Basal-Plane Catalytic Activity of Layered Metallic Transition Metal Ditellurides for the Hydrogen Evolution Reaction
Abstract
1. Introduction
2. Experiments
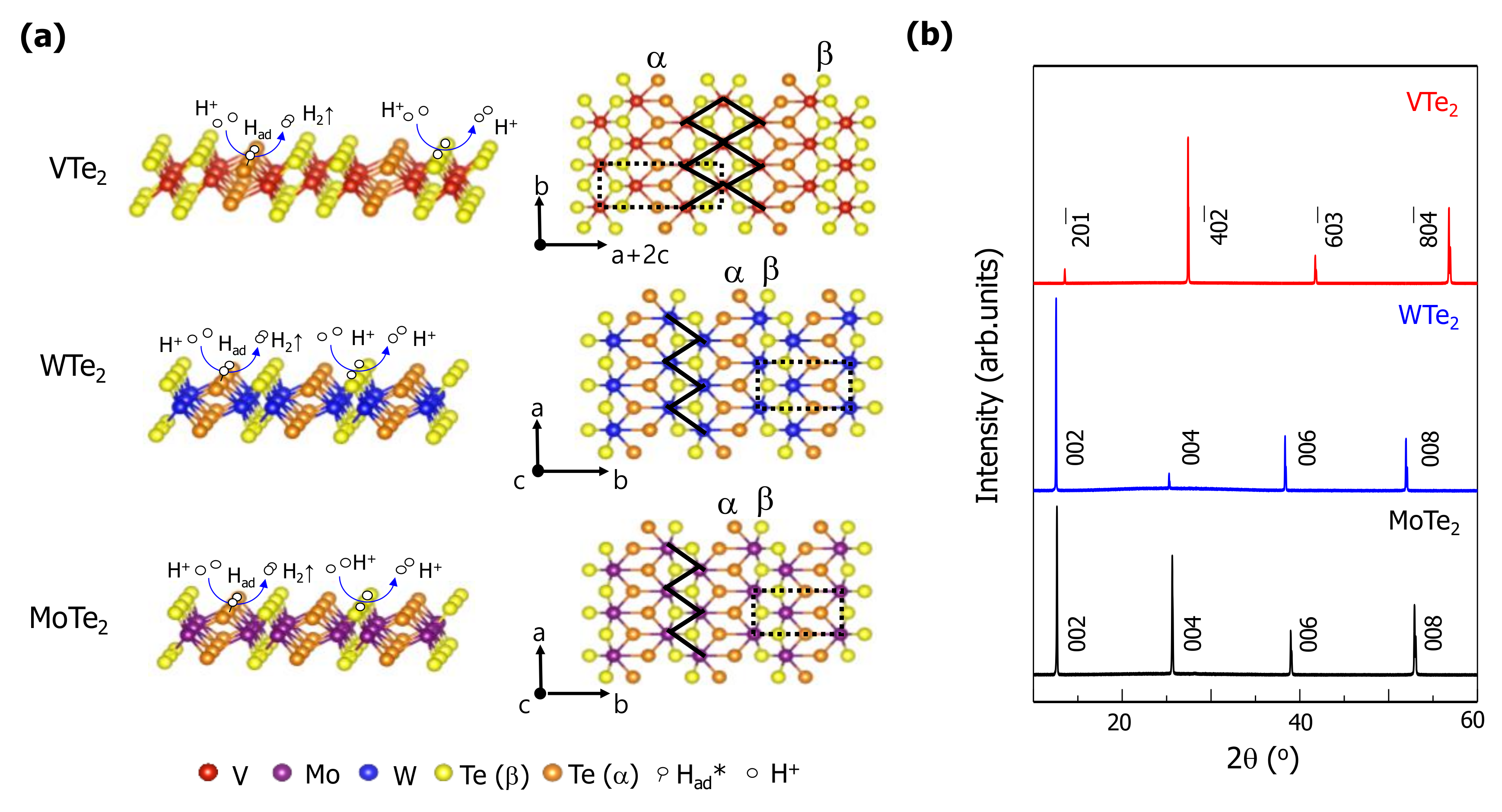
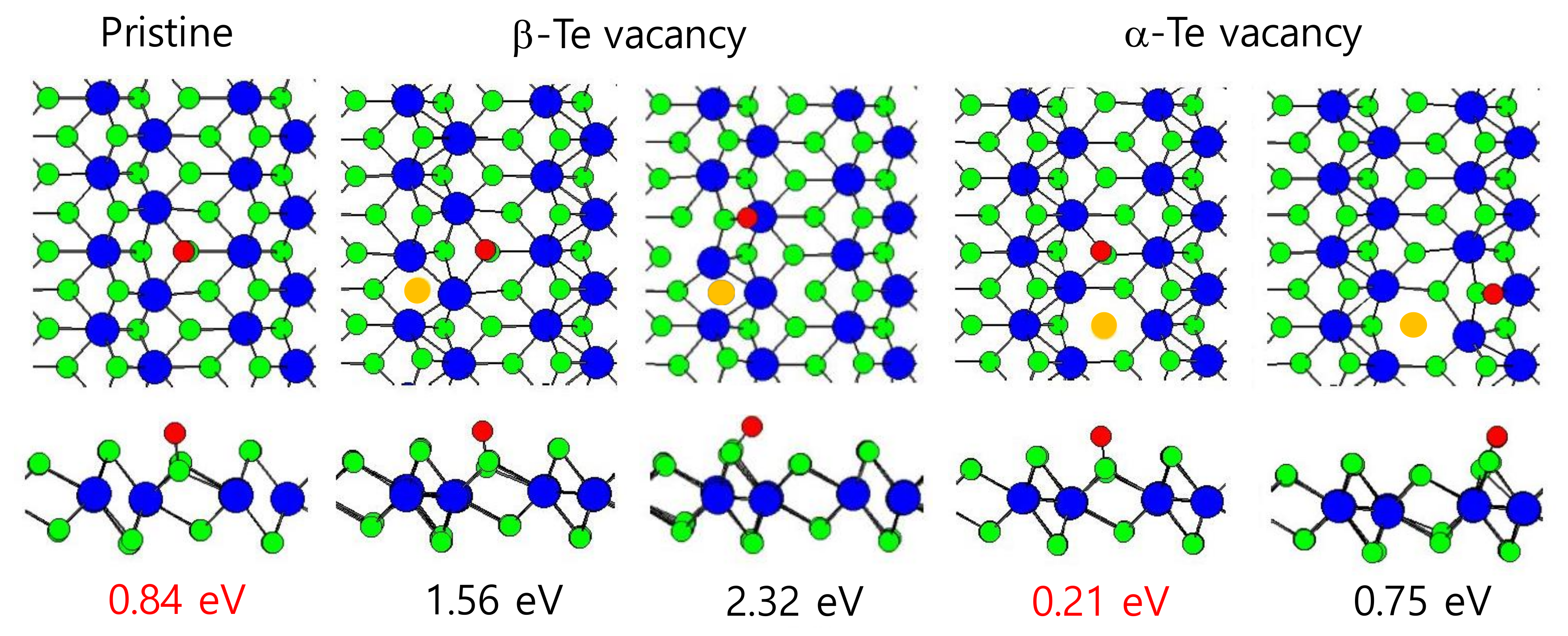
3. Result and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Dong, G.; Fang, M.; Wang, H.; Yip, S.; Cheung, H.Y.; Wang, F.; Wong, C.Y.; Chu, S.T.; Ho, J.C. Insight into the electrochemical activation of carbon-based cathodes for hydrogen evolution reaction. J. Mater. Chem. A 2015, 3, 13080. [Google Scholar] [CrossRef]
- Zhang, L.; Han, L.; Liu, H.; Liu, X.; Luo, J. Potential-cycling synthesis of single platinum atoms for efficient hydrogen evolution in neutral media. Angew. Chem. 2017, 129, 13882. [Google Scholar] [CrossRef]
- Sun, S.W.; Wang, G.F.; Zhou, Y.; Wang, F.B.; Xia, X.H. High-performance Ru@C4N electrocatalyst for hydrogen evolution reaction in both acidic and alkaline solutions. ACS Appl. Mater. Interfaces 2019, 11, 19176–19182. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sofer, Z.; Luxa, J.; Pumera, M. Lithium exfoliated vanadium dichalcogenides (VS2, VSe2, VTe2) exhibit dramatically different properties from their bulk counterparts. Adv. Mater. Interfaces 2016, 3, 1600433. [Google Scholar] [CrossRef]
- Li, C.; Baek, J.B. Recent advances in noble metal (Pt, Ru, and Ir)-based electrocatalysts for efficient hydrogen evolution reaction. ACS Omega 2020, 5, 31–40. [Google Scholar] [CrossRef]
- Tavakkoli, M.; Holmberg, N.; Kronberg, R.; Jiang, H.; Saino, J.; Kauppinen, E.I.; Kallio, T.; Lassonen, K. Electrochemical activation of single-walled carbon nanotubes with Pseudo-atomic-scale platinum for the hydrogen evolution reaction. ACS Catal. 2017, 7, 3121. [Google Scholar] [CrossRef]
- Chen, J.; Yang, Y.; Su, J.; Jiang, P.; Zia, G.; Chen, Q. Enhanced activity for hydrogen evolution reaction over CoFe catalysts by alloying with small amount of Pt. ACS Appl. Mater. Interfaces 2017, 9, 4. [Google Scholar] [CrossRef]
- Jiang, B.; Sun, Y.; Cheng, Y.; Shao, M. Pt nanocrystals on nitrogen-doped graphene for the hydrogen evolution reaction using Si nanowires as a sacrificial template. Nanoscale 2017, 9, 10138–10144. [Google Scholar] [CrossRef]
- Liu, D.; Li, L.; You, T. Superior catalytic performances of platinum nanoparticles loaded nitrogen-doped graphene toward methanol oxidation and hydrogen evolution reaction. J. Colloid Interface Sci. 2017, 487, 330–335. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Zang, X.; Li, X.; Zhu, H. Photo-promoted platinum nanoparticles decorated MoS2@Graphene woven fabric catalyst for efficient hydrogen generation. ACS Appl. Mater. Interfaces 2016, 8, 10866–10873. [Google Scholar] [CrossRef]
- Li, W.; Amiinu, I.S.; Ye, B.; Wang, Z.; Zhu, J.; Kou, Z.; Mu, S. TePtFe nanotubes as high-performing bifunctional electrocatalysts for the oxygen reduction reaction and hydrogen evolution reaction. ChemSusChem 2018, 11, 1328–1333. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, Y.; Okayasu, T.; Yadav, A.P.; Nishikata, A.; Tsuru, T. Dissolution mechanism of platinum in sulfuric acid solution. J. Electrochem. Soc. 2012, 159, F779–F786. [Google Scholar] [CrossRef]
- Seok, J.; Lee, J.; Bae, D.; Ji, B.; Son, Y.; Lee, Y.H.; Yang, H.; Cho, S. Hybrid catalyst with monoclinic MoTe2 and platinum for efficient hydrogen evolution. APL Mater. 2019, 7, 071118. [Google Scholar] [CrossRef]
- Ling, N.; Wang, Z.; Kim, S.; Oh, S.H.; Park, J.H.; Shin, H.; Cho, S.; Yang, H. In operando stacking of reduced graphene oxide for active hydrogen evolution. ACS Appl. Mater. Interfaces 2019, 11, 43460–43465. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.W.; DeLaRiva, A.T.; Challa, S.R.; Datye, A.K. Sintering of catalytic nanoparticles: Particle migration or Ostwald rippening? Acc. Chem. Res. 2013, 46, 1720–1730. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.; Xiong, H.; De La Riva, A.T.; Peterson, E.J.; Pham, H.; Challa, S.R.; Qi, G.; Oh, S.; Wiebenga, M.H.; Hernández, X.I.P.; et al. Thermally stable single-atom platinum-on-ceria catalysts via atom trapping. Science 2016, 353, 150–154. [Google Scholar] [CrossRef]
- Hinnemann, B.; Moses, P.G.; Bonde, J.; Jørgensen, K.P.; Nielsen, J.H.; Horch, S.; Chorkendorff, I.; Nørskov, J.K. Biomimetic hydrogen evolution: MoS2 nanoparticles as catalyst for hydrogen evolution. J. Am. Chem. Soc. 2005, 127, 5308–5309. [Google Scholar] [CrossRef]
- Lee, J.; Kang, S.; Yim, K.; Kim, K.Y.; Jang, H.W.; Kang, Y.; Han, S. Hydrogen evolution reaction at anion vacancy of two-dimensional transition-metal dichalcogenides: Ab initio computational screening. J. Phys. Chem. Lett. 2018, 9, 2049–2055. [Google Scholar] [CrossRef]
- Li, G.; Zhang, D.; Qiao, Q.; Yu, Y.; Peterson, D.; Zafar, A.; Kumar, R.; Curtarolo, S.; Hunte, F.; Shannon, S.; et al. All the catalytic active sites of MoS2 for hydrogen evolution. J. Am. Chem. Soc. 2016, 138, 16632. [Google Scholar] [CrossRef]
- Tsai, C.; Chan, K.; Abild-Pedersen, F.; Nørskov, J.K. Active edge sites in MoSe2 and WSe2 catalysts for the hydrogen evolution reaction: A density functional study. Phys. Chem. Chem. Phys. 2014, 16, 13156–13164. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, H.; Li, S.; Wang, R.; Sun, X.; Zhou, M.; Zhou, J.; Lou, X.W.; Xie, Y. Defect-rich MoS2 ultrathin nanosheets with additional active edge sites for enhanced electrocatalytic hydrogen evolution. Adv. Mater. 2013, 25, 5807–5813. [Google Scholar] [CrossRef] [PubMed]
- Seok, J.; Lee, J.; Cho, S.; Ji, B.; Kim, H.W.; Kwon, M.; Kim, D.; Kim, Y.; Oh, S.H.; Kim, S.W.; et al. Active hydrogen evolution through lattice distortion in metallic MoTe2. 2D Mater. 2017, 4, 025061. [Google Scholar] [CrossRef]
- McGlynn, J.C.; Dankwort, T.; Kienle, L.; Bandeira, N.A.G.; Fraser, J.P.; Gibson, E.K.; Cascallana-Matías, I.; Kamarás, K.; Symes., M.D.; Miras, H.N.; et al. The rapid electrochemical activation of MoTe2 for the hydrogen evolution reaction. Nat. Commun. 2019, 10, 4916. [Google Scholar] [CrossRef] [PubMed]
- Mc Manus, J.B.; Cunningham, G.; McEvoy, N.; Cullen, C.P.; Gity, F.L.; Schmidt, M.; Mullarkey, D.; Shvets, I.V.; Hurley, P.K.; Hallam, T.; et al. Growth of 1T’ MoTe2 by thermally assisted conversion of electrodeposited tellurium films. ACS Appl. Energy Mater. 2019, 2, 521–530. [Google Scholar] [CrossRef]
- Rajamathi, C.R.; Gupta, U.; Kumar, N.; Yang, H.; Sun, Y.; Süß, V.; Shekhar, C.; Schmidt, M.; Blumtritt, H.; Werner, P.; et al. Weyl semimetal as hydrogen evolution catalyst. Adv. Mater. 2017, 29, 1606202. [Google Scholar] [CrossRef]
- Luxa, J.; Vosecký, P.; Mazánek, V.; Sedmidubský, D.; Pumera, M.; Lazar, P.; Sofer, Z. Layered transition-metal ditellurides in electrocatalystic applications—Contrasting properties. ACS Catal. 2017, 7, 5706–5716. [Google Scholar] [CrossRef]
- Kwon, H.; Ji, B.; Bae, D.; Lee, J.H.; Park, H.J.; Kim, D.H.; Kim, Y.M.; Son, Y.W.; Yang, H.; Cho, S. Role of anionic vacancy for active hydrogen evolution in WTe2. Appl. Surf. Sci. 2020, 515, 145972. [Google Scholar] [CrossRef]
- Keum, D.H.; Cho, S.; Kim, J.H.; Choe, D.; Sung, H.; Kan, M.; Kang, H.; Hwang, J.; Kim, S.W.; Yang, H.; et al. Bandgap opening in few-layered monoclinic MoTe2. Nat. Phys. 2015, 11, 482–486. [Google Scholar] [CrossRef]
- Won, D.; Kiem, D.H.; Cho, H.; Kim, D.; Kim, Y.; Jeong, M.Y.; Seo, C.; Kim, J.; Park, J.; Han, M.J.; et al. Polymorphic spin, charge, and lattice waves in vanadium ditelluride. Adv. Mater. 2020, 1906578, 1–7. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. QUANTUM ESPRESSO: A modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter. 2009, 21, 395502. [Google Scholar] [CrossRef]
- Hamann, D.R.; Schlüter, M.; Chiang, C. Norm-conserving pseudopotentials. Phys. Rev. Let. 1979, 43, 1494. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Let. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Kang, S.H.; Yu, H.S.; Kim, H.W.; Ko, W.; Hwang, S.W.; Han, W.H.; Choe, D.H.; Jung, Y.H.; Chang, K.J.; et al. Te vacancy-driven superconductivity in orthorhombic molybdenum ditelluride. 2D Mater. 2017, 4, 021030. [Google Scholar] [CrossRef]
- Ali, M.N.; Xiong, J.; Flynn, S.; Tao, J.; Gibson, Q.D.; Schoop, L.M.; Liang, T.; Haldolaarachchige, N.; Hirschberger, M.; Ong, N.P.; et al. Large, non-saturating magnetoresistance in WTe2. Nature 2014, 514, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Chia, X.; Ambrosi, A.; Lazar, P.; Sofer, Z.; Pumera, M. Electrocatalysis of layered group 5 metallic transition metal dichalcogenides (MX2, M= V, Nb, and Ta; X= S, Se, and Te). J. Mater. Chem. A 2016, 4, 14241. [Google Scholar] [CrossRef]
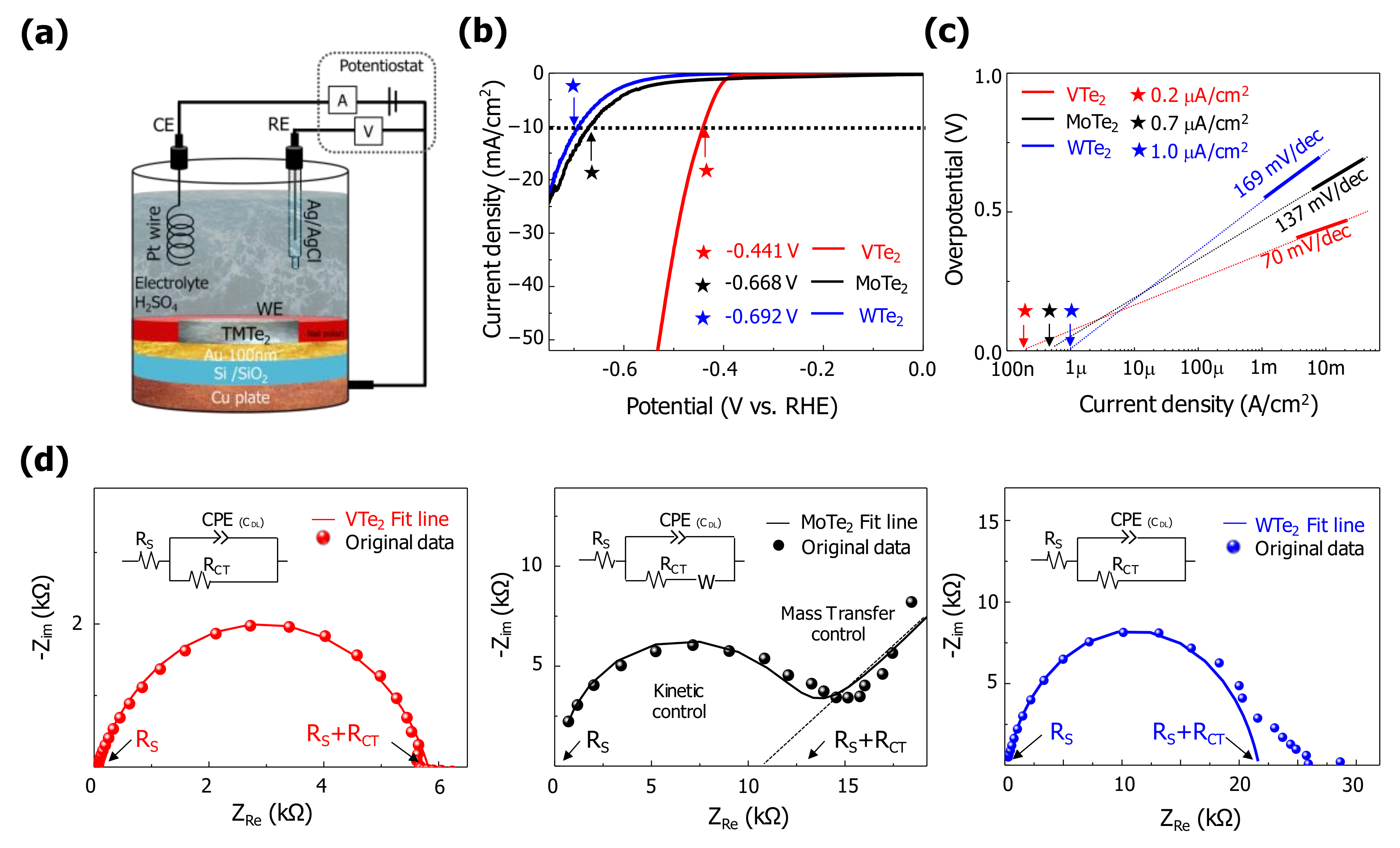

| Catalyst | Overpotential (mV vs. RHE) | Tafel Slope (mV/dec) | Exchange Current Density (mA/cm2) | ⚜ site density (#/nm2) | TOF (ms−) | Double Layer Capacitance (mF/cm2) | Charge Transfer Resistance (k⚜CT) |
|---|---|---|---|---|---|---|---|
| VTe2 | −441 | 70 | 0.2 | 2.92 | 2.14 | 0.03 | 5.8 |
| MoTe2 | −668 | 137 | 0.7 | 4.50 | 4.85 | 0.08 | 13.0 |
| WTe2 | −692 | 169 | 1.0 | 4.51 | 6.92 | 0.06 | 21.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, H.; Bae, D.; Jun, H.; Ji, B.; Won, D.; Lee, J.-H.; Son, Y.-W.; Yang, H.; Cho, S. Basal-Plane Catalytic Activity of Layered Metallic Transition Metal Ditellurides for the Hydrogen Evolution Reaction. Appl. Sci. 2020, 10, 3087. https://doi.org/10.3390/app10093087
Kwon H, Bae D, Jun H, Ji B, Won D, Lee J-H, Son Y-W, Yang H, Cho S. Basal-Plane Catalytic Activity of Layered Metallic Transition Metal Ditellurides for the Hydrogen Evolution Reaction. Applied Sciences. 2020; 10(9):3087. https://doi.org/10.3390/app10093087
Chicago/Turabian StyleKwon, Hagyeong, Dongyeon Bae, Hyeyoung Jun, Byungdo Ji, Dongyeun Won, Jun-Ho Lee, Young-Woo Son, Heejun Yang, and Suyeon Cho. 2020. "Basal-Plane Catalytic Activity of Layered Metallic Transition Metal Ditellurides for the Hydrogen Evolution Reaction" Applied Sciences 10, no. 9: 3087. https://doi.org/10.3390/app10093087
APA StyleKwon, H., Bae, D., Jun, H., Ji, B., Won, D., Lee, J.-H., Son, Y.-W., Yang, H., & Cho, S. (2020). Basal-Plane Catalytic Activity of Layered Metallic Transition Metal Ditellurides for the Hydrogen Evolution Reaction. Applied Sciences, 10(9), 3087. https://doi.org/10.3390/app10093087





