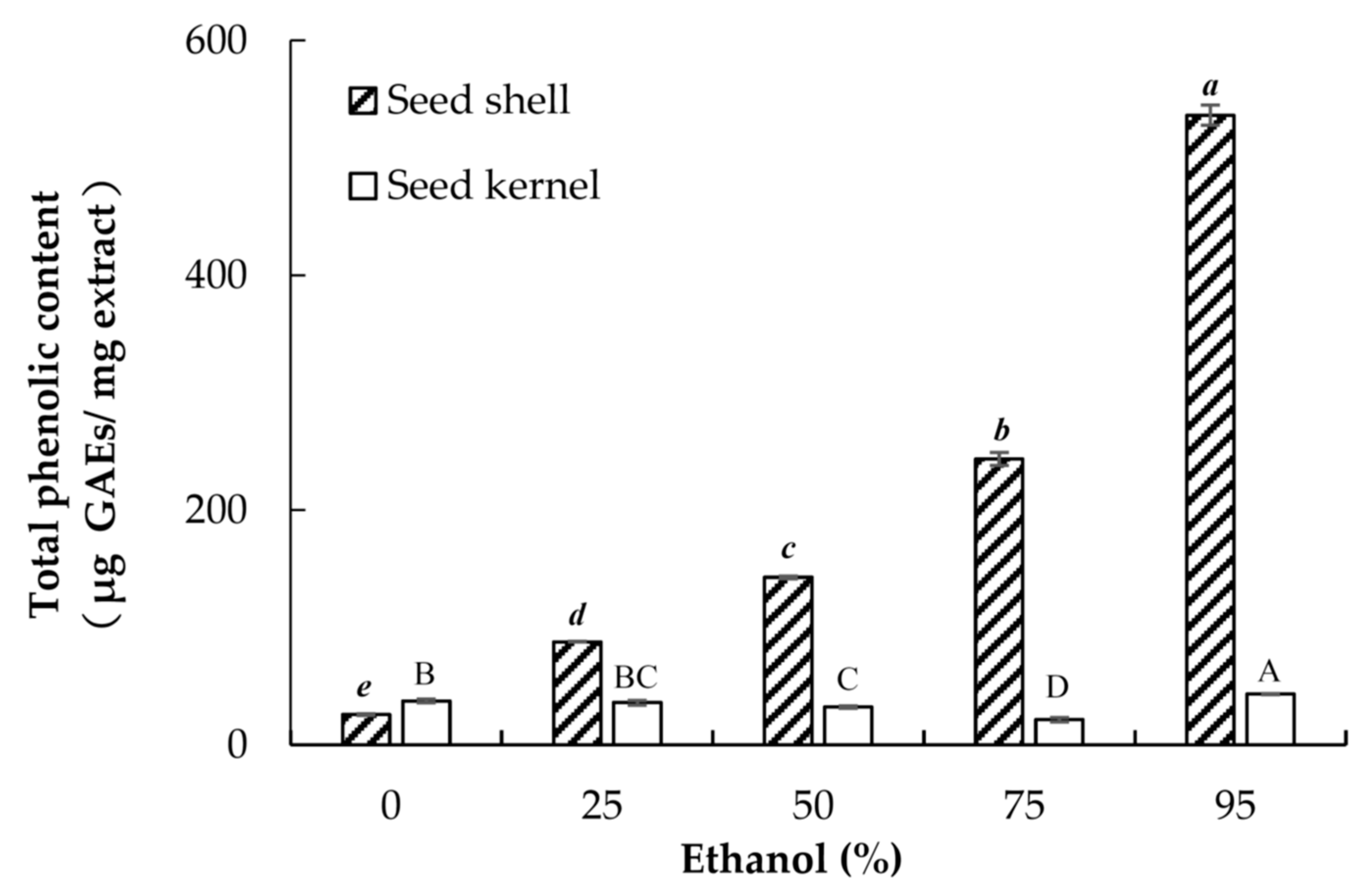
3.5. Determination of the Total Phenolic Content
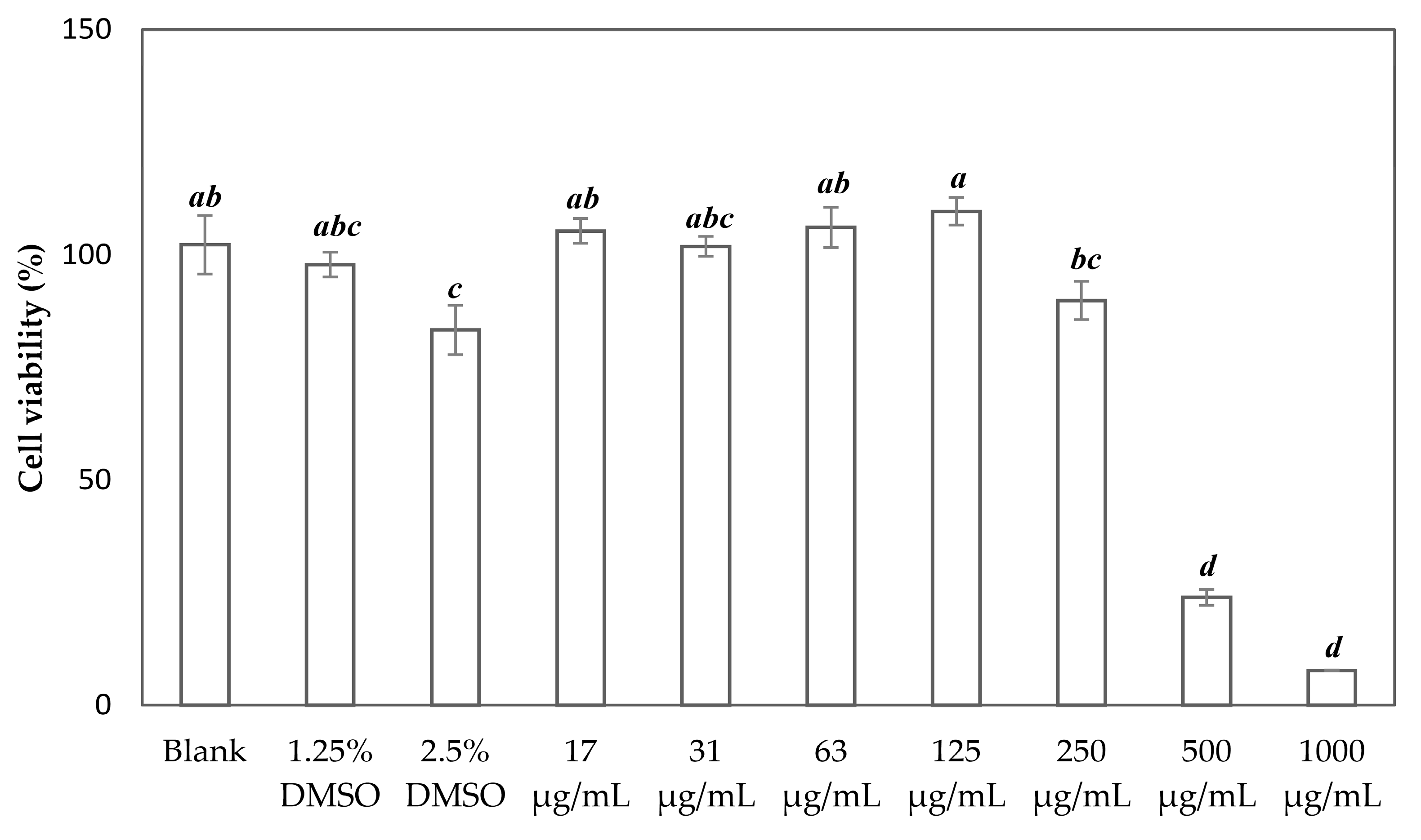
3.6. In Vitro Cytotoxicity Test
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Osman, S.A.; Abdullah, N.N.; Ahmad, S. Antioxidant activity and phytochemical components of Jatropha curcas Linn. root extract. J. Biochem. Microbiol. Biotechnol. 2017, 5, 2–7. [Google Scholar]
- Boudjeko, T.; Ngomoyogoli, J.E.K.; Woguia, A.L.; Yanou, N.N. Partial characterization, antioxidative properties and hypolipidemic effects of oilseed cake of Allanblackia floribunda and Jatropha curcas. BMC Complement. Altern. Med. 2013, 13, 352. [Google Scholar] [CrossRef]
- Augustus, G.D.P.S.; Jayabalan, M.; Seiler, G.J. Evaluation and bioinduction of energy components of Jatropha curcas. Biomass Bioenergy 2002, 23, 161–164. [Google Scholar] [CrossRef]
- Openshaw, K. A review of Jatropha curcas: An oil plant of unfulfilled promise. Biomass Bioenergy 2000, 19, 1–15. [Google Scholar] [CrossRef]
- Rampadarath, S.; Puchooa, D.; Ranghoo-Sanmukhiya, V.M. Antimicrobial, phytochemical and larvicidal properties of Jatropha multifida Linn. Asian Pac. J. Trop. Med. 2014, 7, S380–S383. [Google Scholar] [CrossRef]
- Ling, T.; Hadi, V.; Guiguemde, A.; Landfear, S.M.; Rivas, F. Jatropha Natural Products as Potential Therapeutic Leads. In The Formation, Structure and Activity of Phytochemicals; Springer: New York, NY, USA; Cham, Switzerland, 2015; pp. 77–98. [Google Scholar]
- Ohtani, M.; Nakano, Y.; Sano, R.; Kurata, T.; Demura, T. Toxic Substances in Jatropha Seeds: Biosynthesis of the Most Problematic Compounds, Phorbol Esters. In The Jatropha Genome; Springer: Cham, Switzerland, 2017; pp. 97–111. [Google Scholar]
- Oskoueian, E.; Abdullah, N.; Ahmad, S.; Saad, W.Z.; Omar, A.R.; Ho, Y.W. Bioactive compounds and biological activities of Jatropha curcas L. kernel meal extract. Int. J. Mol. Sci. 2011, 12, 5955–5970. [Google Scholar] [CrossRef]
- Sundari, J.; Selvaraj, R.; Prasad, N.R.; Elumalai, R. Jatropha curcas leaf and bark fractions protect against ultraviolet radiation-B induced DNA damage in human peripheral blood lymphocytes. Environ. Toxicol. Pharmacol. 2013, 36, 875–882. [Google Scholar] [CrossRef]
- Namuli, A.; Abdullah, N.; Sieo, C.C.; Zuhainis, S.W.; Oskoueian, E. Phytochemical compounds and antibacterial activity of Jatropha curcas Linn. extracts. J. Med. Plants Res. 2011, 5, 3982–3990. [Google Scholar]
- Insanu, M.; Dimaki, C.; Wilkins, R.; Brooker, J.; Van der Linde, P.; Kayser, O. Rational use of Jatropha curcas L. in food and medicine: From toxicity problems to safe applications. Phytochem. Rev. 2013, 12, 107–119. [Google Scholar] [CrossRef]
- Aiyelaagbe, O.O.; Hamid, A.A.; Fattorusso, E.; Taglialatela-Scafati, O.; Schröder, H.C.; Müller, W.E. Cytotoxic activity of crude extracts as well as of pure components from Jatropha species, plants used extensively in African traditional medicine. Evid. Based Complement. Altern. Med. 2011, 2011, 134954. [Google Scholar] [CrossRef]
- Devappa, R.K.; Makkar, H.P.; Becker, K. Localisation of antinutrients and qualitative identification of toxic components in Jatropha curcas seed. J. Sci. Food Agric. 2012, 92, 1519–1525. [Google Scholar] [CrossRef]
- Verma, S.; Gupta, A.; Kushwaha, P.; Khare, V.; Srivastava, S.; Rawat, A.K.S. Phytochemical evaluation and antioxidant study of Jatropha curcas seeds. Pharmacogn. J. 2012, 4, 50–54. [Google Scholar] [CrossRef]
- Nithiyanantham, S.; Siddhuraju, P.; Francis, G. A promising approach to enhance the total phenolic content and antioxidant activity of raw and processed Jatropha curcas L. kernel meal extracts. Ind. Crops Prod. 2013, 43, 261–269. [Google Scholar] [CrossRef]
- Fu, R.; Zhang, Y.; Guo, Y.; Liu, F.; Chen, F. Determination of phenolic contents and antioxidant activities of extracts of Jatropha curcas L. seed shell, a by-product, a new source of natural antioxidant. Ind. Crops Prod. 2014, 58, 265–270. [Google Scholar] [CrossRef]
- Islam, A.K.M.A.; Yaakob, Z.; Anuar, N. Jatropha: A multipurpose plant with considerable potential for the tropics. Sci. Res. Essays 2011, 6, 2597–2605. [Google Scholar]
- Pinelo, M.; Manzocco, L.; Nunez, M.J.; Nicoli, M.C. Solvent effect on quercetin antioxidant capacity. Food Chem. 2004, 88, 201–207. [Google Scholar] [CrossRef]
- Tsai, C.C.; Chan, C.F.; Huang, W.Y.; Lin, J.S.; Chan, P.; Liu, H.Y.; Lin, Y.S. Applications of Lactobacillus rhamnosus spent culture supernatant in cosmetic antioxidation, whitening and moisture retention applications. Molecules 2013, 18, 14161–14171. [Google Scholar] [CrossRef]
- Huang, W.Y.; Lee, P.C.; Hsu, J.C.; Lin, Y.R.; Chen, H.J.; Lin, Y.S. Effects of water quality on dissolution of yerba mate extract powders. Sci. World J. 2014, 2014, 768742. [Google Scholar] [CrossRef]
- Chan, C.F.; Wu, C.T.; Huang, W.Y.; Lin, W.S.; Wu, H.W.; Huang, T.K.; Chang, M.Y.; Lin, Y.S. Antioxidation and melanogenesis inhibition of various Dendrobium tosaense extracts. Molecules 2018, 23, 1810. [Google Scholar] [CrossRef]
- Wu, C.T.; Agrawa, D.C.; Huang, W.Y.; Hsu, H.C.; Yang, S.J.; Huang, S.L.; Lin, Y.S. Functionality analysis of spent coffee ground extracts obtained by the hydrothermal method. J. Chem. 2019, 2019, 4671438. [Google Scholar] [CrossRef]
- Chang, M.Y.; Lin, Y.Y.; Chang, Y.C.; Huang, W.Y.; Lin, W.S.; Chen, C.Y.; Huang, S.L.; Lin, Y.S. Effects of infusion and storage on antioxidant activity and total phenolic content of black tea. Appl. Sci. 2020, 10, 2685. [Google Scholar] [CrossRef]
- Huang, W.Y.; Lin, Y.R.; Ho, R.F.; Liu, H.Y.; Lin, Y.S. Effects of water solutions on extracting green tea leaves. Sci. World J. 2013, 2013, 368350. [Google Scholar] [CrossRef]
- De la Rosa, L.A.; Alvarez-Parrilla, E.; Shahidi, F. Phenolic compounds and antioxidant activity of kernels and shells of Mexican pecan (Carya illinoinensis). J. Agric. Food Chem. 2010, 59, 152–162. [Google Scholar] [CrossRef]
- Contini, M.; Baccelloni, S.; Massantini, R.; Anelli, G. Extraction of natural antioxidants from hazelnut (Corylus avellana L.) shell and skin wastes by long maceration at room temperature. Food Chem. 2008, 110, 659–669. [Google Scholar] [CrossRef]
- Musa, K.H.; Abdullah, A.; Jusoh, K.; Subramaniam, V. Antioxidant activity of pink-flesh guava (Psidium guajava L.): Effect of extraction techniques and solvents. Food Anal. Methods 2011, 4, 100–107. [Google Scholar] [CrossRef]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef]
- Rofida, S. Antioxidant activity of Jatropha curcas and Jatropha gossypifolia by DPPH method. Farmasains 2015, 2, 281–284. [Google Scholar]
- Haq, M.N.U.; Wazir, S.M.; Ullah, F.; Khan, R.A.; Shah, M.S.; Khatak, A. Phytochemical and biological evaluation of defatted seeds of Jatropha curcas. Sains Malays. 2016, 45, 1435–1442. [Google Scholar]
- Alimpić, A.Z.; Duletić-Laušević, S.N.; Matevski, V.S.; Marin, P.D. Antioxidant activity of Salvia jurisicii Košanin ethanol extracts. Bot. Serbica 2015, 39, 53–58. [Google Scholar]
- Dhanani, T.; Shah, S.; Gajbhiye, N.A.; Kumar, S. Effect of extraction methods on yield, phytochemical constituents and antioxidant activity of Withania somnifera. Arab. J. Chem. 2017, 10, S1193–S1199. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, P.; Koundal, R.; Agnihotri, V.K. Antioxidant properties and UPLC-MS/MS profiling of phenolics in jacquemont’s hazelnut kernels (Corylus jacquemontii) and its byproducts from western Himalaya. J. Food Sci. Technol. 2016, 53, 3522–3531. [Google Scholar] [CrossRef]
- Ghali, W.; Vaudry, D.; Jouenne, T.; Marzouki, M.N. Assessment of cyto-protective, antiproliferative and antioxidant potential of a medicinal plant Jatropha podagrica. Ind. Crops Prod. 2013, 44, 111–118. [Google Scholar] [CrossRef]
- Nour, V.; Stampar, F.; Veberic, R.; Jakopic, J. Anthocyanins profile, total phenolics and antioxidant activity of black currant ethanolic extracts as influenced by genotype and ethanol concentration. Food Chem. 2013, 141, 961–966. [Google Scholar] [CrossRef]
- Othman, A.R.; Abdullah, N.; Ahmad, S.; Ismail, I.S.; Zakaria, M.P. Elucidation of in-vitro anti-inflammatory bioactive compounds isolated from Jatropha curcas L. plant root. BMC Complement. Altern. Med. 2015, 15, 11. [Google Scholar] [CrossRef]
- Marchesan, S.; Qu, Y.; Waddington, L.J.; Easton, C.D.; Glattauer, V.; Lithgow, T.J.; McLean, K.M.; Forsythe, J.S.; Hartley, P.G. Self-assembly of ciprofloxacin and a tripeptide into an antimicrobial nanostructured hydrogel. Biomaterials 2013, 34, 3678–3687. [Google Scholar] [CrossRef]
- De Oliveira, R.B.; Vaz, A.; Alves, R.O.; Liarte, D.B.; Donnici, C.L.; Romanha, A.J.; Zani, C.L. Arylfurans as potential Trypanosoma cruzi trypanothione reductase inhibitors. Memórias Do Inst. Oswaldo Cruz 2006, 101, 169–173. [Google Scholar] [CrossRef]


| Ethanol conc. (%) | 0 | 25 | 50 | 75 | 95 | |
|---|---|---|---|---|---|---|
| Part | ||||||
| Seed shell | 12.58±0.25 b * | 14.81±0.92 a | 13.35±0.78 a | 8.35±0.29 c | 3.00±0.45 d | |
| Seed kernel | 24.23±0.78 a | 23.27±0.57 a | 20.45±0.26 b | 17.60±0.82 c | 8.19±1.26 d | |
| p value ** | <0.0001 | <0.0001 | 0.0001 | <0.0001 | <0.0001 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.-L.; Wang, W.-H.; Zhong, X.-Y.; Lin, C.-T.; Lin, W.-S.; Chang, M.-Y.; Lin, Y.-S. Antioxidant Properties of Jatropha curcas L. Seed Shell and Kernel Extracts. Appl. Sci. 2020, 10, 3279. https://doi.org/10.3390/app10093279
Huang S-L, Wang W-H, Zhong X-Y, Lin C-T, Lin W-S, Chang M-Y, Lin Y-S. Antioxidant Properties of Jatropha curcas L. Seed Shell and Kernel Extracts. Applied Sciences. 2020; 10(9):3279. https://doi.org/10.3390/app10093279
Chicago/Turabian StyleHuang, Shu-Ling, Wei-Hsiung Wang, Xin-Yi Zhong, Chih-Ting Lin, Wen-Shin Lin, Min-Yun Chang, and Yung-Sheng Lin. 2020. "Antioxidant Properties of Jatropha curcas L. Seed Shell and Kernel Extracts" Applied Sciences 10, no. 9: 3279. https://doi.org/10.3390/app10093279
APA StyleHuang, S.-L., Wang, W.-H., Zhong, X.-Y., Lin, C.-T., Lin, W.-S., Chang, M.-Y., & Lin, Y.-S. (2020). Antioxidant Properties of Jatropha curcas L. Seed Shell and Kernel Extracts. Applied Sciences, 10(9), 3279. https://doi.org/10.3390/app10093279







