Characterization of Antimicrobial Effects of Plasma-Treated Water (PTW) Produced by Microwave-Induced Plasma (MidiPLexc) on Pseudomonas fluorescens Biofilms
Abstract
1. Introduction
2. Materials and Methods
2.1. Plasma Source
2.2. Generation of PTW by the Microwave Plasma
2.3. Bacterial Strains and Growth Conditions
2.4. PTW Treatment of P. fluorescens Biofilms
2.5. Performance of the Colony-Forming Units (CFU) Assay of PTW-Treated Biofilms
2.6. Fluorescence LIVE/DEAD Assay
2.7. XTT Assay
2.8. Fluorescence Microscopy
2.9. Confocal Laser Scanning Microscopy (CLSM)
2.10. Atomic-Force Microscopy (AFM)
3. Results
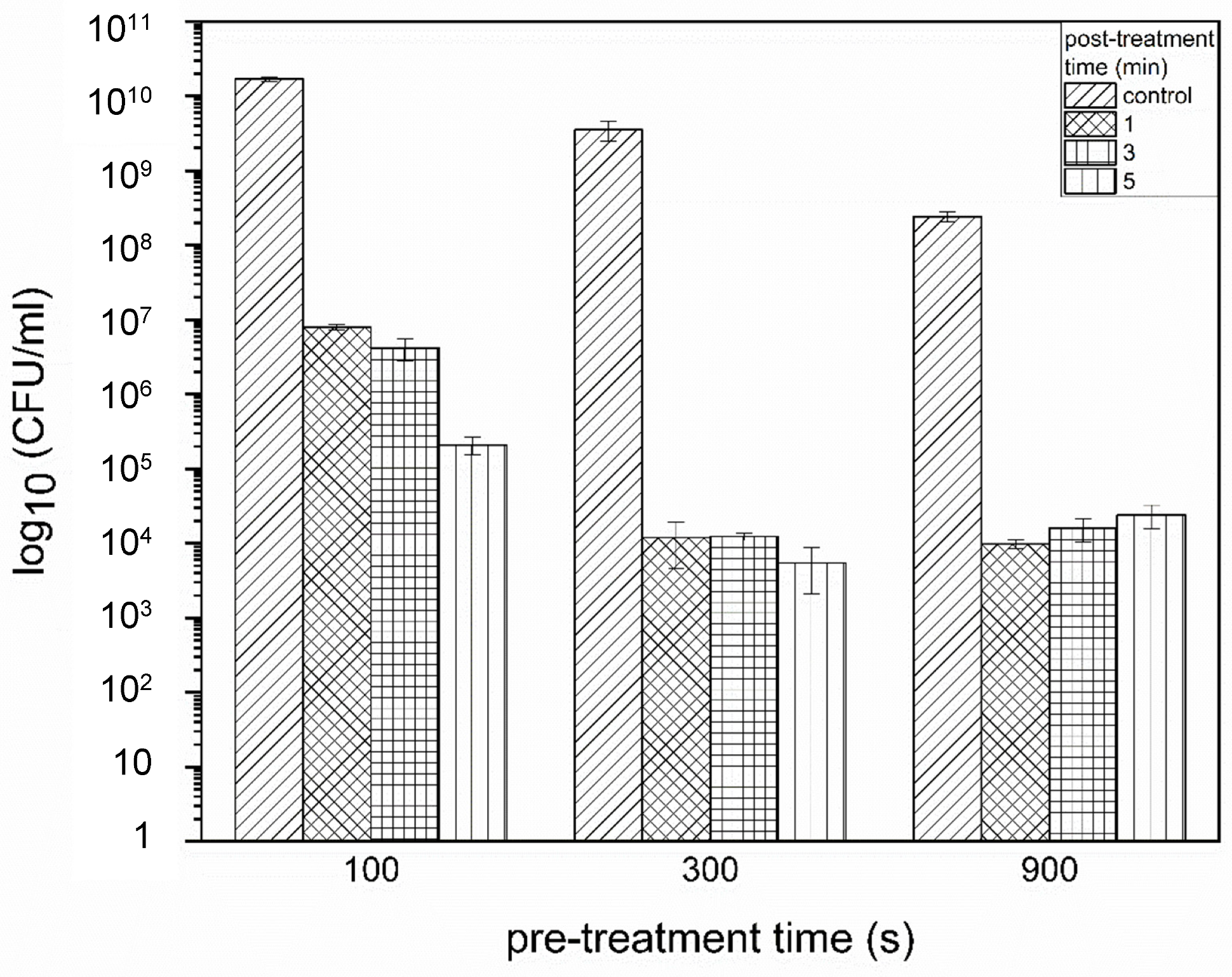
3.1. Impact of the PTW Treatment on the Proliferation Ability of the Cells (CFU)
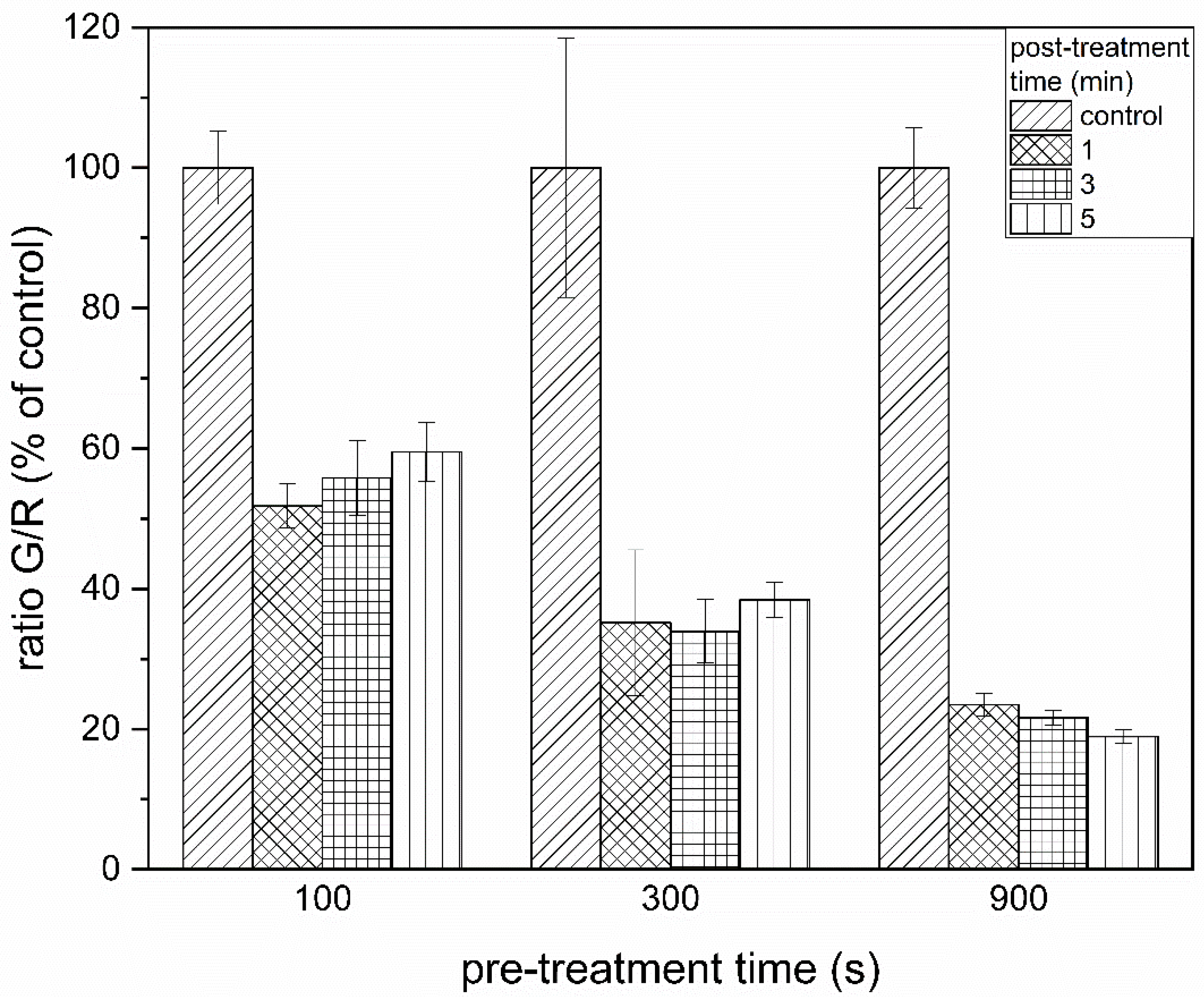
3.2. PTW Treatment of Biofilms Leads to Membrane Damage in Cells
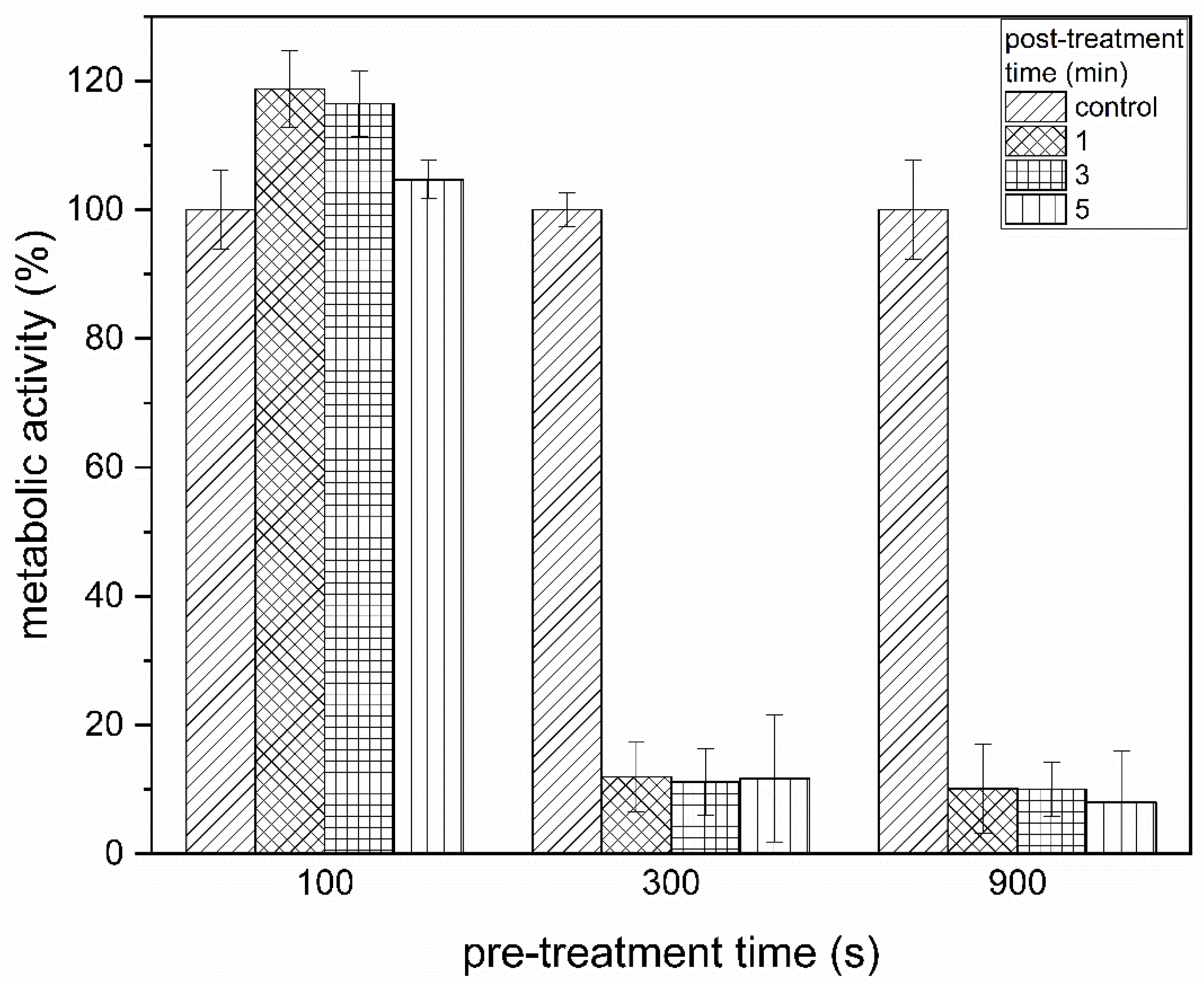
3.3. XTT Assay Revealed a Reduction in the Metabolic Activity of the Cells after PTW Treatment
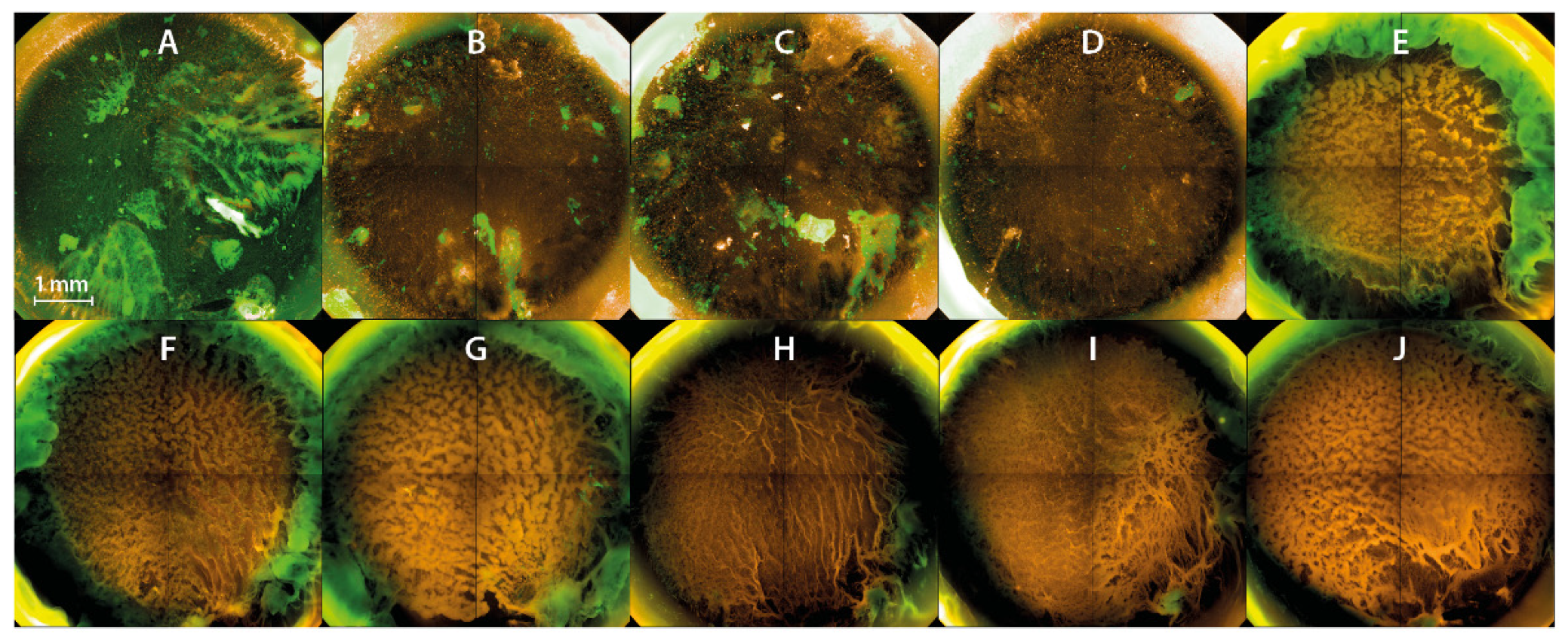
3.4. PTW Treatment of the Biofilms Leads to Inactivation of the Biofilm Layers from the Top in the Fluorescence Microscopy
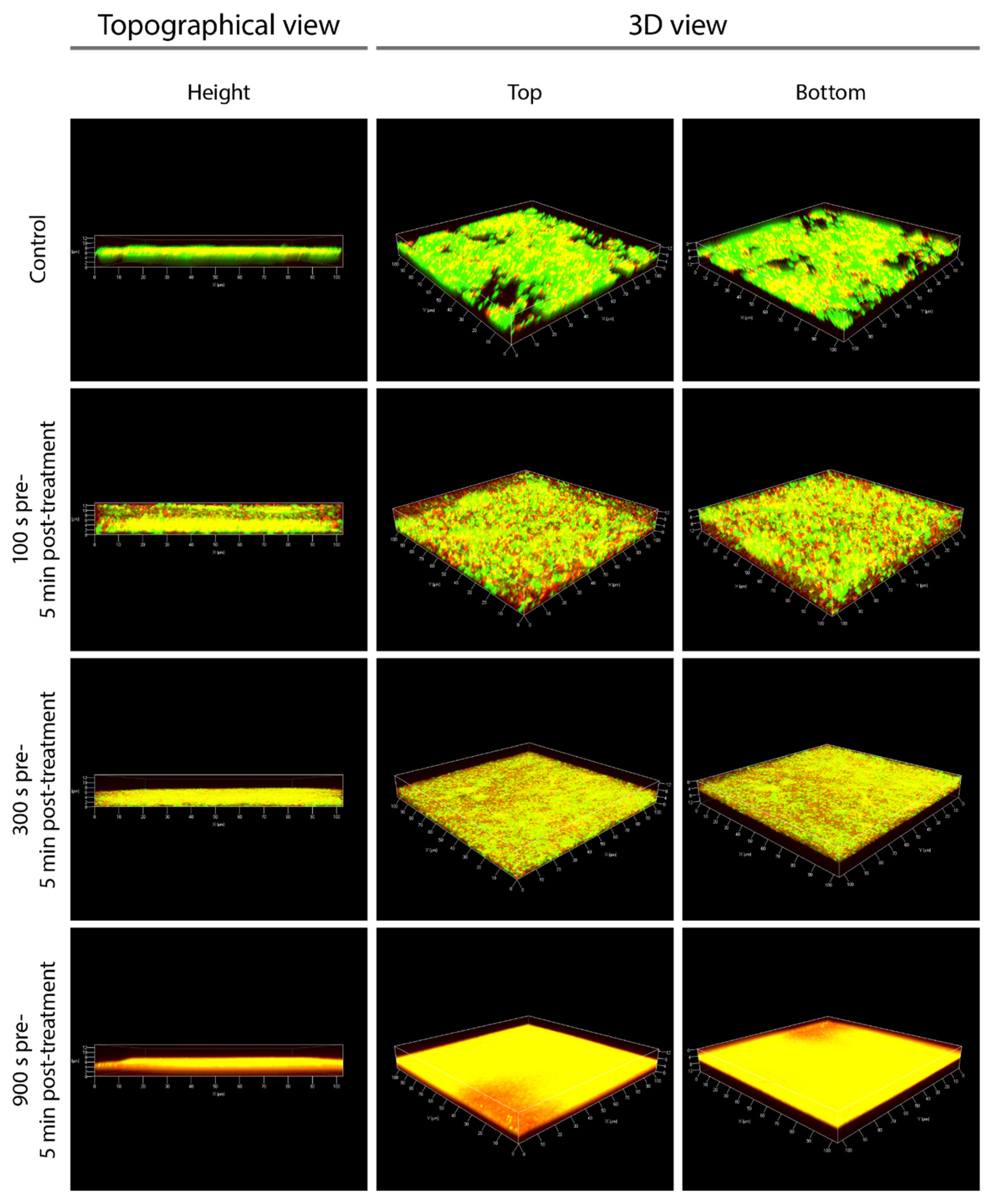
3.5. CLSM Confirms the Detachment of Cell Layers from the Surface of Biofilms
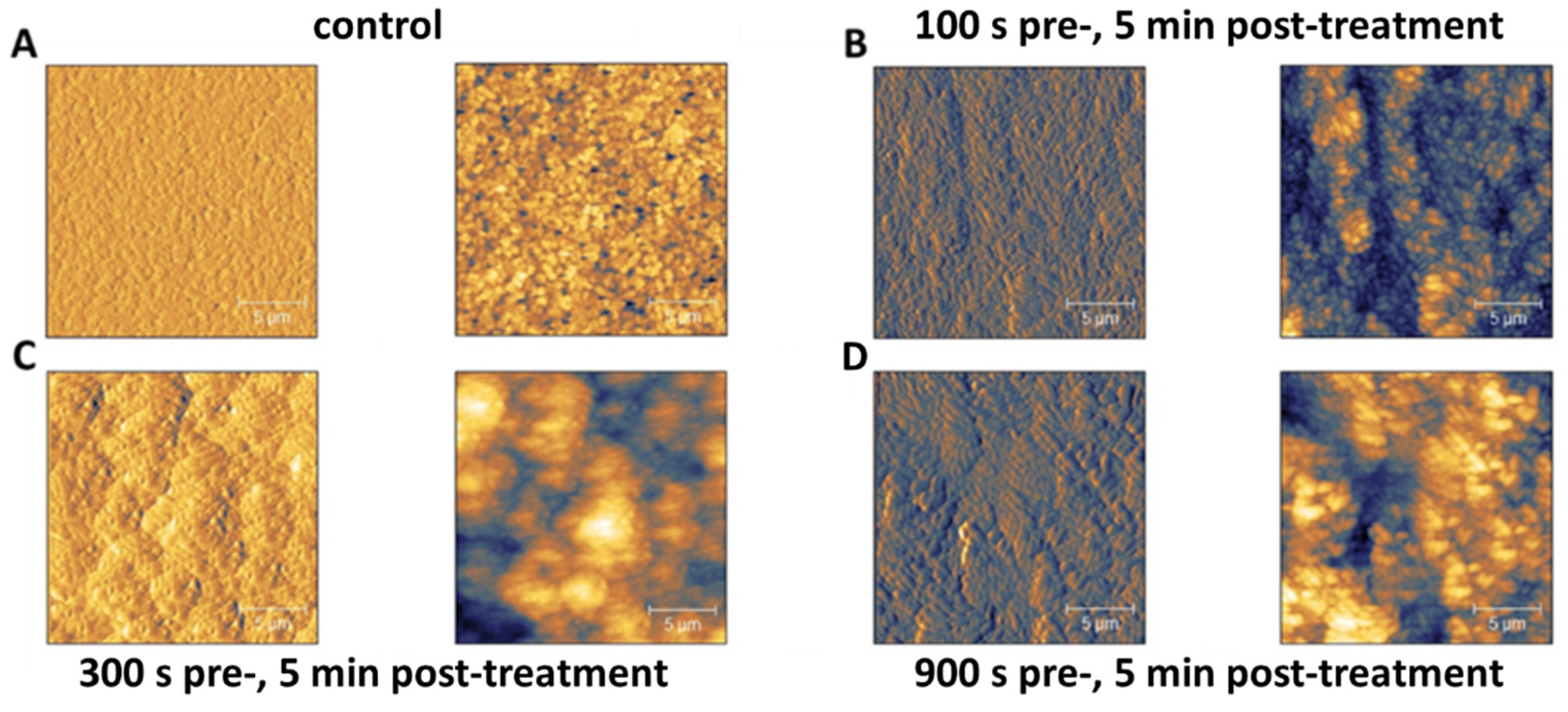
3.6. Enhanced Clustering of Cells and Modification of Physical-Mechanical Properties after PTW Treatment Visible in AFM
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mir, S.A.; Shah, M.A.; Mir, M.M. Understanding the Role of Plasma Technology in Food Industry. Food Bioprocess Technol. 2016, 9, 734–750. [Google Scholar] [CrossRef]
- Schnabel, U.; Handorf, O.; Yarova, K.; Zessin, B.; Zechlin, S.; Sydow, D.; Zellmer, E.; Stachowiak, J.; Andrasch, M.; Below, H.; et al. Plasma-Treated Air and Water-Assessment of Synergistic Antimicrobial Effects for Sanitation of Food Processing Surfaces and Environment. Foods 2019, 8, 55. [Google Scholar] [CrossRef] [PubMed]
- Handorf, O.; Below, H.; Schnabel, U.; Riedel, K.; Ehlbeck, J. Investigation of the chemical composition of plasma treated water by MidiPLexc and its antimicrobial effect on Listeria monocytogenes and Pseudomonas fluorescens monospecies suspension cultures. J. Phys. D Appl. Phys. 2020. [Google Scholar] [CrossRef]
- Schokker, E.P.; van Boekel, M.A.J.S. Kinetics of thermal inactivation of the extracellular proteinase from Pseudomonas fluorescens 22F: Influence of pH, calcium, and protein. J. Agric. Food Chem. 1999, 47, 1681–1686. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, U.; Sydow, D.; Schlüter, O.; Andrasch, M.; Ehlbeck, J. Decontamination of Fresh-Cut Iceberg Lettuce and Fresh Mung Bean Sprouts by Non-Thermal Atmospheric Pressure Plasma Processed Water (PPW). Mod. Agric. Sci. Technol. 2015, 1, 23–39. [Google Scholar]
- Ma, R.N.; Yu, S.; Tian, Y.; Wang, K.L.; Sun, C.D.; Li, X.; Zhang, J.; Chen, K.S.; Fang, J. Effect of Non-Thermal Plasma-Activated Water on Fruit Decay and Quality in Postharvest Chinese Bayberries. Food Bioprocess Technol. 2016, 9, 1825–1834. [Google Scholar] [CrossRef]
- Schnabel, U.; Niquet, R.; Andrasch, M.; Jakobs, M.; Schlüter, O.; Katroschan, K.U.; Weltmann, K.D.; Ehlbeck, J. Broccoli: Antimicrobial efficacy and influences to sensory and storage properties by microwave plasma processed air (PPA) treatment. Plasma Med. 2017, 6, 375–388. [Google Scholar] [CrossRef]
- Schnabel, U.; Schmidt, C.; Stachowiak, J.; Bösel, A.; Andrasch, M.; Ehlbeck, J. Plasma processed air for biological decontamination of PET and fresh plant tissue. Plasma Process. Polym. 2018, 15, 1600057. [Google Scholar] [CrossRef]
- Yong, H.I.; Park, J.; Kim, H.J.; Jung, S.; Park, S.; Lee, H.J.; Choe, W.; Jo, C. An innovative curing process with plasma-treated water for production of loin ham and for its quality and safety. Plasma Process. Polym. 2018, 15, 1700050. [Google Scholar] [CrossRef]
- Schnabel, U.; Andrasch, M.; Stachowiak, J.; Weit, C.; Weihe, T.; Schmidt, C.; Muranyi, P.; Schlüter, O.; Ehlbeck, J. Sanitation of fresh-cut endive lettuce by plasma processed tap water (PPtW)–Up-scaling to industrial level. Innov. Food Sci. Emerg. Technol. 2019, 53, 45–55. [Google Scholar] [CrossRef]
- Xiang, Q.S.; Liu, X.F.; Liu, S.N.; Ma, Y.F.; Xu, C.Q.; Bai, Y.H. Effect of plasma-activated water on microbial quality and physicochemical characteristics of mung bean sprouts. Innov. Food Sci. Emerg. Technol. 2019, 52, 49–56. [Google Scholar] [CrossRef]
- Kramer, A.; Bekeschus, S.; Matthes, R.; Bender, C.; Stope, M.B.; Napp, M.; Lademann, O.; Lademann, J.; Weltmann, K.D.; Schauer, F. Cold Physical Plasmas in the Field of Hygiene-Relevance, Significance, and Future Applications. Plasma Process. Polym. 2015, 12, 1410–1422. [Google Scholar] [CrossRef]
- Bekeschus, S.; Schmidt, A.; Weltmann, K.D.; Woedtke, T. The plasma jet kINPen—A powerful tool for wound healing. Clin. Plasma Med. 2016, 4, 19–28. [Google Scholar] [CrossRef]
- Rhodes, M.E. The Characterization of Pseudomonas-Fluorescens. J. Gen. Microbiol. 1959, 21, 221–263. [Google Scholar] [CrossRef]
- Rajmohan, S.; Dodd, C.E.R.; Waites, W.M. Enzymes from isolates of Pseudomonas fluorescens involved in food spoilage. J. Appl. Microbiol. 2002, 93, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Huang, T.; Dalwadi, H.; Sutton, C.L.; Bruckner, D.; Braun, J. Pseudomonas fluorescens encodes the Crohn’s disease-associated I2 sequence and T-cell superantigen. Infect. Immun. 2002, 70, 6567–6575. [Google Scholar] [CrossRef]
- Alsohim, A.S.; Taylor, T.B.; Barrett, G.A.; Gallie, J.; Zhang, X.X.; Altamirano-Junqueira, A.E.; Johnson, L.J.; Rainey, P.B.; Jackson, R.W. The biosurfactant viscosin produced by Pseudomonas fluorescens SBW25 aids spreading motility and plant growth promotion. Environ. Microbiol. 2014, 16, 2267–2281. [Google Scholar] [CrossRef]
- Martin, N.H.; Murphy, S.C.; Ralyea, R.D.; Wiedmann, M.; Boor, K.J. When cheese gets the blues: Pseudomonas fluorescens as the causative agent of cheese spoilage. J. Dairy Sci. 2011, 94, 3176–3183. [Google Scholar] [CrossRef]
- Miller, A.; Scanlan, R.A.; Lee, J.S.; Libbey, L.M. Volatile Compounds Produced in Sterile Fish Muscle (Sebastes-Melanops) by Pseudomonas-Putrefaciens, Pseudomonas-Fluorescens, and an Achromobacter Species. Appl. Microbiol. 1973, 26, 18–21. [Google Scholar] [CrossRef]
- Ternstrom, A.; Lindberg, A.M.; Molin, G. Classification of the Spoilage Flora of Raw and Pasteurized Bovine-Milk, with Special Reference to Pseudomonas and Bacillus. J. Appl. Bacteriol. 1993, 75, 25–34. [Google Scholar] [CrossRef]
- Ouattara, B.; Simard, R.E.; Holley, R.A.; Piette, G.J.P.; Begin, A. Antibacterial activity of selected fatty acids and essential oils against six meat spoilage organisms. Int. J. Food Microbiol. 1997, 37, 155–162. [Google Scholar] [CrossRef]
- Costerton, J.W.; Cheng, K.J.; Geesey, G.G.; Ladd, T.I.; Nickel, J.C.; Dasgupta, M.; Marrie, T.J. Bacterial Biofilms in Nature and Disease. Annu. Rev. Microbiol. 1987, 41, 435–464. [Google Scholar] [CrossRef] [PubMed]
- Allison, D.G.; Sutherland, I.W. The role of exopolysaccharides in adhesion of freshwater. Microbiology 1987, 133, 1319–1327. [Google Scholar] [CrossRef][Green Version]
- Flemming, H.C.; Neu, T.R.; Wozniak, D.J. The EPS matrix: The “House of Biofilm cells”. J. Bacteriol. 2007, 189, 7945–7947. [Google Scholar] [CrossRef]
- Sun, Y.Y.; Chi, H.; Sun, L. Pseudomonas fluorescens Filamentous Hemagglutinin, an Iron-Regulated Protein, Is an Important Virulence Factor that Modulates Bacterial Pathogenicity. Front. Microbiol. 2016, 7, 1320. [Google Scholar] [CrossRef]
- O’Toole, G.A.; Kolter, R. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: A genetic analysis. Mol. Microbiol. 1998, 28, 449–461. [Google Scholar] [CrossRef]
- Allison, D.G.; Ruiz, B.; SanJose, C.; Jaspe, A.; Gilbert, P. Extracellular products as mediators of the formation and detachment of Pseudomonas fluorescens biofilms. Fems Microbiol. Lett. 1998, 167, 179–184. [Google Scholar] [CrossRef]
- Willcock, L.; Holah, J.; Allison, D.G.; Gilbert, P. Steady-state biofilm and dispersal. Biofilms Community Interact. Control 1997, 23–31. [Google Scholar]
- Handorf, O.; Weihe, T.; Bekeschus, S.; Graf, A.C.; Schnabel, U.; Riedel, K.; Ehlbeck, J. Non-thermal plasma jet treatment negatively affects viability and structure of C. albicans SC5314 biofilms. Appl. Environ. Microbiol. 2018, 84, e01163-18. [Google Scholar] [CrossRef]
- Handorf, O.; Schnabel, U.; Bösel, A.; Weihe, T.; Bekeschus, S.; Graf, A.C.; Riedel, K.; Ehlbeck, J. Antimicrobial effects of microwave-induced plasma torch (MiniMIP) treatment on Candida albicans biofilms. Microb. Biotechnol. 2019, 12, 1034–1048. [Google Scholar] [CrossRef]
- Gränicher, W.H.H. Messung Beendet-Was Nun? Hochschulverlag AG der ETH Zürich: Zurich, Switzerland, 1994; Volume 6, pp. 6–9. [Google Scholar]
- Scudiero, D.A.; Shoemaker, R.H.; Paull, K.D.; Monks, A.; Tierney, S.; Nofziger, T.H.; Currens, M.J.; Seniff, D.; Boyd, M.R. Evaluation of a Soluble Tetrazolium Formazan Assay for Cell-Growth and Drug Sensitivity in Culture Using Human and Other Tumor-Cell Lines. Cancer Res. 1988, 48, 4827–4833. [Google Scholar]
- Schlüter, O.; Ehlbeck, J.; Hertel, C.; Habermeyer, M.; Roth, A.; Engel, K.H.; Holzhauser, T.; Knorr, D.; Eisenbrand, G. Opinion on the use of plasma processes for treatment of foods. Mol. Nutr. Food Res. 2013, 57, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Bourke, P.; Ziuzina, D.; Boehm, D.; Cullen, P.J.; Keener, K. The Potential of Cold Plasma for Safe and Sustainable Food Production. Trends Biotechnol. 2018, 36, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, U.; Niquet, R.; Krohmann, U.; Winter, J.; Schlüter, O.; Weltmann, K.D.; Ehlbeck, J. Decontamination of Microbiologically Contaminated Specimen by Direct and Indirect Plasma Treatment. Plasma Process. Polym. 2012, 9, 569–575. [Google Scholar] [CrossRef]
- Pankaj, S.K.; Bueno-Ferrer, C.; Misra, N.N.; Milosavljevic, V.; O’Donnell, C.P.; Bourke, P.; Keener, K.M.; Cullen, P.J. Applications of cold plasma technology in food packaging. Trends Food Sci. Technol. 2014, 35, 5–17. [Google Scholar] [CrossRef]
- Ziuzina, D.; Petil, S.; Cullen, P.J.; Keener, K.M.; Bourke, P. Atmospheric cold plasma inactivation of Escherichia coli, Salmonella enterica serovar Typhimurium and Listeria monocytogenes inoculated on fresh produce. Food Microbiol. 2014, 42, 109–116. [Google Scholar] [CrossRef]
- Misra, N.N.; Patil, S.; Moiseev, T.; Bourke, P.; Mosnier, J.P.; Keener, K.M.; Cullen, P.J. In-package atmospheric pressure cold plasma treatment of strawberries (vol 125, pg 131, 2014). J. Food Eng. 2015, 161, 95. [Google Scholar] [CrossRef]
- Oehmigen, K.; Winter, J.; Hähnel, M.; Wilke, C.; Brandenburg, R.; Weltmann, K.D.; von Woedtke, T. Estimation of Possible Mechanisms of Escherichia coli Inactivation by Plasma Treated Sodium Chloride Solution. Plasma Process. Polym. 2011, 8, 904–913. [Google Scholar] [CrossRef]
- Van Gils, C.A.J.; Hofmann, S.; Boekema, B.K.H.L.; Brandenburg, R.; Bruggeman, P.J. Mechanisms of bacterial inactivation in the liquid phase induced by a remote RF cold atmospheric pressure plasma jet. J. Phys. D Appl. Phys. 2013, 46, 175203. [Google Scholar] [CrossRef]
- Dolezalova, E.; Lukes, P. Membrane damage and active but nonculturable state in liquid cultures of Escherichia coli treated with an atmospheric pressure plasma jet. Bioelectrochemistry 2015, 103, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Andrasch, M.; Stachowiak, J.; Schlüter, O.; Schnabel, U.; Ehlbeck, J. Scale-up to pilot plant dimensions of plasma processed water generation for fresh-cut lettuce treatment. Food Packag. Shelf Life 2017, 14, 40–45. [Google Scholar] [CrossRef]
- Van Houdt, R.; Michiels, C. Biofilm formation and the food industry, a focus on the bacterial outer surface. J. Appl. Microbiol. 2010, 109, 1117–1131. [Google Scholar] [CrossRef] [PubMed]
- Baum, M.M.; Kainovic, A.; O’Keeffe, T.; Pandita, R.; McDonald, K.; Wu, S.; Webster, P. Characterization of structures in biofilms formed by a Pseudomonas fluorescens isolated from soil. BMC Microbiol. 2009, 9, 103. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef]
- Choi, Y.C.; Morgenroth, E. Monitoring biofilm detachment under dynamic changes in shear stress using laser-based particle size analysis and mass fractionation. Water Sci. Technol. 2003, 47, 69–76. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef]
- Kaplan, J.B. Biofilm Dispersal: Mechanisms, Clinical Implications, and Potential Therapeutic Uses. J. Dent. Res. 2010, 89, 205–218. [Google Scholar] [CrossRef]
- Gibson, H.; Taylor, J.H.; Hall, K.E.; Holah, J.T. Effectiveness of cleaning techniques used in the food industry in terms of the removal of bacterial biofilms. J. Appl. Microbiol. 1999, 87, 41–48. [Google Scholar] [CrossRef]
- Chmielewski, R.A.N.; Frank, J.F. Biofilm Formation and Control in Food Processing Facilities. Compr. Rev. Food Sci. Food Saf. 2003, 2, 22–32. [Google Scholar] [CrossRef]
- Myszka, K.; Czaczyk, K. Bacterial Biofilms on Food Contact Surfaces—A Review. Polish J. Food Nutr. Sci. 2011, 61, 173–180. [Google Scholar] [CrossRef]
- Magder, S. Reactive oxygen species: Toxic molecules or spark of life? Crit. Care 2006, 10, 208. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Wu, H.; Collier-Hyams, L.S.; Hansen, J.M.; Li, T.; Yamoah, K.; Pan, Z.Q.; Jones, D.P.; Neish, A.S. Commensal bacteria modulate cullin-dependent signaling via generation of reactive oxygen species. EMBO J. 2007, 26, 4457–4466. [Google Scholar] [CrossRef] [PubMed]
- Weidinger, A.; Kozlov, A.V. Biological Activities of Reactive Oxygen and Nitrogen Species: Oxidative Stress versus Signal Transduction. Biomolecules 2015, 5, 472–484. [Google Scholar] [CrossRef]
- Picioreanu, C.; van Loosdrecht, M.C.M.; Heijnen, J.J. Two-dimensional model of biofilm detachment caused by internal stress from liquid flow. Biotechnol. Bioeng. 2001, 72, 205–218. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Handorf, O.; Pauker, V.I.; Schnabel, U.; Weihe, T.; Freund, E.; Bekeschus, S.; Riedel, K.; Ehlbeck, J. Characterization of Antimicrobial Effects of Plasma-Treated Water (PTW) Produced by Microwave-Induced Plasma (MidiPLexc) on Pseudomonas fluorescens Biofilms. Appl. Sci. 2020, 10, 3118. https://doi.org/10.3390/app10093118
Handorf O, Pauker VI, Schnabel U, Weihe T, Freund E, Bekeschus S, Riedel K, Ehlbeck J. Characterization of Antimicrobial Effects of Plasma-Treated Water (PTW) Produced by Microwave-Induced Plasma (MidiPLexc) on Pseudomonas fluorescens Biofilms. Applied Sciences. 2020; 10(9):3118. https://doi.org/10.3390/app10093118
Chicago/Turabian StyleHandorf, Oliver, Viktoria Isabella Pauker, Uta Schnabel, Thomas Weihe, Eric Freund, Sander Bekeschus, Katharina Riedel, and Jörg Ehlbeck. 2020. "Characterization of Antimicrobial Effects of Plasma-Treated Water (PTW) Produced by Microwave-Induced Plasma (MidiPLexc) on Pseudomonas fluorescens Biofilms" Applied Sciences 10, no. 9: 3118. https://doi.org/10.3390/app10093118
APA StyleHandorf, O., Pauker, V. I., Schnabel, U., Weihe, T., Freund, E., Bekeschus, S., Riedel, K., & Ehlbeck, J. (2020). Characterization of Antimicrobial Effects of Plasma-Treated Water (PTW) Produced by Microwave-Induced Plasma (MidiPLexc) on Pseudomonas fluorescens Biofilms. Applied Sciences, 10(9), 3118. https://doi.org/10.3390/app10093118







