Effect of Precious Metals on NO Reduction by CO in Oxidative Conditions
Abstract
:1. Introduction
- -
- Direct use without transformation: industrial use (water treatment, supercritical CO2 …);
- -
- Chemical transformation: organic chemical synthesis, methanation, hydrogenation (methanol), reforming: dry (CO2) or steam reforming (CO2 + H2O);
- -
- Biological transformation: microalgae, biocatalysis.
2. Materials and Methods
2.1. Catalysts Preparation
2.2. Characterization of the Catalysts
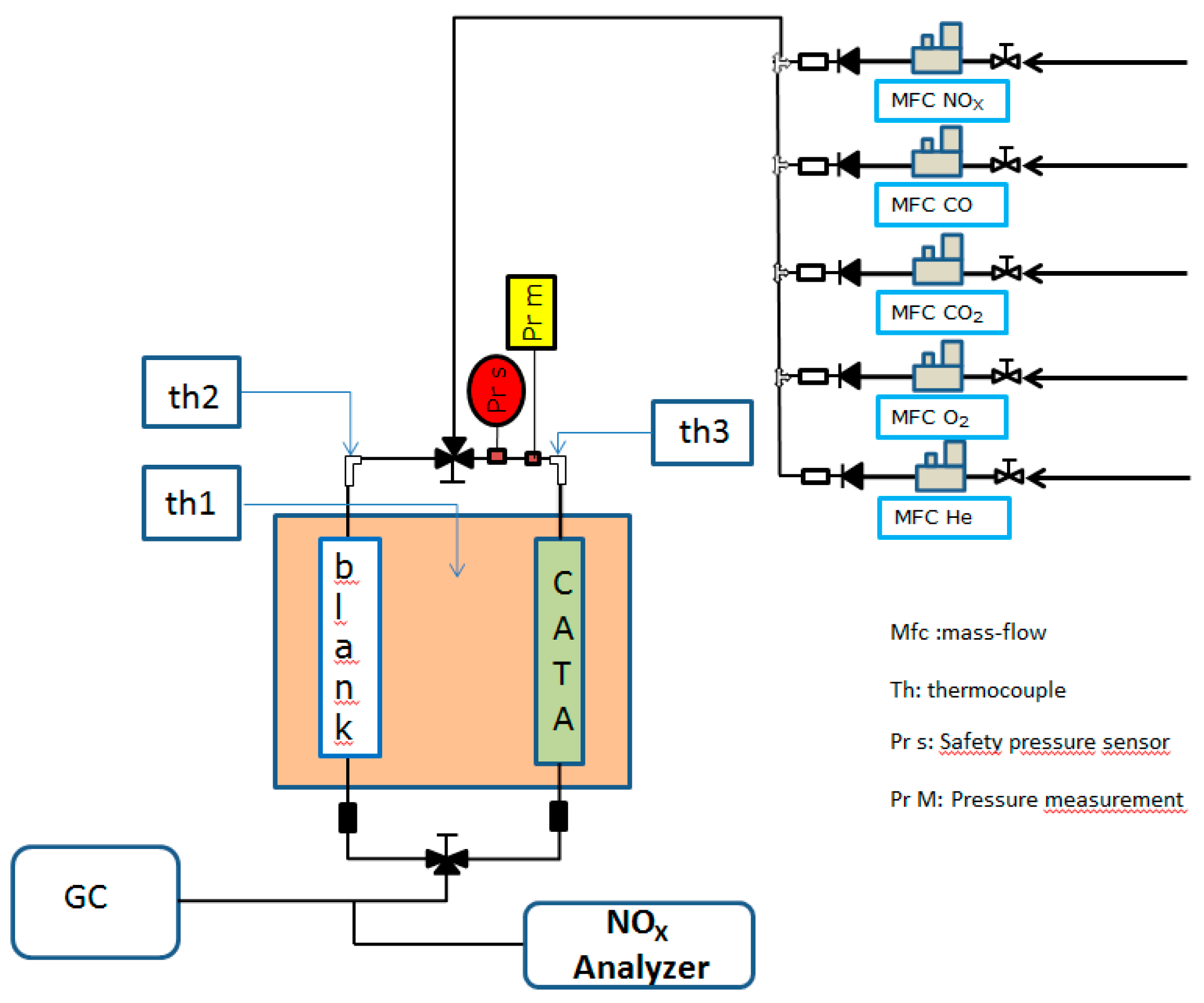
2.3. Catalytic Tests
- -
- Conversion: (%) = where and are number of mole of the corresponding compounds “i” at the inlet and the outlet of the reactor.
- -
- Selectivity: (%) = * 100.
- -
- Yield: (%) = .
3. Results
3.1. Brunauer–Emmett–Teller (BET) Surface Area
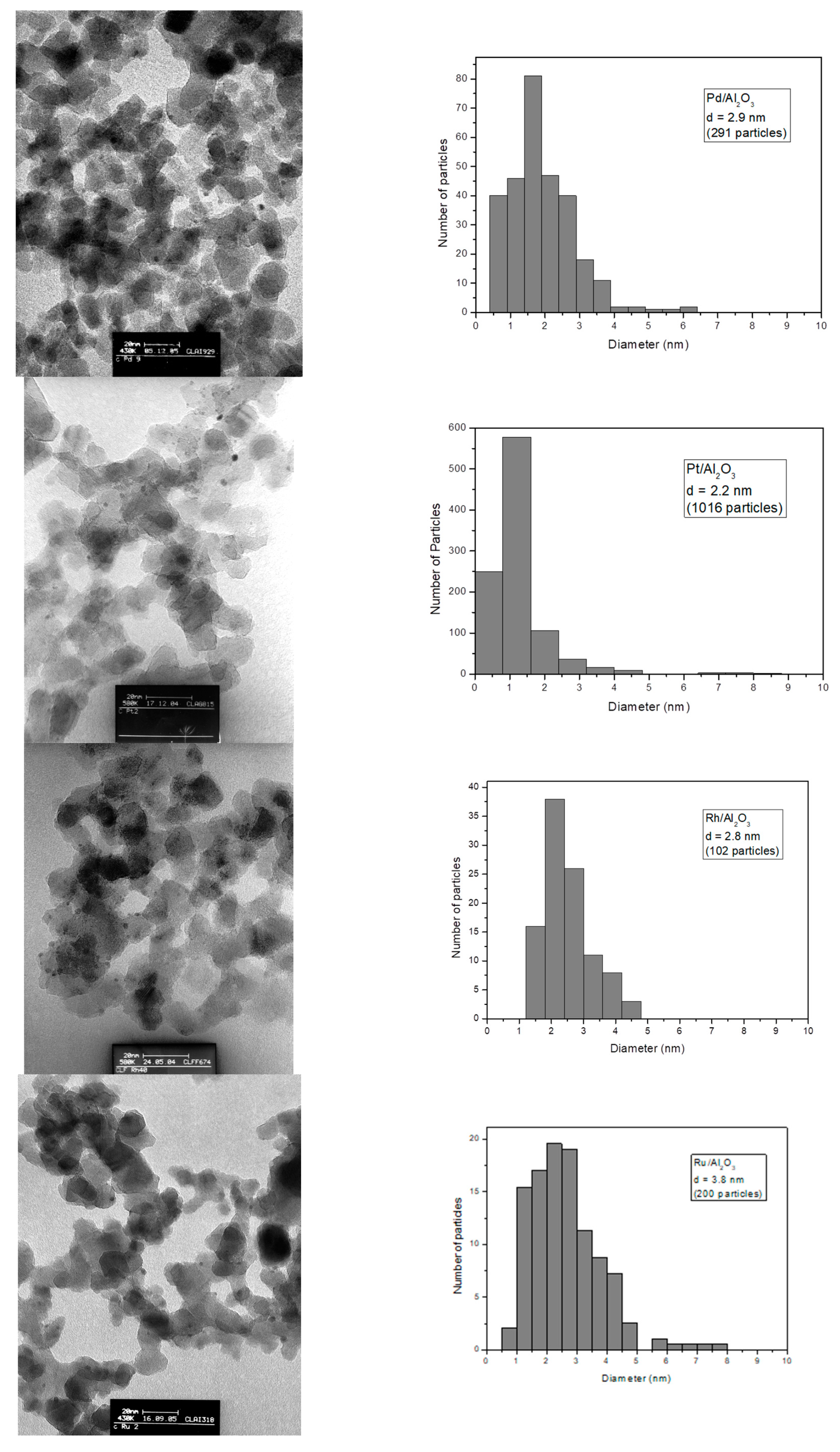
3.2. Transmission Electron Microscopy (TEM) and H2 Chemisorption
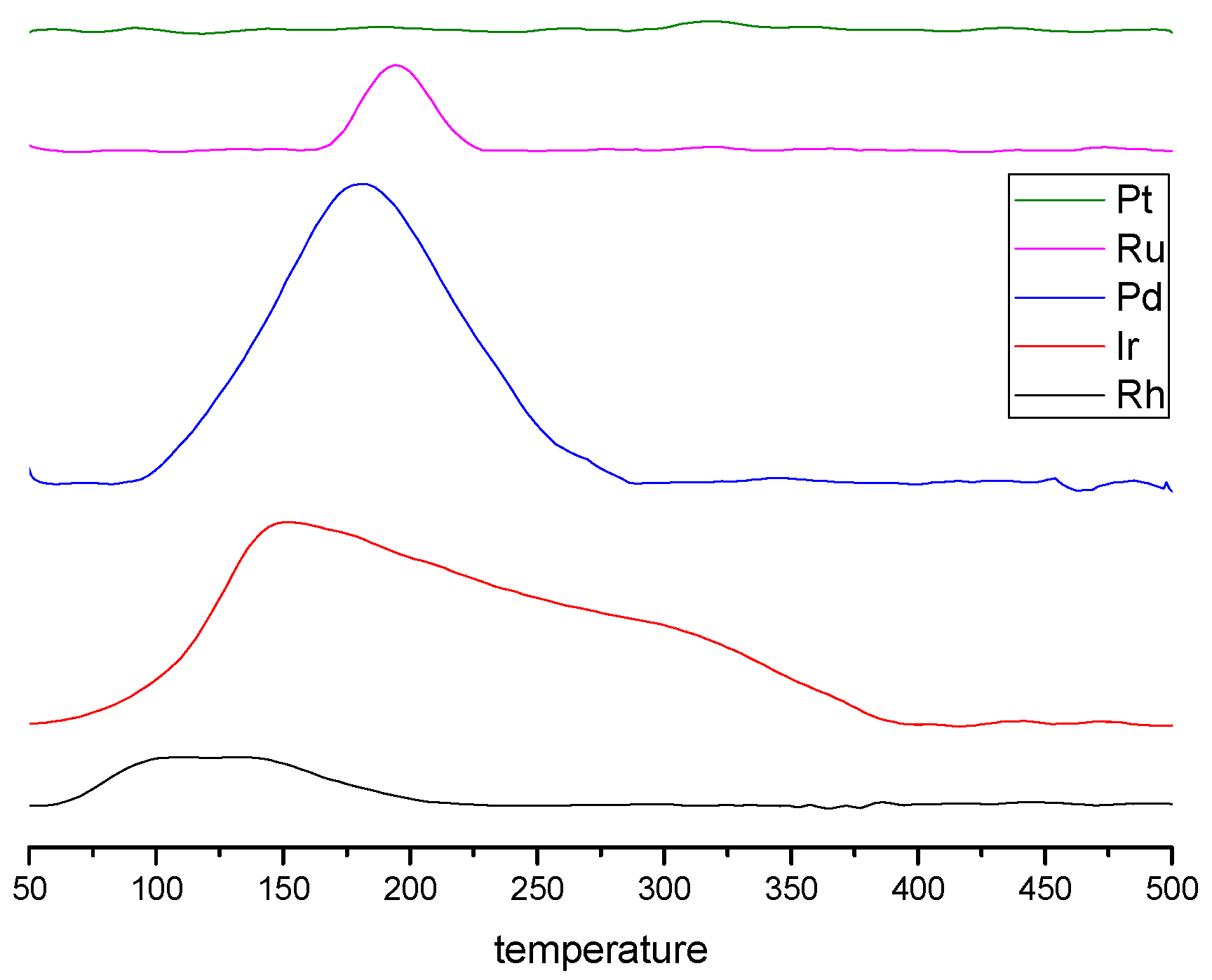
3.3. Temperature Programmed Reduction (TPR) by H2
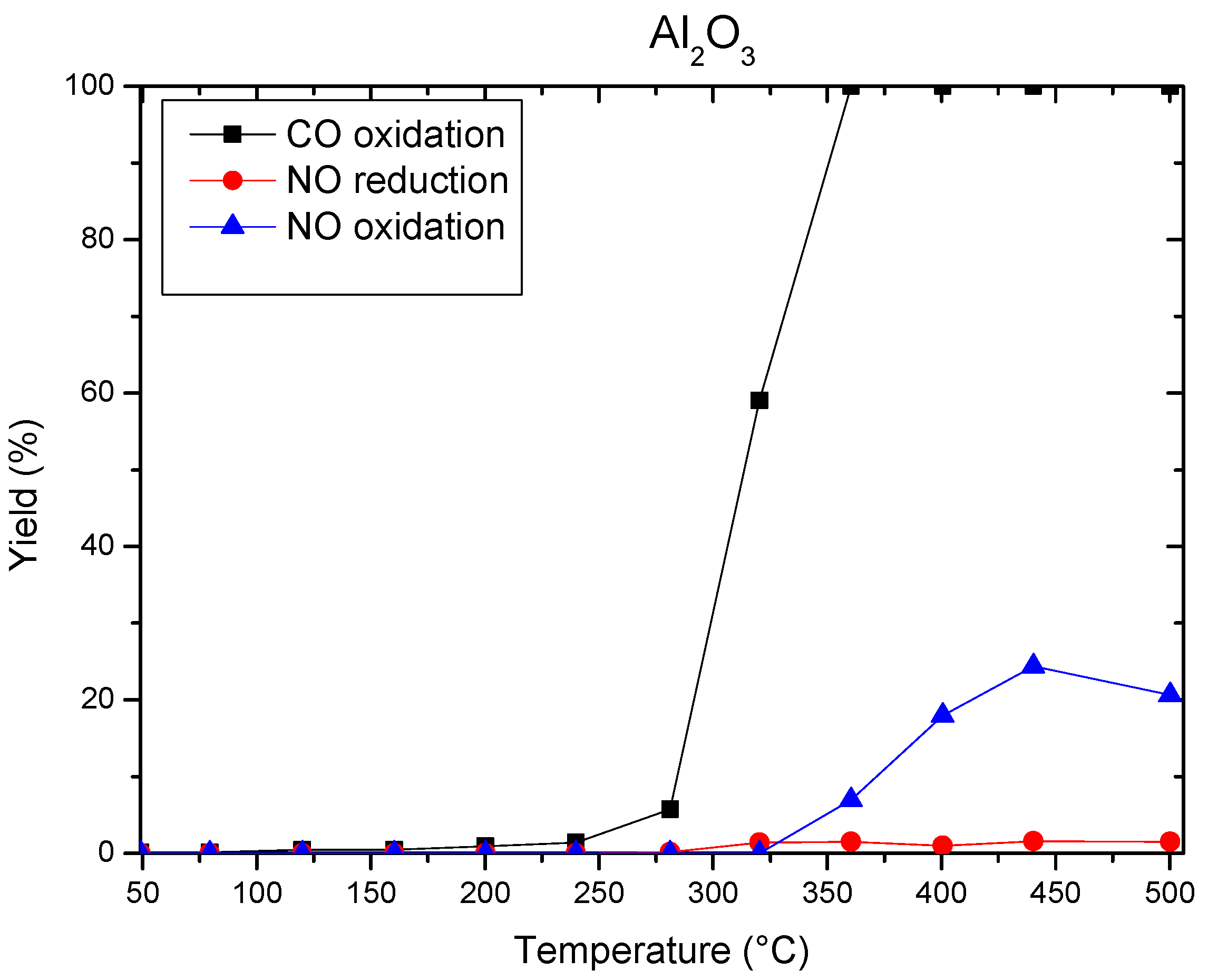
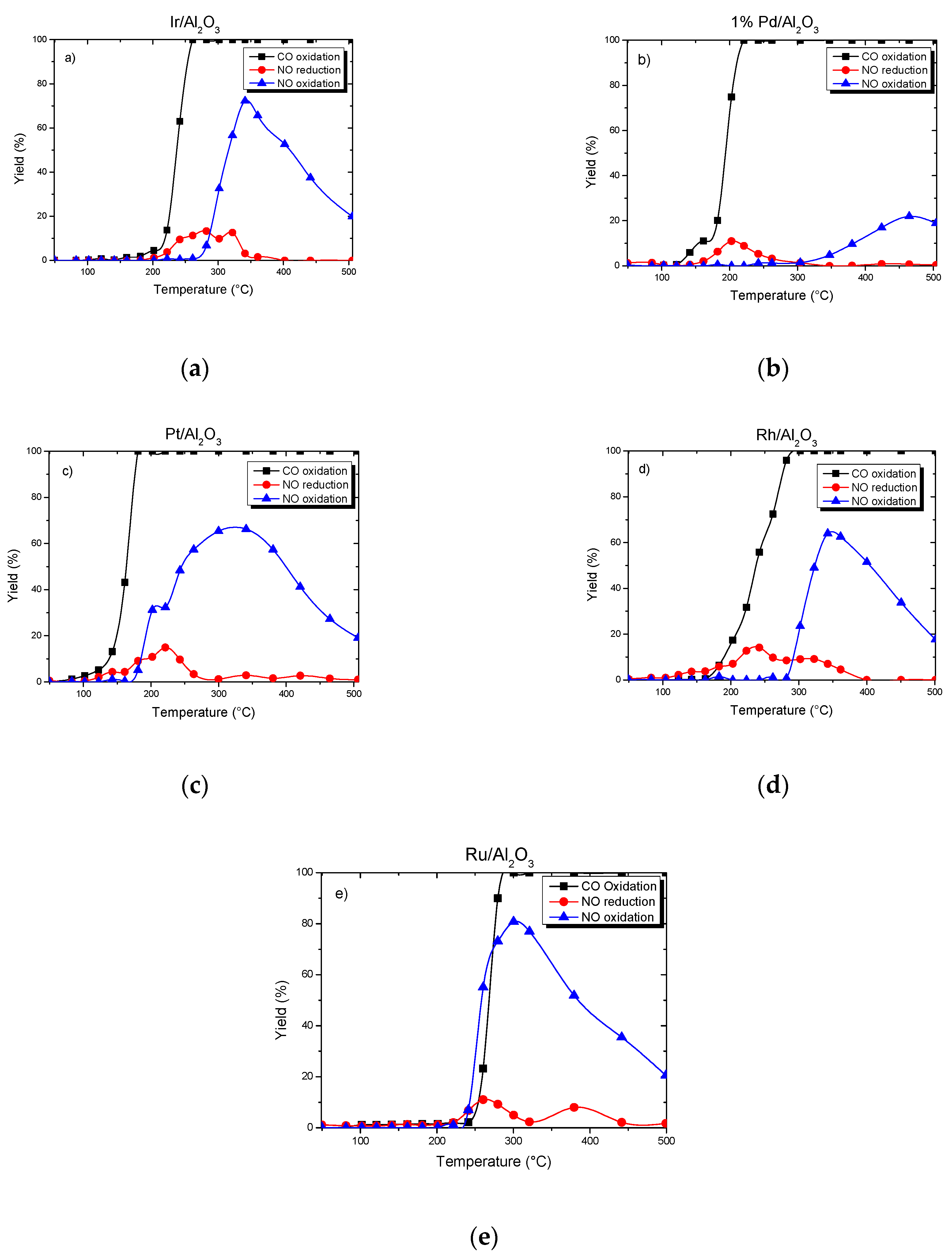
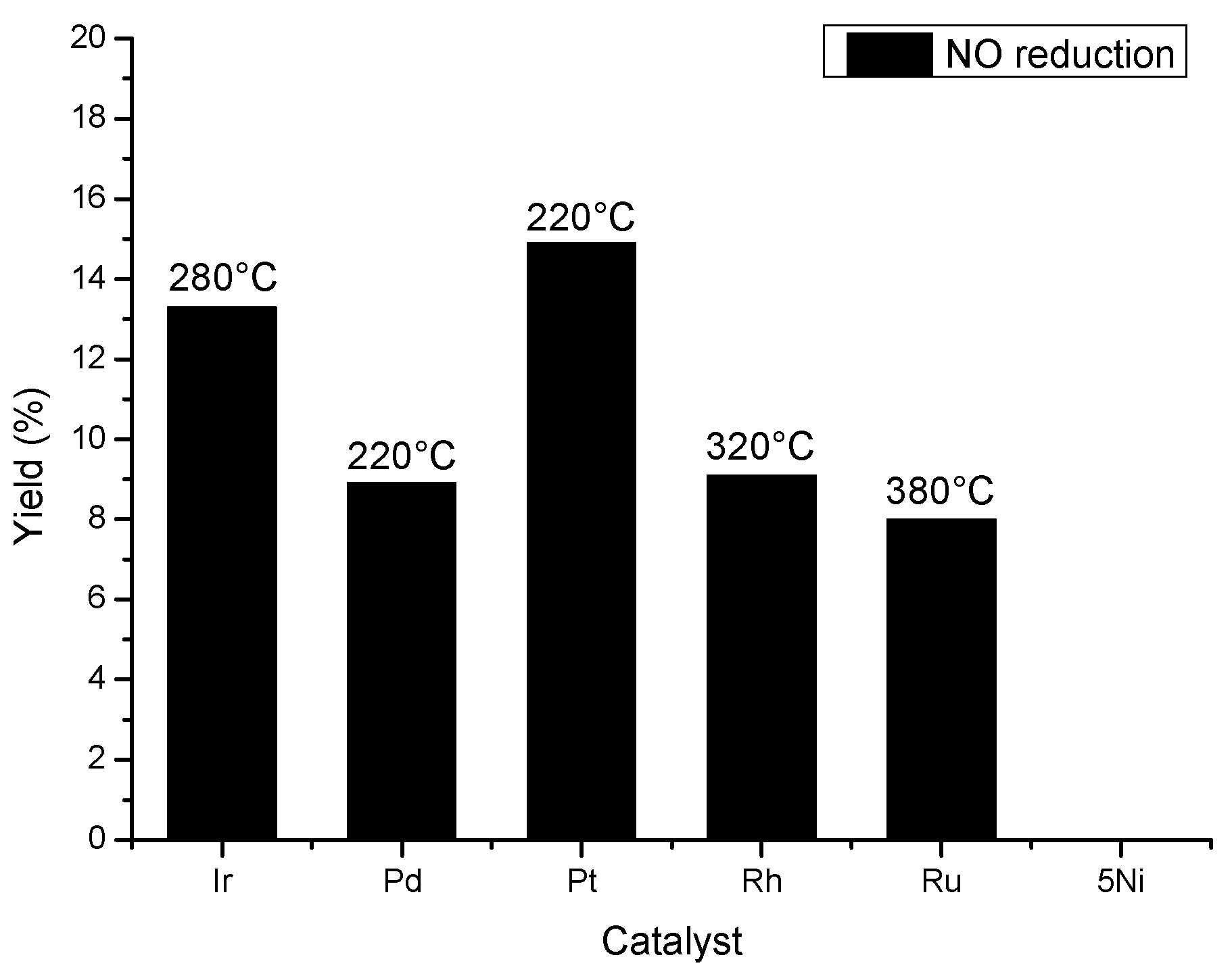
3.4. Catalytic Reaction
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Olivier, J.G.J.; Schure, K.M.; Peters, J.A.H.W. Trends in Global CO2 and Total Greenhouse Gas Emissions: Summary of the 2017 Report; PBL Netherlands Environmental Assessment Agency: The Hague, The Netherlands, 2017. [Google Scholar]
- Dumergues, L.; Favier, B.; Claver, R.A. CO2 Reuse. State of the Art and Expert Opinion Case of Waste Treatment Activities; RECORD: Pau, France, 2014. [Google Scholar]
- Iloeje, C.; Field, R.; Ghoniem, A.F. Modeling and parametric analysis of nitrogen and sulfur oxide removal from oxy-combustion flue gas using a single column absorber. Fuel 2015, 160, 178–188. [Google Scholar] [CrossRef]
- Skalska, K.; Miller, J.S.; Ledakowicz, S. Trends in NOx abatement: A review. Sci. Total. Environ. 2010, 408, 3976–3989. [Google Scholar] [CrossRef] [PubMed]
- Malfoy, P. Reduction Catalytique de NO et N2O par H2, CO ou C3H8; University of Poitiers: Poitiers, France, 1997. [Google Scholar]
- Hegedus, L.L.; Hertz, R.K.; Oh, S.H.; Aris, R.J. Effect of catalyst reactions loading on the simultaneous of NO, CO, and O2. J. Catal. 1979, 57, 513–515. [Google Scholar] [CrossRef]
- Tauster, S.; Murrell, L. The NO-CO reaction in the presence of excess O2 as catalyzed by iridium. J. Catal. 1976, 41, 192–195. [Google Scholar] [CrossRef]
- Shelef, M. The oxidation of CO by O2 and by NO on supported chromium oxide and other metal oxide catalysts. J. Catal. 1968, 12, 361–375. [Google Scholar] [CrossRef]
- Oh, S. Role of NO in inhibiting CO oxidation over alumina-supported rhodium. J. Catal. 1986, 101, 114–122. [Google Scholar] [CrossRef]
- Voltz, S.E.; Morgan, C.R.; Liederman, D.; Jacob, S.M. Kinetic study of carbon monoxide and propylene oxidation on platinum catalysts. Ind. Eng. Chem. Prod. Res. Dev. 1973, 12, 294–301. [Google Scholar] [CrossRef]
- Granger, P.; Parvulescu, V. Catalytic NOxAbatement systems for mobile sources: From three-way to lean burn after-treatment technologies. Chem. Rev. 2011, 111, 3155–3207. [Google Scholar] [CrossRef]
- Barbier, J.; Duprez, D. Steam effects in 3-way catalysis. Appl. Catal. B 1994, 4, 105–140. [Google Scholar] [CrossRef]
- Vedyagin, A.A.; Volodin, A.M.; Stoyanovskii, V.; Kenzhin, R.M.; Slavinskaya, E.; Mishakov, I.V.; Plyusnin, P.E.; Shubin, Y.V. Stabilization of active sites in alloyed Pd–Rh catalysts on γ-Al2O3 support. Catal. Today 2014, 238, 80–86. [Google Scholar] [CrossRef]
- Kobylinski, T.P.; Taylor, B.W. The catalytic chemistry of nitric oxide. J. Catal. 1974, 33, 376–384. [Google Scholar] [CrossRef]
- Taylor, K.C.; Schlatter, J.C. Selective reduction of nitric oxide over noble metals. J. Catal. 1980, 63, 53–71. [Google Scholar] [CrossRef]
- Wang, C.-B.; Lee, H.-G.; Yeh, T.-F.; Hsu, S.-N.; Chu, K.-S. Thermal characterization of titania-modified alumina-supported palladium and catalytic properties for methane combustion. Thermochim. Acta 2003, 401, 209–216. [Google Scholar] [CrossRef]
- Bond, G.C. The origins of particle size effects in heterogenous catalysis. Surf. Sci. 1985, 156, 966–981. [Google Scholar] [CrossRef]
- Ferrer, V.; Finol, D.; Solano, R.; Moronta, A.; Ramos, M. Reduction of NO by CO using Pd–CeTb and Pd–CeZr catalysts supported on SiO2 and La2O3–Al2O3. J. Environ. Sci. 2015, 27, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M. Etude de Catalyseurs Pd/Au Déposés Sur Oxydes Poreux, TiO2 et Composés Organiques Volatils (COV); Littoral University: Dunkerque, France, 2008. [Google Scholar]
- Koopman, P. Characterization of ruthenium catalysts as studied by temperature programmed reduction. J. Catal. 1981, 69, 172–179. [Google Scholar] [CrossRef]
- Zhao, B.; Ran, R.; Cao, Y.; Wu, X.; Weng, D.; Fan, J.; Wu, X. Insight into the effects of different ageing protocols on Rh/Al2O3 catalyst. Appl. Surf. Sci. 2014, 308, 230–236. [Google Scholar] [CrossRef]
- McCabe, R.; Usmen, R.; Ober, K.; Gandhi, H. The Effect of alumina phase-structure on the dispersion of rhodium/alumina catalysts. J. Catal. 1995, 151, 385–393. [Google Scholar] [CrossRef]
- Goula, M.; Charisiou, N.; Papageridis, K.; Delimitis, A.; Papista, E.; Pachatouridou, E.; Iliopoulou, E.; Marnellos, G.; Konsolakis, M.; Yentekakis, I.V. A comparative study of the H2-assisted selective catalytic reduction of nitric oxide by propene over noble metal (Pt, Pd, Ir)/γ-Al2O3 catalysts. J. Environ. Chem. Eng. 2016, 4, 1629–1641. [Google Scholar] [CrossRef]
- Vicerich, M.A.; Benitez, V.M.; Especel, C.; Epron, F.; Pieck, C. Influence of iridium content on the behavior of Pt-Ir/Al2O3 and Pt-Ir/TiO2 catalysts for selective ring opening of naphthenes. Appl. Catal. A Gen. 2013, 453, 167–174. [Google Scholar] [CrossRef]
- Haneda, M.; Fujitani, T.; Hamada, H. Effect of iridium dispersion on the catalytic activity of Ir/SiO2 for the selective reduction of NO with CO in the presence of O2 and SO2. J. Mol. Catal. A Chem. 2006, 256, 143–148. [Google Scholar] [CrossRef]
- Santos, V.P.; Carabineiro, S.A.C.; Tavares, P.B.; Pereira, M.F.R.; Órfão, J.J.M.; Figueiredo, J.L. Oxidation of CO, ethanol and toluene over TiO2 supported noble metal catalysts. Appl. Catal. B 2010, 99, 198–205. [Google Scholar] [CrossRef]
- Almusaiteer, K.; Chuang, S.S. Isolation of active adsorbates for the NO-CO Reaction on Pd/Al2O3by selective enhancement and selective poisoning. J. Catal. 1998, 180, 161–170. [Google Scholar] [CrossRef]
| Metal | Iridium | Palladium | Platinum | Rhodium | Ruthenium |
|---|---|---|---|---|---|
| precursor used | Ir(acac)3 | Pd(acac)2 | Pt(NO2)2(NO3)2 | Rh(NO3)3 | Ru(acac)3 |
| Sample | SBET (m2·g−1) | Vporous (cm3·g−1) | Average Pore Size (nm) |
|---|---|---|---|
| Alumina | 100 | 0.74 | 31 |
| Catalysts | Coding | Metallic Accessibility (%) | <d> a (nm) | <d> b (nm) |
|---|---|---|---|---|
| 1% Ir/Al2O3 | Ir | 42 | 2.2 | - |
| 1% Pd/Al2O3 | Pd | 40 | 2.4 | 2.9 |
| 1% Pt/Al2O3 | Pt | 40 | 2.3 | 2.2 |
| 1% Rh/Al2O3 | Rh | 41 | 2.2 | 2.8 |
| 1% Ru/Al2O3 | Ru | 28 | 3.8 | 3.8 |
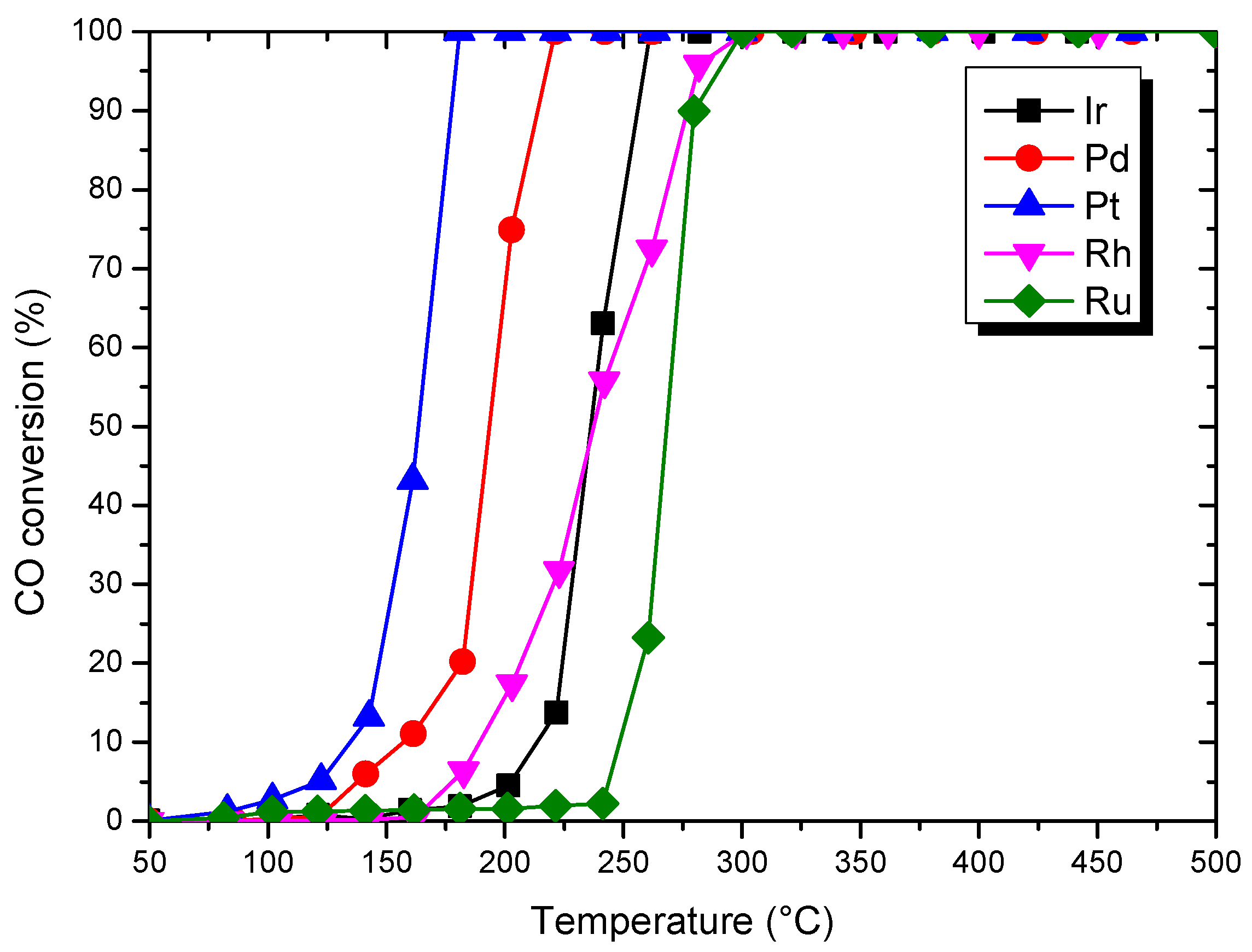
| Catalysts | T50 (°C) | T90 (°C) |
|---|---|---|
| Ir | 236 | 260 |
| Pd | 194 | 212 |
| Pt | 164 | 176 |
| Rh | 236 | 280 |
| Ru | 268 | 280 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akil, J.; Siffert, S.; Laurence, P.-R.; Debecker, D.P.; Devred, F.; Cousin, R.; Poupin, C. Effect of Precious Metals on NO Reduction by CO in Oxidative Conditions. Appl. Sci. 2020, 10, 3042. https://doi.org/10.3390/app10093042
Akil J, Siffert S, Laurence P-R, Debecker DP, Devred F, Cousin R, Poupin C. Effect of Precious Metals on NO Reduction by CO in Oxidative Conditions. Applied Sciences. 2020; 10(9):3042. https://doi.org/10.3390/app10093042
Chicago/Turabian StyleAkil, Joudia, Stéphane Siffert, Pirault-Roy Laurence, Damien P. Debecker, François Devred, Renaud Cousin, and Christophe Poupin. 2020. "Effect of Precious Metals on NO Reduction by CO in Oxidative Conditions" Applied Sciences 10, no. 9: 3042. https://doi.org/10.3390/app10093042
APA StyleAkil, J., Siffert, S., Laurence, P.-R., Debecker, D. P., Devred, F., Cousin, R., & Poupin, C. (2020). Effect of Precious Metals on NO Reduction by CO in Oxidative Conditions. Applied Sciences, 10(9), 3042. https://doi.org/10.3390/app10093042







